CHAPTER 31 Superomedial Pedicle Extension Mastopexy
Key Points
Summary
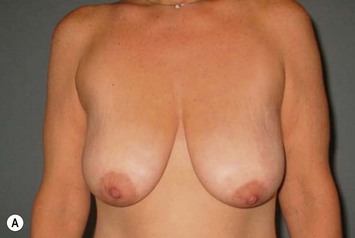
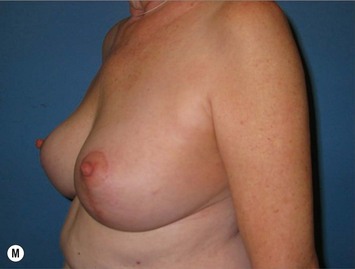
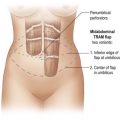
Superomedial pedicle extension flaps essentially involve the rotation of lower pole dermatoglandular tissue attached to the superomedial nipple–areola complex (NAC) pedicle and moved to remote areas in the breast where additional volume is required (Fig. 31.1). All breast reduction and mastopexy techniques rely on repositioning the NAC on a vascularized pedicle, resecting tissue as needed, and reshaping the breast mound with the pedicle and residual breast tissue. Extension flaps and autoaugmentation techniques are relatively new and are slightly more involved. In addition to relying on residual breast tissue for shape and size, they rotate local or distant flaps from one region of the body to fill an area of volume void within the breast. Although distant flaps have been reported, the majority of autoaugmentation techniques are local from the thoracic or abdominal region, or from within the breast. Local ‘non-breast’ options for autoaugmentation techniques typically use fasciocutaneous flaps or perforator flaps from the lateral chest wall or upper abdominal region.1–4 Local breast flaps are dermatoglandular flaps or glandular flaps based off the chest wall or as extension flaps from various NAC pedicles.5–7 Although these autoaugmentation techniques were initially used to improve shape and volume in mastopexy cases, they have become more popular recently out of necessity in the management of the massive weight loss breast, and partial mastectomy defects.
Patient Selection
Numerous options exist for extending pedicles to autoaugment certain locations within the breast. Surgeons will often rely on a technique that they are familiar with and modify their approach to best address the deformity. Various authors have employed central or inferior pedicle techniques, and designed vascularized attachments to provide suspension of the breast mound, and autoaugmentation mainly in the upper pole where volume is often desired.6,7 Although the majority of surgeons still use the