Chapter 42 Superior Semicircular Canal Dehiscence Syndrome
Superior canal dehiscence syndrome (SCDS) has only more recently been described, but during the decade since the initial description,1 the pathophysiology has been elucidated, and curative surgery is now regularly performed at several centers throughout the world. SCDS is characterized by the clinical findings of sound-induced vertigo and eye movements, chronic dysequilibrium, conductive hearing loss, and decreased hearing thresholds for bone conducted sounds, so that patients may experience autophony and may even hear their pulse or eye movements.
Symptoms caused by abnormal openings into the labyrinth have been known for decades. Fenestration of the semicircular canals was known to produce eye movements in response to sound in animals 80 years ago.2 The Tullio phenomenon, or eye movements in response to loud sound, was initially identified in humans with advanced syphilis secondary to gummatous osteomyelitis and labyrinthine fistulas.3 In patients with the Tullio phenomenon, the Hennebert sign (eye movement induced by pressure in the external auditory canal) is also often present. Subsequent reports have identified the Tullio phenomenon in perilymphatic fistula,4 head trauma,5 and cholesteatoma with semicircular canal erosion and fenestration.6 Today it is recognized that all of these causes of the Tullio phenomenon are uncommon compared with SCDS.
The incidence of superior canal dehiscence (SCD), meaning only the anatomic abnormality, regardless of the symptomatic status, has been estimated from a temporal bone library.7 This study of 1000 temporal bones revealed a 0.5% incidence of complete dehiscence of the superior canal into the middle fossa or superior petrosal sinus. In an additional 1.4% of specimens, the bone was 0.1 mm or thinner. The incidence of SCDS is not known with certainty, but it is likely that only a subset of patients with SCD actually experience symptoms.
DIAGNOSTIC EVALUATION
With any patient presenting with complaints of dizziness, a good history is the most effective diagnostic tool. Patients with SCDS usually present with a primary complaint of either autophony or dizziness. There are many possible causes of dizziness, and patient evaluation should focus on narrowing the diagnosis. Vertigo symptoms related to SCDS are usually induced by loud sound or pressure changes and are brief in duration. Dizziness or oscillopsia induced by loud sound are present in 90% of SCDS patients.8 Vestibular symptoms induced by pressure changes such as coughing or straining are present in 73% of patients, with 67% exhibiting pressure-related and sound-related symptoms.8 Chronic dysequilibrium symptoms and cognitive impairment (“brain fog”) may also be attributed to SCDS.
Auditory symptoms are also a common feature of SCDS. Hyperacusis for bone-conducted sound9 is present in 52% of SCDS patients.8 Symptoms often include hearing one’s pulse, eye movements, or the impact of the feet during walking. More uncommon but quite dramatic conductive hyperacusis symptoms include being able to hear others’ bowel sounds when sitting in the same church pew or the motor of a faraway vehicle while sitting on a park bench. Autophony or the patient’s own voice sounding disturbing to them is present in varying degree in 60% of patients.8 Patients with SCDS occasionally can hear in the affected ear a 512 Hz tuning fork placed against the foot or ankle.10
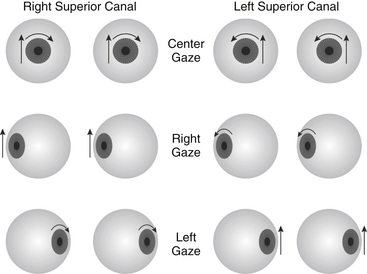
Evoked eye movements in the plane of the superior canal are the hallmark of SCDS11; these are present in 60% of our patients. The eyes should be examined under Frenzel lenses, with infrared video goggles, or by some other means to eliminate the effect of visual fixation. Using an audiometer, pure tones at levels up to 110 dB normal hearing level (nHL) should be delivered in one ear at a time covering the frequency range of 125 to 4000 Hz. Sound-evoked eye movements at one or more frequencies were noted in 82% of SCDS patients using such stimuli.8 Eye movements can also be induced with Valsalva maneuvers (75%) or pressure in the external auditory canal (45%). Depending on the type of stimulus, either excitation or inhibition of the superior canal may occur as shown in Figure 42-1. Pressure-evoked or sound-evoked eye movements almost always occur in the plane of the superior canal as shown in Figure 42-2. In the case of larger dehiscences, eye movements may be shifted out of the superior canal plane12; however, if eye movements are not in this direction, the diagnosis of SCDS should be questioned, and alternative diagnoses of posterior canal dehiscence13 or horizontal canal fistula14 must be considered. Sound-evoked rotation of the head, which also tilts in the plane of the affected superior canal, occurred with tones in 14% of our SCDS patients.
