CHAPTER 24 Suprascapular Nerve Releases
PREOPERATIVE CONSIDERATIONS
Suprascapular nerve (SSN) dysfunction is an uncommon cause of shoulder symptoms and is therefore often overlooked as a potential source of pathology. Compression of the nerve occurring at the suprascapular notch was originally described by Thomas1 in 1936 and later by Kopell and Thompson2 in 1959. However, compression at the spinoglenoid notch was not described until 1982 by Aiello and colleagues.3 Of all causes of shoulder pain, isolated SSN pathology represents just 1% to 2% of cases presenting to shoulder surgeons.4
SSN dysfunction can result from a variety of mechanisms, including direct trauma, indirect trauma (traction), space-occupying lesions (e.g., ganglion cysts, lipomas), repetitive overuse (e.g., overhead sports such as volleyball,5,6 baseball7), rotator cuff tears,8,9 and anatomic variants such as suprascapular artery passage through the suprascapular notch10 and an anomalous transverse ligament.11
Until recently, open decompression has been the mainstay of surgical treatment for this disorder, using an anterior, superior, or posterior approach.12 These approaches inevitably required some degree of muscle splitting or detachment to gain adequate exposure.13 Despite this wide dissection, the supraspinous notch is often difficult to visualize because of its close proximity to the clavicle and its deep position.
ANATOMY AND PATHOANATOMY
Anatomy
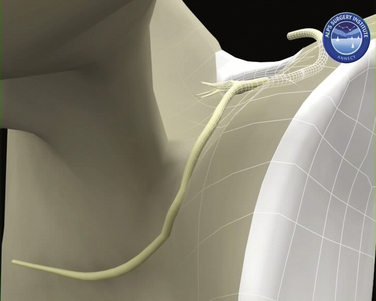
The SSN arises from the upper trunk of the brachial plexus, usually from the ventral fibers of the C5 and C6 nerve roots. The nerve then takes a course through the posterior triangle accompanied by the suprascapular artery and vein, passing inferior and parallel to the posterior belly of the omohyoid and deep to the trapezius. It enters the supraspinous fossa deep to the supraspinatus. As these neurovascular structures approach the suprascapular notch, their courses become briefly divergent so that the nerve takes a path deep to the transverse scapular ligament (TSL), whereas the artery remains superficial to this (Fig. 24-1). Variations have been reported in this arrangement, with the artery passing beneath the ligament in up to 2.5% of shoulders.10
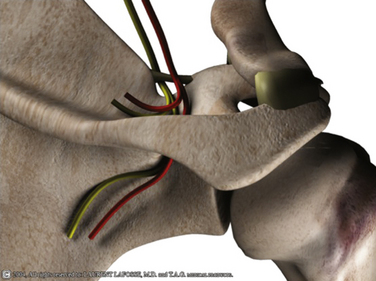
FIGURE 24-1 Three-dimensional representation of SSN and suprascapular artery and their relationship with the TSL.
The transverse scapular ligament spans the suprascapular notch, extending from the base of the coracoid and creating the suprascapular foramen. Because it is a ligament that connects two parts of the same bone, it is considered by some to represent a continuation of the coracoclavicular ligaments.10 Variable degrees of ossification have been reported in the ligament, with complete ossification in 3.7% in one series14 and partial ossification seen in 18%.15 It has also been seen to be bifid or trifid in 3% of cases in the same cadaveric series. It is important to remember, therefore, that in up to 25% of cases, the TSL is represented by an ossified variant or one with multiple bands.
As the nerve passes beneath the TSL, it gives two motor branches to supraspinatus, with the first branch generally being the larger of the two. It has been reported that one of these motor branches can pass superficial to the TSL.16 According to Hilton’s law, the SSN gives articular branches to the acromioclavicular and glenohumeral joints.10 On a microscopic level, it can be seen that the superficial part of the nerve carries afferent fibers (i.e., proprioception, pain), whereas the deeper fibers are responsible for motor innervation.17
The nerve then descends around the lateral margin of the scapular spine (Fig. 24-2) to split into three or four terminal motor branches supplying the infraspinatus. In doing so, it passes beneath the spinoglenoid ligament, forming the soft tissue boundary of the spinoglenoid notch. This quadrangular ligament extends from the posterior glenoid neck and capsule to insert onto the scapular spine.18
A cutaneous branch has been found in 14.7% of cadaveric specimens examined, which supplies the skin overlying the proximal lateral third of the arm.17,19 These cutaneous and articular branches may in part explain the nature and location of pain in some patients with SSN dysfunction.
