Surgery for cancer of the stomach
Modes of spread and areas of potential failure after gastric cancer surgery
In addition, it should be noted that the patterns of failure after gastric cancer surgery have been variously reported using similar classifications but with different definitions. An example is shown in Table 7.1: hepatic and lymph node recurrences are categorised as distant and local failure respectively in the Dutch D1/D2 trial,1,2 but as regional failure in the Intergroup 0116 study.3
Table 7.1
Different definitions of patterns of failure in the Dutch and American trials on gastric cancer surgery
| Pattern of failure | Dutch D1/D2 trial1,2 | US Intergroup 01163 |
| Local | Gastric bed, anastomosis, regional lymph nodes | Gastric bed, anastomosis, residual stomach |
| Regional | Peritoneal carcinomatosa | Liver, lymph nodes, peritoneal carcinomatosa |
| Distant | Liver, lung, ovary and other organs | Outside the peritoneal cavity |
Metastatic pathway
Lymphatic spread is the most common mode of dissemination in gastric cancer. Lymph node metastasis is histologically proven in 10% of T1 tumours, and the rate increases as the invasion deepens, up to 80% of T4a tumours.4,5
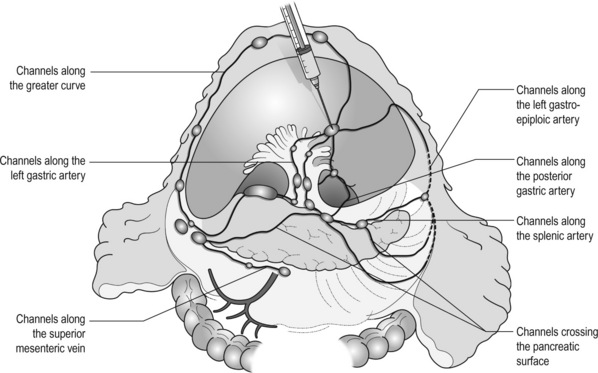
The lymphatic drainage system from the stomach has been well demonstrated in lymphography studies (Fig. 7.1). Unlike other parts of the digestive tract, the stomach has multidimensional mesenteries that contain dense lymphatic networks. Cancer cells can flow out of the stomach through any of these routes and by way of the nearby perigastric nodes, to reach the nodes around the coeliac artery. They then enter the para-aortic nodes and finally flow into the thoracic duct and systemic circulation. Systemic metastasis can occur via this route. In particular, bone marrow carcinomatosis occurs most frequently in cases with extensive nodal disease.6,7
The stomach has the largest number of ‘regional lymph nodes’ of any organ in the human body. After a total gastrectomy with D2 lymphadenectomy, more than 40 lymph nodes can usually be collected with careful retrieval. Of the malignant tumours listed in the UICC/TNM classification,8 stomach cancer requires the largest number of nodes to be removed as a minimal requirement to allow a pN0 diagnosis (16 nodes) and the largest number of positive nodes for the highest N category (pN3b, 16 or more positive nodes).This suggests that lymphatic metastasis from gastric cancer may remain in the dense lymphatic filters for some time and that patients with nodal metastasis can still be cured by adequate dissection.
Peritoneal spread
Peritoneal metastasis is the most common type of failure after radical surgery for gastric cancer.9 Once the tumour penetrates the serosal surface (T4a), cancer cells may be scattered in the peritoneal space. They can be implanted in the gastric bed or any part of the peritoneal cavity and subsequently cause intestinal obstruction or ascites. Peritoneal metastasis is much more common in diffuse-type cancers than the intestinal type10 and later causes peritonitis carcinomatosa, a characteristic recurrent pattern of gastric cancer, which is relatively uncommon in colorectal adenocarcinomas that are mostly of the intestinal type.
Peritoneal lavage cytology is a sensitive test for this metastasis. Almost all patients with positive cytology subsequently develop peritoneal recurrence even after macroscopically curative surgery. In the UICC/TNM 7th edition,8 positive cytology (‘cy+’) has been included in the definition of pM1.
Peritoneal metastasis is refractory to systemic chemotherapy. Intraperitoneal chemotherapy with or without hyperthermia is being vigorously tested in various centres. Some promising results have been reported,11 but the evidence is not yet compelling.
Haematogenous spread
Liver metastasis is relatively uncommon at the time of diagnosis of gastric cancer, but is commonly seen as a part of systemic failure. In the 15-year follow-up report of the Dutch D1/D2 trial, liver metastasis was found, either as the sole site or with other sites in 102 of 319 deaths with recurrence.12 It occurs predominantly in intestinal-type tumours. Unlike in colorectal cancer, liver metastasis from gastric cancer is usually multiple and associated with other modes of spread. Resection is rarely indicated and, even if it is carried out, the prognosis is poor.
Direct extension
Gastric cancer penetrating the serosa sometimes extends to the adjacent organs or structures. When the operation is potentially curative, these may be excised en bloc with the stomach. It is of note that, in a considerable proportion of apparent T4b cases, pathological assessment shows only inflammatory adhesion without direct tumour invasion.13
Intraoperative spillage
Even serosa-negative tumours can recur in the peritoneal cavity after surgery. These cases are usually associated with lymph node metastasis. A possible explanation for this is that during lymph node dissection lymphatic channels were broken and cancer cells in the lymph nodes spilled out. This was proven in a unique study from Korea,14 though it has not been confirmed whether these spilled cells are implanted and grow.
These intraoperative spillages of cancer cells might be prevented by careful non-touch isolation techniques and/or by use of clips or vessel-sealing devices. However, the simplest means to prevent cancer cell implantation during surgery will be peritoneal wash with a large volume of saline before abdominal closure. A small-scale randomised study showed a significant survival benefit of extensive intraoperative peritoneal lavage (EIPL) in gastric cancer patients with positive cytology.15
The concept of radical gastric cancer surgery
Gastric cancer surgery in Japan
The incidence of gastric cancer in Japan is among the highest in the world. Approximately 100 000 new cases are diagnosed every year, accounting for 11% of all cases in the world.16 The age- standardised incidence, however, has been rapidly decreasing as in other countries in the world, probably due to the decreasing infection of Helicobacter pylori. The peak incidence was in the 1950s (male 71, female 37 per 105 population), the time when the mass screening programme was planned and the basic style of radical D2 gastrectomy was proposed (for reference, the incidences in 2010 were 12.4 and 7.1 respectively).
Development of gastric cancer surgery in the West
The Japanese documentation system and excellent treatment results influenced the Western concept of radical surgery for gastric cancer. Some surgeons visited Japanese institutions to convince themselves of the feasibility and efficacy of the technique and have successfully reproduced the results in the West.17,18 However, most non-specialist surgeons could not overcome their scepticism and were reluctant to practise this aggressive surgery in their patients. An important obstacle is the difficulty in directly comparing the results between Japan and the West due to the following two issues.
Different disease hypotheses
A hypothesis that gastric cancer in the West may be a different disease to that in Japan prevails and prevents positive discussion to advance optimal treatment for gastric cancer patients on a global level. In the studies biologically analysing and comparing surgical specimens, no evidence has been shown to support the hypothesis.19,20 The following are the currently discussed differences.
