Surgery for cancer of the oesophagus
Introduction
While treatment for cancer of the oesophagus is multidisciplinary, surgery is still the primary mode of therapy. In the UK, 70% of patients now present with adenocarcinoma of the lower oesophagus or gastro-oesophageal junction, which represent a different disease from the previously more common squamous carcinoma. This chapter discusses the surgical management of adenocarcinoma of the lower oesophagus and the cardia (Siewert types 1 and 2), which are frequently staged and treated as oesophageal cancers, but not subcardial tumours (Siewert type 3), which are described elsewhere (Chapter 7).
Unfortunately, the disease often presents late when increasing dysphagia has developed over several months. As a result of poor fitness or unresectable disease only 30–40% of patients are suitable for radical, potentially curative treatment, whilst the majority receive non-surgical therapies with the aim of palliation. Outcome is strongly stage dependent; whilst early tumours have excellent results with surgery alone, the majority with transmural or node-positive tumours benefit from multimodality therapy, combining surgery with neoadjuvant chemotherapy or chemoradiotherapy (Chapter 9). The multidisciplinary team must exercise judgment in the choice of the appropriate combination of therapies for each individual patient. This will depend on patient age, fitness, symptoms, prognosis and evidence base, as well as the overall stage and histopathology.
Surgical pathology
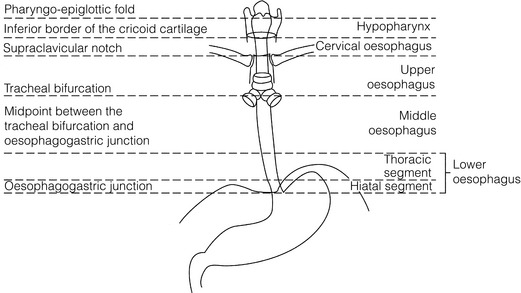
Squamous cell carcinoma arising in the cervical and thoracic oesophagus and adenocarcinoma arising in the thoracic oesophagus and cardia differ in their mode of spread and response to therapeutic modalities. It is essential that the anatomical divisions of the oesophagus are described such that the different therapeutic surgical procedures adopted for tumours at each site can be understood (Fig. 5.1).
Surgical anatomy
The TNM classification (Version 7, released in 2009)1 combines the salient features of the staging process. This classification has divided the oesophagus into discrete anatomical regions (Fig. 5.1) and is described in Chapter 2.
Blood supply and lymphatic drainage
The lymphatics of the oesophagus are distributed predominantly in the form of a submucosal plexus and a paraoesophageal plexus. Both plexuses receive lymph from all parts of the respective layers of the oesophageal wall. The plexuses communicate through penetrating vessels that traverse the longitudinal and circular muscle walls. The paraoesophageal plexus drains into the paraoesophageal lymph nodes, which are situated on the surface of the oesophagus, and also into perioesophageal lymph nodes, situated in close proximity to the oesophagus. Lymphatics also drain from the perioesophageal nodes to the lateral oesophageal nodes or directly from the paraoesophageal to the lateral oesophageal nodes, skipping the perioesophageal group2 (Box 5.1).
Preoperative surgical preparation
Meticulous preoperative evaluation to accurately stage the tumour and estimate surgical risk is a crucial prerequisite to successful surgical outcome in this disease (see also Chapters 3 and 4).
Nutritional support
Malnutrition is associated with loss of tissue function, leading to many potential complications during the postoperative period, such as wound breakdown, respiratory failure secondary to poor respiratory muscle function, deep vein thrombosis and infective complications.3
Perioperative enteral nutrition with the addition of nutrients that can modulate the immune system, termed ‘immunonutrition’, has been proposed to further reduce postoperative complications and improve outcome.5
Preoperative nutritional support
Placement of a feeding jejunostomy at the time of surgery is routine in many units. Although mortality related specifically to feeding jejunostomy is less than 1%,6 it is not without morbidity, both in-hospital and longer term (from adhesions). Nevertheless, establishing enteral nutrition in patients with complications following oesophagectomy, when they are most catabolic septic and ill, is a major therapeutic problem and avoiding re-operation has significant advantages.
Routine preoperative and postoperative feeding by jejunostomy in every patient has yet to be proven efficacious on current evidence.7 Furthermore, a recent randomised controlled study comparing standard perioperative nutrition with immunonutrition failed to demonstrate any clinical advantage.7
A variety of other routes of nutrition have been assessed, with a recent systematic review showing no strong direct evidence to support one particular route.8 Nasojejunal and nasoduodenal tubes are associated with a significant rate of dislodgement.8
Respiratory care
Optimisation of respiratory function is vital in preventing serious pulmonary complications associated with prolonged surgery and thoracotomy (see Chapter 4). Smoking must be stopped as early as possible, ideally 6 weeks prior to surgery, with the aid of nicotine replacement. Preoperative physiotherapy with coughing exercises and effective use of the diaphragm by restoration of muscle strength through ambulation is encouraged. High-risk patients should also be provided with vigorous physiotherapy with or without bronchodilators prior to surgery. Orodental hygiene should be undertaken, removing any source of chronic sepsis that could disseminate infection to the tracheobronchial tree during intubation. Many surgeons advocate routine use of an incentive spirometer; they are inexpensive, and used properly they help to set goals for patients that can be measured, albeit indirectly.
Surgical objectives
Survival is related to the stage of disease. Patients with stage I disease can expect a 5-year survival of greater than 80%,9 emphasising the importance of early detection. Resection alone, therefore, must be the chosen method of therapy in fit patients with T1 tumours of the middle and lower thirds of the oesophagus. In stage III disease, surgery alone produces poor results with prolonged survival for only 10–20% of cases.10 It appears that both neoadjuvant chemotherapy and radiotherapy provide a benefit for these patients.11 Further randomised trials must be completed in order to outline the optimal therapeutic strategy.
Survival following surgical resection for all stages of tumour has improved over the past 20 years, with morbidity and mortality falling. The reasons for this are listed in Box 5.2 and were well described in the COG Guidance Report on Upper GI Cancers.12 Many studies have confirmed that results parallel experience in managing this condition,13 and poor results occur when experience is limited.14,15 There is now overwhelming evidence to confirm the influence of surgeon case volume on the outcome of site-specific cancer surgery.14–16 Centralisation of oesophagogastric resection in specialist units in the UK has provided sufficient caseload to support strong multidisciplinary teams. Other reasons for improved outcome include better patient selection, earlier diagnosis by open-access endoscopy, surveillance of Barrett’s oesophagus, and improved preoperative, operative and postoperative management.
Principles of oesophagectomy
Rules on resection margins
The majority of authors favour a subtotal oesophagectomy to optimise longitudinal margins and take into account submucosal spread of both squamous and adenocarcinomas. There has been much debate around what length of macroscopically normal oesophagus to allow for complete resection margins. In squamous carcinoma this pertains especially to the proximal margin, whereas in adenocarcinoma the distal margin (gastric) is usually the greater concern. Skinner17 advocated a minimum resection margin of 10 cm from the palpable edge of the tumour. However, this figure does not take into account the nature, location and pattern of occurrence of the primary cancer. Neither does it discriminate between in vivo margins and margins measured by the histopathologist after shrinkage has occurred during fixation.18
Primary tumours with multicentric lesions require more extensive longitudinal margins. In squamous cancers, three representative patterns of presentation are encountered (Fig. 5.2).19 Failure to take these into account may explain high R1 rates (40%) when the oesophageal resection margin is limited to only 4 cm; compared to this, R1 was 17% when the margin was 10 cm. A 10-cm resection margin is a goal to attain in both directions if this is possible. In practice, this rule can rarely be achieved. A 10-cm margin either side of a 6-cm tumour would require an overall length of specimen exceeding that of the normal human oesophagus. It is the authors’ opinion that when only a short resection margin can be obtained through the thoracic exposure, a cervical phase with near total oesophagectomy is advisable.
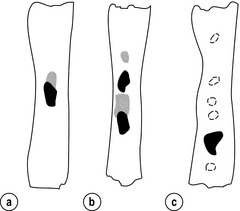
Figure 5.2 (a) A single cancer. (b) Multifocal cancer. (c) Intramural lymphovascular spread. There is a high risk of positive resection margins in (b) and (c). Shaded areas represent submucosal spread.
Adenocarcinoma of the lower oesophagus commonly infiltrates the gastric cardia, fundus and lesser curve. Extensive sleeve resection of the lesser curve and fundus with the formation of a tubular conduit is necessary to minimise positive distal resection margins. Other studies have demonstrated that patients with microscopically positive margins undergoing palliative resection died of other manifestations before clinical evidence of locoregional recurrence.20,21 A tumour-free surgical margin is therefore not the only important factor to be considered in radical surgery. Nevertheless, it should remain the main goal of every operation.
A clear radial resection margin is equally important and is accepted as an independent prognostic factor for oesophageal cancer, with a definition of R1 being less than 1 mm clear margin.22,23 The potential benefits of extended lymphadenectomy, discussed later, only pertain if the primary tumour has been completely excised (R0). Radical en-bloc resection techniques, outlined below in operative description, aim to produce a clear radial resection margin. Roder et al.24 showed a statistically significant difference between R0 and R1 (microscopic residual disease) or R2 (macroscopic residual disease) resections for squamous cell carcinoma in a series of 204 resections with 5-year survival rates of 35% and < 10%, respectively. Lerut et al.25 demonstrated a 20% 5-year survival for R0 vs. zero 5-year survival for R1 and R2 resections in advanced stage III and stage IV adenocarcinomas and squamous cell carcinomas.