Pathoanatomy
Rengachary and associates20 have described six different types of suprascapular notches of varying depths and profiles, ranging from a wide depression on the superior border of the scapula to complete osseous enclosure. The morphology of these notches may predispose some patients to developing SSN pathology by virtue of having a narrower and therefore more constrictive notch, or one that is entirely osseous. They also offered a second mechanism to explain local nerve trauma through the principle of the sling effect. They proposed that this is caused by the change in angulation of the nerve as it passes through the foramen, especially with the arm in hyperabduction or depression combined with retraction. Kopell and Thompson2 preferred a traction friction mechanism of injury to the nerve as it passes through the notch.
Repetitive overuse is postulated to result in chronic irritation of the SSN, particularly in those who regularly engage in overhead activities.12 This is because these activities exacerbate traction on the sites of nerve fixation and expose the nerve to compressive and frictional forces on any sharp turns made by the nerve. Movements involving scapular depression and retraction, hyperabduction, or cross-arm adduction with forward flexion20,25 all place increased tension on the nerve in the notch.
Glenohumeral joint dislocations, scapular fractures, and penetrating injuries can all result in direct or indirect traumatic injuries to the SSN. Iatrogenic injuries have been reported in surgery for distal clavicle resection, shoulder stabilization, and positioning of spinal patients for procedures.12
HISTORY AND PHYSICAL EXAMINATION
The principal complaint is usually one of deep diffuse pain or aching, often localized posteriorly or laterally in the proximal arm. Some have described this pain as burning in nature, which can radiate down the arm or into the neck. The onset of symptoms is usually insidious in nature, however, if trauma has been a precipitating feature; the cause is commonly compression at the level of the TSL.4 Patients also complain of weakness, particularly in movements involving external rotation and abduction and their symptoms are typically exacerbated with overhead activities. However, all these symptoms can be difficult to differentiate from those of rotator cuff tears or impingement, which may be coexistent. As such, pathology related to the SSN continues to be underdiagnosed. Symptoms from nerve compression at the suprascapular notch tend to be more severe than those compressive causes occurring distally.26 Because of the poorly defined nature of the symptoms, patients frequently present many months after symptoms have begun and, in many cases, after having had previous surgery. These patients remain troubled by persistent symptoms; by this stage, their pain has become chronic and muscle atrophy has become apparent. One of the most important aspects in the management of SSN neuropathy is to detect it early to prevent it reaching this late stage.
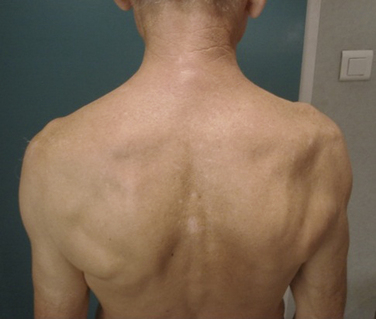
Clinical examination reveals only a subtle loss of external rotation and abduction strength in the early stages of compression. Atrophy is seen later in the course of the disorder; it affects the supraspinatus and infraspinatus in cases of proximal nerve entrapment at the suprascapular notch, although in some cases isolated infraspinatus wasting is seen with proximal compression.4 Nerve compression at the level of the spinoglenoid notch causes only infraspinatus atrophy. Tenderness may found in the suprascapular notch, but this is a relatively nonspecific sign. Long-standing compression can cause profound weakness in external rotation and abduction, with marked muscle atrophy, although deltoid mass should be reasonably maintained (Fig. 24-3).
