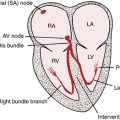
Chapter 15 Supraventricular Arrhythmias, Part II Atrial Flutter and Atrial Fibrillation
Please go to expertconsult.com for supplemental chapter material.
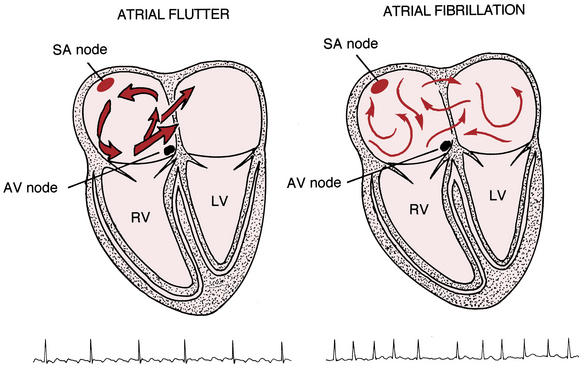
The supraventricular (common narrow complex) tachycardias discussed so far have organized atrial activity (manifest by discrete P waves) and, with some notable exceptions, 1:1 atrioventricular (AV) conduction.∗ Atrial fibrillation (AF) and atrial flutter (AFL) are two related (and sometimes missed or mistaken) arrhythmias with very rapid atrial rates that greatly exceed the ventricular rate (Fig. 15-1). This finding implies that some degree of physiologic (functional) AV block† is almost always present. Furthermore, both arrhythmias involve reentrant mechanisms with impulses rapidly and continuously spinning around, “chasing their tails,” in the atrial muscle itself (see Fig. 15-1). Therefore, instead of true P waves, one sees continuous F (flutter) or f (fibrillatory) waves.
Atrial Flutter
Location of Conduction Pathways
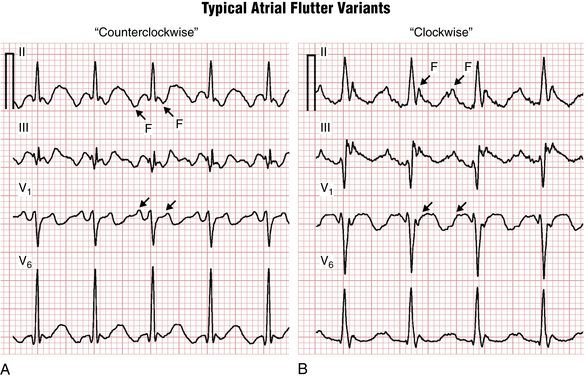
The classic “sawtooth” pattern of F waves that are predominantly negative in leads II, III, and aVF and positive in V1 with a very regular ventricular (QRS) rate of about 150/min (functional 2:1 AV block) is suggestive of the counterclockwise (common) type of typical right atrial flutter (Fig. 15-2A). Less frequently the same circuit gets initiated in the opposite direction, producing “clockwise” flutter. The polarity of the F waves will then be reversed: positive in leads II, III, and aVF, and negative in lead V1 (Fig. 15-2B). Clockwise and counterclockwise flutter can occur in the same patient and both are usually isthmus-dependent.‡ Clinically, the development of atrial flutter most often indicates the presence of underlying structural/electrical atrial disease.
Conduction to the Ventricles
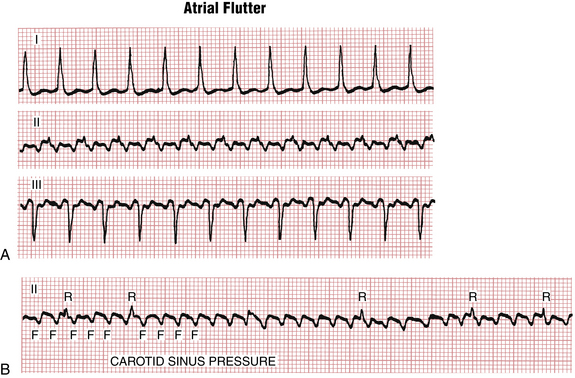
The atrial rate during typical atrial flutter, as noted, is around 300 cycles/min (range usually between about 240 to 330 cycles/min). Slower rates can be due to drugs that slow atrial conduction. Fortunately, the AV node cannot conduct electrical signals at that rate to the ventricles—although a bypass tract in the Wolff-Parkinson-White (WPW) syndrome (see Chapter 12) can! Thus, with atrial flutter, physiologic AV block develops (usually with a 2:1 A/V ratio) (Figs. 15-2 and 15-3). In the presence of high vagal tone, AV nodal disease, or AV nodal blocking drugs (e.g., beta blockers, digoxin, and certain calcium channel antagonists) higher degrees of AV block can be seen, for example with a 4:1 conduction ratio (Figs. 15-3 and 15-4).
