Chapter 31 Surgery for Cochlear Implantation
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
There have been remarkable advances in cochlear implantation. Although the first attempt to stimulate the auditory system electrically occurred nearly 2 centuries ago, the development of a cochlear prosthesis to restore hearing to patients with sensorineural hearing loss has happened only over the past 5 decades. The early pioneering work of Simmons, Michaelson, and House provided the stimulus to encourage others, including Bonfai, Chouard, Clark, Eddington, and the Hochmairs.1 The initial acceptance of cochlear implants was slow; safety and efficacy were the concerns of the early investigators, and the greatest champions of the implant were the patients themselves. Time and technology have increased the benefits gained by most patients from their cochlear implants. As a result, cochlear implants have become the most successful prosthesis ever used to attempt to restore a sensory deficit.
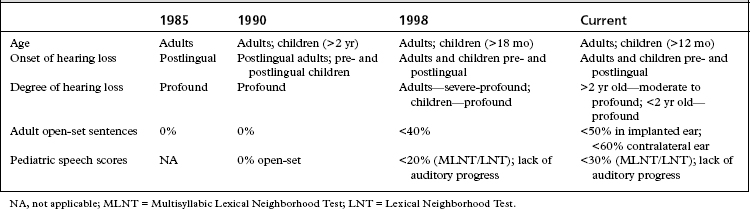
Incremental improvements in technology have resulted in the approval of multiple devices over the years. As performance with cochlear implants has improved over the years, the criteria for cochlear implantation have expanded (Table 31-1). In more challenging situations, bilateral cochlear implantation has been shown to provide additional benefit beyond a single cochlear implant, and is now considered an accepted medical practice outside of research protocols.2 Prospective studies continue to examine the benefits of bilateral cochlear implantation in adults and children.
MEDICAL EVALUATION
Two crucial factors influencing auditory performance after cochlear implantation include age of onset of deafness and duration of profound hearing loss. The ideal adult candidate has profound acquired sensorineural hearing loss. A period of auditory experience adequate for development of normal speech, speech perception, and language offers a significant advantage in learning to use the implant. These postlingually deafened patients represent most adults undergoing cochlear implantation. In these patients, there is a significant correlation between duration of profound hearing loss and performance.3 Patients with prolonged auditory deprivation receive similar auditory information as do other implant patients, but are unable to use the information as effectively; this is thought to be due to the loss of central auditory processing. A few adult implant recipients are congenitally or prelingually deafened, with prolonged auditory deprivation and little to no experience with sound. These patients typically have greater difficulty assimilating the new auditory information, and generally have performed less well than patients with some degree of auditory memory.
Physical Examination
It is important to identify preoperatively any external or middle ear diseases, including perforations of the tympanic membrane, that must be treated before cochlear implantation. For young children, the size of the implant in relation to the size of the child’s skull must be evaluated, and issues involved with skull maturation must be considered. The distance between the cochlear promontory and the mastoid cortex, the approximate sites of the electrode array, and the receiver-stimulator increases about 1.7 cm from birth to adulthood, with one half of the increase occurring during the first 2 years of life.4 The electrodes must be long enough to tolerate the increase in height and width of the skull that occurs with the child’s growth. The accommodation occurs through the gradual straightening of the excess electrode length within the air-containing mastoid cavity.5
Imaging
Preoperative imaging completes the candidacy evaluation process and assists in surgical planning. Choice of imaging modality varies at individual implant centers. High-resolution computed tomography (CT) scanning of the temporal bone and magnetic resonance imaging (MRI) are currently used. CT scanning provides excellent detail of inner ear morphology, the course of the intratemporal facial nerve, and aeration of the mastoid. Limitations of CT include the inability to evaluate retrocochlear pathology and cochlear patency in patients with fibrosis of the cochlea as a precursor to cochlear ossification. The ability to detect an absent cochlear nerve also is limited. MRI addresses these limitations,6 but this imaging modality incurs additional expense and may require general anesthesia in young patients.