DIAGNOSTIC IMAGING
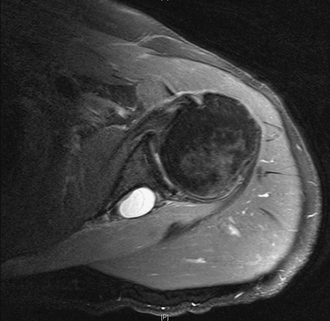
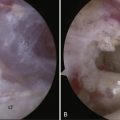
Magnetic resonance imaging (MRI) remains the best imaging tool in suspected SSN pathology because of its soft tissue resolution. It can be used to visualize the course of the nerve and integrity of the rotator cuff, demonstrate the presence of fatty infiltration, and is very helpful for identifying space-occupying lesions. The presence of muscle edema on MRI scanning has been suggested as a more sensitive and specific early sign of SSN entrapment than that of muscle atrophy and fatty infiltration.27 Caution should be exercised in interpreting the clinical significance of a cyst because the presence of a cyst does not always indicate whether there is SSN compression (Fig. 24-4). If CT and MRI fail to show a ganglion cyst or other space-occupying lesion and there remains a clinical suspicion of SSN pathology, it is our practice to request a nerve conduction study (NCS) and electromyography (EMG). This not only provides a diagnosis but also locates the level of the lesion. It is important to state the suspected pathology and need for SSN testing on the request card because this nerve is not always routinely sought with NCS and EMG. Positive findings include denervation potentials, fibrillations, reduced amplitude of evoked potentials, and prolonged motor latencies. However, as an added complication, SSN dysfunction can present with a normal NCS.
A further diagnostic aid is the use of a local anesthetic injection placed ideally under radiologic guidance. This can help confirm the diagnosis if it successfully relieves the patients symptoms but, given the difficulty of locating this notch, a negative test does not exclude SSN pathology.28
INDICATIONS AND CONTRAINDICATIONS
Indications for surgical release of SSN include the following:
Compressive lesions such as spinoglenoid cysts and lipomas respond better to operative than nonoperative intervention.29 We prefer to operate on these early rather than subject the patient to a protracted period of physiotherapy. This is particularly true for space-occupying lesions found by MRI, with an accompanying abnormal NCS EMG result. This also allows us to treat the commonly associated intra-articular pathologies at the same time (e.g., posterosuperior labral lesions in the case of spinoglenoid cysts).
In the case of large and massive rotator cuff tears, there remain unanswered questions in terms of suprascapular nerve release. The first question relates to whether the cuff tear itself and subsequent retraction lead to SSN dysfunction through a traction or kinking mechanism. Mallon and colleagues8 have demonstrated the presence of SSN neuropathy as proven on NCS and EMG in a series of eight patients with massive rotator cuff tears. These patients also had MRI changes of fatty infiltration; of the eight patients, four underwent débridement and partial repair of their cuff tear. Subsequent EMG testing in two patients demonstrated good signs of nerve recovery.
It has been proposed by some authors that the improvement experienced by some patients with massive rotator cuff tears is partly the result of the partial or complete decompression of the tethered SSN, which occurs by reducing the retraction of the supraspinatus tear.8,30 Vad and associates31 have also demonstrated that peripheral neuropathy can be found in 28% of full-thickness rotator cuff tears presenting with shoulder muscle atrophy; of these neuropathies, the SSN is involved in 29%. Costouros and coworkers32 have found that 27% of patients (7 of 26) with massive rotator cuff tears accompanied by muscular atrophy and fatty degeneration on MRI were also found to have SSN neuropathy on EMG testing. Of their patients, 6 underwent arthroscopic rotator cuff repair and all went on to demonstrate clinical and EMG improvements in SSN and shoulder function. They now recommend routine EMG testing in cases of massive rotator cuff tears, given the high prevalence of associated SSN neuropathy and the likely improvement with surgical repair. Along with Albritton and colleagues,30 they have postulated that the cuff retraction results in tethering of the nerve at the suprascapular notch, and this gives rise to a subsequent neuropathy. By reducing the cuff, the nerve is moved more laterally, thereby releasing its tension.
The second question relates to whether the reduction of a large to massive cuff tear can itself cause SSN dysfunction through advancement of the cuff. Warner and associates16 have demonstrated in their anatomic study that neither the supraspinatus nor infraspinatus could be advanced more than 3 cm before being restricted by tension in the motor branches of the SSN, even after a modified deinsertion-advancement technique. Furthermore, when no release was performed, only 1 cm of lateral advancement was possible before the nerve came under tension. This study found that in the process of lateral advancement, the motor branches to the supraspinatus pivoted 180 degrees around the SSN pedicle, which remained fixed in the fossa. In the same study, they noted that releasing the TSL affords further cuff advancement before tension again becomes a limiting factor.