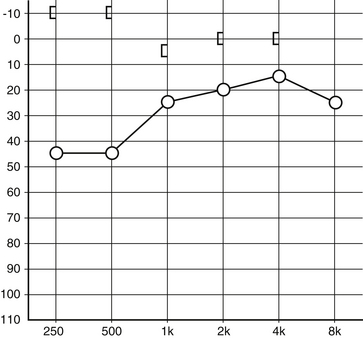
The audiogram (Fig. 42-3) is an important part of the SCDS evaluation, and a few patients have auditory symptoms in the absence of any vestibular signs or symptoms.8,10,15,16 Conductive hearing loss is often largest at lower frequencies,15–17 and bone conduction thresholds are often less than 0 dB nHL (conductive hyperacusis). Because of the conductive hearing loss and normal appearance of the ear, some patients with primarily auditory symptoms have historically been misdiagnosed as having otosclerosis.10 The key differences are (1) that conductive hyperacusis does not occur in otosclerosis, and (2) that the acoustic stapedial reflex, which is often normal in SCD, should be absent in an ear affected with otosclerosis.
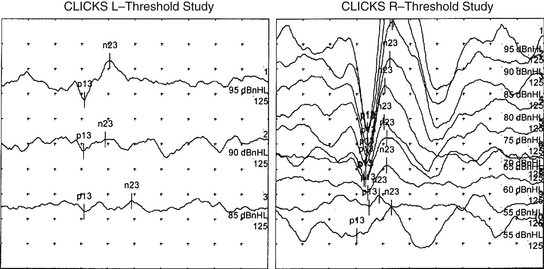
Vestibular evoked myogenic potential (VEMP) thresholds are usually decreased in patients with SCDS. These potentials are most commonly measured from the sternocleidomastoid muscles using averaged electromyography in response to multiple loud clicks or tone bursts delivered to the ear (Fig. 42-4). The reflex is thought to be activated by sound transmitted through the stapes footplate to the saccule and inferior vestibular nerve.18 Decreased VEMP thresholds are indicative of SCDS. For air-conducted 500 Hz tone bursts, we have found that cervical VEMP thresholds were 80 to 95 dB sound pressure level (SPL) for 13 patients with SCDS (83.85 ± 1.40 dB SPL, mean ± SD), 20 to 30 dB lower than in normal control subjects (110.25 ± 1.28 dB SPL).19 It has been argued that VEMP is better than 90% sensitive and specific for SCD,20 whereas other series have found the sensitivity and specificity closer to 80%.21 The VEMP is not measurable in all patients and is especially likely to be absent in patients who have had previous middle ear surgery. The VEMP threshold may also be decreased in other conditions, such as enlarged vestibular aqueduct syndrome.22
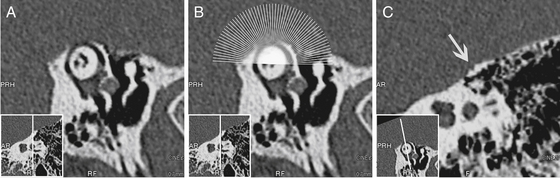
For the diagnosis of SCD to be considered, imaging of the temporal bone with computed tomography (CT) must show the absence of bone over the superior canal. If the superior canal appears surrounded with bone on CT, the diagnosis of SCDS is excluded; however, the appearance of a dehiscence on CT does not rule out thin bone covering the superior canal below the resolution of the scanner. CT is a highly sensitive test for SCD, but it is not specific.21 Optimal imaging uses high-resolution CT formatted in the plane of the superior canal.21,23 The term high-resolution has been applied to a wide variety of CT scanning parameters, which continue to change as technology is updated. In a review of temporal bone CT scans done in the general population, 9% of scans had apparent SCD with one observer identifying 12%.24 Many of these are likely false dehiscences caused by the limits of resolving thin bone because the incidence of SCD in a survey of temporal bones was only 0.7%.7
Images should be reconstructed in the plane of the superior canal and orthogonal to it so that any dehiscence can be definitively shown (Fig. 42-5). Even optimized scans are not without the risks of false-positive findings, however, so the diagnosis of SCD must never be based on a CT scan alone. A finding of SCD on CT should be considered in the context of findings on physical examination, VEMP, and audiogram, and the patient’s symptoms before concluding that the patient has SCDS.