Complete agenesis of the cochlea and an abnormal acoustic nerve resulting from congenital malformation, trauma, or surgery are contraindications for cochlear implant placement.7 Cochlear hypoplasia (present in Mondini’s deformity) is not a contraindication for cochlear implantation. Adults and children with incomplete congenital cochlear malformations have received implants successfully.8 Ossification or fibrous occlusion of the cochlea or the round window does not exclude a patient from implantation, but it may influence outcome. Occlusion of the cochlea may lead to partial insertion of the electrode carrier. MRI has become more useful than CT in the evaluation of the membranous inner ear in detecting cochlear fibrosis.
Promontory Evaluation
A few implant teams perform an electric stimulation test at either the promontory or the round window membrane.9 A positive response is a perception of sound on stimulation. Some investigators do not believe that such testing is crucial in the selection of candidates because patients with a negative response, particularly at the promontory, may respond to intracochlear stimulation with an implant. Promontory stimulation may be helpful in cases of suspected cochlear nerve agenesis.
SURGICAL TECHNIQUE
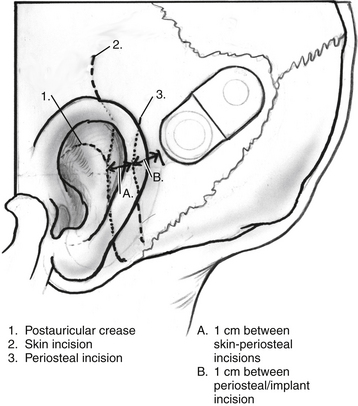
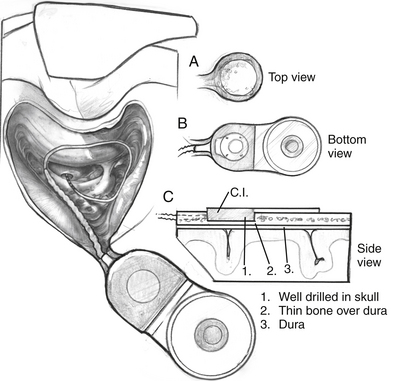
Surgery is performed with the patient under general anesthesia with the use of continuous intraoperative facial nerve monitoring. Perioperative antibiotics are routinely administered before making the initial incision. Many different incisions have been designed to allow placement of the receiver-stimulator (Fig. 31-1). Generally, the skin flap must be large enough to cover the receiver-stimulator completely. The length of the incision and size of the skin flap have been reduced over the years. By reducing incision and flap size, there is less interruption of the vascular supply to the operative field; this seems to correlate with fewer wound and flap complications. Skin and periosteal incisions should overlap by at least 1 cm; the skin incision should be at least 1 cm anterior to the front edge of the receiver-stimulator (Fig. 31-5).
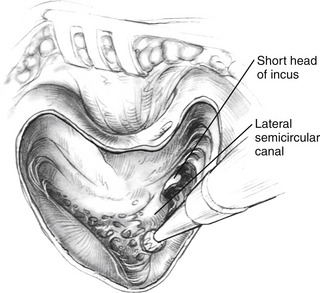
All implant systems use the transmastoid, facial recess approach to the round window and scala tympani. The mastoidectomy is done using conventional burrs and suction-irrigation techniques (Fig. 31-2). In contrast to surgery for chronic otitis media, the superior and posterior mastoid cortical margins are not saucerized. The margins can be undercut to create a bony overhang that stabilizes the coiled electrode within the mastoid cavity. The bone removal extends back to the sigmoid, but retraction of the sigmoid is not required unless it is far forward. Enough of the bone in the attic is removed so that the top of the incus can be clearly seen. The incus should not be dislocated or removed because this method does not increase surgical exposure. The short process of the incus and its buttress are important landmarks in the development of the facial recess. The posterior bony ear canal wall is thinned without exposing the overlying vascular strip tissue. Thinning of the bony ear canal is necessary because in viewing the round window area, the direction of vision is parallel to the external auditory canal.