TREATMENT OPTIONS
Conservative Management
The natural history of SSN neuropathy remains unknown and its outcome is probably related to the specific cause. Consequently, it is difficult to know how long to pursue a course of nonoperative management although most would agree that in the absence of a space-occupying lesion, it is reasonable to prescribe 6 months of physiotherapy and activity modification. Patients whose neuropathy is caused by repetitive use or overhead activities show good results with a nonoperative approach.33
Thereafter, surgical decompression is indicated for those who fail conservative treatment.34 Conversely, Post35 has reported good results after early release of the SSN and found that early atrophy and pain subsided. Once advanced atrophy has developed, this is largely irreversible.
Arthroscopic Release
The goal of surgery is to improve pain and symptoms, prevent irreversible muscle atrophy, and improve strength. Despite open surgery being an accepted method of treatment,13,36,37 we think that arthroscopy has several advantages:
The location and type of release we perform are determined by the preoperative history and examination, anatomy, and pathology, as revealed by imaging and NCS EMG findings.
Position and Portals
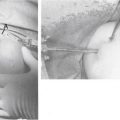
The patient is placed in the beach chair position with the arm held in 60 degrees of flexion and with 3 kg of longitudinal traction. This traction increases the size of the subacromial space, permitting improved instrument access to the suprascapular notch. The portals we use to perform our releases include the standard posterior soft spot portal, required to visualize the glenohumeral joint and subacromial space. A posterolateral portal can be used to gain access to the posterosuperior labrum and can be used subacromially or intra-articularly as a transcuff portal. A lateral subacromial portal and anterolateral portal are also used. Lafosse and associates have described38 a new superior portal, which is now required, known as the G or SSN portal. It is positioned between the clavicle and scapular spine, approximately 7 cm medial to the lateral border of the acromion (Fig. 24-5). This portal is approximately 2 cm medial to the classic Neviaser portal. These portals are created using an outside-in technique.
Transverse Scapular Ligament Release
The procedure commences with a glenohumeral joint inspection through the posterior A portal (see Fig. 24-5). Once completed, the arthroscope is introduced into the subacromial space and a lateral C portal is created. Using a radiofrequency probe or shaver, the soft tissue of the anteromedial bursa is cleared. The arthroscope is then moved to the lateral C portal and an anterolateral D portal is created 1 cm lateral to the anterolateral corner of the acromion. This portal is ideal for continuing the medial dissection with the shaver and radiofrequency probe toward the suprascapular notch.
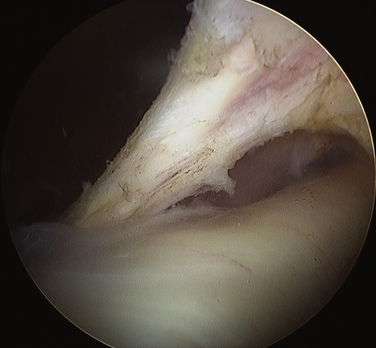
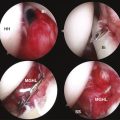
Next, it is important to identify landmarks. This is done by following the coracoacromial ligament down to the coracoid anterior to the anterior border of supraspinatus. Then, the coracoclavicular ligaments are identified (trapezoid followed more medially the conoid) by continuing the dissection posteriorly and medially (Fig. 24-6). The medial border of the conoid ligament coincides with the lateral attachment of the TSL; the two are considered continuous. It is of great assistance during this dissection to remain anterior to the muscle belly of supraspinatus and to use this border of the muscle as a landmark as it appears to disappear medially.
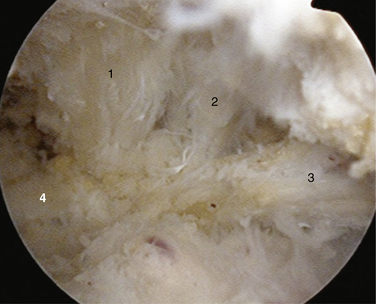
FIGURE 24-6 View of the ligaments leading to the TSL. 1, Conoid. 2, Trapezoid. 3, Coracoacromial. 4, TSL.