Resection of lymph nodes
Lymph node involvement is another independent variable for prognosis, in terms of both locoregional recurrence and survival. Patients with higher nodal burdens have worse outcomes after resection. The Worldwide Esophageal Cancer Collaboration has used pooled data from centres that have undertaken radical lymphadenectomy to report in detail the relationship between nodal involvement and survival.26
Nodal tiers
Lymph node tiers draining oesophageal cancer have been described according to the anatomy of the lymphatic drainage of the oesophagus.2,27,28 The extent of lymphadenectomy associated with resection of these tiers is demonstrated in Box 5.1 and Fig. 5.3.

Figure 5.3 Extent of resection and fields of lymph node dissection routinely carried out for cancer of the oesophagus.
The fields of nodal dissection should not be confused with the histopathological staging of nodal involvement (see Chapter 3, Table 3.3).
In the past some authors felt that lymph node metastases were simply markers of systemic disease and their removal conferred no benefit.23 Others contended that cure could be obtained in some patients with positive nodes by a radical lymphadenectomy with clear resection margins.29 There has been increasing support for radical lymphadenectomy over the last 5 years.30
The rationale for lymphadenectomy
Optimal staging: Radical lymphadenectomy allows more accurate pathological staging.25,31–32 TNM7 relies not only on the finding of positive lymph nodes, but on how many are found (N1, 1–2; N2, 3–6; N3, ≥ 7). Without an adequate lymphadenectomy, the patient is deprived of the most accurate prognosis available, and the patient’s unit is deprived of a quality benchmark. Quality control is not possible as the baseline information, accurate pathological stage, is missing. If an inadequate lymphadenectomy is undertaken, survival will be worse than predicted for stage (the phenomenon of stage migration).
Locoregional tumour control: Locoregional tumour control is an important goal in treating oesophageal carcinoma; recurrent locoregional mediastinal disease can be very difficult to palliate. As Lerut et al. emphasise, long-term palliation is a significant benefit for those patients who are not cured by surgery.25 It is impossible to separate potential benefits of radical lymphadenectomy from radical en-bloc resection, as already mentioned.
Radical en-bloc resection with lymphadenectomy has been associated with prolonged tumour-free survival; this is partly a result of consistently clear resection margins and partly a result of complete removal of involved nodes. Nodal and local tumour recurrence is less common after radical en-bloc resection in a number of retrospective series.25,31,33–35 Single-institution comparative studies have also shown better survival.36,37 Altorki et al. described a local recurrence rate of only 9.7% and a 33% 5-year survival for node-positive oesophageal cancer patients using a three-field lymphadenectomy and en-bloc resection.38
Locoregional recurrence may be further reduced by induction therapy, whether chemotherapy alone or chemoradiotherapy, with fewer positive nodes as well as an increase in R0 resection, consistent with improved disease-free and overall survival in some randomised trials.39
Improved cure rate: It is extremely difficult to demonstrate that radical lymphadenectomy improves cure rate in a conventional randomised controlled trial. Patients allocated to less than radical lymphadenectomy would be understaged, so no baseline would exist and the groups would not be comparable. Furthermore, most patients now have induction therapy that changes their nodal status during treatment. There are therefore very few randomised trials in the literature.40–42
Despite the limitations, there are indications from the Dutch trial,40,43 comparing radical transthoracic and transhiatal resection, that patients with a limited number of positive nodes (1–8) had a significantly better survival following radical transthoracic oesophagectomy compared with less radical transhiatal resection. This group of patients would be expected to benefit from extended lymphadenectomy if it conferred survival advantage. Node-negative patients did well and those with a higher nodal burden did poorly, irrespective of the radicality of surgery (see Fig. 5.4). Another smaller, retrospective comparison showed improved survival with radical en-bloc resection compared with transhiatal resection in patients with T3N1 disease with fewer than eight nodes involved.44
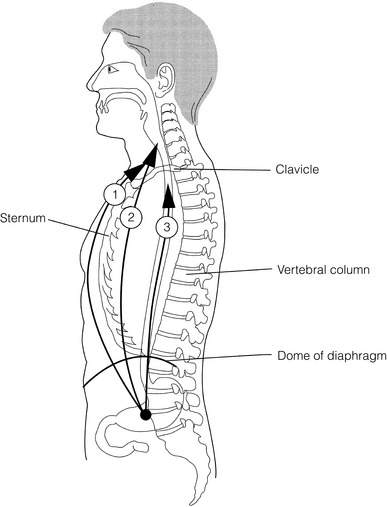
Figure 5.4 Three routes of oesophageal reconstruction: (1) presternal route; (2) retrosternal route; (3) posterior mediastinal route.
The role of radical lymphadenectomy in early-stage disease remains in question, and depends on the precise depth of invasion of the primary tumour.40 There is some evidence that even patients with early-stage carcinoma, in whom a significant proportion can have nodal involvement, could benefit from extensive resection with lymphadenectomy.45
The most important recent evidence in support of radical lymphadenectomy comes from the Worldwide Esophageal Cancer Collaboration, who reported on their multi-institution international database comprising over 4600 resections for oesophageal cancer in patients who had not had induction therapy.46 They showed that prognosis was highly dependent on the number of lymph nodes involved; patients with more than three nodes involved had a 50% likelihood of systemic disease and patients with more than eight nodes involved had almost 100% likelihood of systemic disease.30,47
Even more important was their finding that survival depended not only on how many nodes were involved, but also on how many were removed at resection.47 The number of lymph nodes removed was the third strongest predictor of survival after depth of invasion and number of nodes involved. This finding has been corroborated in an analysis of the SEER (Surveillance, Epidemiology and End Results) database, both for oesophageal cancer48 and gastric cancer.49 It does appear from these data that the number of nodes resected has an effect on survival.
Harvested nodal counts of 2347 and 3048 have been suggested as the optimal number, with caveats given that this is a goal and not an expected target in all patients. The recommendation for adequate staging for TNM7 is a minimum of six nodes. The AJCC (American Joint Committee on Cancer Staging) suggest 18 lymph nodes as the minimum number for accurate staging.50 The authors’ view is that this is too low.
The role of the more extensive three-field dissection in oesophageal malignancy is less clear. Five-year survival rates showed no significant difference between two-field and three-field dissection for lower-third squamous tumours;32 the same pertains to patients with adenocarcinoma of the lower oesophagus. Patients with cancer of the upper thoracic oesophagus (third field) may benefit from dissection in the neck.24,38,51
Summary
There is little justification for oesophagectomy to be performed with intent to cure without any attempt to clear the first level of lymph nodes. Patients with either squamous carcinoma or adenocarcinoma of the oesophagus affecting the upper, middle and lower regions have mediastinal lymph node metastases in over 70% of cases.21,31,32,52 Over three-quarters of patients presenting with lower-third tumours have positive upper abdominal lymph nodes.53 In order to perform a potentially curative resection for carcinoma in the middle and lower thirds, a dissection of abdominal and mediastinal lymph nodes is therefore essential.
Method of reconstruction of the oesophagus
After resection of the cervical, thoracic or abdominal oesophagus, one of three main paths can be used for reconstruction: presternal, retrosternal and posterior mediastinal (Fig. 5.4).
Presternal route: This route is mentioned for historic completeness. It is approximately 2 cm longer than the retrosternal route, which in turn is approximately 2 cm longer than the posterior mediastinal route. The only indication for using this route is in the situation of multiple previous reconstructions that have compromised the other two routes.
Retrosternal route (anterior mediastinal): The potential space between the sternum and the anterior mediastinum is easily opened up through effective dissection. There is reported to be a lower incidence of cervical anastomotic dehiscence compared with that of the presternal route. Its major disadvantage stems from the unnatural position of the cervical oesophagus in front of the trachea, which can result in an unpleasant sensation on swallowing.
This route is used for reconstruction following emergency treatment of anastomotic dehiscence or the dehiscence of a gastric substitute that has caused posterior mediastinal sepsis. After incomplete resection (R1 and R2) there is some evidence that a retrosternal conduit would be preferable to the posterior mediastinal route.54
Organ of reconstruction
Reconstruction with stomach: The method of reconstruction should be kept as simple as possible, to minimise complications. The oesophageal replacement is determined by the site of the primary lesion. The stomach is the preferred option as this organ is easy to prepare and involves only one anastomosis.
1. The use of isoperistaltic stomach maintaining vascular supply. The right gastroepiploic and the right gastric artery and veins are vital to the viability of the stomach when used as an oesophageal substitute. The greater omentum is opened and the entire course of the right gastroepiploic artery is carefully identified and preserved. The vascular arcade is interrupted at the junction where the right gastroepiploic artery meets the left. The short gastric vessels are divided and ligated (Fig. 5.5).
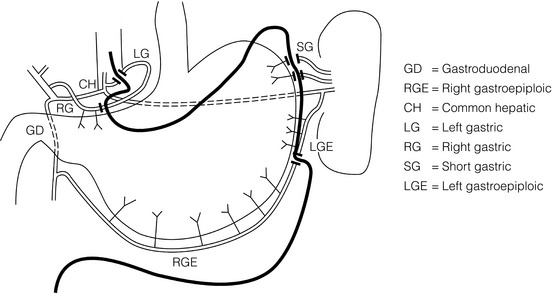
Figure 5.5 Main arteries of the stomach and points of division of vessels and stomach for oesophageal substitution.