Proximal location: It has been repeatedly highlighted that Western gastric cancers are predominantly located in the proximal stomach while Japanese tumours are found mostly in the distal stomach. This might suggest that these are different diseases. However, this needs careful consideration.
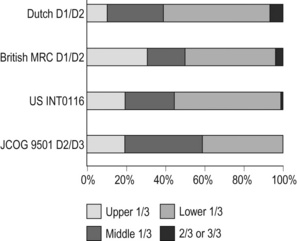
Adenocarcinoma of the lower oesophagus and the oesophagogastric junction is one of the most rapidly increasing malignant tumours in the West, especially among white males.21 This trend, together with the rapid decrease of distal gastric cancers, makes it plausible that Western gastric cancer arises mostly in the proximal stomach. However, the lower oesophageal adenocarcinoma is a new, distinct disease with a different aetiology and contrasting patient backgrounds,22 and therefore should be considered separately from ‘classical’ gastric cancer. In the three large-scale Western surgical trials, the Dutch D1/D2,1 the British MRC D1/D223 and United States INT0116 studies,3 the proportion of proximal third tumours was 10.3%, 30.5% and 19.5% respectively, and was not significantly different from that (19.1%) in the Japanese D2/D3 study24 (Fig. 7.2). This suggests that, as far as surgically targeted gastric cancers are concerned, tumour location is not largely different between the West and Japan. The apparent predominance of proximal tumours in the West may be a simple reflection of the mixture of different diseases, i.e. increasing oesophageal and decreasing gastric adenocarcinomas.
Patient factor: Western patients with gastric cancer are on average 10 years older, much more likely to be obese and more frequently have comorbidities, especially of cardiovascular diseases, than their Japanese counterparts.25 Although this does not mean that the disease is different, it certainly affects surgical process and outcomes. In particular, obesity hampers the completion of extended lymphadenectomy for gastric cancer, even in specialist Japanese centres. It has been shown to be an independent risk factor for postoperative morbidity.26
Role of radical surgery in Western practice
Due to the decreased incidence and the technically demanding therapeutic requirements, gastric cancer in the West is today considered as a disease that should be treated in specialist centres. Several studies have shown relationships between the hospital/surgeon volume of gastric cancer treatment and operative mortality.27 Given the accelerated ‘proximal shift’ of the disease and the increasing surgical risks in Western patients, the trend of centralisation will further progress.
Principles of radical gastric cancer surgery
Resection margins
Proximal resection margin is the main determinant in selecting a total or distal gastrectomy. During surgery for T2 or deeper tumours, the resection line should be determined with a sufficient margin from the palpable edge of the tumour. A 5-cm margin has traditionally been recommended.30 In some guidelines, even 8 cm is recommended for diffuse-type tumours,31 but this would necessitate most tumours of the gastric body requiring a total gastrectomy or oesophagogastrectomy.
Type of gastrectomy
Common types of gastrectomy for gastric cancer are as follows.
Total gastrectomy: This involves removal of the whole stomach including the cardia (oesophagogastric junction) and the pylorus. It is indicated for tumours arising at or invading the proximal stomach.
Distal (subtotal) gastrectomy: This involves removal of the stomach including the pylorus but preserving the cardia. Two-thirds or more of the stomach is usually removed for gastric cancer. It is indicated for middle or lower third tumours with sufficient resection margins mentioned above.
Proximal gastrectomy: This involves removal of the stomach including the cardia but preserving the pylorus. It is indicated for proximal tumours with or without oesophageal invasion, where more than half of the distal stomach can be preserved.
Other resections for T1 tumours: Pylorus-preserving gastrectomy (PPG) is indicated for T1 tumours in the gastric body having negligible possibility of metastasis to the peripyloric lymph nodes. About a 3-cm pyloric cuff with the right gastric artery is preserved.
Total gastrectomy ‘de principe’ for distal cancers: Some European surgeons have argued that all cancers of the stomach, even those in the distal third, should be treated by total gastrectomy. This principle is based on the experience of frequent involvement of proximal resection margin and consequent anastomotic local recurrence. Theoretically, total gastrectomy ensures more certain negative margins and sufficient lymphadenectomy. In addition, the possible occurrence of multicentric cancer in the gastric stump can be prevented. On the other hand, total gastrectomy is associated with a higher operative morbidity and mortality, increased risk of long-term nutritional problems and impaired quality of life as compared to distal gastrectomy.
The policy of total gastrectomy de principe should be abandoned for the following reasons:
1. Provided the rules on safe margins of resection listed above are adhered to, a positive proximal resection margin is rare. If the margins are still positive, this usually indicates an aggressive and extensive malignancy and the resection line involvement will not be a major determinant of prognosis.
2. The lymph nodes that can be removed only by total gastrectomy, station nos. 2 (left cardia), 4sa (upper greater curve), 10 (splenic hilum) and 11d (distal splenic artery), are seldom involved in distal gastric cancers. If they are involved, again this indicates an aggressive malignancy and extended surgery would not alter survival outcome.
3. The incidence of second primary cancer in the gastric stump is low. Long-term surveillance by endoscopy may detect a new lesion that can be removed by endoscopic submucosal dissection.
Lymphadenectomy
Lymph node metastasis is the most common mode of spread in gastric cancer. Histological nodal metastasis has been proven in 80% of T4a/T4b tumours, and even T1 tumours have a 10% probability of lymph node metastasis (T1a 3%, T1b 18%).4,5 Unlike hepatic and other distant metastases, lymph node metastasis from gastric cancer can be surgically removed for potential cure as long as it is confined to the regional area.The optimal extent of lymphadenectomy, however, has been controversial.
Lymph node groups in the former Japanese classifications
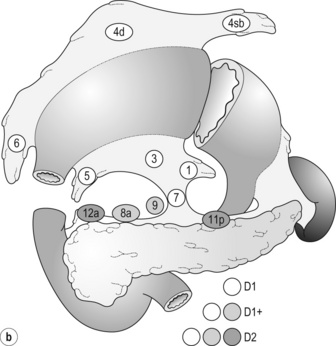
Japanese surgeons and pathologists have extensively investigated the distribution of lymph node metastasis. They have recorded it using standardised anatomical station numbers (Fig. 7.3). They then classified the stations into three groups, basically according to the incidence of metastasis (N groups 1–3). As the pattern of lymph node metastasis varies with the location of the primary tumour, N groups 1–3 were separately defined depending on the primary tumour location. These numbers of nodal groups were also used to express the grade of nodal disease (N1–3) and the extent of lymphadenectomy (D1–3), e.g. cancer with metastasis to a node in the second group was designated as N2, and complete dissection of up to the second group nodes was defined as D2.