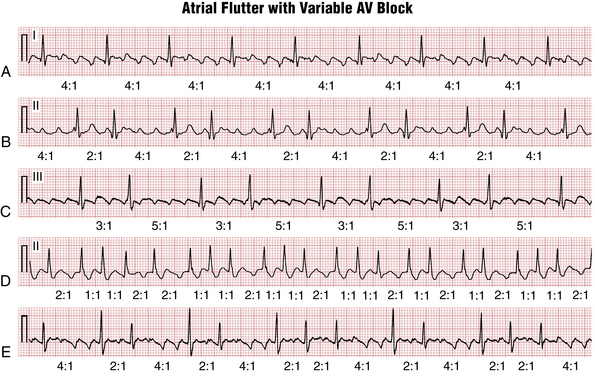
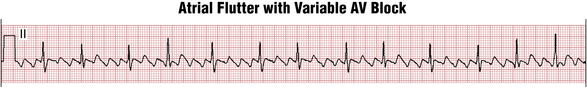
Often the AV nodal conduction shows more complex patterns and the degree of AV block varies in a periodic way, producing flutter/QRS ratios with repeating patterns (Fig. 15-4) of RR intervals (group beating). This phenomenon is believed to be due to multiple levels of block within the conduction system. Variable AV block may be due to other mechanisms (e.g., AV Wenckebach) and produce noninteger ratios of F waves to QRS complexes (Fig. 15-5).
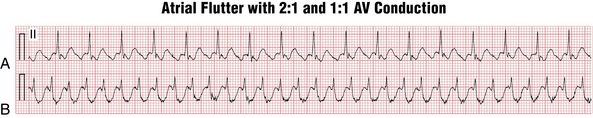
On the other hand, atrial flutter with 1:1 AV conduction (Fig. 15-6), although uncommon, can occur in three major settings:
• In high catecholamine states (strenuous physical activity, infection, with high fever, shock, etc.)
• With certain antiarrhythmic medications (such as flecainide) that slow down the flutter rate (for example, from 300 to 250/min or less) to the point where 1:1 conduction through AV node becomes possible
• In the presence of a bypass tract (WPW pattern) capable of rapid conduction (short refractory period)
Atrial flutter with sustained 1:1 AV conduction represents an emergency situation, requiring consideration of immediate synchronized electrical cardioversion because of the dangerously rapid ventricular rate.
Atrial Fibrillation
Unlike atrial flutter, the reentrant waves of atrial fibrillation (AF) cannot be localized to any repetitive and stable circuit in the atria. Most cases of AF are thought to originate in the area of pulmonary vein–left atrial junctions. With time, more and more of the atrial tissue becomes involved in the active maintenance of the arrhythmia, associated with the simultaneous formation of multiple unstable reentrant circuits throughout the atria (see Fig 15-1).
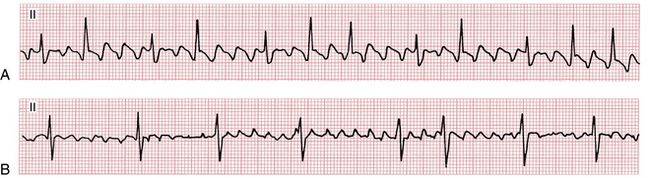
Milder degrees of atrial activity “disorganization” or drugs that slow atrial activation may produce coarse AF with high amplitude f waves resembling atrial flutter (Fig. 15-7).
Key Point
Usually, the single best lead to identify the diagnostic irregular atrial activity of AF is lead V1, where irregular f waves are likely to be most clearly seen (Fig. 15-8).