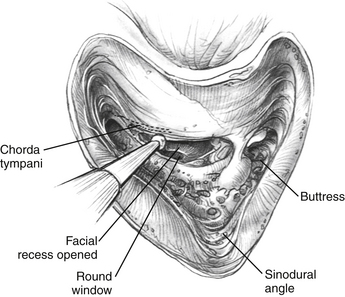
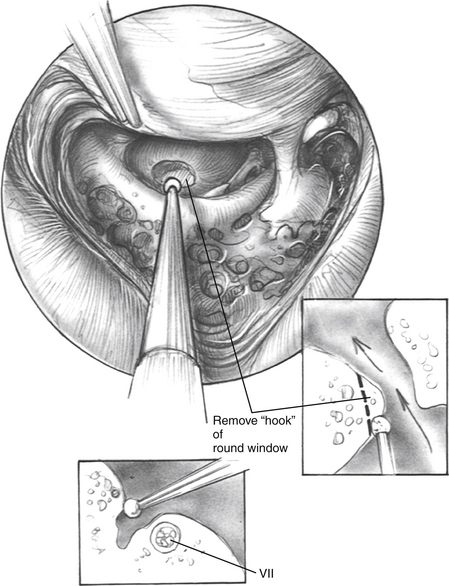
The facial recess is opened (Fig. 31-3). The facial nerve is carefully skeletonized at the mastoid genu to avoid exposure of the nerve sheath. When the facial recess is opened, the lip of the round window niche is usually visible just inferior to the stapedius tendon and oval window. To get a good look at the round window, one must open the facial recess more inferiorly and posteriorly. Usually, removing the chorda tympani is unnecessary for adequate visualization of the round window niche area. If the facial recess is very restricted, the chorda can be removed, but because the chorda enters the middle ear at the level of the annulus, care must be taken not to damage the tympanic membrane.
In some cases, the round window niche and membrane are replaced with new bone growth.10 This condition is more common in patients whose deafness is attributable to meningitis, rather than to other diseases.11 In these cases, the surgeon must drill forward along the basal coil for 4 to 5 mm. Usually, the new bone is white and can be demarcated from the surrounding otic capsule. Following this white plug of bone with the drill usually leads to the patent scala, allowing placement of the electrode array.12 If new bone growth completely obliterates the scala tympani, the surgeon can drill superiorly and possibly enter a patent scala vestibuli.13,14 In cases with complete ossification of the cochlea, the surgeon may choose to perform a canal wall down mastoidectomy and close the ear canal. A trough around the modiolus is created, and the electrode is placed in it.15,16
When drilling the round window niche or attempting to create an opening into the scala tympani through new bone growth, the surgeon must direct the burr anteriorly toward the nose (Fig. 31-4). Drilling superiorly may lead to damage to the basilar membrane and osseous spiral lamina, which may result in the loss of ganglion cells. If the surgeon directs the burr inferiorly, a hypotympanic air cell may be accidentally entered, and the active electrode would be placed improperly into this area. Postoperatively, these cases may fail to stimulate. Temporal bone imaging shows that the active electrode is extracochlear. Revision surgery with placement of the electrode array into the scala tympani remedies this situation. If the surgeon is uncertain of the placement of the electrode, an intraoperative anteroposterior transorbital plain film can be taken to check the electrode position.