inferior pedicle breast reduction technique,8 there is a growing trend toward the more superiorly based pedicle designs. It is felt that the superomedial pedicle technique can afford more reliable cosmetic outcomes, shorter operating times, and less pseudoptosis.9,10 The superomedial pedicle is a relatively short, well-vascularized pedicle that can easily rotate into position.
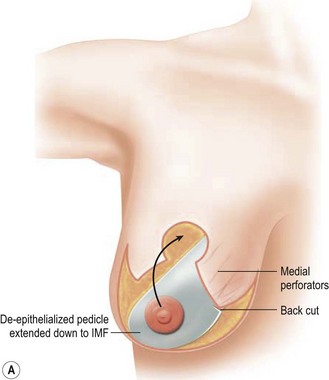
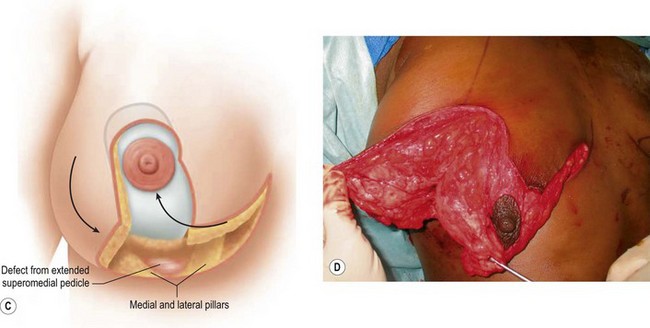
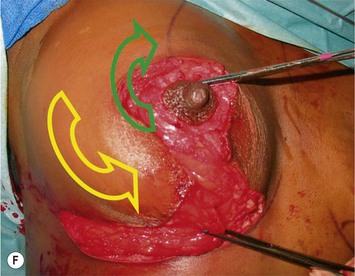
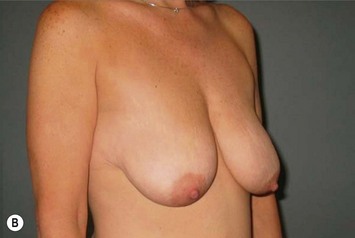
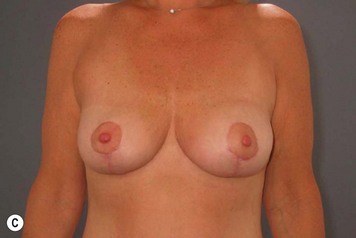
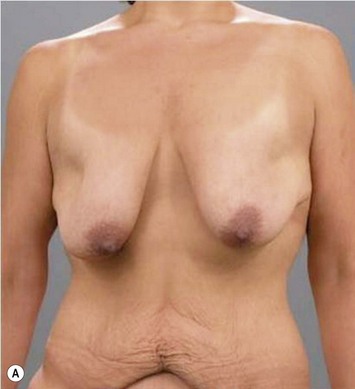
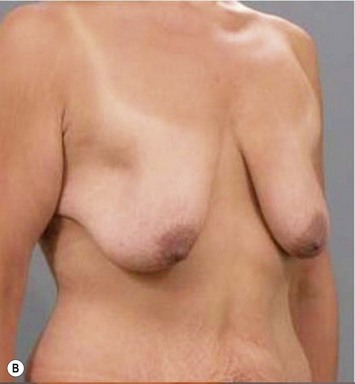
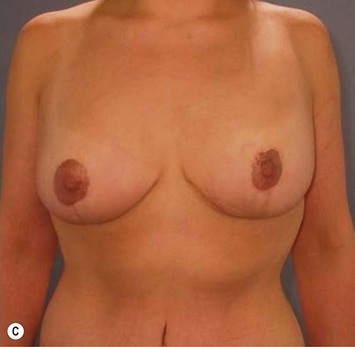
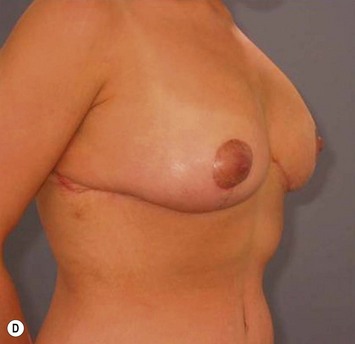
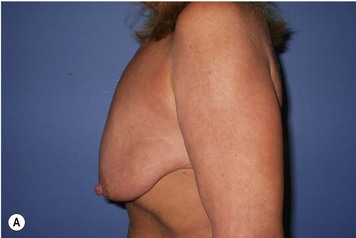
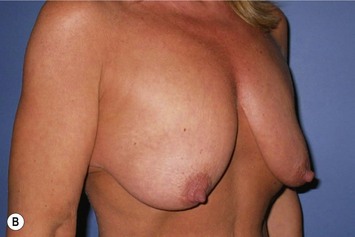
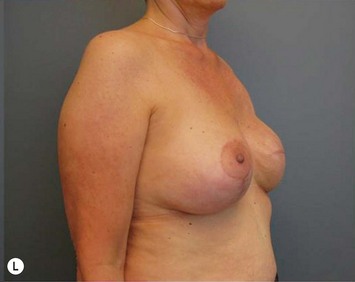
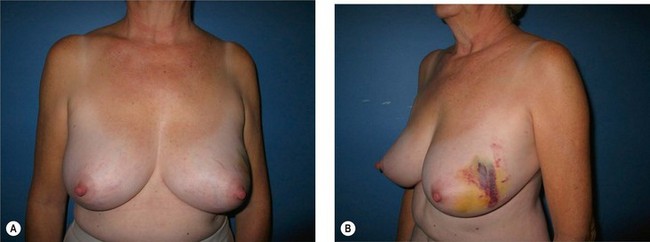
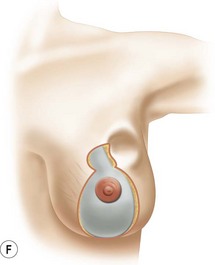
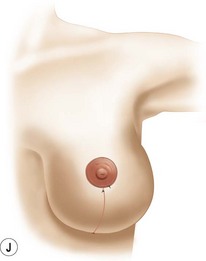
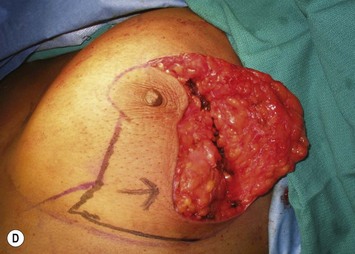
Breast reduction techniques using superiorly based pedicles, whether superior or superomedial, both have the excess dermatoglandular tissue resected in an ‘en bloc’ wedge type fashion from the lower pole. This tissue is subsequently very amenable to being used as a vascularized extension of the NAC pedicle design for rotation to fill the upper or outer breast quadrant. Graf initially described the use of this lower pole breast tissue as a weaving technique through the pectoralis muscles to autoaugment the breast mound in mastopexy techniques.11 Although it can also be used as an extended superior pedicle flap, this chapter is going to focus on it being rotated as an extended superomedial pedicle. Many of the advantages of the superomedial technique as described by Hall-Findlay can also be applied to the extended superomedial technique.10,12 The extended pedicle is easily rotated laterally and superiorly with the NAC to provide elevation of the nipple and autoaugmentation of the upper or lateral breast. This is a more natural arc of rotation than the extended superior pedicle where the nipple needs to move superiorly, and the pedicle extends