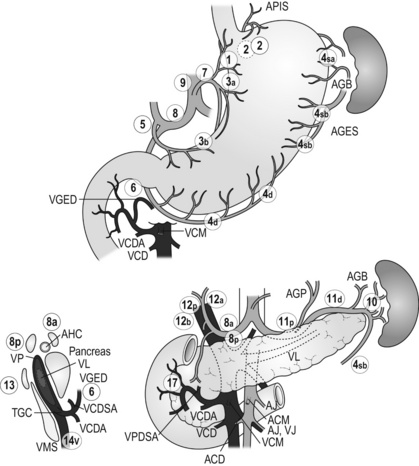
Figure 7.3 Station numbers of lymph nodes around the stomach. Modified from the Japanese classification of gastric carcinoma, 3rd English edn.39 ACM, A. colica media; AGB, Aa. gastrica breves; AGES, A. gastroepiploica sinistra; AHC, A. hepatica communis; AJ, A. jejunalis; APIS, A. phrenic inferior sinistra; TGC, truncus gastrocolicus; VCD, V. colica dextra; VCDA, V. colica dextra accessoria; VCM: V. colica media; VGED, V. vastroepiploica dextra; VJ:V. jejunalis; VL, V. lienalis; VMS, V. mesenterica superior; VP, V. portae; VPDSA, V. pancreaticoduodenalis inferior anterior.
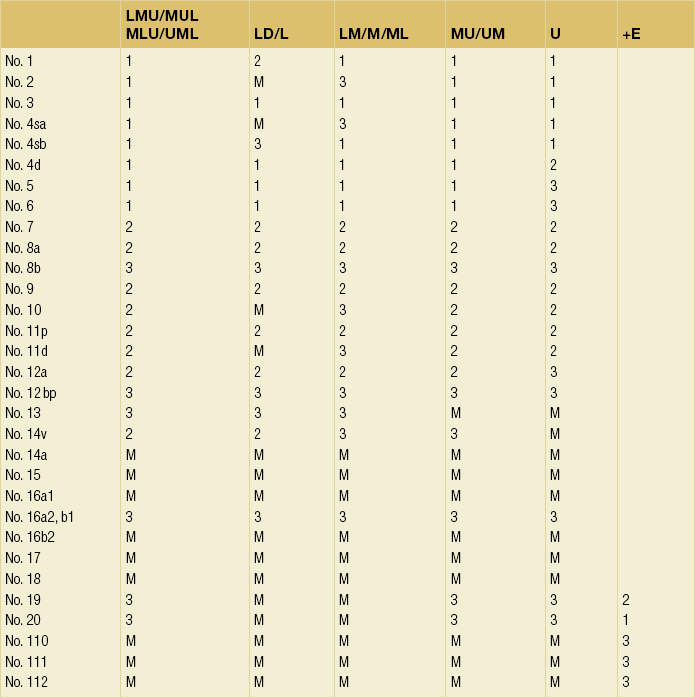
Since its first edition published in 1962, the Japanese Classification of Gastric Carcinoma (JCGC) has undergone periodic revisions, and each time the definitions of the lymph node groups have been slightly modified. The Dutch and MRC D1/D2 trials were conducted using the N and D (‘R’ at that time) definitions of the 11th edition of the JCGC34 (Table 7.2), while the Taipei D1/D3 trial35 and the Japanese D2/D3 trial24 used the 12th edition,36 in which the lymph nodes were grouped from N1 to N4. In the 13th edition,37 the nodal grouping was completed, with four groups (N1–3 and ‘M’) in five categories of the primary tumour location (Table 7.3). This definition was based on the ‘dissection efficiency index’ of each lymph node station,38 calculated using the incidence of metastasis and survival data of a large number of patients. As compared to the 11th edition, D2 lymphadenectomy defined in the 13th edition required more extensive dissection, e.g. for distal third tumours, station nos. 11p, 12a and 14v were included as N2 nodes.
Table 7.2
Lymph node groups used in the Dutch and MRC D1/D2 trials (Japanese classification, 11th edn)

A, lower third; M, middle third; C, upper third.
*Resection or non-resection of these nodes does not affect the D number.
†These nodes should be excised if the primary tumour site is the MC. If the primary tumour site is MA or M, removal is optional.
‡In proximal gastrectomy, non-resection of these nodes does not affect the D number.
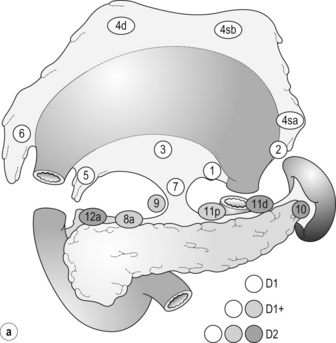
Outside Japan, it is widely believed that Japanese N1 nodes are perigastric and N2 nodes are those along the coeliac artery and its branches. Although this expression roughly reflects the nodal groups, it is apparently incorrect in terms of the original concept of grouping based on the primary tumour location. The misunderstanding seems to be due to the over-complicated definitions of the JCGC. In the latest 14th edition (third English edition39), the traditional nodal grouping system has been abandoned, and the simplified ‘D’ has been defined according to the type of gastrectomy (Fig. 7.4).
New definition of lymphadenectomy
The new ‘D’ definitions in the Japanese Treatment Guidelines32 are simple, practical and mostly compatible with those in the 13th edition, with only some exceptions. D1, D1 + and D2 (D3 is no longer included) are defined for the two major types of gastrectomy, total and distal, regardless of the tumour location. It should be noted that the lymph nodes along the left gastric artery (no. 7), which used to be classified as N2 for tumours in any location, are now included in the D1 category for any type of gastrectomy. This is based not only on the previously mentioned efficacy index analysis, but also on the view that surgery for gastric carcinoma should as a minimum include the division of the left gastric artery at its origin.
D2 lymphadenectomy – evidence
In Japan, the benefits of D2 over less extensive dissections have never been tested in a randomised study. Instead, D2 was compared with more extensive surgery, D2 plus para-aortic nodal dissection (PAND), in a well-designed, multicentre randomised controlled trial (RCT).24 This confirmed that D2 and D2 + PAND were performed with low operative mortality (0.8%) by specialist surgeons,40 but failed to show a survival benefit of PAND. They have abandoned this super-extended lymphadenectomy as a means of prophylaxis, but they still never consider that D2 may not be superior to D1. A single institutional RCT in Taipei showed a significant survival benefit of D2/3 over D1,35 and this is so far the only RCT that showed superiority of extended lymphadenectomy for gastric cancer.
In these trials, total gastrectomy in the D2 arm was performed with pancreatosplenectomy, which caused high morbidity and mortality. Despite these negative results for D2, the Dutch group continued the follow-up of the patients for 15 years and finally published remarkable results.12 They compared the recurrence of gastric cancer in both arms on the basis of autopsy findings and found a significantly lower rate of gastric cancer death in D2 than in D1 patients. They concluded the study by stating that D2 should be performed as potentially curative surgery for gastric cancer.
Number of lymph nodes and extent of lymphadenectomy
In the Western literature, the definition of extent of lymphadenectomy for gastric cancer is often ambiguous. The number of retrieved lymph nodes is sometimes used as a surrogate for ‘D’, e.g. extended lymphadenectomy means retrieval of 25 or more nodes.41 This is a useful method to retrospectively assess the volume of lymphadenectomy in a gastrectomy where anatomical information of dissected lymph nodes is unavailable.
1. Who picked up the nodes from which condition of the specimen for what goal. More nodes are retrieved when a surgeon tries to pick up as many nodes as possible in a fresh specimen than when a pathologist picks up swollen nodes from a formalin-fixed specimen up to the minimal requirement number.
2. Disease stage. In advanced disease with multiple lymph node metastases, the nodes are hard and easily recognised, even if they are small.