COMPLICATIONS
The risks of the implant procedure are the same as the risks for chronic otitis media surgery and include infection, facial paralysis, cerebrospinal fluid drainage, meningitis, and the usual risks of anesthesia. All of these risks are remote in chronic otitis media surgery and have proved to be so in implant surgery as well.14,17 Failure of the incision to heal and associated minor infections are the most common problems associated with implant surgery.18 In a few patients in whom the internal receiver has been placed too close to the wound’s edge, or in patients in whom the flap over the internal receiver is too thin, the internal receiver has extruded. As noted earlier, at least 1 cm must be maintained between the incision and the edge of the internal receiver. The ideal thickness for the flap is 6 to 7 mm. Although too thin a flap may necrose, too thick a flap may diminish device performance by decreasing the transcutaneous transmission of information.
Problems with the facial nerve can occur as the result of surgery and stimulation.19 Good surgical landmarks must be maintained when the facial recess is created. Although the facial nerve is identified, it usually does not have to be uncovered with the facial recess approach. Adequate irrigation at the facial recess must be maintained to help dissipate the heat generated by the turning shaft of the diamond burr used to create the exposure of the round window and entrance into the scala tympani, especially in drill-out cases. To help alleviate the problem of the drill shaft turning against the facial nerve, a drill such as the Skeeter® drill manufactured by Medtronic, Inc., Jacksonville, FL could be used. The width of the drill bit shaft is smaller, and a sleeve around most of the length of the shaft protects the surrounding tissues as well. In cases in which the heat from the rotating burr shaft has led to facial nerve problems, the paralysis has been temporary, and has resolved over several weeks to several months. Facial nerve paralysis has occurred in patients with congenital malformation of the cochlea and in patients who have undergone radical mastoidectomy many years before the implant procedure. In these cases, the facial nerve, either because it is congenitally displaced or because it is exposed by previous surgery, is at greater risk.20 The use of facial nerve monitoring may decrease the chance of facial nerve trauma.
Meningitis is serious complication that is more frequent in patients with cochlear implants. Children with cochlear implants are at an elevated risk for meningitis compared with the general population.21,22 In addition, children implanted with a positioner distributed with Advanced Bionics Corporation devices before July 2002 are at greater risk than children without positioners. Streptococcus pneumoniae is the primary bacterial isolate in this patient group. In light of this increased risk, it is strongly advised that all patients undergoing cochlear implantation be vaccinated against S. pneumoniae in accordance with current Centers for Disease Control and Prevention guidelines (Table 31-2). Cochlear implant recipients, along with their families, educators, daycare providers, and health care providers, need to be aware of the signs of meningitis. Otitis media should be promptly diagnosed and treated with the appropriate antibiotics.
TABLE 31-2 Centers for Disease Control and Prevention Guidelines for Immunization in Patients with Cochlear Implants
| Age (yr) | Vaccine∗ |
|---|---|
| STREPTOCOCCUS PNEUMONIAE | |
| <2 | Prevnar |
| 2-5 | Prevnar and Pneumovax |
| >5 | Pneumovax |
| HAEMOPHILUS INFLUENZAE | |
| <5 | Hib |
∗ Prevnar, 7-valent pneumococcal conjugate vaccine (PCV-7); Pneumovax, 23-valent pneumococcal polysaccharide vaccine (PPV-23); Hib, Haemophilus influenzae type B conjugate.
REVISION COCHLEAR IMPLANTATION
Revision surgery accounts for approximately 5% to 10% of adult and pediatric cochlear implant surgeries in many centers. The reasons for revision fall into the above-listed categories. Performance has been shown to be good after revision surgery, including cases that require replacement of the cochlear implant.23
BILATERAL COCHLEAR IMPLANTATION
Most recipients of cochlear implants have undergone unilateral cochlear implantation. Because there are definite advantages to binaural acoustic hearing, one would expect to find advantage with bilateral cochlear implantation. This has been found to be the case in numerous studies.24–27 Patients undergoing bilateral cochlear implantation have shown improved sound localization and speech detection in noise. Binaural summation, or the ability of two ears to hear the sound that a single ear cannot, has also been shown in most binaural cochlear implant recipients.