2. Excision of the lesser curvature. Cancers of the lower two-thirds of the oesophagus require clearance of the lesser curve lymph nodes as well as the left gastric, common hepatic and proximal splenic artery lymph nodes. The left gastric artery should be ligated at its origin and resection of the proximal half of the lesser curvature of the stomach, including the cardia, is performed. The right gastric artery contributes to the maintenance of the gastric intramural vascular network and should be preserved if possible. Although the width of gastric conduit appeared not to impact on outcome in one study,56 the authors recommend using a gastric tube of 5 cm width or greater to minimise the risk of ischaemia as described by Akiyama et al.57 in the 1970s.
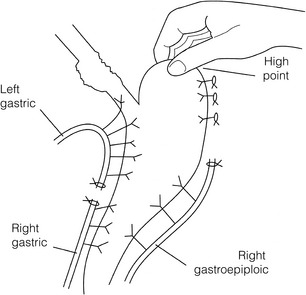
3. Preservation of the intramural vascular arcade. Extensive intramural arterial anastomoses between the vascular arcades of the lesser and greater curvatures exist. This has been well demonstrated by el-Eishi et al.58 and Thomas et al.59 This vascular network must be preserved during resection of the left gastric area of the lesser curvature and the cardia of the stomach. The extent of the resection of the lesser curvature is determined by a line connecting the highest point of the fundus (Fig. 5.6) and the lesser curvature at the junction of the right and left gastric arteries. This allows the removal of all potentially involved lymph nodes, yet preserves the arterial network to the fundus. There is no evidence that the trunk and descending branches of the left gastric artery running along the lesser curve need to be preserved and, from an oncological point of view, it is important that these are excised with the specimen. Care should be taken to ligate the short gastric vessels away from the greater curvature of the stomach to avoid damage to the intramural network and to preserve the extramural vascular network as well. The right gastroepiploic artery provides an adequate blood flow to maintain vascularity in the region of the fundus, which is the area used for anastomosis.
4. The high point of the stomach. The stomach is a flexible and capacious organ; its high point is the logical and sensible place at which to fashion an anastomosis with the remaining oesophagus. It is easily identified by applying traction with the surgeon’s fingers in an upward direction after all preparations have been completed. The stomach is transected as described previously (Fig. 5.6).
5. Gastric drainage. Pyloroplasty or pyloromyotomy after gastric reconstruction is contentious. There is some evidence that pyloroplasty reduces the incidence of gastric outlet obstruction.60 As short-term complications of pyloroplasty are minimal, the authors routinely perform a pyloroplasty to prevent the life-threatening early complications of gastric stasis and aspiration as well as late vomiting and bloatedness.46,61
1. Kocher manoeuvre. This manoeuvre allows the distance between the first part of the duodenum and the hiatus to be reduced.
2. Excision of the lesser curve of the stomach. When the lesser curve of the stomach is unusually short, an increase in length of the gastric substitute can be obtained, by dividing the lesser curve between curved clamps, before its resection. If absolutely necessary, a tense right gastric artery may be sacrificed by division at the level of the pylorus.
3. Incision of the serosa on the gastric wall. Multiple incisions placed in the gastric serosa may lengthen the stomach. A longitudinal incision placed along the resection line allows this to occur. The indications for this procedure are extremely rare.
Reconstruction with colon: The principal indication for the use of colonic interposition is for tumours requiring an extensive oesophageal as well as gastric resection, although with thorough staging few of these patients are suitable for resection. A small proportion of patients presenting with oesophageal malignancy will have had a previous gastric resection for peptic ulcer disease, precluding the use of stomach as the oesophageal substitute. The numbers of patients such as this are diminishing. The choice of an oesophageal replacement under these circumstances lies between colon and jejunum. The colon is often recommended because of its advantage in having a greater capacity as a reservoir than the jejunum.
Indications for colonic reconstruction (Box 5.3): It is preferable to use the colon in an isoperistaltic fashion. Unfortunately the vascular pattern of the colon varies and careful selection of the correct vascular pedicle to ensure viability of the transverse colon is essential. Each case requires evaluation on its own merit because of variations in anatomy. Not infrequently, the marginal artery is found to be of insufficient calibre to maintain viability of the transposed colon. Although the vascular appearance determines the appropriate colonic segment for use in each individual, the two possibilities for effective use of isoperistaltic colon are: (a) transverse colon based on the left colic vessels; (b) right colon based on the middle colic vessels.
The disadvantage of transverse colon is that an abnormally narrow marginal artery may exist at the splenic flexure, compromising the blood supply of the proximal colonic segment. Preoperative assessment by angiography of the colonic vascular pathway has been suggested,62 but careful intraoperative observation of the vascular anatomy with temporary occlusion of vessels before division is a simple manoeuvre that is effective in most cases.
Surgical technique: Preoperative mechanical bowel preparation is necessary, as is oral antibiotic cover to sterilise the bowel for 48 hours prior to surgery. The omentum is freed from the transverse colon and the hepatic and splenic flexures, while the entire colon is mobilised so that it can be placed outside the abdominal cavity for inspection of its vascular blood supply. Mobilising the sigmoid colon provides additional length so that the transverse colon can be tunnelled into the chest, to reach the neck. The proximal colon should be divided and, after anastomosis to the oesophagus, placed on sufficient stretch to prevent redundancy within the chest or in the substernal area. The colon should then be anchored in the straightened position by sutures to the crural margin of the hiatus, although not circumferentially. Continuity of the large bowel is re-established by end-to-end anastomosis, which is conveniently performed before the colo-jejunostomy or colo-gastrostomy for anatomical reasons. An excellent technical description of the use of various segments of colon has been provided by DeMeester et al.63 O’Sullivan and colleagues have described a useful refinement whereby resection of a short length of colon at either end of the chosen graft, leaving redundant mesentery, maximises blood supply at the critical points of the graft.64
Reconstruction with jejunum: Replacement of the lower oesophagus is accomplished using either a Roux-en-Y technique or by segmental interposition. Replacement of the upper oesophagus is accomplished by free jejunal transfer with microvascular anastomosis of the jejunal pedicle to neck vessels. It is sometimes possible to create a long loop for replacement of the entire thoracic oesophagus, particularly when the proximal jejunum has adapted after previous gastric surgery. The jejunum should be considered the third choice, after stomach and colon.
The technique of microvascular free jejunal transfer for reconstruction of the upper oesophagus is well described elsewhere.65 The specific indications for such a reconstruction are usually after pharyngo-laryngectomy performed for carcinoma of the hypopharynx, postcricoid region and cervical oesophagus. The operation is usually performed with a radical neck dissection as part of the primary treatment programme or as palliative surgery following recurrence after radiotherapy.
Open surgical approaches to oesophagectomy
Two-phase subtotal oesophagectomy via a right thoracotomy for carcinomas of the middle and lower thirds of the oesophagus
The abdominal oesophagus is dissected from the hiatus, taking a cuff of hiatal muscle if the tumour is located here, beginning the dissection into the chest. Anteriorly, all tissue posterior to the pericardium is taken en bloc, making the dissection plane for the thoracic phase clear. The right and left pleura at this point at this point are entered and resected en bloc if they are going to resect the oesophagus within its partial pleural envelope. There must be sufficient room at the hiatus for the conduit to lie unimpeded, so sometimes partial division of the right crus of the diaphragm is necessary.64
Combined synchronous two-team oesophagectomy: Modification of the standard access for oesophagectomy has been described wherein mobilisation of the stomach and abdominal oesophagus proceeds synchronously with mobilisation of the thoracic oesophagus via a right anterolateral thoracotomy using a second operating team.66,67 A reduction in operating and anaesthetic time was suggested as a possible reason for decreased operative morbidity and mortality rates in Hong Kong Chinese patients. Patients in the study had a lower incidence of pulmonary and cardiovascular disease than those with oesophageal cancer in the West.
Three-phase subtotal oesophagectomy for tumours of the upper middle third of the oesophagus
Exposing and dividing the oesophagus in the neck certainly provides excellent access for anastomosis, although it does not allow resection of much more oesophagus than can be removed by the two-phase approach. This is because the cervical oesophagus is relatively short and it is difficult to perform an anastomosis unless a stump of oesophagus is left, hence the term subtotal oesophagectomy. McKeown69 recommended cervical anastomosis on the grounds that a leak in the neck is less catastrophic than a thoracic leak. This is probably an overstatement and is now of less significance as overall true oesophageal anastomotic leakage is uncommon (ideally less than 5%). The three-phase operation takes longer to complete and is also associated with early postoperative difficulty in swallowing. This is probably because of the extensive proximal mobilisation of the cervical oesophagus. Proponents of the three-phase operation claim that a more complete oesophagectomy is achieved. If the tumour cannot be resected with an adequate proximal longitudinal margin then the three-phase technique ought to be employed.
Left-sided subtotal oesophagectomy for lower-third oesophageal cancers
A left-sided approach has been popular in the past for lower-third tumours, predominantly amongst thoracic surgeons, initially using a thoracotomy and phrenotomy, and later a thoraco-abdominal incision, crossing the costal arch.70,71 Exposure is not adequate to perform a thorough abdominal lymphadenectomy without dividing the costal arch, and indeed at least one author has described a higher positive resection margin with the left thoracotomy approach.72 A left-sided approach is absolutely contraindicated if the tumour is situated at or above the aortic arch as exposure of this part of the oesophagus is inadequate.