DIFFERENTIAL DIAGNOSIS
The conductive hearing loss component of SCDS can appear similar to otosclerosis because both occur in adulthood in ears that appear normal on physical examination.10 The audiograms differ in that SCDS patients often have conductive hyperacusis (see Fig. 42-3), and if there is no previous history of middle ear surgery, the acoustic reflex is often intact. Otosclerosis is not associated with decreased VEMP thresholds, vertigo symptoms, or CT findings of SCD.
Meniere’s disease is characterized by the triad of low-frequency hearing loss, vertigo, aural fullness, and tinnitus.25 Although the hearing loss in Meniere’s disease is classically sensorineural, conductive hearing loss has also been described.26 The attacks of vertigo associated with Meniere’s disease usually are severe and last hours with normal periods between attacks. The dizziness associated with SCDS can be chronic, but there are also often short periods of vertigo associated with exposure to noise or pressure changes; a careful history usually differentiates these two conditions.
Autophony is often the predominant symptom in patients with a patulous eustachian tube,27 but it can also be the most disturbing symptom in SCDS. One distinguishing feature between the two conditions is that patients with patulous eustachian tube typically have autophony for their own breath sounds, whereas patients with SCDS usually do not.27 Also, a history of vertigo symptoms and hyperacusis of bone-conducted sound are not typical of a patulous eustachian tube. The audiogram, VEMP, and CT typically differentiate a patulous eustachian tube and SCDS.
A perilymphatic fistula is a leak of perilymph somewhere in the vestibular labyrinth, and the leak creates an abnormal compliance that allows fluid to move and stimulate the vestibular end organs in response to sound or pressure changes. Perilymph fistula along with fenestrations of other semicircular canals are often considered in the differential diagnosis of SCDS.28 The term perilymphatic fistula is usually used to describe a fistula into the middle ear through the round or oval window. The perilymphatic fistula diagnosis is most clear in the presence of recent stapes surgery, temporal bone fracture, or barotrauma injury. In these cases, acute vertigo is usually accompanied by a sensorineural hearing loss. A fistula in the horizontal canal can be acquired in cases of cholesteatoma or prior mastoidectomy.29 Spontaneous perilymphatic fistula is a controversial diagnosis, which if considered at all should be considered only after all other possible causes are excluded.30
Just as it is possible for patients with SCDS to have prior incorrect diagnoses, patients with other disorders are occasionally diagnosed with SCDS. In recent years, the SCDS diagnosis has become well publicized with the disorder featured on nationally televised news programs and Internet forums. Radiologists are also aware of the diagnosis, and 12% of temporal bone CT scans of the general population may be read as showing SCD,24 even though the true prevalence of the disorder is likely less than 1%.7
The most common cause of spontaneous (nonpositional) vertigo is migraine-associated vertigo.31 The incidence of migraine is 17.6% of women and 5.7% of men,32 and approximately 25% of migraine patients report some vertigo.33 Migraine is much more common than SCDS, and inevitably we have found some patients with radiographically apparent SCD whose symptoms were nonspecific and better explained by migraine. Particularly challenging are patients who have specific symptoms of SCDS and migraine. It may be difficult to determine if their sound sensitivity is due to one more than the other. Chronic dysequilibrium may be related to migraine, or it may be due to the constant transmission of intracranial pressure pulsations through the dehiscence to the labyrinth. The physiologic disturbances of the labyrinth caused by SCDS could serve as triggers to exacerbate migraine in susceptible individuals. The neurotologist must also consider, however, that failure to recognize and treat coexistent migraine can lead to disappointing results in SCDS surgery, as it can with other causes of vertigo as well.