below the nipple and is then tucked superiorly. Dermal release below the nipple is often required when using the extended superior pedicle, which could potentially compromise viability of the extension. The extended superomedial pedicle takes tissue from the lower pole where volume is often less desirable, and rotating it to the upper pole where there is often a volume void. This also leaves a lower pole defect, amenable to parenchymal plication with the added benefits of glandular reshaping, narrowing the breast mounds, tightening the IMF and minimizing recurrent ptosis (Fig. 31.1). This extended superomedial technique can be used with a vertical or a standard Wise mastopexy skin pattern.
Indications
Benefits
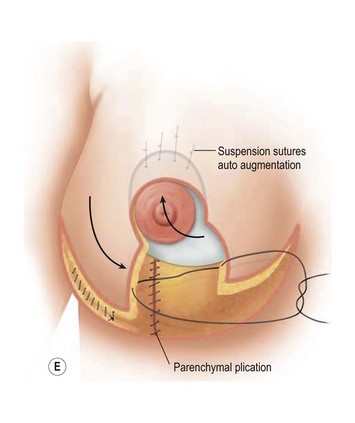
The benefits of using an extended superomedial pedicle include the ability to use local breast parenchyma, without the morbidity of a donor site, and provide vascularized tissue to areas in the breast where there is a volume void. The arc of rotation is ideal for transfer to the lateral or upper breast region. Volume is taken from the lower pole where it is often excessive, and transferred to the upper pole where it is often deficient. The rotation advancement technique allows lower pole glandular plication, upper pole autoaugmentation, as well as suspension of the breast if necessary (Fig. 31.1). This autoaugmentation technique will often give the appearance of an augmented breast without the need for implant placement in women who desire upper pole fullness. Since this technique does not bring in tissue from outside the breast, patients who have insufficient breast volume and still desire upper pole fullness will require implant augmentation or alternative techniques. Since this technique is based on a well-vascularized dermatoglandular pedicle, placement of a submuscular or even subglandular implant is still an option if necessary. The theoretical, although unproven, advantages of using the lower pole as a donor site along with glandular plication with the superomedial technique is to reduce the incidence of pseudoptosis.
Operative Technique
Marking
The patient is marked preoperatively in the standing position.