The left thoraco-abdominal approach is still appropriate for selected patients to resect tumours that have limited but significant involvement of the cardia73 and for patients requiring extended total gastrectomy. A good operative description is given by Sundaresan.70 It is important to use a circumferential incision to divide the diaphragm rather than a radial incision that denervates part of the left diaphragm. A paravertebral catheter can be used to provide good analgesia for this unilateral, single-dermatome incision.74 The Japanese Clinical Oncology Group trial has shown that, for proximal gastric cancer, the left thoraco-abdominal approach has a higher complication rate and no survival benefit compared to the alternative transhiatal approach to gastrectomy.75
Transhiatal oesophagectomy for upper- and lower-third tumours of the oesophagus
Controversy still exists about the role of oesophagectomy without thoracotomy in oesophageal cancer surgery. Proponents of the technique argue that outcome is dependent on the stage at presentation rather than the operative technique employed. Opponents claim improvements in survival for some undergoing radical en-bloc resection.25,35 The original technique was a blind procedure, defying the fundamental principle that surgery should always be carried out under direct vision.76–78 Nevertheless, refinements to the technique have been made and the operation has developed and gained many advocates.79 Orringer et al. have published a series of over 2000 transhiatal resections that demonstrates an improvement in outcomes over 30 years with refinements of technique.80 Data on resection margins and lymph node harvest are not supplied. When questioned about the absence of radical lymphadenectomy in these patients, Dr Orringer gave the caveat that if nodal disease is suspected by postneoadjuvant staging, he would opt for a transthoracic approach with formal lymphadenectomy.80 The issue of routine radical lymphadenectomy has been discussed earlier in this chapter.
A modified technique of transhiatal oesophagectomy under direct vision has been described81 using a modification of the transhiatal technique described by Pinotti.81 Almost the entire procedure is undertaken under direct vision, ensuring adequate local clearance by avoiding direct contact with the tumour, and the anastomosis performed in the neck as a combined synchronous operation. The authors demonstrated no evidence of proximal or distal resection margin involvement with the tumour and an acceptable morbidity and mortality.
Details of the surgical procedure are clearly described elsewhere.82 At present there are selected indications for transhiatal oesophagectomy:
• Carcinoma of the hypopharynx and cervical oesophagus. If the tumour is localised the incidence of mediastinal metastases is low. In this situation oesophagectomy without thoracotomy can be safely performed by blunt dissection. Radical neck dissection with pharyngolaryngo-oesophagectomy is carried out at the same time and reconstruction fashioned using the stomach through the posterior mediastinal route.
• Intraepithelial squamous carcinoma of the oesophagus. These tumours rarely disseminate via the lymphatics.19 With substantial progress in endoscopic techniques using epithelial dye staining and endoscopic ultrasonography, early tumours can be more accurately staged. When tumour penetration is confined to the epithelial layer, resection by transhiatal oesophagectomy is entirely feasible (Chapter 6).
• Patients with high-grade dysplasia within a Barrett segment, in whom endoscopic mucosal resection and/or radiofrequency ablation is not an option, in the absence of invasion or nodal disease.
The strongest evidence so far comes from the Dutch trial,40,43 which included 220 patients with adenocarcinoma of the middle and lower oesophagus. Significantly more nodes were dissected in the thoracic approach, but pulmonary complications were also greater. Significance was reached for an increased survival in the radical transthoracic approach, which continued with long-term data. Interestingly, patients with lower oesophageal tumours (Siewert type 1) and those with a low burden (1–8 nodes) of nodal disease appeared to benefit from extended transthoracic oesophagectomy, as discussed earlier in the chapter.
Minimally invasive surgical approaches to oesophagectomy
It is entirely appropriate that much energy has been invested in the search for the ‘holy grail’ of a radical oesophageal resection that can be undertaken with minimum trauma of access. However, outcomes from series of minimal access procedures must be comparable with the best outcomes from open surgery. Low and colleagues report a consecutive series of 340 patients, with an anastomotic leak rate of 3.4% and an in-hospital mortality of 0.3%, outcomes with which few if any minimally invasive series can compare. However, there were a variety of open procedures used by Low and colleagues, the majority being left thoraco-abdominal.85
Minimally invasive three-stage procedures
The first publications from Luketich et al.86,87 describing the total minimally invasive thoracoscopic, laparoscopic oesophagectomy with cervical anastomosis held out the potential of being the minimal access approach for some time. The procedure was technically challenging, even if a mini-laparotomy was added for gastric tube formation,88 and the learning curve was steep. For many surgeons who do not routinely perform a cervical anastomosis for lower-third tumour excision, adopting this operation would add, in their view, unnecessary complexity and risk. It was not worth adopting a three-stage approach to avoid the technical challenge of a minimally invasive intrathoracic anastomosis.
Despite enormous interest in the procedure, few centres other than Pittsburgh have published their results.88–93 Some authors reported significant anastomotic leak rates87,88 and highlighted an increased conduit necrosis rate.89 This was of sufficient concern that attempts were made by two centres to improve gastric conduit vascularity through ischaemic conditioning,94–96 although there are no randomised studies to show this to be effective. Non-randomised comparative studies from single institutions suggested that the three-stage total minimimally invasive procedure had equivalent but not better outcomes than open surgery.92–94 A multicentre feasibility study (Eastern Cooperative Oncology Group ECOG 2202) comprising 106 patients in 16 institutions suggests that short-term outcomes are acceptable.97
To the authors’ knowledge only one multicentre randomised controlled trial, comparing three-stage minimally invasive oesophagectomy with open surgery, has been conducted and recently published (TIME trial).98
Minimally invasive two-stage procedures
It is significant that Luketich and colleagues describe a change in practice, away from a cervical phase, favouring a minimally invasive two-stage laparoscopic, thoracoscopic procedure with a mini-thoracotomy, first reported in 2006.99 Levy and colleagues report disadvantages with the cervical phase including recurrent nerve injury, perturbations in pharyngeal transit and swallowing dysfunction even in the absence of overt recurrent laryngeal nerve injury.100 Thoracoscopic, laparoscopic two-stage oesophagectomy has become their preferred approach, but they caution a steep learning curve and emphasise the critical importance of appropriate thoracic port placement. There are few large series of this procedure, and no randomised studies on which to recommend this approach.
Minimally invasive hybrid procedures
There are two main hybrid approaches that are commonly undertaken. The first is a three-stage procedure with a thoracoscopic oesophageal mobilisation combined with an open abdominal phase and a cervical anastomosis. Smithers et al. have published series of this procedure in a non-randomised comparison with totally minimally invasive oesophagectomy.92 The advantage of this approach is extracorporeal gastric conduit formation together with manual passing of the conduit through the mediastinum to the neck, avoiding traction injury, carefully presenting the conduit to the neck. Disadvantages are those of a cervical phase mentioned above as well as the need for an epidural for analgesia.
The second hybrid procedure is a two-stage approach with a laparoscopic abdominal phase and an open thoracic phase. There are few series of this approach published, but a prospective randomised trial (MIRO trial) has been completed at the time of writing, awaiting publication of early outcomes.101 The potential advantages of this approach are, again, extracorporeal gastric conduit formation, ideal conditions for performing an anastomosis and gentle handling of the conduit within the chest. Another advantage is that an epidural catheter can be avoided if a paravertebral catheter is used for analgesia.102,103
While we do not have robust evidence to recommend minimal access approaches to oesophagectomy in favour of open procedures at the time of writing, there is evidence that Health Related Quality of Life appears to be well preserved, at least in the early postoperative period, in particular with total minimally invasive approaches,104 although this may be a short-lived advantage, with one series reporting significantly more delayed gastric emptying.90
Overview of minimally invasive approaches
The many individual series of hybrid or totally minimally invasive resection have been reviewed collectively,46,58–61 showing no advantage, or a relatively small advantage of a minimally invasive approach. There may be better evidence in the near future from prospective randomised trials,98,101 but in the absence of this quality of evidence, the authors’ opinion is that totally minimally invasive and hybrid approaches to oesophagectomy have yet to prove themselves superior to open surgery.105
Current practice
Mamidanna et al.106 have recently published HES (Hospital Episode Statistics) data showing that, of the 7502 oesophageal resections undertaken in England between April 2005 and March 2010, 15.5% were performed with a minimally invasive component. This has risen to 24.7% for the year 2009–10, a fourfold increase over the decade. These procedures were not uniformly distributed amongst surgeons or trusts. The authors examined whether there were differences in short-term outcome between minimally invasive and open procedures.
Rice and Blackstone107 raise significant questions about minimally invasive approaches to oesophagectomy that arise from this study and list them in their editorial.
Technique of anastomosis
One-, two- and three-layer anastomoses have been described, but no conclusive randomised controlled studies have been reported. A two-layer oesophagogastric anastomosis is advocated by Akiyama,19 who emphasises the absence of a serosal layer, which he believes would reinforce strength at the anastomotic site. He therefore advocates a carefully preserved adventitia, which provides sufficient strength to support sutures.
Stapling devices have been developed for ease of introduction and application, with a low-profile head that permits a larger-diameter anvil to be introduced into the oesophageal stump. A larger-diameter anastomosis is thereby fashioned, reducing the rate of benign anastomotic stricture formation, most commonly seen with a staple ring diameter of 25 mm or less.9,52,108 The staple head can now also be inserted transorally, allowing a double-staple technique, similar to that in colorectal surgery, to be used.