COMBINED ELECTROACOUSTIC STIMULATION
Cochlear implantation has been very successful in providing meaningful auditory information to patients who meet the current cochlear implant criteria. Criteria for cochlear implantation continue to expand as the performance of patients with cochlear implants improves. As patients with more residual hearing have been implanted, it has been found that hearing may be preserved in a many of these patients. This has led to the development of a new model of cochlear stimulation. Shorter cochlear implant electrodes are placed atraumatically into the basal turn of the cochlea to provide electric stimulation for high-frequency sound representation. When low-frequency hearing is preserved, a hearing aid is used to provide acoustic information to these frequencies. This strategy has been termed hybrid stimulation or combined electroacoustic stimulation (EAS).28 EAS has been shown to provide improvement of recognition of speech in noise and improved appreciation of music.29 Both of these factors were linked to the ability to distinguish fine pitch differences because of preserved residual low-frequency acoustic hearing.
Appropriate surgical technique must be used to maximize the potential for hearing preservation. Implantation for EAS must be treated as carefully as a stapes procedure where preservation of hearing is essential. The scala tympani must be accessed through an appropriately placed cochleostomy or round window insertion to avoid damage to the basilar membrane and stria vascularis. A slow rotation diamond burr should be used for the cochleostomy. Contact between the burr and the endosteum of the cochlea should be limited because the energy transmitted to the cochlea is similar to drill contact with the incus.30 The electrode must be oriented appropriately in relation to the modiolus. Aspiration of perilymph must be avoided, and a gentle insertion technique must be used to avoid rapid changes to cochlear fluids during insertion.
Electrode design is also essential for the EAS strategy. The ideal electrode would provide extensive coverage of the cochlea and produce little to no shift in hearing thresholds. Shorter electrodes with fewer contacts provide less auditory benefit than longer electrodes.29,31 Generally, the longer the electrode, the more potential there is for trauma during insertion with resultant hearing loss. Optimally, the electrode should be long enough to provide similar benefit as a standard cochlear implant if a total hearing loss occurs as a result of implantation. This ideal electrode design has not yet been established. A single one-size-fits-all electrode may not be appropriate for all patterns and degrees of hearing loss.
1. Luxford W., Brackmann D.E. The history of cochlear implants. In: Gray R.F., editor. Cochlear Implants. London: Croom Helm; 1985:1-26.
2. Balkany T., Hodges A., Telischi F., et al. William House Cochlear Implant Study Group: Position statement on bilateral cochlear implantation. Otol Neurotol. 2008;29:107-108.
3. Dowell R.C., Mecklenburg D.J., Clark G.M. Speech recognition for 40 patients receiving multichannel cochlear implants. Arch Otolaryngol Head Neck Surg. 1986;112:1054-1059.
4. O’Donoghue G.M., Jackler R.K., Jenkins W.M., et al. Cochlear implantation in children: The problem of head growth. Otolaryngol Head Neck Surg. 1986;94:78-81.
5. Marks D.R., Jackler R.K., Bates G.J., et al. Pediatric cochlear implantation: Strategies to accommodate for head growth. Otolaryngol Head Neck Surg. 1989;101:38-46.
6. Adunka O.F., Jewells V., Buchman C.A. Value of computed tomography in the evaluation of children with cochlear nerve deficiency. Otol Neurotol. 2007;28:597-604.
7. Shelton C., Luxford W.M., Tonokawa L.L., et al. The narrow internal auditory canal in children: A contraindication to cochlear implants. Otolaryngol Head Neck Surg. 1989;100:227-231.
8. Slattery W.H.3rd, Luxford W.M. Cochlear implantation in the congenital malformed cochlea. Laryngoscope. 1995;105:1184-1187.
9. Waltzman S.B., Cohen N.L., Shapiro W.H., et al. The prognostic value of round window electrical stimulation in cochlear implant patients. Otolaryngol Head Neck Surg. 1990;103:102-106.