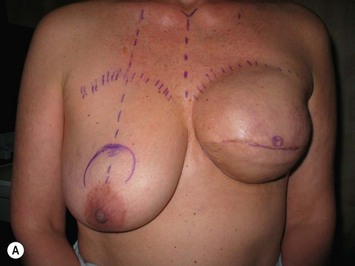
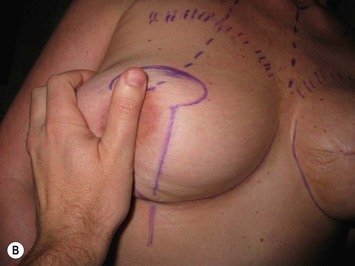
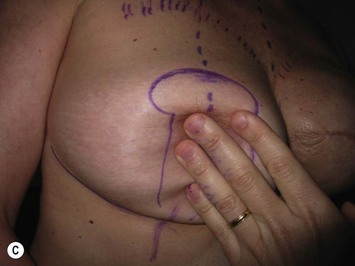
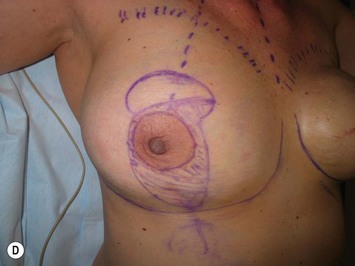
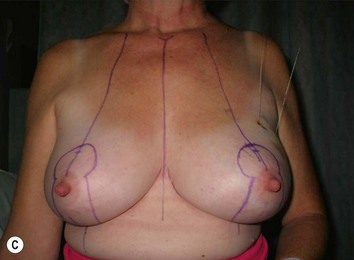
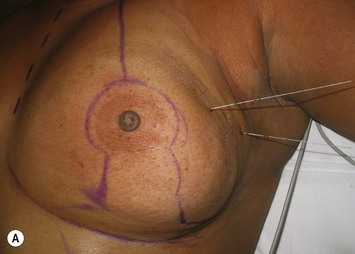
The midaxial line of the breast and the existing inframammary fold (IMF) is marked. The proposed new nipple location is determined typically 1–2 cm above the IMF. This typically results in a suprasternal notch to nipple distance of 20–23 cm, depending on the height of the patient, level of the IMF, and desired size after the mastopexy. If the IMF is loose or low, the desired level of the IMF is also marked and taken into consideration when deciding on the nipple position. A mosque-shaped areolar pattern is then drawn, as described previously,12,14 with a diameter of about 5 cm, and length of the periareolar scar of less than 16 cm. Relatively conservative glandular displacement techniques are then performed off the breast meridian to decide on the medial and lateral pillar positions and width of the extended pedicle (Fig. 31.5). Horizontal Wise pattern markings are typically included about 10 cm beneath the top of the proposed NAC location. The superomedial pedicle is drawn and extended down to within the vertical markings stopping about 2–3 cm above the IMF in a ‘U’ shaped pattern.
Surgical technique
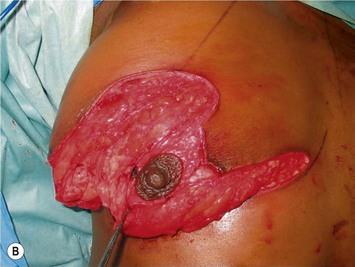
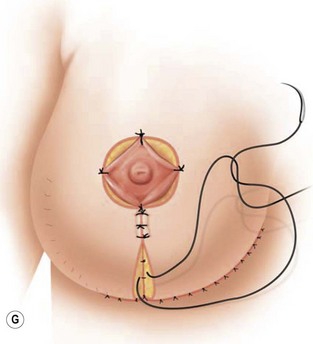
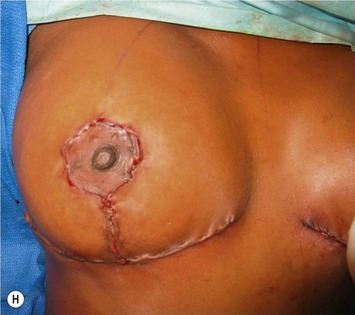
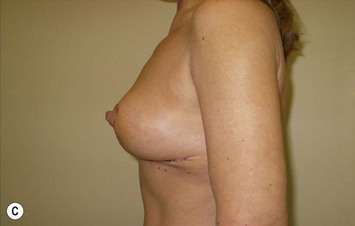
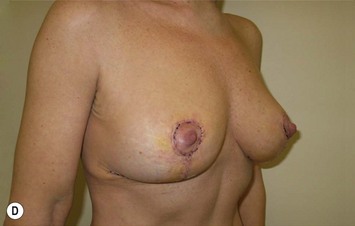
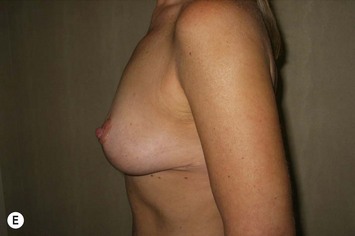
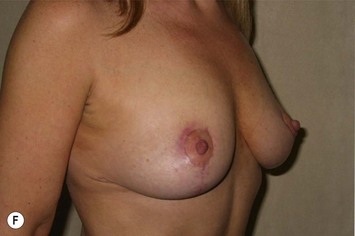
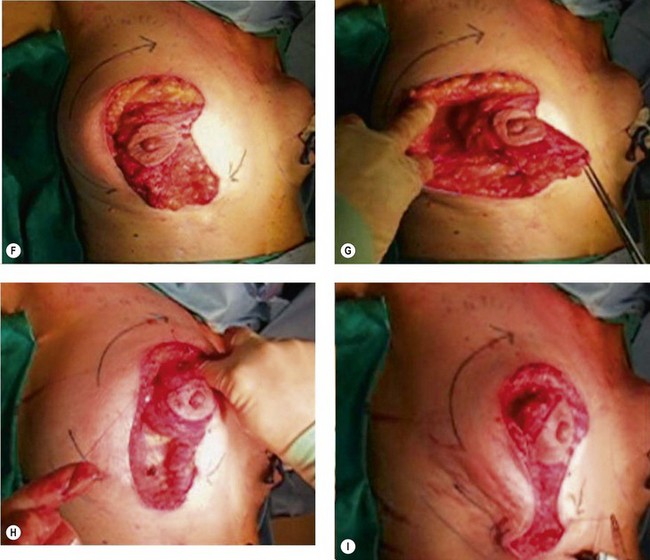
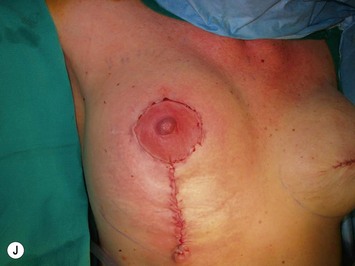
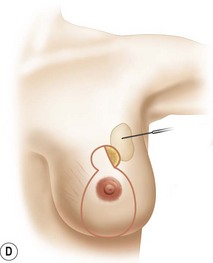
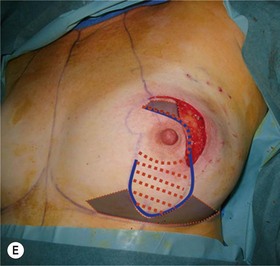