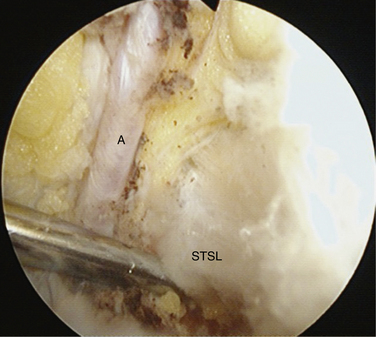
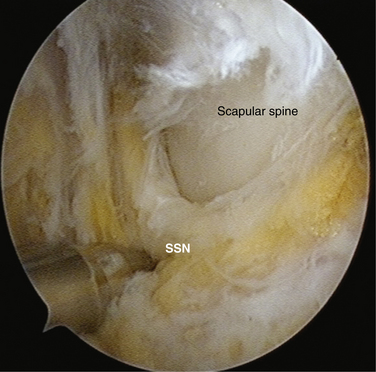
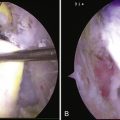
To aid in the exposure and definition of the TSL, a SSN (G) portal is created using a spinal needle with an outside-in technique (see earlier). A knife is used to incise the skin, after which a blunt trocar is inserted to determine the path for a specialized TSL-cutting scissor (Fig. 24-7). This instrument is blunt at its tip to allow gentle dissection and has a side cutting arm. The tip is used to displace the tissues around the notch carefully. It is usually necessary to retract the supraspinatus muscle and fat around the TSL posteriorly to get a good view of the TSL, suprascapular artery, and SSN (Fig. 24-8). The suprascapular artery should now be seen passing superiorly to the TSL, with the nerve passing underneath. The instrument is then introduced directly into the suprascapular notch and the nerve is displaced medially by the blunt tip, thereby protecting it (Fig. 24-9). The TSL is divided and the mobility of the nerve assessed with the blunt tip to ensure that an adequate release has been accomplished (Figs. 24-10 and 24-11). A normal blunt-tipped trocar followed by a meniscectomy punch can be used as alternatives, although this combination does not provide the same protection to the nerve as it is being cut. If there is any bony narrowing of the foramen or ligament ossification, it can be dealt with now by performing a notchplasty with a burr. Others have reported using a Kerrison punch rongeur or osteotome as alternatives for the notchplasty.39,40
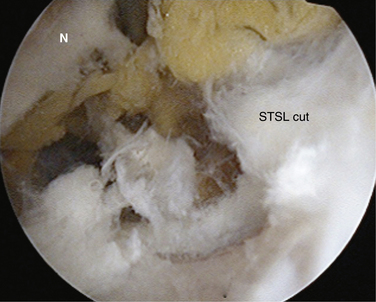
FIGURE 24-10 Ligament now divided, liberating the nerve. The SSN has moved superiorly from its previous site of entrapment.
Our early experiences with this technique involved 10 patients (8 men and 2 women) with EMG-proven SSN compression, a mean age of 50.4 years (range, 36 to 73 years), and an average 15-month follow-up.38 All patients had posterior shoulder pain and weakness, with symptoms on overhead activity, and had failed 6 months of conservative management. They had supraspinatus and infraspinatus atrophy and weakness in abduction and external rotation. All patients underwent SSN release and were reviewed 6 months postoperatively with repeat EMG examination in 8 of 10 patients; 7 had normalization of nerve conduction velocity, latency, and amplitude and an increase in their Constant score from 60.3 to 83.4.
Spinoglenoid Notch Decompression
Extra-Articular Subacromial Technique at the Posterior Interval.
Because of the high incidence of capsulolabral pathology associated with spinoglenoid cysts, a detailed glenohumeral joint arthroscopy is performed assessing these structures. In the absence of any intra-articular pathology, the arthroscope is moved subacromially to the C portal. Using the posterolateral B portal as a working portal, the connective tissue surrounding the posterosuperior cuff and lateral aspect of the scapula spine is removed using a shaver and radiofrequency device. A probe is then used from the A portal to retract the infraspinatus, allowing better access to the spinoglenoid notch deep to the scapula spine. By careful soft tissue dissection, the cyst can be visualized, opened, and excised. Once this is completed, the decompressed suprascapular nerve can be demonstrated.41
Intra-Articular Technique
Compressive lesions were demonstrated in one series to have an improved outcome when decompressed surgically, especially in the cases of spinoglenoid cysts and compressive lesions of the suprascapular notch.29
Cadaveric studies have demonstrated that the distance of the SSN in the suprascapular notch from the supraglenoid tubercle is 3.0 cm and the distance from the glenoid rim to the nerve at the base of the spine of the scapula is 1.8 cm.42 These distances are important, especially if an intra-articular approach is taken to decompress the SSN, releasing retracted rotator cuff tears.