3. Patient factor. In obese patients, the lymph nodes are not easily recognised.
Splenectomy
Proximal gastric cancer may metastasise to the splenic hilar nodes (no. 10) via the gastrosplenic ligament (no. 4sa) and/or the left gastroepiploic lymphatics (no. 4sb). The incidence of no. 10 metastasis increases up to 25–30% when the tumour invades the greater curvature of the upper gastric body.38 These can be completely cleared by splenectomy, and up to 25% of the patients having positive no. 10 nodes survive more than 5 years. Total gastrectomy with splenectomy can be performed by specialist surgeons without increasing mortality.40 In this situation the author advocates gastrectomy with splenectomy.
Although a number of observational studies have demonstrated a lack of survival benefit or even a negative prognostic effect of splenectomy,43,44 these are all heavily biased, retrospective comparisons and cannot advocate spleen preservation. Medium-sized RCTs were conducted in Chile46 (N = 187) and Korea47 (N = 207) to compare total gastrectomy (TG) and TG + splenectomy (TGS). In both studies, TGS did not increase operative mortality by experienced surgeons, and although the 5-year survival rate of TGS was higher than that of TG, the difference was not statistically significant. They concluded that splenectomy was not justified. These are negative studies, but are still unconvincing.
In a multicentre RCT conducted in Japan,48 503 patients with proximal gastric cancer were randomised to receive TG or TGS during a curative operation, and are currently being followed up until the final survival analysis with complete 5-year results, which is scheduled in 2014. The TGS showed higher operative morbidity (23.6%) than TG (16.7%), but similar mortality (0.4% vs. 0.8%).49
Distal pancreatectomy
Distal pancreatectomy and splenectomy (DP + S) used to be a part of D2 gastrectomy for proximal gastric cancer regardless of the presence or absence of pancreatic invasion of the tumour, and actually was performed in the Dutch and British D1/D2 trials.1,23 The aim of the routine use of this aggressive procedure was complete dissection of the lymph nodes along the splenic artery (nos. 11p and 11d) and those in the splenic hilum (no. 10).
It is of note that pathological assessment in apparent T4b cases shows that the adhesion to the other organ is often inflammatory rather than neoplastic.13 In order to avoid unnecessary DP + S in ambiguous cases, it may be worthwhile surgically separating the adhesion without DP + S, paying special attention not to injure the pancreatic parenchyma.
Extended resections
En bloc resection of involved adjacent organs
When a distal tumour invades the pancreatic head or extends to the duodenum for a long distance intramurally, a pancreatoduodenectomy may enable en bloc tumour resection. However, this operation is rarely indicated because such tumours are frequently associated with other non-curative factors such as peritoneal disease. Although some case series from high-volume centres suggest survival benefit in R0 resection, the selection criteria are difficult to define.52
Extended lymphadenectomy
An Italian group of surgeons are still performing this surgery, aimed at improving the prognosis of patients.53
The retropancreatic lymph nodes (no. 13) are not regional nodes of gastric cancer and the prognosis of patients with positive no. 13 nodes is extremely poor. However, for distal tumours invading the duodenum, no. 13 nodes are considered as regional nodes according to the TNM rules, and indeed some patients with a pyloric cancer invading the duodenum survive after dissection of positive no. 13 nodes.54
Resection of liver metastases
Unlike for colorectal cancer, liver resection for gastric cancer is rarely indicated. In the literature, only some case series from Japanese high-volume centres suggest possible survival benefit in selected cases.55,56 In Koga et al.’s study,56 for example, of 5520 patients who underwent gastric cancer surgery during a 20-year period, 121 (2.2%) had synchronous liver metastases and 126 (2.3%) developed metachronous ones, and only 42 patients underwent liver resection, of whom eight had survived more than 5 years at the time of analysis. In these reports, the authors’ proposals for selection criteria of liver resection are not consistent. They mostly agree that liver resection should be considered for solitary liver tumours without other non-curative factors.
Technique of gastric resection with D2 lymphadenectomy
For a proximal gastric cancer invading the oesophagus, either transhiatal (TH) or left thoracoabdominal (LTA) approach is selected. LTA provides excellent exposure of the paracardiac area and lower mediastinum, but is associated with an increased morbidity. A Japanese randomised trial compared TH and LTA for Siewert type 2 and 3 gastric cancer invading the oesophagus within 3 cm and showed no survival improvement but increased morbidity in LTA approach.57
Procedure of D2 lymphadenectomy
Kocherisation: Mobilisation of the duodenum facilitates a safe and smooth procedure for the subsequent infrapyloric lymphadenectomy. The assistant should hold the descending portion of the duodenum to stretch the parietal peritoneum. The peritoneum close to the duodenum should be incised and the incision extended along the duodenum. The assistant should then ‘roll up’ the duodenum and proceed to the back of the pancreatic head, staying close to the posterior pancreatic fascia, and mobilise the pancreatic head. The para-aortic area should be palpated and, if suspicious nodes exist, they should be sampled.
Omentectomy: Though omentectomy is not necessarily a part of D2 dissection, it is usually performed for T3/T4 tumours to remove possible tumour spread into the omenta. The omentum is removed from the right side of the transverse colon and the duodenum. It is then dissected along the transverse colon toward the lower pole of the spleen.
Division of left gastroepiploic vessels: At the lower splenic hilum and the pancreatic tail, the left gastroepiploic artery (LGEA) arises from the end of the splenic artery, sometimes as a branch of the lower polar splenic artery. The LGEA and the vein of the same name should be ligated and then cut. As the lymph nodes along the LGEA (no. 4sb) are rarely metastatic from distal gastric tumours, the dissection does not have to include the trunk of this artery. However, tumours in the gastric body, especially those located on the greater curvature, may metastasise to the splenic hilar nodes (no. 10) via no. 4sb. The LGEA should be dissected at the origin in these cases.
Infrapyloric node dissection (no. 6): In distal gastric cancers, a precise dissection of no. 6 lymph nodes is essential because they are most frequently involved and the dissection of positive nodes can still bring cure.
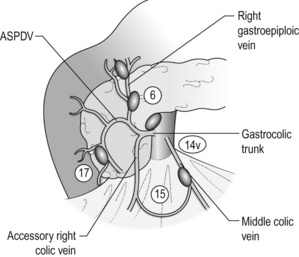
The (second) assistant should hold the transverse colon and gently stretch the mesocolon. The middle colic vein should be identified and pursued, to the approach of the gastrocolic venous junction point (Fig. 7.5). Identify the accessory right colic vein (ARCV), right gastroepiploic vein (RGEV), gastrocolic trunk and the anterior superior pancreaticoduodenal vein (ASPDV). The middle colic vein usually drains directly into the superior mesenteric vein (SMV).
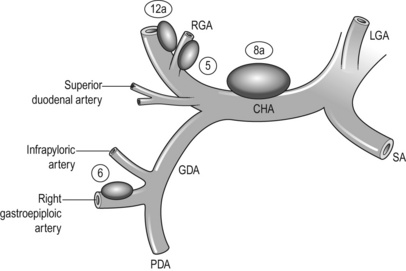
Then, the gastric antrum should be pulled up and the gastroduodenal artery (GDA) identified between the duodenum and the pancreas. The GDA is exposed distally as far as the origin of the right gastroepiploic artery (RGEA) (Fig. 7.6). The infrapyloric artery arises either from the GDA or from the RGEA. The RGEA and the infrapyloric artery should be ligated and cut together or separately at their origin.