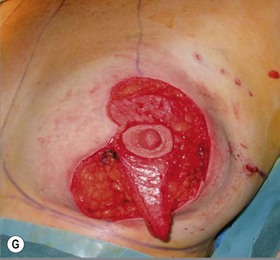
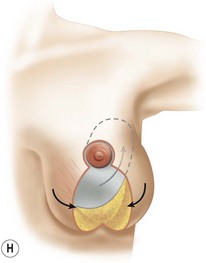
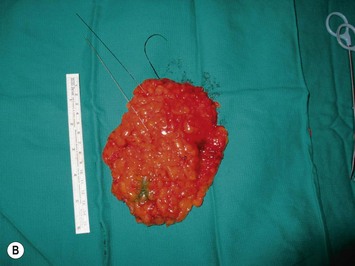
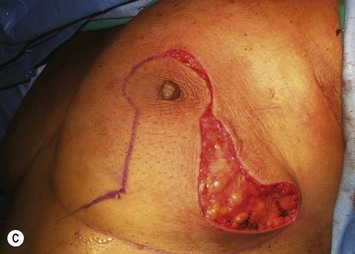
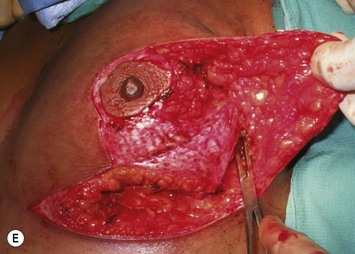
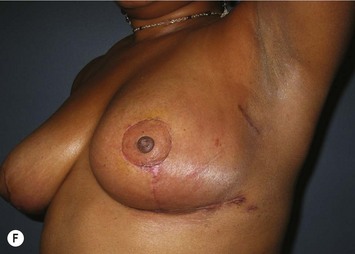
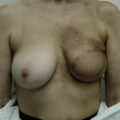
The NAC is incised and the superomedial pedicle as well as the extended pedicle is de-epithelialized down to just above the IMF. The dermis is then released creating the outline of the pedicle. The pedicle is back-cut medially only as much as is necessary to minimize restriction of rotation while keeping the pedicle base as wide as possible (Fig. 31.1). Parenchyma is then incised almost down to the level of the chest wall with no undermining inferiorly. The lateral parenchymal extension is then transected from the lateral pillar up to the superior markings and minimal undermining is performed superiorly above the proposed nipple position to create a pocket for the autoaugmentation. Elevation of the pedicle off the chest wall is required inferiorly for rotation into the upper pole; however, central attachments are left intact to maximize pedicle viability. This allows for safe pedicle back-cut for nipple inset when necessary. Once the dermatoglandular pedicle has been created, the excess skin and parenchyma is resected depending on size and shape desired. The pedicle is then rotated superiorly and suspended as needed to fill the upper pole (Fig. 31.1D, E). It is important to minimize excessive tension or torsion on the pedicle which could impair perfusion of the extension. Parenchymal plication is performed by bringing the medial and lateral pillar together with 2-0 polydioxanone suture to reshape, and narrow the lower pole of the breast. This can also redefine and tighten the IMF at a higher elevation if necessary. Lateral