Anastomotic leakage is more frequent in the neck than the chest, although the related mortality rates have not been shown to differ between these anastomotic sites.109 Leakage rate does not depend on suture material or the technical modalities used to perform the anastomosis. Indeed, there is no evidence that the overall decrease in anastomotic complications is related to the use of a specific conduit approach or route of reconstruction; it is more likely due to progress made in general perioperative management.110
The semi-mechanical anastomosis, with a side-to-side configuration, a linear stapled posterior wall and a hand-sewn anterior wall has been described by Collard et al.111 and by Orringer et al.112 with an associated reduction in cervical anastomotic leak rate and long-term stricture rate. However, the majority of these anastomoses have been performed during transhiatal oesophagectomy for lower-third tumours, so that sufficient length of cervical oesophagus could be left without compromising resection margins. The authors would caution against this anastomosis if the purpose of the cervical dissection is primarily proximal clearance; in this situation a high anastomosis using interrupted sutures to a short cervical oesophagus allows more proximal clearance. The authors have many years’ experience of wrapping the anastomosis with transposed omentum.
Postoperative management
A detailed account of immediate postoperative care after oesophageal cancer surgery is described in Chapter 4, and a summary is given in Boxes 5.4 and 5.5. Meticulous attention to the maintenance of fluid balance and respiratory care is essential in the immediate postoperative period. Adequate pain control via a thoracic epidural and physiotherapy are crucial. It is the authors’ routine practice to enterally feed patients undergoing oesophagectomy in the postoperative period, commencing feeding via jejunostomy early postoperatively. Early mobilisation as part of an enhanced recovery programme is important in preventing venous thrombosis and pulmonary embolus. It also enhances ventilation, clearance of sputum and early bowel movement. Removal of the chest drains by the fifth postoperative day helps in mobilisation, especially once free oral fluids have recommenced.
The role of routine postoperative radiological imaging of the oesophageal anastomosis has become clearer. There is no evidence that the routine use of contrast radiology is of any value in patients who are asymptomatic in the postoperative phase.113,114 Patients who are clinically well should be started on oral feeding, while video endoscopy or contrast radiology should be reserved for patients showing signs of sepsis, pleural effusion or haemodynamic instability. Non-ionic contrast media may pick up gross leaks, but if no leak is shown should be followed up by barium investigations or an endoscopy to exclude a small leak.
Postoperative complications
Postoperative complications may be subdivided into those that are common to any major surgical procedure in an elderly population and those specific to oesophageal resection. The complication rate of oesophageal surgery is relatively high, in the region of 30–40%. Some studies have found increased morbidity rates following neoadjuvant therapy, particularly respiratory problems after chemoradiotherapy. This seems to be further compounded with salvage oesophagectomy after definitive chemoradiotherapy for squamous carcinoma.115 Early recognition of complications and rapid proactive management are essential to achieve good results for all patients. It has been proposed that postoperative complications are not only associated with poor early outcome, but also, possibly through immunosuppression, with early death from cancer recurrence.116
General complications
These complications (see also Chapter 4) may be minimised by improved preoperative patient evaluation. Respiratory complications constitute the largest proportion of this group. Pain is the major contributor to decreased ventilation and atelectasis, which leads to bronchopneumonia and respiratory failure. Extensive lymphadenectomy can cause poor lymphatic drainage of the pulmonary alveoli, leading to parenchymal fluid retention and a consequent acute pulmonary oedema. Significant respiratory complications occur in approximately 24% of cases following subtotal oesophagectomy.117
Major haemorrhage is uncommon and as a result of meticulous technique and the use of new techniques, such as the ultrasonic scalpel during gastric mobilisation, routine blood loss is less than 500 mL, with only a small minority of patients requiring transfusion. Secondary haemorrhage is also rare and is almost always associated with a mediastinal infection from a specific complication such as an anastomotic leakage. The value of minimisation of surgical blood loss should not be underestimated. Perioperative blood transfusion is a significant predictor of decreased overall survival.118
Specific complications
Anastomotic leakage and leakage from the gastric conduit
Anastomotic leakage is influenced by a variety of factors, including cancer hypermetabolism, malnutrition, anastomotic vascular deficit, anastomotic tension and surgical technique. The incidence of anastomotic leakage has decreased significantly over the last 10 years and rates of well under 5% should be expected.110,113,119
Dehiscence of the gastric resection line is rare but requires re-exploration as the extent of leakage is frequently large.113
Chylothorax
The thoracic duct can often be damaged during mobilisation of advanced oesophageal cancers, whether via a right thoracotomy or through the transhiatal route. A comprehensive review reports chylothorax occurring in up to 10% of patients after blunt transhiatal oesophagectomy.120 An incidence of 2–3% during open resection is commonly reported.121 Accidental damage to the thoracic duct can be prevented by identification during dissection, as previously described, and ligating the duct low in the inferior mediastinum on the right lateral aspect of the descending thoracic aorta. Chylothorax usually presents in the first 7 days after surgery, when the patient has commenced oral intake, or jejunostomy feeds, especially of fat-containing nutrients. A massive increase in chest drainage occurs, that if left untreated results in malnutrition and significant immune suppression, with a markedly reduced CD4 count, from the subsequent white cell loss. It is difficult to predict whether a chylous leak will spontaneously heal despite attempts to quantify the size of the leak.122 Immediate re-exploration is therefore recommended for major leaks, as the damaged thoracic duct is usually easily identified, following a bolus of cream, at the time of re-exploration.121 Leaks of less than 500 mL/day may resolve with enteral feeding using medium-chain triglycerides. Prolonged total parenteral nutrition has been used but patients who rapidly become malnourished are prone to nosocomial infections and frequently require a long hospital stay. Prophylactic antibiotic cover with co-trimoxazole for Pneumocystis is essential for the lymphopenic patient.123 On rare occasions, chylothorax can be resistant to treatment, whether re-exploration or conservative therapy. This is often due to abnormal lymph anatomy around the hiatus. The author has documented up to three large ducts in the posterior mediastinum in such patients. Pleuroperitoneal shunting has resulted in successful outcomes in resistant chylous leaks. This allows reabsorption of chyle and prevents the sequelae of immune suppression.
Recurrent laryngeal palsy
The incidence of recurrent laryngeal palsy has increased over recent years due to the increase of cervical oesophagogastric anastomoses. It is extremely rare when the anastomosis is constructed in the apex of the chest via the thoracotomy route for subtotal oesophagectomy. If the palsy is transient but unilateral, the opposite cord may well compensate. If the palsy is permanent, Teflon injection of the cord or a formal thyroplasty can restore adequate voice volume and a satisfactory cough.124
Duodeno-gastro-oesophageal reflux
Acid or alkaline reflux is common125,126 and, although it may be controlled by motility agents and acid suppressants, can be troublesome. There is some evidence that performing a modified fundoplication as an antireflux manoeuvre at the time of oesophagectomy is effective in controlling postoesophagectomy reflux in the majority of patients.127
Overall results of single-modality resectional therapy
Overall results of surgical therapy in oesophageal cancer can be analysed in terms of hospital mortality and patient survival. Assessment of quality of life (patient-related outcomes) as an outcome measure is essential as there is increasing evidence relating it to overall survival.128 The fact that it takes 9 months for quality of life to recover following surgery illustrates the scale of trauma that oesophagectomy produces. Very few new data have become available on single-modality surgery for oesophageal cancer. Increasingly, published results include patients subjected to multimodality treatments.
Hospital mortality
Although individual units have achieved considerably better results, three comprehensive reviews during the last two decades shed some light on trends in both hospital mortality and overall survival.8,129,130
This may be attributed to improvements in anaesthesia, surgical technique, perioperative care, and the specialisation and centralisation of oesophageal cancer services. No evidence has been provided to relate tumour biology to mortality rate following oesophageal resection and there is no difference in mortality rates between resections for squamous cell carcinoma and adenocarcinoma. Overall mortality rates in many series can be confusing because of variations in definitions. ‘In-hospital’ and not 30-day mortality rates should be quoted in all papers, but unfortunately this continues not to be the case. Series from specialist centres in the last few years cite operative hospital mortality rates of less than 5%.31,51,113 This includes a huge series of over 20 000 oesophagectomies from China.131 There is no longer any place for the occasional oesophagectomist in the management of this disease. There is clearly still further room for improvement as data from a large multicentre UK audit recently reported mortality rates of over 10%.132
Comparisons of hospital mortality rates for different resection techniques reveal only minor differences. In the review by Muller et al.,119 the lowest mortality rate was for transhiatal oesophagectomy, with a median figure of 8%. These data, however, are not strictly comparable because transhiatal resection was the most recent surgical development and therefore benefited from the experience of recent advances in perioperative care.
Rigorous preoperative assessment will continue to reduce hospital mortality from this major thoraco-abdominal operation (see Chapter 4).
Survival figures
In a review of the 1980s, Muller et al. found that 56% of all resected patients survived the first postoperative year, 34% the second, 25% the third, 21% the fourth and 20% the fifth year after resection. It was depressing to note that these figures were very similar to those collected by Earlam and Cunha Melo, revealing that despite improved hospital mortality, the overall long-term prognosis had remained unchanged. No differences in the 5-year survival rates were noted between different techniques of resection but en-bloc resections showed a significantly better long-term prognosis.28,77 Data from the Dutch trial40 revealed 5-year survival was 36% and 34% after transhiatal and transthoracic resection, respectively. There is some evidence to suggest that adenocarcinomas tend to fare worse than squamous lesions, although this may simply reflect the more advanced stage at which these lesions tend to present.72 With increasing numbers of early tumours being diagnosed on surveillance programmes for Barrett’s oesophagus, this hypothesis will be tested. The primary determinants of overall outcome appear to be the stage of the tumour and the cell type.