SUMMARY AND CONCLUSIONS
Suprascapular nerve pathology is an uncommon cause of shoulder pain and weakness but should be suspected particularly in younger patients who fail to respond to conventional therapies. Careful history and examination can yield vital clues to the diagnosis, which can be confirmed by MRI imaging and NCS EMG. Half of patients who have had surgical decompression of the SSN in one meta-analysis had persistent muscle atrophy,4 suggesting that early surgical intervention may be advisable to prevent irreversible nerve damage and muscle changes.
We believe that arthroscopic release of the suprascapular nerve provides a controlled reproducible technique that is minimally invasive and takes minimal time. It can be performed as day case surgery and our early results have been encouraging. We are currently undertaking a study in over 100 patients to determine whether SSN release should form part of routine rotator cuff surgery for retracted tears.
1. Thomas A. La paralysie du muscle sous-epineux. Presse Med. 1936;64:1283-1284.
2. Kopell HP, Thompson WA. Pain and the frozen shoulder. Surg Gynecol Obstet. 1959;109:92-96.
3. Aiello I, Serra G, Traina GC, Tugnoli V. Entrapment of the suprascapular nerve at the spinoglenoid notch. Ann Neurol. 1982;12:314-3146.
4. Zehetgruber H, Noske H, Lang T, Wurnig C. Suprascapular nerve entrapment. A meta-analysis. Int Orthop. 2002;26:339-343.
5. Dramis A, Pimpalnerkar A. Suprascapular neuropathy in volleyball players. Acta Orthop Belg. 2005;71:269-272.
6. Sandow MJ, Ilic J. Suprascapular nerve rotator cuff compression syndrome in volleyball players. J Shoulder Elbow Surg. 1998;7:516-521.
7. Cummins CA, Bowen M, Anderson K, Messer T. Suprascapular nerve entrapment at the spinoglenoid notch in a professional baseball pitcher. Am J Sports Med. 1999;27:810-812.
8. Mallon WJ, Wilson RJ, Basamania CJ The association of suprascapular neuropathy with massive rotator cuff tears: a preliminary report. J Shoulder Elbow Surg, 15; 2006:395-398.
9. Asami A, Sonohata M, Morisawa K. Bilateral suprascapular nerve entrapment syndrome associated with rotator cuff tear. J Shoulder Elbow Surg. 2000;9:70-72.
10. Tubbs RS, Smyth MD, Salter G, Oakes WJ Anomalous traversement of the suprascapular artery through the suprascapular notch: a possible mechanism for undiagnosed shoulder pain. Med Sci Monit, 9; 2003:116-119.
11. Alon M, Weiss S, Fishel B, Dekel S. Bilateral suprascapular nerve entrapment syndrome due to an anomalous transverse scapular ligament. Clin Orthop Relat Res. 1988;234:31-33.
12. Safran MR Nerve injury about the shoulder in athletes, part 1: suprascapular nerve and axillary nerve. Am J Sports Med, 32; 2004:803-819.
13. Callahan JD, Scully TB, Shapiro SA, Worth RM. Suprascapular nerve entrapment. A series of 27 cases. J Neurosurg. 1991;74:893-896.
14. Hrdlicka A The scapula: visual observations. Am J Phys Anthropol, 29; 1942:73-94.
15. Ticker JB, Djurasovic M, Strauch RJ, et al. The incidence of ganglion cysts and other variations in anatomy along the course of the suprascapular nerve. J Shoulder Elbow Surg. 1998;7:472-478.
16. Warner JP, Krushell RJ, Masquelet A, Gerber C Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator-cuff tears. J Bone Joint Surg Am, 74; 1992:36-45.
17. Horiguchi M. The cutaneous branch of some human suprascapular nerves. J Anat. 1980;130(pt 1):191-195.
18. Plancher KD, Peterson RK, Johnston JC, Luke TA. The spinoglenoid ligament. Anatomy, morphology, and histological findings. J Bone Joint Surg Am. 2005;87:361-365.