parenchymal plication is performed in a horizontal orientation as needed. Once the breast mound has been shaped, the skin envelope is redraped and any excess skin removed (Fig. 31.1F, G). Lateral breast skin is advanced medially to redefine the lateral breast and eliminate the lateral breast roll. Although this is often performed through a vertical only skin take out pattern, the horizontal component is usually required in the massive weight loss breast to redefine the lateral mound and remove the excess skin.
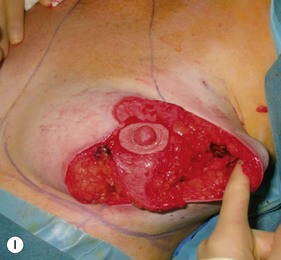
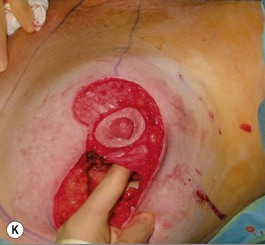
Oncoplastic reduction techniques
On examination of the partial mastectomy defect, the goals are to:
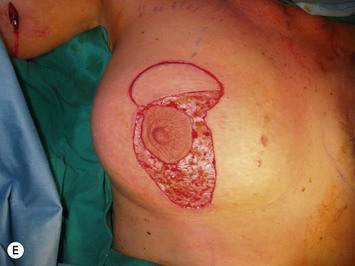
Numerous options exist for defects in the lateral part of the breast mound. When the defect is below the level of the nipple or if the defect is relatively small, it can be filled with residual breast tissue using almost any pedicle or reduction technique. If the breasts are small to moderate sized, an extended superomedial dermatoglandular pedicle can be rotated into the defect, closing the remaining breast in a vertical mastopexy fashion. The nipple is incised and the lower pole is de-epithelialized. If it appears as though additional tissue is required from the lower pole to fill a relatively high outer quadrant defect, then the extended superomedial pedicle is created. The size is determined by the amount of tissue available, and the size of the defect. The pedicle is then rotated into the defect and secured with suture fixation (Fig. 31.6). Excess breast tissue is not resected until a decision has been made how to fill the dead space, rotate the nipple and reshape the breast mound. Parenchymal plication and skin redraping are then performed as previously described.