PREOPERATIVE DECISION MAKING
The Dizziness Handicap Inventory (DHI)34 is an instrument that may be helpful in gauging vestibular symptom severity. This questionnaire grades dizziness symptoms on a scale from 0-100. It has previously been validated for surgical treatment of benign paroxysmal positional vertigo,35 acoustic neuroma surgery,36,37 and ablative procedures for Meniere’s disease.38 We measured the DHI in 19 patients with SCDS before they underwent SCD repair via a middle fossa approach. The average preoperative DHI score was 44 ± 24 (mean ± SD)39; this compares with the handicap caused by untreated primary benign paroxysmal positioning vertigo, in which the DHI score averaged 38.5 in one series,40 and with the handicap caused by active Meniere’s disease, in which the DHI score averaged 39.6 ± 21.1 in another series.41 The comparisons indicate a high degree of dizziness handicap for SCDS patients who seek surgical treatment.
Auditory symptoms are the primary complaint in a significant fraction of SCDS patients.16 Autophony, or the abnormal sound of the one’s own voice, can often be quite disabling, especially in patients for whom singing or speaking is important. There is no medical treatment for autophony symptoms resulting from SCDS because the sound transmission is via bone, not the eustachian tube. For SCDS patients who are significantly disturbed by autophony, surgery is the only option for relief. With conductive hyperacusis or abnormally high sensitivity to other bone-conducted sounds, patients may hear sounds emanating from within their body, such as their pulse or the motion of their eyes. These symptoms can also be very annoying to many patients, and surgery is the only option for relief.
Conductive hearing loss is a common symptom in SCDS,42 but because it is often limited to the low frequencies and usually affects only one ear, many patients do not have a significant disability because of it. In most patients, the conductive hearing loss improves with surgery,42 and resolution of a large sensorineural hearing loss has been reported.43 Plugging of SCD also carries a risk of hearing loss, however, and this risk is greater in patients who have had previous inner ear surgery for the problem, including stapes surgery.42 For this reason, patients who have hearing loss as their primary symptom of SCDS should first be encouraged to consider nonsurgical options, such as a hearing aid.
Patients should be advised on their likely postoperative course and possible complications of SCD plugging as part of the preoperative decision-making process. Although dizziness symptoms are often the motivation for surgery, it is common for imbalance symptoms to be worse during the immediate postoperative period. These symptoms improve as the patient adapts; in about one third of patients, a physical therapist is involved in vestibular rehabilitation. Plugging of the superior canal causes a vestibular sensory deficit owing to the hydrodynamic insufficiency of the canal. This deficit is permanent, as can be shown with head thrust testing in the superior canal plane (rotating the head quickly nose down and rolling toward the ipsilateral side in the superior canal plane).3 Patients can adapt very well to this single-canal insufficiency, however, because low-frequency, low-acceleration head movements still generate useful inhibitory signals from the contralateral posterior canal. These are silenced only for high-frequency, high-acceleration head movements, which are rarer. Vestibular physical therapy can take advantage of the function of the contralateral posterior canal and of other gaze-stabilizing mechanisms in promoting compensation for the loss caused by SCD plugging. In our experience, the compensated state after SCD plugging allows the patient to lead a much more active lifestyle than did the SCDS condition.
Surgery for SCD plugging shares the risk of perioperative complications common to any middle fossa approach.44 Cerebrospinal fluid leak may occur if the dura is violated, especially if air cells into the mastoid are exposed during surgery, or if there is a tegmen dehiscence. Intracranial hematoma is a rare postoperative complication that can occur after any middle fossa surgery. The patient’s mental status should be closely monitored during the acute postoperative period, and the onset of unusually severe pain should be a warning sign. If this complication occurs, the patient must be returned to the operating room quickly for hematoma evacuation to prevent more serious sequelae.
The risk of sensorineural hearing loss has previously been discussed, and is likely higher in patients with previous inner ear surgery.42 Patients should be carefully counseled about this risk.