Suprapyloric nodes dissection (no. 5) and transection of the duodenum: The assistant pulls down the pylorus and the duodenum to stretch the suprapyloric area. The right gastric artery (RGA) and the superior duodenal arteries (SDAs) are identified and the serosa between them incised. The previously placed gauze is encountered, protecting the GDA and CHA.
Exposure of the oesophageal hiatus: The assistant pulls down the stomach to stretch the lesser omentum. It is incised close to the liver, and the incision extended toward the right cardia. The accessory left hepatic artery is sometimes encountered and can be dissected. If it is large and replaces the proper left hepatic artery then it should be preserved. In this case, the division of the left gastric artery needs special attention, as described later.
Dissection of the upper border of the pancreas (nos. 8a, 9, 11p and 12a): This is the core of D2 lymphadenectomy. The nerve tissue surrounding the major arteries in this area does not have to be removed in lymphadenectomy for gastric cancer because the lymphatic tissue between the nerve and the arterial adventitia is sparse and the perineural infiltration at this level is very rare.
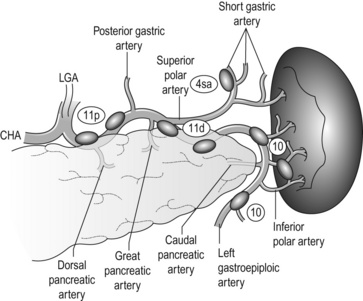
The SpA originating from the coeliac artery immediately passes behind the pancreas, then reappears on the upper border of the pancreas and winds towards the spleen (Fig. 7.7). The left side of the coeliac artery and the proximal part of the SpA are dissected. In distal gastrectomy for distal tumours, dissection around the proximal 4–5 cm of the artery is sufficient. Note that there are lymphatic channels from the infrapyloric no. 6 area to the splenic artery nodes crossing the surface of the pancreas, and the no. 11p nodes often have metastases from pyloric tumors (Fig. 7.1).
Dissection of the upper lesser curvature nodes (nos. 1 and 3a): The lymph nodes along the lesser curve (no. 3) are most frequently involved with tumours of the gastric body, and therefore complete removal is essential. The assistant should pull down the stomach. The lesser omental surface is lifted and incised close to the gastric wall, and then peeled away towards the cardia. The anterior trunk of the vagal nerve is cut and the left cardia nodes (no. 1) dissected. The posterior vagal trunk is then cut and the stomach reflected. Complete the no. 1 and 3 dissection by removing the lymphatics on the posterior aspect of the cardia.
Total gastrectomy
Dissection of the upper greater curvature nodes (nos. 2 and 4sa): Following the division of the LGEA at its origin, the upper stomach is raised to inspect the splenic hilum from inside the lesser sac. The wall of the left bottom of the lesser sac is the dorsal gastric mesentery, which connects the upper greater curve of the stomach, the spleen, and the pancreatic body and tail. The gastrosplenic ligament is part of the dorsal mesentery.
Dissection along the distal splenic artery (no. 11d) and splenic hilum (no. 10): Following the 11p dissection, the procedure is continued along the SpA towards the spleen. The winding SpA gives off several branches to both the pancreas and the stomach (Fig. 7.7). The great pancreatic artery, though not so large as its name suggests, is an important blood supply to the pancreatic tail. The caudal pancreatic artery arises near the splenic hilum. These pancreatic branches are preserved.
Modified surgery for early gastric cancer
Lymph node metastasis from early gastric cancer
There is a clear correlation between the depth of tumour invasion and the incidence of lymph node metastasis in gastric cancer. This is true between any two adjacent T categories, and even in the same T1 category, mucosal (T1a) and submucosal (T1b) tumours show clearly different chances of metastasis. However, the reported incidence of lymph node metastasis from pT1 tumours has not been consistent.5,58 This is probably due to differences in the pathological process. In Japan the sectioning method of gastric cancer has been standardised by the Japanese Classification of Gastric Carcinomas,39 and all lesions in the surgically resected specimens are sectioned at 5-mm intervals. With sectioning at wider intervals, or with only one or two central sectioning, the deepest invasion of a lesion can be overlooked and the T category would be under-staged. Then a group of tumours diagnosed as T1a may include some deeper ones having a greater chance of lymph node metastasis and the ‘incidence of lymph node metastasis from T1a’ will be reported as higher. With sectioning at smaller intervals, deeper invasion may be detected in a tumour and a diagnosis of deeper T category is given, resulting in a lower incidence of metastasis in more strictly staged T1a tumours.
On the other hand, lymph node retrieval and the sectioning method of the nodes will also change the results. By meticulous retrieval and multiple sectioning, the chance of detection of metastasis increases (by 20% according to one report59) and the incidence of metastasis in T1 tumours is reported as higher.
Considering these possibilities, the incidence of lymph node metastasis is broadly estimated to be 3% and 20% from T1a and T1b tumours respectively. It should be noted that once the tumour invades the proper muscle layer, the incidence increases up to 50%.38
Limited lymphadenectomy
The Japanese treatment guidelines define D1 and D1 + lymphadenectomy for tumours clinically diagnosed as T1. Since most metastasis from T1 is limited to the perigastric nodes and no. 7 and 8a nodes, D1 is sufficient to remove all possibly positive nodes. The problem is that the preoperative diagnosis of T1 is not always accurate: even for endoscopists who have diagnosed more than 100 ECGs, more than 10% of tumours they diagnose as T1 are pathologically T2 or deeper.60 Surgeons should always bear this in mind and be ready to perform D2 whenever deeper invasion is suspected during surgery.
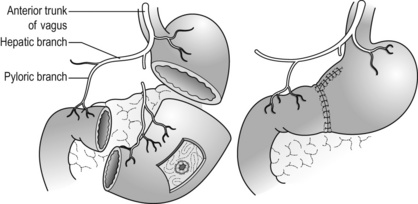
Pylorus-preserving gastrectomy (PPG) (Fig. 7.8)
PPG was originally developed as surgery for peptic ulcer disease,61 but due to its excellent functional results it is currently applied for selected cases of EGC. A short cuff (usually 3 cm) of the pyloric antrum is preserved together with the pyloric branch of the anterior vagal nerve and the infrapyloric artery. The upper gastric remnant should be large enough to function as a reservoir. For these restrictions, the dissection of no. 1 and 5 nodes is incomplete compared to standard distal gastrectomy. Therefore, PPG is indicated for middle gastric tumours that have very low possibility of metastasis to these nodes, i.e. clinical T1N0 in the middle portion of the stomach with the distal tumour border at least 4 cm proximal to the pylorus.
The quality of life after PPG is superior to that after distal gastrectomy. Low incidence of early and late dumping syndromes, low incidence of iron-deficiency anaemia and less body weight loss are reported.62,63 These are attributable to less rapid gastric emptying and prolonged acid contact of dietary iron. On the other hand, some patients suffer from early satiety and reflux due to malfunction of the pylorus and/or too small gastric remnant.