Pitfalls and How to Correct
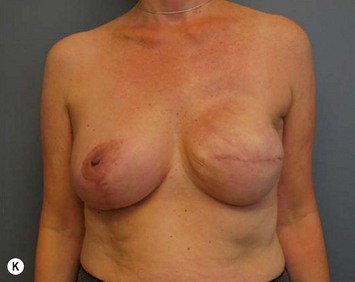
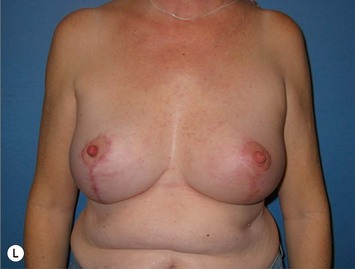
The extended superomedial technique has been used at our institution on 53 patients for all the above mentioned indications. We reported favorable results with mastopexy techniques in the massive weight loss patients,10 as well as for the reconstruction of partial mastectomy defects.13 Fat necrosis remains a concern, especially when length is required for the extended pedicle. This is minimized by keeping the superomedial pedicle as wide as possible, and preserving central perforating vessels if the arc of rotation allows. We have experienced some early edema and fat necrosis in four patients at the most distal portion of the flap; however, this did resolve with time. Recurrent ptosis is another issue which is minimized using this technique, however, not prevented. Despite overcorrection and extensive glandular manipulation, revision rate is up to 10% especially in the MWL breast when implants are also used.10
1 Hamdi M, Van Landuyt K, Blondeel P, et al. Autologous breast augmentation with the lateral intercostal artery perforator flap in massive weight loss patients. J Plast Rec Aesth Surg. 2007;62:65-70.
2 Wallach SG, Zienowicz RJ. Augmentation mammaplasty by reverse abdominoplasty (AMBRA) and transabdominoplasty breast augmentation (TABA): Alternative options for breast augmentation, American Society for Aesthetic Plastic Surgery annual meeting May 1–6, 2008 in San Diego, CA.
3 Kwei S, Borud L, Lee BT. Mastopexy with autologous augmentation after massive weight loss: The intercosal artery perforator (ICAP) flap. Ann Plast Surg. 2006;57(4):361-365.
4 Graf RM, Mansur AE, Tenius FP, Ono MC, Romano GG, Cruz GA. Mastopexy after massive weight loss: extended chest wall-based flap associated with a loop of pectoralis muscle. Aesth Plast Surg. 2008;32(2):371-374.
5 Botti G. Vertical scar mammaplasty: stable padding of the superior pole by means of posterioly based pedicle autoprosthesis. Aesth Surg J. 1999;19:116-123.
6 Rubin JP, Khachi G. Mastopexy after massive weight loss: dermal suspension and selective autoaugmentation. Clin Plast Surg. 2008;35(1):123-129.
7 Hurwitz DJ. Single staged total body lift after massive weight loss. Ann Plast Surg. 2004;52:435-441.
8 Rohrich RJ, Gosman AA, Brown SA, Reisch J. Mastopexy preferences: a survey of board-certified plastic surgeons. Plast Reconstr Surg. 2006;118:1631.
9 Davison SP, Mesbahi AN, Ducic I, Sarcia M, Dayan J, Spear SL. The versatility of the superomedial pedicle with various skin reduction patterns. Plast Reconstr Surg. 2007;120:1466.
10 Losken A, Holtz DJ. Versatility of the superomedial pedicle in managing the massive weight loss breast: the rotation-advancement technique. Plast Reconstr Surg. 2007;120:1060.
11 Graf R, Reis de Araujo LR, Ripple R, et al. Reduction mammaplasty and mastopexy using the vertical scar and thoracic wall flap technique. Aesth Plast Surg. 2003;27:6-12.
12 Hall-Findlay EJ. A simplified vertical reduction mammaplasty: shortening the learning curve. Plast Reconstr Surg. 1999;104(3):748-759.
13 Losken A, Styblo TM, Carlson GW, Jones G, Amerson B. Management algorithm and outcome evaluation of partial mastectomy defects treated using reduction or mastopexy techniques. Ann Plast Surg. 2007;3(59):235.
14 Lejour M. Vertical mammaplasty and liposuction of the breast. Plast Reconstr Surg. 1994;94:100.