10. Green J.D.Jr., Marion M.S., Hinojosa R. Labyrinthitis ossificans: Histopathologic consideration for cochlear implantation. Otolaryngol Head Neck Surg. 1991;104:320-326.
11. Novak M.A., Fifer R.C., Barkmeier J.C., et al. Labyrinthine ossification after meningitis: Its implications for cochlear implantation. Otolaryngol Head Neck Surg. 1990;103:351-356.
12. Balkany T., Gantz B., Nadol J.B.Jr. Multichannel cochlear implants in partially ossified cochleas. Ann Otol Rhinol Laryngol Suppl. 1988;135:3-7.
13. Steenerson R.L., Gary L.B. Multichannel cochlear implantation in children with cochlear ossification. Am J Otol. 1999;20:442-444.
14. Webb R.L., Lehnhardt E., Clark G.M., et al. Surgical complications with the cochlear multiple-channel intracochlear implant: Experience at Hannover and Melbourne. Ann Otol Rhinol Laryngol. 1991;100:131-136.
15. Gantz B.J., McCabe B.F., Tyler R.S. Use of multichannel cochlear implants in obstructed and obliterated cochleas. Otolaryngol Head Neck Surg. 1988;98:72-81.
16. Lambert P.R., Ruth R.A., Hodges A.V. Multichannel cochlear implant and electrically evoked auditory brainstem responses in a child with labyrinthitis ossificans. Laryngoscope. 1991;101:14-19.
17. Cohen N.L., Hoffman R.A. Complications of cochlear implant surgery in adults and children. Ann Otol Rhinol Laryngol. 1991;100:708-711.
18. Haberkamp T.J., Schwaber M.K. Management of flap necrosis in cochlear implantation. Ann Otol Rhinol Laryngol. 1992;101:38-41.
19. Niparko J.K., Oviatt D.L., Coker N.J., et al. Facial nerve stimulation with cochlear implantation. VA Cooperative Study Group on Cochlear Implantation. Otolaryngol Head Neck Surg. 1991;104:826-830.
20. House J.R.3rd, Luxford W.M. Facial nerve injury in cochlear implantation. Otolaryngol Head Neck Surg. 1993;109:1078-1082.
21. Reefhuis J., Honein M.A., Whitney C.G., et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:435-445.
22. Biernath K.R., Reefhuis J., Whitney C.G., et al. Bacterial meningitis among children with cochlear implants beyond 24 months after implantation. Pediatrics. 2006;117:284-289.
23. Cullen R.D., Fayad J.N., Luxford W.M., et al. Revision cochlear implant surgery in children. Otol Neurotol. 2008;29:214-220.
24. Litovsky R., Parkinson A., Arcaroli J., et al. Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study. Ear Hear. 2006;27:714-731.
25. Litovsky R.Y., Johnstone P.M., Godar S.P. Benefits of bilateral cochlear implants and/or hearing aids in children. Int J Audiol. 2006;45(Suppl 1):S78-S91.
26. Litovsky R.Y., Johnstone P.M., Godar S., et al. Bilateral cochlear implants in children: Localization acuity measured with minimum audible angle. Ear Hear. 2006;27:43-59.
27. Peters B.R., Litovsky R., Parkinson A., et al. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol. 2007;28:649-657.
28. Soriano A.F., Helfrich B., Chan D.C., et al. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999;59:6178-6184.
29. Gantz B.J., Turner C., Gfeller K.E. Acoustic plus electric speech processing: Preliminary results of a multicenter clinical trial of the Iowa/Nucleus Hybrid implant. Audiol Neurotol. 2006;11(Suppl 1):63-68.
30. Pau H.W., Just T., Bornitz M., et al. Noise exposure of the inner ear during drilling a cochleostomy for cochlear implantation. Laryngoscope. 2007;117:535-540.
31. Gantz B.J., Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus Hybrid implant. Acta Otolaryngol. 2004;124:344-347.