CHAPTER 72 Suprarenal (adrenal) gland
The suprarenal (adrenal) glands lie immediately superior and slightly anterior to the upper pole of either kidney (see Fig. 74.5A,B). Golden yellow in colour, each gland possesses two functionally and structurally distinct areas, an outer cortex and an inner medulla. The glands are surrounded by connective tissue containing perinephric fat, enclosed within the renal fascia, and separated from the kidneys by a small amount of fibrous tissue.
The dimensions of the suprarenal glands in adults in vivo have been defined by Vincent and colleagues (1994) using computed tomography (CT). The mean transverse dimensions of the body of the suprarenal gland are 61 mm (right) and 79 mm (left) and the mean transverse dimensions of suprarenal limbs are 28 mm (right) and 33 mm (left). No individual suprarenal limb should measure more than 6.5 mm in transverse section. The suprarenal glands each weigh approximately 5 g (the medulla contributes about one-tenth of the total weight).
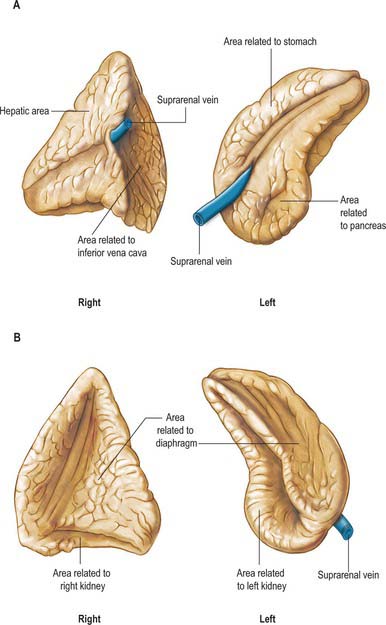
The glands are macroscopically slightly different in external appearance (Fig. 72.1). The right gland is pyramidal in shape and has two well developed lower projections (limbs) giving a cross-sectional appearance similar to a broad-headed arrow. The left gland has a more semilunar form and is flattened in the anteroposterior plane. The left gland is marginally larger than the right. The bulk of the right suprarenal sits on the apex of the right kidney and usually lies slightly higher than the left gland, which sits on the anteromedial aspect of the upper pole of the left kidney.
RIGHT SUPRARENAL GLAND
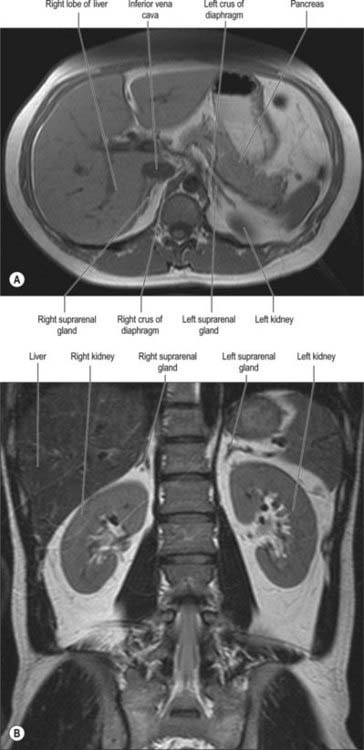
The right suprarenal gland lies posterior to the inferior vena cava, from which it is only separated by a thin layer of fascia and connective tissue. It lies posterior to the right lobe of the liver and anterior to the right crus of the diaphragm and superior pole of the right kidney (Fig. 72.2). Its inferior surface is referred to as the base and adjoins the anterosuperior aspect of the superior pole of the right kidney. It often overlaps the apex of the upper pole of the right kidney as the two lower projections (limbs) straddle the renal tissue. The anterior surface faces slightly laterally and possesses two distinct facets. The medial facet is somewhat narrow, runs vertically and lies posterior to the inferior vena cava. The lateral facet is triangular and lies in contact with the bare area of the liver. The lowest part of the anterior surface may be covered by peritoneum, reflected onto it from the inferior layer of the coronary ligament. At this point it may lie posterior to the lateral border of the second part of the duodenum. Below the apex, near the anterior border of the gland, the hilum lies in a short sulcus from which the right suprarenal vein emerges to join the inferior vena cava. This vein is particularly short, which makes surgical resection of the gland potentially hazardous, because ligation may be difficult. The vein may be avulsed from the inferior vena cava during surgery or occasionally by high-energy deceleration injuries. The posterior surface is divided into upper and lower areas by a curved transverse ridge. The large upper area is slightly convex and rests on the diaphragm. The small lower area is concave and lies in contact with the superior aspect of the upper pole of the right kidney. The medial border of the gland is thin and lies lateral to the right coeliac ganglion and the right inferior phrenic artery as the artery runs over the right crus of the diaphragm.