Local tumour resection based on sentinel lymph node diagnosis
If the sentinel lymph nodes from a small gastric cancer are accurately detected and their negativity can be confirmed, gastrectomy can be omitted and local tumour resection is sufficient for cure. However, the lymphatic network surrounding the stomach is very complex and accurate identification of the sentinel nodes is not easy. Several prospective studies have failed to detect the ‘lymph nodes that first receive metastasis’.64 At present, this strategy still remains investigational.
Reconstruction after gastric resection
1. Safety of surgery. In gastrectomy for advanced tumours (curative or palliative) or for patients having high operative risk, any procedure after removal of the tumour should be simple and safe, so as not to prolong the surgical time or increase the postoperative complications.
2. Possible local recurrence. In locally advanced tumours with wide serosal involvement and/or apparent nodal metastasis, gastric bed recurrence should be prepared for and a reconstruction with the least risk of obstruction should be selected.
3. Long-term quality of life. In gastrectomy with a high probability of cure, a reconstruction to maximise quality of life should be selected. Reflux of bile and alkaline duodenal juices into the oesophagus should be prevented. At the same time, long-term nutritional status should be considered.
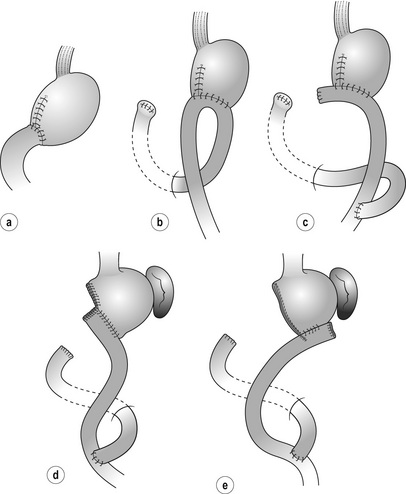
Reconstruction after distal gastrectomy (Fig. 7.9)
Roux-en-Y
Indication: Advantages of R-Y over B-I are absence of duodenal juice reflux, safe anastomosis with very low leak rate, and low risk of obstruction at gastric bed recurrence. Weak points include loss of easy endoscopic access to the duodenal papilla and possible nutritional problems due to non-physiological food passage, though no clear evidence has proven this. R-Y procedure involves jejunum and this may cause adhesive obstruction or internal hernia in the future. So-called ‘Roux-en-Y syndrome’, characterised by chronic abdominal pain and nausea that are aggravated by meals and associated with malnutrition, used to be reported mainly after ulcer surgery, but has not been observed as a serious problem lately.
1. tumours involving the gastric body for which the remnant stomach after resection is small;
2. patients who suffer from reflux esophagitis before surgery;
3. patients with high operative risks for whom anastomotic leak must be prevented;
4. locally advanced disease with high risk of gastric bed recurrence.
In special patients with biliary tract problems or duodenal pathological conditions that require endoscopic access or follow-up, B-I reconstruction might be considered.
Procedure: The duodenum is transected using a linear-type stapler and many surgeons add covering seromuscular stitches. The jejunum 20–30 cm distal to the Treiz ligament is divided and the jejunal limb is pulled up either via the ante- or retrocolic route. In T4a/T4b tumours that have a significant risk of local recurrence involving the mesocolon, the antecolic route is preferred. Gastrojejunostomy is achieved either by stapler or hand-sewing; different anastomotic sites and jejunal directions are selected accordingly (Fig. 7.9d,e). The jejunojejunostomy is made 40 cm distal to the gastrojejunostomy. The mesentery holes are closed to prevent internal hernia.
Billroth I
Indication and procedure: Advantages of B-I over R-Y are physiological food passage, simple anastomosis without jejunal manipulation, and preserved endoscopic access to the duodenal papilla. B-I is useful for distal tumours at an early stage for which a relatively large proximal stomach can be preserved and recurrence is not thought likely. Weak points of B-I are duodenal juice reflux, possible anastomotic leak and possible obstruction at recurrence. Thus, B-I should be avoided in cases with high operative risks, small remnant stomach, preoperative presence of oesophageal reflux or locally advanced disease.
Reconstruction after total gastrectomy (Fig. 7.10)
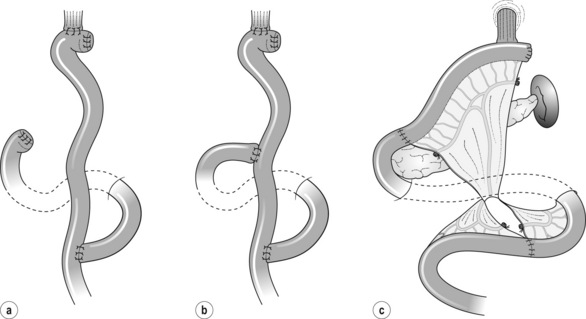
Figure 7.10 Reconstructions after total gastrectomy. (a) Roux-en-Y. (b) Double tract Roux-en-Y. (c) Jejunal interposition.
Roux-en-Y
Indication: There is virtually no contraindication for R-Y. In rare cases where endoscopic access to the duodenum needs to be maintained for biliary tract problems, a jejunoduodenostomy is added at about 30–40 cm from oesophagojejunostomy (double-tract R-Y, Fig. 7.10b).
Procedure: The jejunum is transected 20–30 cm from the Treiz ligament. Either the ante- or retrocolic route is selected according to the criteria mentioned in the section on distal gastrectomy. The retrocolic pathway provides the shortest route and the least tension on the limb mesentery, especially in obese patients with a large omental residue.
Jejunal interposition
This method is employed to maintain the physiological food passage to the duodenum.
Reconstruction after proximal gastrectomy (Fig. 7.11)
Indication: The original method was described by Merendino and Dillard in 1955 for reflux disease.67 The interposed isoperistaltic jejunum prevents gastric juice reflux to the oesophagus, and strangely anastomotic ulcers do not appear. This reconstruction method is applicable to proximal gastrectomy for small tumours of the oesophagogastric junction in which resection of the oesophagus and stomach can be minimal. Note that even a short segment of the interposed jejunum could cause early dumping syndrome due to rapid food inflow.
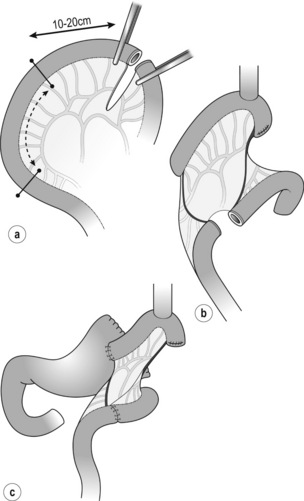
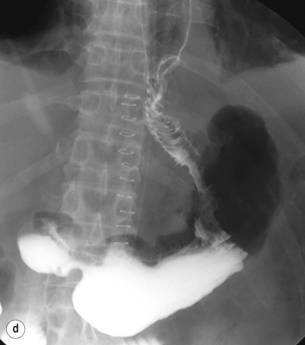
Figure 7. 11 Jejunal interposition after proximal gastrectomy. (a) Jejunal interposition. (b) Oesophagojejunostomy. (c) Jejunogastrostomy. (d) Post operative Gastrografin swallow. Modified from Katai H, Sano T, Fukagawa T et al. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg 2003; 90:850–3.