Trying to identify improvements in overall survival for adenocarcinoma over time from surgery alone is difficult because of the now widespread use of neoadjuvant treatment for locally advanced disease. Over the past decade the continued reporting of results combining both adenocarcinoma and squamous cell cancers has made interpretation even more confounding. Most recent ‘surgery-alone’ series reflect operations for very early disease that would not allow for comparisons with the earlier publications. However, as discussed earlier there are data from the Dutch trial that revealed 5-year survival for adenocarcinoma was 34% and 36% after transhiatal and transthoracic resection, respectively.40 The long-term results of the OEO2 study showed that for adenocarcinoma the unimodality surgery patients had a 5-year survival of 17.6%.133 A population-based study from Sweden evaluated survival with resection alone from 1997 to 2005: the 5-year survival for adenocarcinoma was 28.3% for the 2001–2005 cohort.134 The Worldwide Esophageal Cancer Collaboration (WECC) data included surgery-alone patients from several decades and produced separate survival curves for adenocarcinoma and squamous cancer.135 Five-year survival for adenocarcinoma was approximately 80% for TNM7 stages 0 and 1A, approximately 64% for stage 1B, 50% for 2A, 40% for 2B and 25% for 3A.135
Overall survival is therefore strongly stage dependent. There are many case series describing stage-specific survival but there has been no systematic review of these reports. The authors’ published results confirm a greater than 90% 5-year survival for stage 0 and stage 1 disease. For stage 2a, 2b and stage 3 disease, 5-year survival is 60%, 19% and 15% respectively.31 Other specialist units have achieved similar results with resection and two-field lymphadenectomy as unimodality therapy.17,31,38,40,41,45,51 The poor outcome for patients with node-positive disease has led to multimodality therapy becoming the standard of care for these patients. A recent meta-analysis demonstrated a greater 2-year survival benefit for both neoadjuvant chemoradiotherapy and chemotherapy, although the standardisation of the staging investigations and surgical resection has been questioned in many of these trials115 (see Chapter 9).
Summary and future research
The main areas of progress and interest in surgery for oesophageal cancer have been the introduction of a multidisciplinary approach, improved disease staging, the development of new surgical and endoscopic techniques for the management of early tumours including minimally invasive oesophagectomy, and the introduction of multimodality therapy for locally advanced disease. The future of oesophageal cancer surgery will be based on procedures tailored to the individual patient. Certain patients with early adenocarcinoma may initially undergo endoscopic resection to identify those requiring a formal oesophagectomy. These patients may undergo sentinel node mapping53 such that patients potentially can be spared radical node dissection. Patients with locally advanced adenocarcinoma, particularly those with a low burden of nodal disease, will be targeted with increasingly effective multimodality regimens including, based on the Dutch trial, a radical en-bloc oesophagectomy with two-field lymph node dissection. Neoadjuvant regimes should be tailored, possibly by genetic profiling, to determine the best therapeutic strategy for each patient. Despite all this, significant improvements in long-term outcome for oesophageal cancer will only be achieved if focus is placed on earlier detection of what continues to be a very aggressive disease.
References
1. Sobin, L.H., Gospodarowicz, M.K., Witteking, C.h. TNM classification of malignant tumours, 7th ed. Oxford: Wiley-Blackwell; 2009.
2. Japanese Society for Oesophageal Diseases. Guidelines for the clinical and pathological studies on carcinoma of the oesophagus. Part 1: clinical classification. Jpn J Surg. 1976; 6:64–78.
3. Tetteroo, G.W., Wagenvoort, J.H., Castelein, A., et al, Selective decontamination to reduce Gram-negative colonisation and infections after oesophageal resection. Lancet. 1990;335(8691):704–707. 1969068
4. Moore, F.A., Feliciano, D.V., Andrassy, R.J., et al, Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216(2):172–183. 1386982 This meta-analysis emphasises the benefits of enteral feeding in the perioperative period.
5. Gianotti, L., Braga, M., Nespoli, L., et al, A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer [see comment]. Gastroenterology. 2002;122(7):1763–1770. 12055582
6. Couper, G., Jejunostomy after oesophagectomy: a review of evidence and current practice. Proc Nutr Soc 2011; 70:316–320. 21781359
7. Sultan, J., Griffin, S.M., Di Franco, F., et al, Randomized clinical trial of omega-3 fatty acid-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br J Surg. 2012;99(3):346–355. 22237467
8. Markides, G.A., Alkhaffaf, B., Vickers, J., Nutritional access routes following oesophagectomy – a systematic review. Eur J Clin Nutr 2011; 65:565–573. 21407246
9. Griffin, S.M., Woods, S.D., Chan, A., et al, Early and late surgical complications of subtotal oesophagectomy for squamous carcinoma of the oesophagus. J R Coll Surg Edinb. 1991;36(3):170–173. 1920231
10. Lerut, T., Oesophageal carcinoma – past and present studies. Eur J Surg Oncol. 1996;22(4):317–323. 8783643
11. Gebski, V., Burmeister, B., Smithers, B.M., et al, Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a metaanalysis. Lancet Oncol. 2007;8(3):226–234. 17329193
12. Department of Health. Guidance on commissioning cancer services. Improving outcomes in uppergastrointestinal cancers. The manual. London: NHS Executive, 2001; .
13. Sutton, D.N., Wayman, J., Griffin, S.M., Learning curve for oesophageal cancer surgery. Br J Surg. 1998;85(10):1399–1402. 9782024
14. Finlayson, E.V., Goodney, P.P., Birkmeyer, J.D., et al, Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138(7):721–726. 12860752
15. Kuo, E.Y., Chang, Y., Wright, C.D., et al, Impact of hospital volume on clinical and economic outcomes for esophagectomy. Ann Thorac Surg. 2001;72(4):1118–1124. 11603422
16. Begg, C.B., Cramer, L.D., Hoskins, W.J., et al, Impact of hospital volume on operative mortality for major cancer surgery [see comment]. JAMA. 1998;280(20):1747–1751. 9842949
17. Skinner, D.B., En bloc resection for neoplasms of the esophagus and cardia. J Thorac Cardiovasc Surg. 1983;85(1):59–71. 6848888
18. Siu, K.F., Cheung, H.C., Wong, J., et al, Shrinkage of the esophagus after resection for carcinoma. Ann Surg. 1986;203(2):173–176. 3947154
19. Akiyama, H. Surgery for cancer of the oesophagus. Baltimore: Williams & Wilkins; 1990.
20. Mandard, A.M., Chasle, J., Marnay, J., et al, Autopsy findings in 111 cases of esophageal cancer. Cancer. 1981;48(2):329–335. 6453643
21. Sons, H.U., Borchard, F., Cancer of the distal esophagus and cardia. Incidence, tumorous infiltration, and metastatic spread. Ann Surg. 1986;203(2):188–195. 3947155
22. Dexter, S.P., Sue-Ling, H., McMahon, M.J., et al, Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut. 2001;48(5):667–670. 11302966
23. Pultrum, B.B., Honing, J., Smit, J.K., et al, A critical appraisal of circumferential resection margins in esophageal carcinoma. Ann Surg Oncol 2010; 17:812–820. 19924487
24. Roder, J.D., Busch, R., Stein, H.J., et al, Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg. 1994;81(3):410–413. 8173915
25. Lerut, T., De Leyn, P., Coosemans, W., et al, Surgical strategies in esophageal carcinoma with emphasis on radical lymphadenectomy. Ann Surg. 1992;216(5):583–590. 1444650 This important publication sets out the evidence for radical en-bloc resection with lymphadenectomy, demonstrating a reduction in operative mortality during the previous decade and excellent survival in relatively advanced disease.
26. Rice, T.W., Rusch, V.W., Apperson-Hansen, C., et al, Worldwide esophageal cancer collaboration. Dis Esophagus 2009; 22:1–8. 19196264
27. Sato, T., Sacamoto, K. Illustrations and photographs of surgical oesophageal anatomy, specially prepared for lymph node dissection. In: Sato T., Sacamoto K., eds. Colour atlas of surgical anatomy for oesophageal cancer. Toyko: Springer; 1992:25–90.
28. Tanabe, G., Baba, M., Kuroshima, K., et al, Clinical evaluation of the esophageal lymph flow system based on RI uptake of dissected regional lymph nodes following lymphoscintigraphy [in Japanese]. Nippon Geka Gakkai Zasshi. 1986;87(3):315–323. 3713683
29. Orringer, M.B., Marshall, B., Iannettoni, M.D., Transhiatal esophagectomy for benign and malignant esophageal disease. World J Surg. 2001;25(2):196–203. 11338022
30. Peyre, C.G., Hagen, J.A., DeMeester, S.R., et al, Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008; 248:979–985. 19092342
31. Dresner, S.M., Griffin, S.M., Pattern of recurrence following radical oesophagectomy with two-field lymphadenectomy. Br J Surg. 2000;87(10):1426–1433. 11044172
32. Akiyama, H., Tsurumaru, M., Udagawa, H., et al, Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220(3):364–373. 8092902
33. Hagen, J.A., DeMeester, S.R., Peters, J.H., et al, Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg 2001; 234:520–530. 11573045
34. Sihvo, E.I., Luostarinen, M.E., Salo, J.A., Fate of patients with adenocarcinoma of the esophagus and the esophagogastric junction: a population-based analysis. Am J Gastroenterol 2004; 99:419–424. 15056079
35. Clark, G.W., Peters, J.H., Ireland, A.P., et al, Nodal metastasis and sites of recurrence after en bloc esophagectomy for adenocarcinoma. Ann Thorac Surg. 1994;58(3):646–654. 7944684
36. Altorki, N.K., Girardi, L., Skinner, D.B., En bloc esophagectomy improves survival for stage III esophageal cancer. J Thorac Cardiovasc Surg 1997; 114:948–955. 9434690
37. Hagen, J.A., Peters, J.H., DeMeester, T.R., Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 1993; 106:850–858. 8231207
38. Altorki, N., Kent, M., Ferrara, C., et al, Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236(2):177–183. 12170022
39. Cunningham, D., Allum, W.H., Stenning, S.P., MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355:11–20. 16822992
40. Hulscher, J.B., van Sandick, J.W., de Boer, A.G., et al, Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347(21):1662–1669. 12444180 This trial suggests that both techniques are safe but that there is lower morbidity in the transhiatal group and a trend to longer survival in the extended transthoracic groups.