LEFT SUPRARENAL GLAND
The left suprarenal gland lies closely applied to the left crus of the diaphragm and is separated from it only by a thin layer of fascia and connective tissue (Fig. 72.2). The medial aspect is convex whilst the lateral aspect is concave since it is shaped by the medial side of the superior pole of the left kidney. The superior border is sharply defined while the inferior surface is more rounded. The anterior surface has a large superior area covered by peritoneum on the posterior wall of the lesser sac, which separates it from the cardia of the stomach and sometimes from the posterior aspect of the spleen. The smaller inferior area is not covered by peritoneum and lies in contact with the pancreas and splenic artery. The hilum faces inferiorly from the medial aspect and is near the lower part of the anterior surface. The left suprarenal vein emerges from the hilum and runs inferomedially to join the left renal vein. The posterior surface is divided by a ridge into a lateral area adjoining the kidney and a smaller medial area which lies in contact with the left crus of the diaphragm. The convex medial border lies lateral to the left coeliac ganglion, and the left inferior phrenic and left gastric arteries, which ascend on the left crus of the diaphragm.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
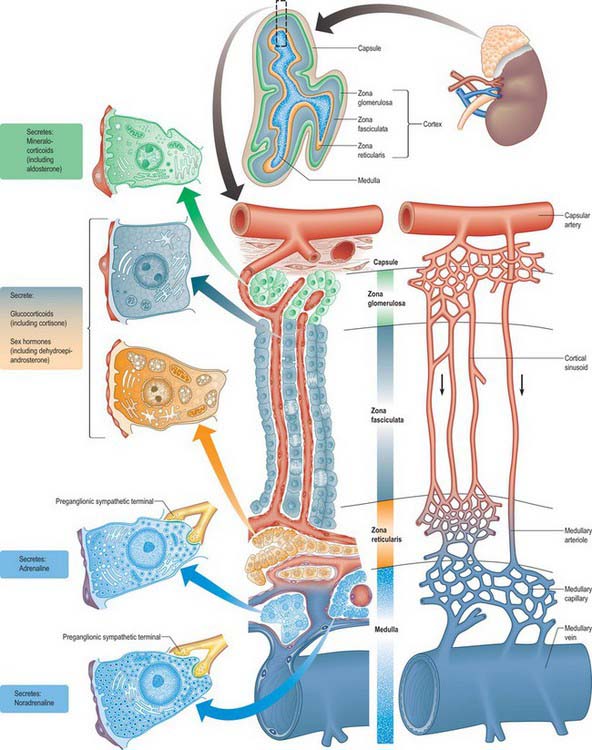
The suprarenal glands have an extensive vascular supply in relation to their size. Each gland is supplied by superior, middle and inferior suprarenal arteries, whose main branches may be duplicated or even multiple (Fig. 72.3). They ramify over the capsule before entering the gland to form a subcapsular plexus, from which fenestrated sinusoids pass around clustered glomerulosal cells and between columns in the zona fasciculata to a deep plexus in the zona reticularis. Venules from this plexus pass between medullary chromaffin cells to medullary veins, which they enter between prominent bundles of smooth muscle fibres. Some relatively large arteries bypass this indirect route and pass directly to the medulla (Fig. 72.4). At operation the arteries are rarely obvious, but the veins deserve specific attention (see below).