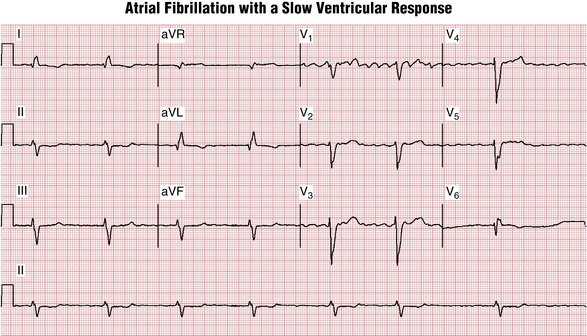
Figure 15-8 Atrial fibrillation (not flutter) is present with a very slow ventricular response. The fibrillation waves are best seen in lead V1. There is an atypical left bundle branch block (LBBB) pattern (see Chapter 7). The rsR′ in lateral leads (e.g., V6 here) is highly suggestive of prior myocardial infarction (MI). A QR (or rsR′) complex is also present in leads I and aVL, also consistent with underlying MI. Left axis deviation and a long QT interval are noted as well. The patient had chronic heart failure due to severe coronary artery disease with prior “silent” MIs. The slow ventricular response raises the question of drug effect or excess (e.g., digoxin) or intrinsic atrioventricular (AV) node disease (see Chapter 17).
Conduction Properties
In AF, the AV node gets bombarded with highly disorganized impulses of different amplitude at rates of up to 400 to 600/min. Most of the signals are blocked in the node and only a fraction conduct to the ventricles (see Figs. 15-7, B, and 15-8). Still, in the absence of AV nodal disease or certain drugs, the ventricular heart rate in AF is much higher than with sinus rhythm (e.g., usually the mean QRS rate in AF is over 100 beats/min at rest, with inappropriate increases during exercise).
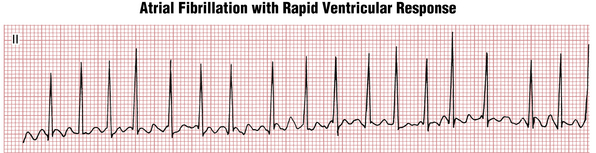
Due to random penetration of the impulses through the AV node, the RR intervals in AF are haphazardly irregular. However, when the ventricular rate gets very fast, this RR irregularity may become more difficult to appreciate; sometimes the rhythm appears regular and may be confused with other tachyarrhythmias such as PSVT (Figs. 15-9 and 15-10).
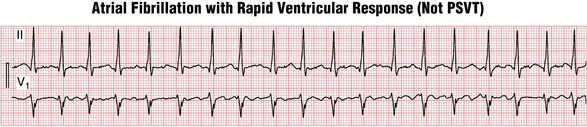
Figure 15-10 Atrial fibrillation with a rapid ventricular response. At rapid rates, the RR interval variability may be more subtle, leading to a mistaken diagnosis of paroxysmal supraventricular tachycardia (PSVT). See also Figure 15-9.
AF or flutter can occur with complete heart block, in which case the ECG will show a regular very slow ventricular response (Fig. 15-11).
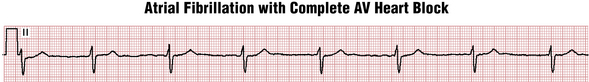
Figure 15-11 Complete atrioventricular (AV) heart block (see Chapter 17) can occur with underlying atrial fibrillation (or flutter); the ventricular response will be very slow, usually 50 beats/min or less, and regular. In this case, the narrow QRS complex indicates that the escape rhythm is in the nodal area. Such patients usually require both permanent pacing and anticoagulation.
Clinicians should also be aware that AF can be also masked by a ventricular pacemaker (see Chapter 21).
Atrial Fibrillation vs. Atrial Flutter: Differential Diagnosis
Although atrial flutter almost always occurs in the setting of structural heart disease, AF can develop in normal hearts. However, patients presenting with atrial flutter have an increased chance of showing AF on follow-up. AF and flutter can occur in the same patient, transitioning from one to the other. In such case it is often said that AF “organizes” into atrial flutter or atrial flutter “degenerates” into AF. However, at any given time there is usually one or the other (but not both) rhythms present. Although electrocardiographically these rhythms can appear quite similar, it is important to differentiate between them because of the differences in management.
The differential diagnosis of AF and flutter is based on the differences in their mechanisms (Table 15-1). The distinction is important because of the different therapeutic implications of the two arrhythmias, particularly the consideration of radiofrequency (RF) ablation as first-line therapy in atrial flutter.