The age and general state of health of the patient should also be considered in the decision to undergo surgery. In older patients, it is more difficult to elevate the middle fossa dura without tearing the dura and causing cerebrospinal fluid leak.45 Language impairment caused by damage of the dominant temporal lobe must be considered. Postoperative vestibular adaptation and recovery can be a longer and more difficult process in older patients.
OPERATIVE TECHNIQUE
Two approaches to plugging the SCD have been described. The middle cranial fossa approach was described first,1 and this technique is detailed in the following paragraphs. An alternative approach that has been described more recently is SCD plugging via a transmastoid approach. Advocates of the transmastoid approach have noted that it avoids a craniotomy, involves no temporal lobe retraction, and may lead to better stability of the canal plug. Most otolaryngologists are more familiar with mastoidectomy.46,47 The transmastoid approach was initially described in two patients in 2001, and although these patients were relieved of vertigo symptoms, one patient experienced significant sensorineural hearing loss after surgery.48 More recently, additional reports of transmastoid SCD plugging have been published with minimal morbidity and improvement in symptoms.46,47,49
We favor the middle fossa approach over the transmastoid approach for several reasons. The transmastoid approach does not allow direct confirmation of the dehiscence, and transmastoid plugging of a superior canal that was later found to be intact has been described.46 The transmastoid approach may be impossible in patients with a low-hanging dura or extensive tegmen dehiscences.46 In the transmastoid approach, the plug is also placed closer to the sensory epithelia of the ampulla and the utricle; this may be more traumatic to these structures, risking disturbance of their baseline firing rates. Opening the superior canal distal to the dehiscence may place the plug into the common crus, causing loss of sensory function of the posterior canal as well.50 Finally, the transmastoid approach requires drilling, irrigation, and suctioning on the bony canal. When the canal is opened, these manipulations could contaminate or remove perilymph from the canal and cause collapse of the membranous labyrinth or serous labyrinthitis.
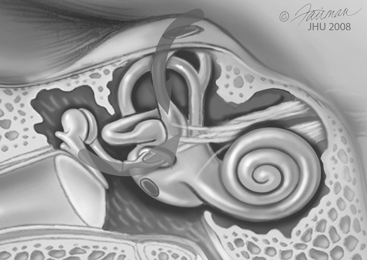
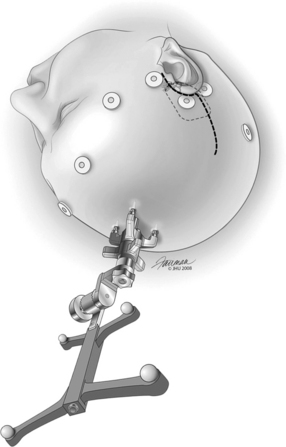
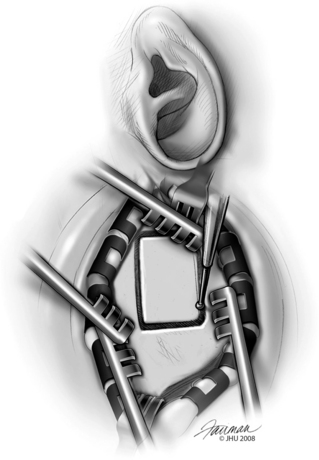
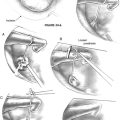
An area of the scalp away from the area of the middle fossa approach incision is prepared and sterilely draped for placement of the reference frame. In positioning of the reference frame, the surgeon should anticipate the position of the eventual incision; the location of the surgeon’s hands during surgery; the location of the microscope; the location of the navigation system; and the patient’s anatomy, including the thickness of the bone and the location of the superior sagittal sinus. When the site is chosen, a 1 cm incision is made, a small patch of periosteum is cleared from the bone, and the reference frame is anchored (Fig. 42-6). The reference frame is registered with the fiducial markers to allow navigation during surgery. Typically, the precision of the navigation registration is 1 mm.