41. Goldminc, M., Maddern, G., Le Prise, E., et al, Oesophagectomy by a transhiatal approach or thoracotomy: a prospective randomized trial. Br J Surg. 1993;80(3):367–370. 8472154
42. Kato, H., Watanabe, H., Tachimori, Y., et al, Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. Ann Thorac Surg. 1991;51(6):931–935. 2039322
43. Omloo, J.M., Lagarde, S.M., Hulscher, J.B., et al, Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007; 246:992–1000. 18043101
44. Johansson, J., DeMeester, T.R., Hagen, J.A., et al, En bloc vs transhiatal esophagectomy for stage T3 N1 adenocarcinoma of the distal esophagus. Arch Surg 2004; 139:627–631. 15197089
45. Kato, H., Tachimori, Y., Mizobuchi, S., et al, Cervical, mediastinal, and abdominal lymph node dissection (three-field dissection) for superficial carcinoma of the thoracic esophagus. Cancer. 1993;72(10):2879–2882. 8221552
46. Law, S., Cheung, M.C., Fok, M., et al, Pyloroplasty and pyloromyotomy in gastric replacement of the esophagus after esophagectomy: a randomized controlled trial. J Am Coll Surg. 1997;184(6):630–636. 9179120
47. Peyre, C.G., Hagen, J.A., DeMeester, S.R., et al, The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008; 248:549–556. 18936567
48. Schwarz, R.E., Smith, D.D., Clinical impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg 2007; 11:1384–1393. 17764019
49. Smith, D.D., Schwarz, R.R., Schwarz, R.E., Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005; 23:7114–7124. 16192595
50. Rizk, N., Venkatraman, E., Park, B., et al, The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2006; 132:1374–1381. 17140960
51. Lerut, T., Coosemans, W., De Leyn, P., et al, Is there a role for radical esophagectomy. Eur J Cardiothorac Surg. 1999;16(Suppl. 1):S44–S47. 10536946
52. Siewert, J.R., Roder, J.D., Lymphadenectomy in oesophageal cancer surgery. Dis Esophagus 1992; 2:91–97. 18197944
53. Lamb, P.J., Griffin, S.M., Burt, A.D., et al, Sentinel node biopsy to evaluate the metastatic dissemination of oesophageal adenocarcinoma. Br J Surg. 2005;92(1):60–67. 15584066
54. Gawad, K.A., Hosch, S.B., Bumann, D., et al, How important is the route of reconstruction after esophagectomy: a prospective randomized study. Am J Gastroenterol. 1999;94(6):1490–1496. 10364012
55. Bartels, H., Thorban, S., Siewert, J.R., Anterior versus posterior reconstruction after transhiatal oesophagectomy: a randomized controlled trial. Br J Surg. 1993;80(9):1141–1144. 8402115 These trials confirm that the mediastinal route is the preferred route for reconstruction after curative resection.
56. Tabira, Y., Sakaguchi, T., Kuhara, H., et al, The width of a gastric tube has no impact on outcome after esophagectomy. Am J Surg. 2004;187(3):417–421. 15006575
57. Akiyama, H., Miyazono, H., Tsurumaru, M., et al, Use of the stomach as an esophageal substitute. Ann Surg 1978; 188:606–610. 718285
58. el-Eishi, H.I., Ayoub, S.F., el-Khalek, M.A., The arterial supply of the human stomach. Acta Anat (Basel). 1973;86(3):565–580. 4785693
59. Thomas, D.M., Langford, R.M., Russell, R.C., et al, The anatomical basis for gastric mobilization in total oesophagectomy. Br J Surg. 1979;66(4):230–233. 454988
60. Khan, O.A., Manners, J., Rengarajan, A., et al, Does pyloroplasty following esophagectomy improve early clinical outcomes? Interact Cardiovasc Thorac Surg. 2007;6(2):247–250. 17669829
61. Cheung, H.C., Siu, K.F., Wong, J., Is pyloroplasty necessary in esophageal replacement by stomach? A prospective, randomized controlled trial. Surgery. 1987;102(1):19–24. 3296264
62. Ventemiglia, R., Khalil, K.G., Frazier, O.H., et al, The role of preoperative mesenteric arteriography in colon interposition. J Thorac Cardiovasc Surg. 1977;74(1):98–104. 875446
63. DeMeester, T.R., Johansson, K.E., Franze, I., et al, Indications, surgical technique, and long-term functional results of colon interposition or bypass. Ann Surg. 1988;208(4):460–474. 3178334
64. Maguire, D., Collins, C., O’Sullivan, G.C., How I do it – Replacement of the oesophagus with colon interposition graft based on the inferior mesenteric vascular system. Eur J Surg Oncol 2001; 27:314–315. 11393186
65. Sasaki, T.M., Baker, H.W., McConnell, D.B., et al, Free jejunal graft reconstruction after extensive head and neck surgery. Am J Surg. 1980;139(5):650–653. 7468913
66. Nanson, E.M., Synchronous combined abdominothoraco- cervical (oesophagectomy). Aust N Z J Surg. 1975;45(4):340–348. 1061553
67. Chung, S.C., Griffin, S.M., Wood, S.D., et al, Two team synchronous esophagectomy. Surg Gynecol Obstet. 1990;170(1):68–69. 2294632
68. Hayes, N., Shaw, I.H., Raimes, S.A., et al, Comparison of conventional Lewis–Tanner two-stage oesophagectomy with the synchronous two-team approach. Br J Surg. 1995;82(3):426. 7796043 This small randomised trial demonstrated higher complication and mortality rates in Western patients operated on by the synchronous technique.
69. McKeown, K.C., The surgical treatment of carcinoma of the oesophagus. A review of the results in 478 cases. J R Coll Surg Edinb. 1985;30(1):1–14. 3989754
70. Sundaresan, S. Left thoracoabdominal incision. Operat Tech Thorac Cardiovasc Surg. 2003; 8:71–85.
71. Matthews, H.R., Steel, A., Left-sided subtotal oesophagectomy for carcinoma. Br J Surg. 1987;74(12):1115–1117. 3427357
72. Molina, J.E., Lawton, B.R., Myers, W.O., et al, Esophagogastrectomy for adenocarcinoma of the cardia. Ten years’ experience and current approach. Ann Surg. 1982;195(2):146–151. 7055390
73. Forshaw, M.J., Gossage, J.A., Ockrim, J., et al, Left thoracoabdominal esophagogastrectomy: still a valid operation for carcinoma of the distal esophagus and esophagogastric junction. Dis Esophagus. 2006;19(5):340–345. 16984529
74. Kelly, F.E., Murdoch, J.A., Sanders, D.J., et al, Continuous paravertebral block for thoraco-abdominal oesophageal surgery. Anaesthesia 2005; 60:98–99. 15601286
75. Sasako, M., Sano, T., Yamamoto, S., et al, Left thoracoabdominal approach versus abdominal–transhiatal approach for gastric cancer of the cardia or sub-cardia: a randomised controlled trial. Lancet Oncol. 2006;7(8):644–651. 16887481
76. Le Quesne, L.P., Ranger, D., Pharyngolaryngectomy, with immediate pharyngogastric anastomosis. Br J Surg. 1966;53(2):105–109. 4952325
77. Turner, G.G. Excision of thoracic oesophagus for carcinoma with construction of an extra thoracic gullet. Lancet. 1933; 1:1315–1316.
78. Ong, G.B. Carcinoma of the hypo-pharynx and cervical oesophagus. In: Smith R.E., ed. Progress in clinical surgery. London: J & A Churchill; 1969:155–178.
79. Orringer, M.B., Sloan, H., Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg. 1978;76(5):643–654. 703369
80. Orringer, M.B., Marshall, B., Chang, A.C., et al, Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007; 246:363–372. 17717440
81. Alderson, D., Courtney, S.P.. Kennedy RH. Radical transhiatal oesophagectomy under direct vision Br J Surg 1994; 81:404–407. 8173913
82. Pinotti, H.W., A new approach to the thoracic esophagus by the abdominal transdiaphragmatic route. Langenbecks Arch Chir. 1983;359(4):229–235. 6855378
83. Chu, K.M., Law, S.Y., Fok, M., et al, A prospective randomized comparison of transhiatal and transthoracic resection for lower-third esophageal carcinoma. Am J Surg. 1997;174(3):320–324. 9324146
84. Jacobi, C.A., Zieren, H.U., Muller, J.M., et al, Surgical therapy of esophageal carcinoma: the influence of surgical approach and esophageal resection on cardiopulmonary function. Eur J Cardiothorac Surg. 1997;11(1):32–37. 9030787 These two small randomised studies failed to demonstrate differences in cardiopulmonary complications between the transhiatal and transthoracic approaches.