Oesophagogastrostomy
Indication: Although this is a simple and safe reconstruction after proximal gastrectomy, some patients suffer from severe reflux oesophagitis and cannot quit medication. This is indicated for proximal gastrectomy in which the abdominal oesophagus is preserved. Reconstruction after lower oesophagectomy using a gastric tube is described in Chapter 5.
Procedure: Oesophagogastrostomy is performed on the anterior wall of the remnant stomach, usually using a circular stapler. A hand-sewing method is not very difficult, and the soft opening of the anastomosis instead of the fixed ring of the stapler seems to function as a protector for the reflux. Many surgeons add some extra stitches between the oesophagus and the stomach to prevent reflux.
Early postoperative complications
Use of prophylactic drains after gastrectomy is controversial. There is no evidence that drains decrease postoperative complications or death.69 Distal gastrectomy without intraoperative problems usually needs no drains. However, in total gastrectomy or extended D2 dissection along the upper border of the distal pancreas, a suction drain along the pancreas gives useful information on pancreatic fistula or early anastomotic leak.
Pancreatic fistula
It is controversial as to whether the use of somatostatin analogues prevents pancreatic fistula. RCTs for pancreatic surgery showed inconsistent results, and meta-analyses did not support a positive effect.70 No RCT has been conducted on this subject in gastrectomy.
Late sequelae and complications
Side-effects and postprandial sequelae
Late dumping syndrome or reactive hypoglycaemic attacks
In a large-scale investigation into dumping syndrome after gastrectomy (N = 1153),71 early dumping syndrome was more commonly experienced than late dumping syndrome (68% vs. 38%) and the incidence varied according to the type of gastrectomy but not the postoperative period. Early and late dumping syndromes have different aetiologies and appear independently. During follow-up, a careful history should be taken and appropriate dietary advice should be given.
References
1. Bonenkamp, J.J., Songun, I., Hermans, J., et al, Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345(8952):745–748. 7891484
2. Dikken, J.L., Jansen, E.P., Cats, A., et al, Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010; 28:2430–2436. 20368551
3. Macdonald, J.S., Smalley, S.R., Benedetti, J., et al, Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345:725–730. 11547741
4. Maruyama, K., Gunven, P., Okabayashi, K., et al, Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg 1989; 210:596–602. 2818028
5. Gotoda, T., Yanagisawa, A., Sasako, M., et al, Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225. 11984739 Detailed analysis of 5265 early gastric cancers that provided the grounds of expanded indications for endoscopic submucosal dissection.
6. Yoshikawa, K., Kitaoka, H., Bone metastasis of gastric cancer. Jpn J Surg 1983; 13:173–176. 6632389
7. Kobayashi, M., Okabayashi, T., Sano, T., et al, Metastatic bone cancer as a recurrence of early gastric cancer: characteristics and possible mechanisms. World J Gastroenterol 2005; 11:5587–5591. 16237749
8. Sobin L., Gospodarowicz M., Wittekind C., eds. TNM classification of malignant tumours, 7th ed, International Union Against Cancer, 2009.
9. Yoo, C.H., Noh, S.H., Shin, D.W., et al, Recurrence following curative resection for gastric carcinoma. Br J Surg 2000; 87:236–242. 10671934
10. Marrelli, D., Roviello, F., de Mazoni, G., et al, Different patterns of recurrence in gastric cancer depending on Lauren’s histological type: longitudinal study. World J Surg 2002; 26:1160–1165. 12209247
11. Ishigami, H., Kitayama, J., Kaisaki, S., et al, Phse II study of weekly intravenous and Intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010; 21:67–70. 19605503
12. Songun, I., Putter, H., Kranenbarg, E.M.K., et al, Surgical treatment of gastric cancer; 15-year follow-up results of the randomized nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11:439–449. 20409751 Long-term follow-up of an RCT finally showed significantly fewer gastric cancer deaths after D2 dissection.
13. Martin, R.C., Jaques, D.P., Brennan, M.F., et al, Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg 2002; 236:159–165. 12170020
14. Han, T.S., Kong, S.H., Lee, H.J., et al, Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol 2011; 18:2818–2825. 21455599
15. Kuramoto, M., Shimada, S., Ikeshima, S., et al, Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009; 250:242–246. 19638909 A small-scale RCT showed that extensive peritoneal lavage before closure reduced peritoneal recurrence.
16. Ferlay, J., Shin, H.R., Bray, F., et al, Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. 21351269
17. Sue-Ling, H.M., Johnston, D., Martin, I.G., et al, Gastric cancer: a curable disease in Britain. Br Med J 1993; 307:591–596. 8401015
18. McCulloch, P., How I do it: D2 gastrectomy. Eur J Surg Oncol 2002; 28:738–743. 12431471
19. McCulloch, P.G., Ochiai, A., O’Dowd, G.M., et al, Comparison of the molecular genetics of c-erb-B2 and p53 expression in stomach cancer in Britain and Japan. Cancer 1995; 75:920–925. 7842412
20. Livingstone, J.I., Yasui, W., Tahara, E., et al, Are Japanese and European gastric cancer the same biological entity? An immunohistochemical study. Br J Cancer 1995; 72:976–980. 7547252
21. Pohl, H., Welch, G., The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005; 97:142–146. 15657344
22. DeMeester, S.R., Adenocarcinoma of the esophagus and cardia: a review of the disease and its treatment. Ann Surg Oncol 2006; 13:12–30. 16378161
23. Cuschieri, A., Fayers, P., Fielding, J., et al, Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial The Surgical Cooperative Group. Lancet 1996; 347:995–999. 8606613
24. Sasako, M., Sano, T., Yamamoto, S., et al, D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453–462. 18669424
25. Davis, P.A., Sano, T., The difference in gastric cancer between Japan, USA and Europe: What are the facts? What are the suggestions? Crit Rev Oncol Hematol 2001; 40:77–94. 11578917
26. Tsujinaka, T., Sasako, M., Yamamoto, S., et al, Influence of overweight on surgical complications for gastric cancer: results from a randomized control trial comparing D2 and extended para-aortic D3 lymphadenectomy (JCOG9501). Ann Surg Oncol 2007; 14:355–361. 17146738
27. Birkmeyer, J.D., Siewers, A.E., Finlayson, E.V.A., et al, Hospital volume and surgical mortality in the United States. N Engl J Med 2002; 346:1128–1137. 11948273
28. Jackson, C., Cunningham, D., Oliveira, J., Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(S4):iv34–iv36. 19454457
29. NCCN Clinical Practice Guidelines in Oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
30. Hornig, D., Hermanek, P., Gall, F.P. The significance of the extent of proximal margins on clearance in gastric cancer surgery. Scand J Gastroenterol. 1977; 22(Suppl. 133):69–71.
31. Moehler, M., Al-Batran, S.E., Andus, T., et al, German S3-guideline “Diagnosis and treatment of esophagogastric cancer” [in German]. Z Gastroenterol 2011; 49:461–531. 21476183
32. Japanese Gastric Cancer Association, Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123. 21573742 The JGCA totally renewed the concept of D2 lymphadenectomy.