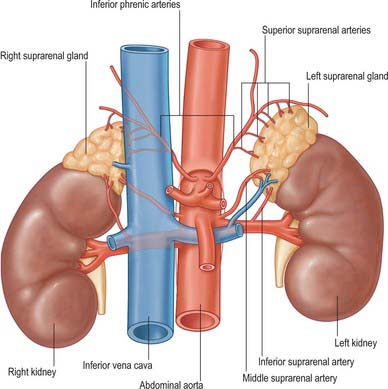
Fig. 72.3 Arterial supply and venous drainage of the suprarenal glands.
(From Drake, Vogl and Mitchell 2005.)
MICROSTRUCTURE
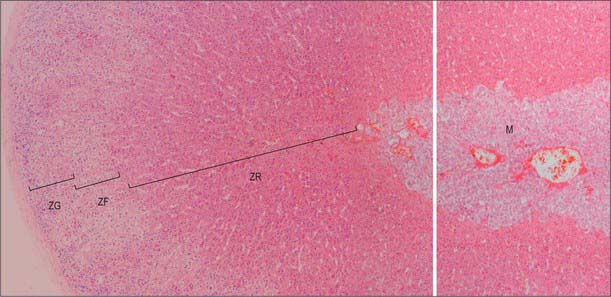
In section, the suprarenal gland has an outer cortex, which is yellowish in colour and forms the main mass, and a thin medulla, forming about one-tenth of the gland, which is dark red or greyish, depending on its content of blood (see Figs 72.4, 72.5). The medulla is completely enclosed by cortex, except at the hilum. The gland has a thick collagenous capsule from which trabeculae extend deep into the cortex. The capsule contains a rich arterial plexus which supplies branches to the gland.
Suprarenal cortex
The suprarenal cortex consists of the zona glomerulosa, zona fasciculata and zona reticularis (Figs 72.4, 72.5). The outer subcapsular zona glomerulosa consists of a narrow region of small polyhedral cells in rounded clusters. The cells have deeply staining nuclei and a basophilic cytoplasm containing a few lipid droplets. Ultrastructurally, the cytoplasm displays abundant smooth endoplasmic reticulum, a feature typical of cells that synthesize steroids. The broader intermediate zona fasciculata consists of large polyhedral basophilic cells arranged in straight columns, two cells wide, with parallel fenestrated venous sinusoids between them. The cells contain many lipid droplets and large amounts of smooth endoplasmic reticulum. The innermost part of the cortex, the zona reticularis, consists of branching interconnected columns of rounded cells whose cytoplasm also contains smooth endoplasmic reticulum, many lysosomes and aggregates of brown lipofuscin pigment that accumulate with age.
SUPRARENAL GLAND EXCISION
During laparoscopic surgery the anterior, transperitoneal approach is most commonly used. The patient is placed in the lateral decubitus position. On the left side, the splenic flexure is mobilized inferiorly, revealing the kidney. The lateral phrenicocolic ligament is then fully divided, which allows the spleen to be mobilized medially exposing the splenorenal ligament. Division of the splenorenal ligament (when the patient is in the lateral decubitus position) allows the spleen to ‘drop’ medially. This exposes the plane between the splenic hilum and medial border of the left kidney, and is where the pancreatic tail is identified as it runs to the hilum of the spleen. The renal fascia is divided to expose the suprarenal gland lying over the superomedial aspect of the upper pole of the kidney. On the right side, the right triangular ligament of the liver is divided, which allows the liver to be retracted caudally. In most individuals the inferior vena cava is easily identified behind the peritoneum lateral to the second part of the duodenum. The peritoneal reflection on the inferior border of the liver is divided from the inferior vena cava to the lateral border of the liver, exposing the suprarenal gland in the angle between the inferior vena cava and liver. The peritoneal reflection along the lateral aspect of the inferior vena cava is divided and the gland is then mobilized laterally exposing the suprarenal vein, which often emerges from the posterior aspect of the inferior vena cava. Once the suprarenal vein has been divided, the small middle suprarenal arteries, which run from the aorta to the gland behind the inferior vena cava, are easily identified. The gland is then usually mobilized very easily in a lateral direction. However, it can lie either behind the inferior vena cava or under the liver, in which case dissection at the junction of the liver and inferior vena cava often becomes very difficult.