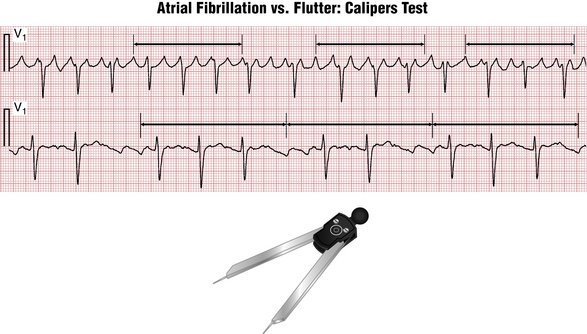
• Atrial flutter has a single, stable reentrant pathway. Therefore, all flutter (F) waves look exactly the same in both shape and duration. A simple, accurate way—the “caliper (calipers) test”—to check for this finding is to measure the interval containing several consecutive clearly visible atrial waves with ECG calipers and move it along the tracing. In case of atrial flutter, the subsequent F wave intervals will “map out” perfectly. In AF, the shape and polarity of f waves often vary over the length of the tracing. Even if the shape of f waves appears similar, their timing will be “off” (Fig. 15-12).
• The propagation velocity of the signal through atrial tissue is limited and it takes a certain amount of time, termed the “cycle length” (usually at least 180 msec, equivalent to 4.5 small “boxes”), for the flutter signal to make a full circle through the atrium. Therefore, atrial waves due to flutter generally cannot appear closer than 4 boxes apart. If they do—it suggests AF. But “coarse” AF can be present with cycle lengths of 180 msec or greater.
• In atrial flutter there is usually either a fixed ratio of F/QRS waves (2:1, 4:1) or a group beating due to a “patterned” ventricular response (e.g., 2:1-4:1). In AF, the QRS interbeat intervals are completely erratic. But be careful: if the ventricular rate is fast, this variability may be subtle.
TABLE 15-1 Differential Diagnosis of Atrial Fibrillation versus Atrial Flutter
| Feature | Atrial Flutter | Atrial Fibrillation |
|---|---|---|
| Atrial wave morphology | Identical from one F wave to another | f waves varying in shape and polarity |
| Atrial wave timing | Identical, i.e., “maps out” throughout the tracing | Variable, i.e., does not map out |
| Atrial wave cycle length | F-F intervals ≥180 msec (4.5 small boxes) | Variable f-f intervals can be shorter than 180 msec (4.5 small boxes) |
| Ventricular (QRS) response | Constant (2:1, 4:1) F/R ratio or shows group beating due to patterned response (2:1-2:1-4:1) or Wenckebach conduction | Completely irregular (no pattern) unless complete heart block or ventricular pacing is present |
Atrial Fibrillation and Flutter: Overview of Major Clinical Considerations
In some patients, AF occurs paroxysmally and may last only minutes or less, hours, or days. Some patients may experience only one episode or occasional episodes, whereas others have multiple recurrences. In some patients, AF is more persistent and may become permanent (chronic), lasting indefinitely (Table 15-2).
TABLE 15-2 Clinical Classification of Atrial Fibrillation (AF) Based on Duration
| Type | Description |
|---|---|
| Paroxysmal | Recurrent AF (≥2 episodes) that terminates spontaneously in less than 7 days (usually less than 48 hours) |
| Persistent | AF that is sustained beyond 7 days, or lasting less than 7 days but necessitating pharmacologic or electrical cardioversion |
| Long-standing persistent | Continuous AF present for longer than 1 year |
| Permanent | AF lasting for more than 1 year in a patient in whom the decision has been made not to pursue restoration of sinus rhythm by any means |
During the episodes, some patients are quite symptomatic (typically complaining of palpitations, fatigue, dyspnea, lightheadedness, or chest pain), whereas others have no specific complaints. Syncope can occur, usually as the result of the spontaneous postconversion pauses upon arrhythmia termination (see “tachy-brady” syndrome, Chapter 13).
Changes in autonomic tone may provoke AF in susceptible individuals. Sometimes the arrhythmia is related to increased sympathetic tone (e.g., occurring during exercise or with emotional excitement). At other times, AF may occur in the context of high vagal tone (e.g., with sinus bradycardia during sleep).
Chapter 24 includes a summary of important causes and contributors to AF (and flutter).
AF and flutter have two major clinical implications:
1. First and foremost is the increase in thromboembolic risk (most importantly, stroke). Therefore, whenever AF or flutter is present on the ECG, the anticoagulation status of the patient should be reviewed and appropriate treatment promptly initiated. Anticoagulation should not be delayed pending rate control. Remember that recurrent paroxysmal arrhythmia does not decrease thromboembolic risk compared to its persistent or chronic form.