19. Ajmani ML. The cutaneous branch of the human suprascapular nerve. J Anat. 1994;185(pt 2):439-442.
20. Rengachary SS, Burr D, Lucas S, et al Suprascapular entrapment neuropathy: a clinical, anatomical, and comparative study. Part 2anatomical study. Neurosurgery, 5; 1979:447-451.
21. Fritz RC, Helms CA, Steinbach LS, Genant HK Suprascapular nerve entrapment: evaluation with MR imaging. Radiology, 182; 1992:437-444.
22. Tirman PF, Feller JF, Janzen DL, et al Association of glenoid labral cysts with labral tears and glenohumeral instability: radiologic findings and clinical significance. Radiology, 1903; 1994:653-658.
23. Skirving AP, Kozak TK, Davis SJ. Infraspinatus paralysis due to spinoglenoid notch ganglion. J Bone Joint Surg Br. 1994;76:588-591.
24. Barwood SA, Burkhart SS, Lo IK Arthroscopic suprascapular nerve release at the suprascapular notch in a cadaveric model: an anatomic approach. Arthroscopy, 232; 2007:221-225.
25. Ferretti A, Cerullo G, Russo G. Suprascapular neuropathy in volleyball players. J Bone Joint Surg Am. 1987;69:260-263.
26. Romeo AA, Rotenberg DD, Bach BRJr. Suprascapular neuropathy. J Am Acad Orthop Surg. 1999;76:358-367.
27. Ludig T, Walter F, Chapuis D, et al. MR imaging evaluation of suprascapular nerve entrapment. Eur Radiol. 2001;11:2161-2169.
28. Garcia G, McQueen D. Bilateral suprascapular-nerve entrapment syndrome. Case report and review of the literature. J Bone Joint Surg Am. 1981;63:491-492.
29. Antoniou J, Tae SK, Williams GR, et al. Suprascapular neuropathy. Variability in the diagnosis, treatment, and outcome. Clin Orthop Relat Res. 2001;386:131-138.
30. Albritton MJ, Graham RD, Richards RS2nd, Basamania CJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg. 2003;12:497-500.
31. Vad VB, Southern D, Warren RF, et al. Prevalence of peripheral neurologic injuries in rotator cuff tears with atrophy. J Shoulder Elbow Surg. 2003;12:333-336.
32. Costouros JG, Porramatikul M, Lie DT, Warner JJ. Reversal of suprascapular neuropathy following arthroscopic repair of massive supraspinatus and infraspinatus rotator cuff tears. Arthroscopy. 2007;231:1152-1161.
33. Martin SD, Warren RF, Martin TL, et al. Suprascapular neuropathy. Results of non-operative treatment. J Bone Joint Surg Am. 1997;79:1159-1165.
34. Cummins CA, Messer TM, Nuber GW. Suprascapular nerve entrapment. J Bone Joint Surg Am. 2000;82:415-424.
35. Post M Diagnosis and treatment of suprascapular nerve entrapment. Clin Orthop Relat Res (368); 1999:92-100.
36. Clein LJ. Suprascapular entrapment neuropathy. J Neurosurg. 1975;43:337-342.
37. Topper SM. The utility of spine surgery instrumentation in decompression of the suprascapular notch. Am J Orthop. 1998;27:151-152.
38. Lafosse L, Tomasi A, Corbett S, et al Arthroscopic release of suprascapular nerve entrapment at the suprascapular notch: technique and preliminary results. Arthroscopy, 23; 2007:34-42.
39. Agrawal V. Arthroscopic decompression of a bony suprascapular foramen. Arthroscopy. 2009;25:325-328.
40. Ghodadra N, Nho SJ, Verma NN, et al. Arthroscopic decompression of the suprascapular nerve at the spinoglenoid notch and suprascapular notch through the subacromial space. Arthroscopy. 2009;25:439-445.
41. Werner CM, Nagy L, Gerber C. Combined intra- and extra-articular arthroscopic treatment of entrapment neuropathy of the infraspinatus branches of the suprascapular nerve caused by a periglenoidal ganglion cyst. Arthroscopy. 2007;23:328.
42. Bigliani LU, Dalsey RM, McCann PD, April EW. An anatomical study of the suprascapular nerve. Arthroscopy. 1990;6:301-305.