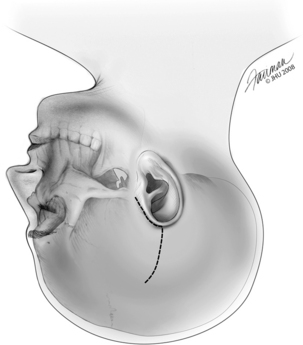
The incision is marked on the scalp extending from the helical root around the helix to a location over the external auditory canal, and then superiorly (Fig. 42-7). Hair around the area of the planned incision is shaved, and the area is infiltrated with 1% lidocaine with 1:100,000 epinephrine. The skin is sterilized widely enough to include the previously placed reference frame in the field and to be prepared for the rare case in which a craniotomy may need to be enlarged to control bleeding or evacuate a hematoma. After the skin incision is completed, bleeding is controlled using Raney clips along the skin edges. A piece of temporalis fascia is harvested for later use in plugging the superior canal, and for repair of any tegmen defects that may be encountered or cerebrospinal fluid leak that may occur (Fig. 42-8). Afterward, the temporalis muscle is divided, and the area of the craniotomy is exposed.
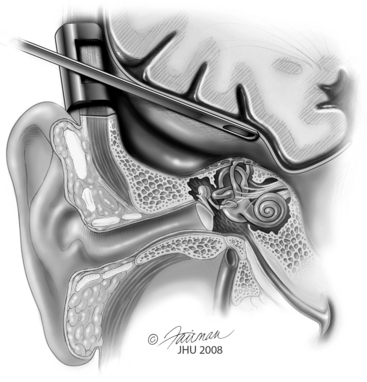
The intraoperative navigation system is used to plan the craniotomy. The trajectory view mode is used to “sight” a line from the surface of the skull to the dehiscence, and the craniotomy is centered here on the skull. The lower border of the craniotomy is placed just high enough to avoid the mastoid air cells, also located with the navigation probe. If a navigation system is not used, the craniotomy should be centered on the external auditory canal. This is slightly different from the placement used for drilling of the internal auditory canal, where the craniotomy is placed with its center anterior to the external auditory canal because of the more anterior location of the internal auditory canal relative to the labyrinth. The width and height of the craniotomy is enough to accommodate the Fisch retractor, typically 3 cm wide × 4 cm high (Fig. 42-9). Care is taken to ensure the anterior and posterior cuts of the craniotomy are parallel to facilitate stable placement of the Fisch retractor.
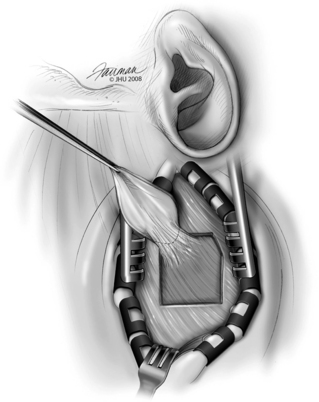
The Fisch middle cranial fossa retractor is placed and used to elevate the dura gently off the floor of the middle fossa (Fig. 42-10). Dura here can be very thin, especially if tegmen dehiscences are also present, and we find that large cotton balls soaked in saline are the least traumatic means for the dural elevation. A hemostatic agent such as dry microfibrillar collagen (Avitene) or absorbable gelatin sponge (Gelfoam) mixed as a paste with thrombin is generously applied in advance of the cotton balls. The image navigation system is frequently useful during the exploration to identify the precise location of the superior canal and its dehiscence. The surgeon is careful to suction only on the cotton balls and not to suction the area of the dehiscence directly because of the risk that this poses for removing excessive perilymph or for tearing the membranous labyrinth, which could cause sensorineural hearing and vestibular loss.