33. Bozzetti, F., Marubini, E., Bonfanti, G., et al, Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 1999; 230:170–178. 10450730
34. Japanese Research Society for Gastric Cancer, The general rules for the gastric cancer study in surgery and pathology. Jpn J Surg 1981; 11:127–138. 7300058
35. Wu, C.W., Hsiung, C.A., Lo, S.S., et al, Nodal dissection for patients with gastric cancer: a randomized controlled trial. Lancet Oncol 2006; 7:309–315. 16574546
36. Japanese Gastric Cancer Association, Japanese classifications of gastric carcinoma 1st English ed. Kanehara, Tokyo, 1995.
37. Japanese Gastric Cancer Association, Japanese classification of gastric carcinoma, 2nd English edn. Gastric Cancer 1998; 1:8–24. 11957039
38. Sasako, M., McCulloch, P., Kinoshita, T., et al, New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 1995; 82:346–351. 7796005 A new concept to evaluated lymphadenectomy was proposed based on the incidence of metastasis and survival of patients with positive nodes.
39. Japanese Gastric Cancer Association, Japanese classification of gastric carcinoma, 3rd English edn. Gastric Cancer 2011; 14:101–112. 21573743
40. Sano, T., Sasako, M., Yamamoto, S., et al, Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy – Japan Clinical Oncology Group Study 9501. J Clin Oncol 2004; 22:2767–2773. 15199090
41. Siewert, J.R., Böttcher, K., Stein, H.J., et al, Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998; 228:449–461. 9790335
42. Fujita, J., Kurokawa, Y., Sugimoto, T., et al, Survival benefit of bursectomy in patients with resectable gastric cancer: interim analysis results of a randomized controlled trial. Gastric Cancer 2012; 15:42–48. 21573917
43. Griffith, J.P., Sue-Ling, H.M., Martin, I., et al, Preservation of the spleen improves survival after radical surgery for gastric cancer. Gut 1995; 36:684–690. 7797117
44. Wanebo, H.J., Kennedy, B.J., Winchester, D.P., et al, Role of splenectomy in gastric cancer surgery: adverse effect of elective splenectomy on long-term survival. J Am Coll Surg 1997; 185:177–184. 9249086
45. Maehara, Y., Moriguchi, S., Yoshida, M., et al, Splenectomy does not correlate with length of survival in patients undergoing curative total gastrectomy for gastric carcinoma Univariate and multivariate analyses. Cancer 1991; 67:3006–3009. 2044047
46. Csendes, A., Burdiles, P., Rojas, J., et al, A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery 2002; 131:401–407. 11935130
47. Yu, W., Choi, G.S., Chung, H.Y., Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 2006; 93:559–563. 16607678
48. Sano, T., Yamamoto, S., Sasako, M., Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan Clinical Oncology Group study JCOG 0110-MF. Jpn J Clin Oncol 2002; 32:363–364. 12417603
49. Sano, T., Sasako, M., Shibata, T., et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma (JCOG0110): analyses of operative morbidity, operation time, and blood loss. J Clin Oncol. 2010; 28(Suppl.):15s. [abstr 4020].
50. Kodera, Y., Sasako, M., Yamamoto, S., et al, Identification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancer. Br J Surg 2005; 92:1103–1109. 16106493
51. Maruyama, K., Sasako, M., Kinoshita, T., et al, Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 1995; 19:532–536. 7676695
52. Roberts, P., Seevaratnam, R., Cardoso, R., et al, Systematic review of pancreaticoduodenectomy for locally advanced gastric cancer. Gastric Cancer. 2012;15(Suppl. 1):S108–S115. 21870150
53. Roviello, F., Pedrazzani, C., Marrelli, D., et al, Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol 2010; 36:439–446. 20392590
54. Tokunaga, M., Ohyama, S., Hiki, N., et al, Therapeutic value of lymph node dissection in advanced gastric cancer with macroscopic duodenum invasion: is the posterior pancreatic head lymph node dissection beneficial? Ann Surg Oncol 2009; 16:1241–1246. 19224285
55. Sakamoto, Y., Sano, T., Shimada, K., et al, Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol 2007; 95:534–539. 17219383
56. Koga, R., Yamamoto, J., Ohyama, S., et al, Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol 2007; 37:836–842. 17928333
57. Sasako, M., Sano, T., Yamamoto, S., et al, Left thoracoabdominal approach versus abdominal-transhiatal approach for cardia or subcardia cancer: a randomised controlled trial. Lancet Oncol 2006; 7:644–651. 16887481
58. Hayes, N., Karat, D., Scott, D.J., et al, Radical lymphadenectomy in the management of early gastric cancer. Br J Surg 1996; 83:1421–1423. 8944462
59. Natsugoe, S., Aikou, T., Shimada, M., et al, Occult lymph node metastasis in gastric cancer with submucosal invasion. Surg Today. 1994;24(10):870–875. 7894183
60. Choi, J., Kim, S.G., Im, J.P., et al, Endoscopic prediction of tumor invasiondepth in early gastric cancer. Gastrointest Endosc 2011; 73:917–927. 21316050
61. Maki, T., Shiratori, T., Hatafuku, T., et al, Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery 1967; 61:838–842. 5338114
62. Nunobe, S., Sasako, M., Saka, M., et al, Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer 2007; 10:167–172. 17922094
63. Tomikawa, M., Korenaga, D., Akahoshi, T., et al, Quality of life after laparoscopy-assisted pylorus-preserving gastrectomy: an evaluation using a questionnaire mailed to the patients. Surg Today. 2012;42(7):625–632. 22527179
64. Isozaki, H., Kimura, T., Tanaka, N., et al, An assessment of the feasibility of sentinel lymph node-guided surgery for gastric cancer. Gastric Cancer 2004; 7:149–153. 15449202
65. Zong, L., Chen, P., Billroth I vs. Billroth II vs. Roux-en-Y following distal gastrectomy: a meta-analysis based on 15 studies. Hepatogastroenterology 2011; 58:1413–1424. 21937419
66. Gertler, R., Rosenberg, R., Feith, M., et al, Pouch vs. no pouch following total gastrectomy: meta-analysis and systematic review. Am J Gastroenterol 2009; 104:2838–2851. 19672251 A meta-analysis showed clinical benefits of pouch formation after total gastrectomy.
67. Merendino, K.A., Dillard, D.H., The concept of sphincter substitution by and interposed jejunal segment for anatomic and physiologic abnormalities at the esophagogastric junction: with special reference to reflux esophagitis, cardiospasm and esophageal varices. Ann Surg 1955; 142:486–509. 13249345
68. Katai, H., Sano, T., Fukagawa, T., et al, Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg 2003; 90:850–853. 12854112
69. Kim, J., Lee, J., Hyung, W.J., et al, Gastric cancer surgery without drains: a prospective randomized trial. J Gastrointest Surg 2004; 8:727–732. 15358335 A high-volume single institutional RCT showed that routine use of drain is not necessary after D2 gastrectomy.
70. Zeng, Q., Zhang, Q., Han, S., et al, Efficacy of somatostatin and its analogues in prevention of postoperative complications after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. Pancreas 2008; 36:18–25. 18192875
71. Mine, S., Sano, T., Tsutsumi, K., et al, Large-scale investigation into dumping syndrome after gastrectomy for gastric cancer. J Am Coll Surg 2010; 211:628–636. 20829078 Direct questionnaire to 1153 gastrectomised patients with various postoperative periods revealed actual conditions of dumping syndrome.