2. The second important clinical implication is the development of chronic heart failure. It can occur immediately due to decreased cardiac output from lack of atrial contraction, and the rapid rate that may be associated with ventricular ischemia. These pathophysiologic events can produce severe shortness of breath and even acute pulmonary edema. Furthermore, long-term (weeks to months) continuation of a rapid uncontrolled ventricular rate can lead to development of a tachycardia-induced cardiomyopathy with ventricular dilatation and decrease in the systolic function.
Treatment of Atrial Fibrillation/Flutter: Acute and Long-Term Considerations
Rate Control
Rate control centers on limiting the ventricular response, without attempts at restoring sinus rhythm. Rate control can be achieved by using AV nodal blocking agents (beta blockers, calcium channel blockers, digoxin) or AV junctional (AVJ) ablation. The criteria of “optimal” rate control are currently under investigation.
Rate control is usually the preferred treatment option in patients with the following:
• New-onset AF within the first 24 hours (which has approximately 50% chance of terminating spontaneously)
• Reversible acute illness when achievement and maintenance of sinus rhythm are unlikely until the cause is corrected (e.g., hyperthyroidism, metabolic abnormalities, especially hypokalemia, alcohol withdrawal, acute infection)
• Asymptomatic patients who can tolerate a lifetime of anticoagulation
Rhythm Control
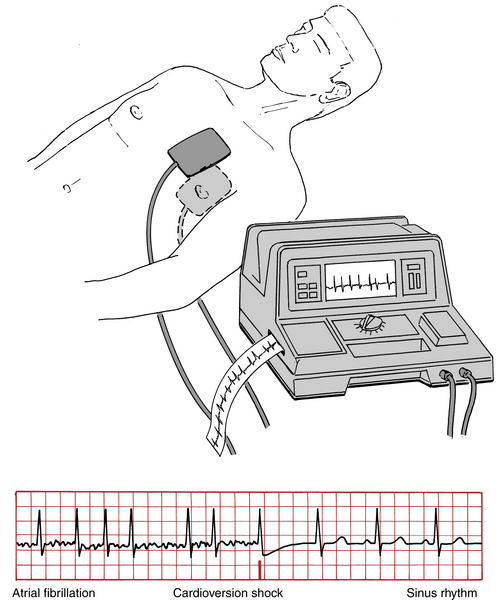
Direct current (electrical) cardioversion (DCCV) is a safe and reliable method of restoring sinus rhythm in AF and flutter. A properly timed direct current shock administered through pads on anterior and posterior chest (Fig. 15-13) depolarizes the whole heart, disrupting reentrant circuits and allowing the sinus node to regain control of the atria. It is important to “synchronize” the shock with ventricular depolarization (R wave on the ECG). Unsynchronized shocks if delivered in the ventricular vulnerable period can induce ventricular fibrillation.
Sinus rhythm maintenance can sometimes be achieved with antiarrhythmic drugs (see Chapter 10); drugs most often used are class I agents flecainide and propafenone, or class III agents sotalol, amiodarone, dofetilide, and dronedarone). Unfortunately, antiarrhythmic drugs are only modestly effective in maintaining sinus rhythm. Furthermore, most require monitoring for ECG changes that may forecast electrical instability: for example, QRS interval widening (flecainide, propafenone) and QT(U) interval prolongation with the risk of torsades de pointes (Chapter 16; sotalol, dofetilide, amiodarone, dronedarone, dofetilide). Another major limitation is that none maintains sinus rhythm reliably enough to discontinue anticoagulation. Dronedarone is contraindicated with advanced heart failure.
Choosing between rate and rhythm control and medication options, along with making decisions about the indications for and timing of AF ablation, needs to be personalized. This field represents one of the most active areas of contemporary cardiology research.
∗ This term means that there is one P wave for every QRS complex, except in some cases of atrial tachycardia with AV block. Note also that in paroxysmal supraventricular tachycardia due to AV nodal reentry (AVNRT), the retrograde P waves may be hidden in the QRS.
† Functional AV block (usually 2:1) refers to physiologic limitations of the AV node in conducting excessively rapid stimuli due to its inherent refractoriness. In contrast, organic AV block (see Chapter 17) refers to impaired conduction in the AV node area associated with intrinsic (e.g., disease processes; excess vagal tone) or extrinsic factors (e.g., drugs) that impair conduction.
‡ The cavo-tricuspid isthmus is the most common area around which atrial flutter develops. However, it can develop around other atrial obstacles such as scars after cardiac surgery, or areas of fibrosis following pericarditis or even following ablation procedures in the left atrium for atrial fibrillation.