85. Low, D.E., Kunz, S., Schembre, D., et al, Esophagectomy – it’s not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg 2007; 11:1395–1402. 17763917
86. Luketich, J.D., Schauer, P.R., Christie, N.A., et al, Minimally invasive esophagectomy. Ann Thorac Surg 2000; 70:906–911. 11016332
87. Luketich, J.D., velo-Rivera, M., Buenaventura, P.O., et al, Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003; 238:486–494. 14530720
88. Palanivelu, C., Prakash, A., Senthilkumar, R., et al, Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position – experience of 130 patients. J Am Coll Surg 2006; 203:7–16. 16798482
89. Berrisford, R.G., Wajed, S.A., Sanders, D., et al, Short term outcomes following total minimally invasive oesophagectomy. Br J Surg 2008; 95:602–610. 18324607
90. Nafteux, P., Moons, J., Coosemans, W., et al, Minimally invasive oesophagectomy: a valuable alternative to open oesophagectomy for the treatment of early oesophageal and gastro-oesophageal junction carcinoma. Eur J Cardiothorac Surg. 2011;40(6):1455–1464. 21514837
91. Nguyen, N.T., Hinojosa, M.W., Smith, B.R., et al, Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg 2008; 248:1081–1091. 19092354
92. Smithers, B.M., Gotley, D.C., Martin, I., et al, Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 2007; 245:232–240. 17245176
93. Zingg, U., McQuinn, A., DiValentino, D., et al, Minimally invasive versus open esophagectomy for patients with esophageal cancer. Ann Thorac Surg 2009; 87:911–919. 19231418
94. Berrisford, R.G., Veeramootoo, D., Parameswaran, R., et al, Laparoscopic ischaemic conditioning of the stomach may reduce gastric-conduit morbidity following total minimally invasive oesophagectomy. Eur J Cardiothorac Surg 2009; 36:888–893. 19615914
95. Nguyen, N.T., Nguyen, X.M., Reavis, K.M., et al, Minimally invasive esophagectomy with and without gastric ischemic conditioning. Surg Endosc. 2012;26(6):1637–1641. 22179469
96. Nguyen, N.T., Longoria, M., Sabio, A., et al, Preoperative laparoscopic ligation of the left gastric vessels in preparation for esophagectomy. Ann Thorac Surg 2006; 81:2318–2320. 16731189
97. Luketich, J., Pennathur, A., Catalano, P.J., et al. Results of a phase II multicenter study of minimally invasive esophagectomy (Eastern Cooperative Oncology Group Study E2202). J Clin Oncol ASCO Annual Meeting Proceedings. 2009; 27:4516.
98. Biere, S.S., Maas, K.W., Bonavina, L., et al, Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial). BMC Surg 2011; 11:2. 21226918
99. Bizekis, C., Kent, M.S., Luketich, J.D., et al, Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2006; 82:402–406. 16863737
100. Levy, R.M., Wizorek, J., Shende, M., et al, Laparoscopic and thoracoscopic esophagectomy. Adv Surg 2010; 44:101–116. 20919517
101. Briez, N., Piessen, G., Bonnetain, F., et al, Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial – the MIRO trial. BMC Cancer 2011; 11:310. 21781337
102. Berrisford, R.G., Sabanathan, S.S., Mearns, A.J., et al, Pulmonary complications after lung resection: the effect of continuous extrapleural intercostal nerve block. Eur J Cardiothorac Surg 1990; 4:407–410. 2223115
103. Sabanathan, S., Mearns, A.J., Bickford Smith, P.J., et al, Efficacy of continuous extrapleural intercostal nerve block on post-thoracotomy pain and pulmonary mechanics. Br J Surg 1990; 77:221–225. 2180536
104. Parameswaran, R., Blazeby, J.M., Hughes, R., et al, Health-related quality of life after minimally invasive oesophagectomy. Br J Surg 2010; 97:525–531. 20155792
105. Berrisford, R.G., Editorial comment: Totally minimally invasive three-stage oesophagectomy – an achievable goal or a step too far? Eur J Cardiothorac Surg 2011; 40:1464–1465. 21511486
106. Mamidanna, R., Bottle, A., Aylin, P., et al, Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg 2012; 255:197–203. 22173202
107. Rice, T.W., Blackstone, E.H., Minimally invasive versus open esophagectomy for cancer: more questions than answers. Ann Surg 2012; 255:204–205. 22241287
108. Dresner, S.M., Lamb, P.J., Wayman, J., et al, Benign anastomotic stricture following transthoracic subtotal oesophagectomy and stapled oesophago- gastrostomy: risk factors and management. Br J Surg. 2000;87(3):362–373. 10718969
109. Egberts, J.H., Schniewind, B., Bestmann, B., et al, Impact of the site of anastomosis after oncologic esophagectomy on quality of life – a prospective, longitudinal outcome study. Ann Surg Oncol. 2008;15(2):566–575. 17929101
110. Lerut, T., Coosemans, W., Decker, G., et al, Anastomotic complications after esophagectomy. Dig Surg. 2002;19(2):92–98. 11978992
111. Collard, J.M., Romagnoli, R., Goncette, L., et al, Terminalized semimechanical side-to-side suture technique for cervical esophagogastrostomy. Ann Thorac Surg 1998; 65:814–817. 9527220
112. Orringer, M.B., Marshall, B., Iannettoni, M.D., Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 2000; 119:277–288. 10649203
113. Griffin, S.M., Lamb, P.J., Dresner, S.M., et al, Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg. 2001;88(10):1346–1351. 11578290
114. Lamb, P.J., Griffin, S.M., Chandrashekar, M.V., et al, Prospective study of routine contrast radiology after total gastrectomy. Br J Surg. 2004;91(8):1015–1019. 15286964
115. Smithers, B.M., Cullinan, M., Thomas, J.M., et al, Outcomes from salvage esophagectomy post definitive chemoradiotherapy compared with resection following preoperative neoadjuvant chemoradiotherapy. Dis Esophagus. 2007;20(6):471–477. 17958721
116. Lagarde, S.M., de Boer, J.D., ten Kate, F.J., et al, Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg. 2008;247(1):71–76. 18156925
117. Tandon, S., Batchelor, A., Bullock, R., et al, Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth. 2001;86(5):633–638. 11575337
118. Dresner, S.M., Lamb, P.J., Shenfine, J., et al, Prognostic significance of peri-operative blood transfusion following radical resection for oesophageal carcinoma. Eur J Surg Oncol. 2000;26(5):492–497. 11016472
119. Muller, J.M., Erasmi, H., Stelzner, M., et al, Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77(8):845–857. 2203505
120. Wemyss-Holden, S.A., Launois, B., Maddern, G.J., Management of thoracic duct injuries after oesophagectomy. Br J Surg. 2001;88(11):1442–1448. 11683738
121. Merigliano, S., Molena, D., Ruol, A., et al, Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg. 2000;119(3):453–457. 10694603
122. Dugue, L., Sauvanet, A., Farges, O., et al, Output of chyle as an indicator of treatment for chylothorax complicating oesophagectomy. Br J Surg. 1998;85(8):1147–1149. 9718017
123. Thaker, H., Snow, M.H., Spickett, G., et al, Pneumocystis carinii pneumonia after thoracic duct ligation and leakage. Clin Infect Dis. 2001;33(11):E129–E131. 11692316
124. Griffin, S.M., Chung, S.C., van Hasselt, C.A., et al, Late swallowing and aspiration problems after esophagectomy for cancer: malignant infiltration of the recurrent laryngeal nerves and its management. Surgery. 1992;112(3):533–535. 1519169
125. Dresner, S.M., Griffin, S.M., Wayman, J., et al, Human model of duodenogastro-oesophageal reflux in the development of Barrett’s metaplasia. Br J Surg. 2003;90(9):1120–1128. 12945080
126. Aly, A., Jamieson, G.G., Reflux after oesophagectomy. Br J Surg. 2004;91(2):137–141. 14760658
127. Aly, A., Jamieson, G.G., Pyragius, M., et al, Antireflux anastomosis following oesophagectomy. Aust N Z J Surg. 2004;74(6):434–438. 15191475
128. Blazeby, J.M., Brookes, S.T., Alderson, D., The prognostic value of quality of life scores during treatment for oesophageal cancer. Gut. 2001;49(2):227–230. 11454799
129. Earlam, R., Cunha-Melo, J.R., Oesophageal squamous cell carcinoma: I. A critical review of surgery. Br J Surg. 1980;67(6):381–390. 6155968
130. Jamieson, G.G., Mathew, G., Ludemann, R., et al, Postoperative mortality following oesophagectomy and problems in reporting its rate. Br J Surg. 2004;91(8):943–947. 15286953
131. Liu, J.F., Wang, Q.Z., Ping, Y.M., et al, Complications after esophagectomy for cancer: 53-year experience with 20,796 patients. World J Surg. 2008;32(3):395–400. 18188641
132. McCulloch, P., Ward, J., Tekkis, P.P., Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. Br Med J. 2003;327(7425):1192–1197. 14630753
133. Allum, W.H., Stenning, S.P., Bancewicz, J., et al, Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067. 19770374
134. Rutegard, M., Charonis, K., Lu, Y., et al, Population- based esophageal cancer survival after resection without neoadjuvant therapy: an update. Surgery. 2012;152(5):903–910. 22657730
135. Rice, T.W., Rusch, V.W., Ishwaran, H., et al, Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116(16):3763–3773. 20564099