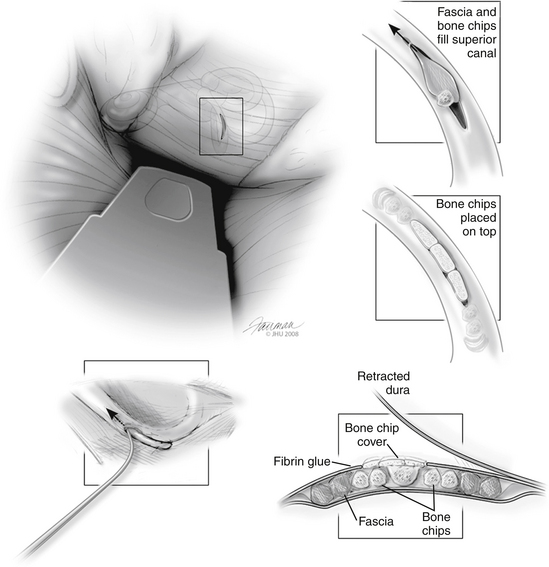
When the SCD has been identified, attention is immediately shifted toward plugging the dehiscence (Fig. 42-11). Small pieces are already prepared from the previously harvested temporalis fascia. These moist pieces of fascia slide into the two open lumina of the bony superior canal with gentle pressure from a curved pick. Several pieces are placed in each end to push the plugs several millimeters beyond the dehiscence; this is done to prevent a recurrence if further bone erosion occurs from the ends of the present dehiscence. Hydraulic pressure tends to push previously placed pieces of fascia out of one end of the dehiscence while the other is being packed. We look for this as the final confirmation that the correct holes are being plugged. Care must be taken that one end is not left open because its fascia is displaced. To prevent this, when the fascia is in place, bone chips matching the diameter of the canal are firmly lodged to “cork” each end of the dehiscence. The auditory brainstem response is carefully monitored during this process, and any degradation of the response serves as a warning that too much pressure may be built up within the inner ear.
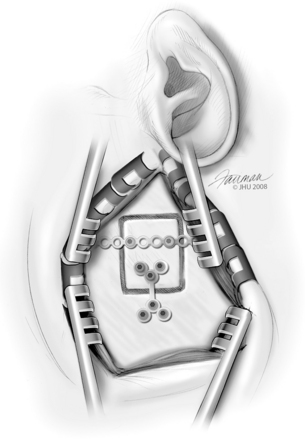
Closure is achieved by anchoring the previously harvested bone flap in place using titanium plates (Fig. 42-12). A burr may be used to recess the plates into the bone so that they are not palpable postoperatively. The temporalis muscle is reapproximated with absorbable sutures, and the skin is closed with staples or nylon suture or both. A drain is not typically used.
LONG-TERM RESULTS
In our experience, most patients are extremely satisfied with the surgery. Relief of dizzy symptoms has been documented by measuring the DHI34 before SCD plugging surgery and 3 months afterward. On average, DHI improved by 26 points, with patients with more severe dizziness (preoperative DHI ≥30) improving by an average of 39 points.39 This improvement is greater than the mean improvement seen after surgical labyrinthectomy for Meniere’s disease, which decreased DHI score by 17, and after vestibular neurectomy, which decreased DHI score by 16.38
The results for improving hearing with SCD surgery are less clear. Dramatic results have been observed in individual patients,43 but are uncommon. In a series of six patients with an air-bone gap before SCD plugging who had no previous history of ear surgery, four (66%) had at least partial closure of the air-bone gap after surgery.42 In patients with previous middle cranial fossa or stapes surgery, the risk of hearing loss was high in this series. In a more recent study from our institution, the average patient experienced a 10 dB improvement in air conduction hearing, although individual results varied from a 45 dB gain to a 45 dB hearing loss.21 There has even been a report of improvement in sensorineural hearing loss after SCD surgery.43
1. Minor L.B., Solomon D., Zinreich J.S., Zee D.S. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249-258.
2. Tullio P. Das Ohr und die Entstehung de Sprache und Schrift. Berlin: Urban Scharzenberg; 1929.
3. Mayer O., Fraser J.S. Pathological changes in the ear in late congenital syphilis. J Laryngol Otol. 1936;51:683-714.
4. Fox E.J., Balkany T.J., Arenberg I.K. The Tullio phenomenon and perilymph fistula. Otolaryngol Head Neck Surg. 1988;98:88-89.
5. Kacker S.K., Hinchcliffe R. Unusual Tullio phenomena. J Laryngol Otol. 1970;84:155-166.
6. Ishizaki H., Pyykko I., Aalto H., Starck J. Tullio phenomenon and postural stability: Experimental study in normal subjects and patients with vertigo. Ann Otol Rhinol Laryngol. 1991;100:976-983.
7. Carey J.P., Minor L.B., Nager G.T. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol Head Neck Surg. 2000;126:137-147.
8. Minor L.B. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717-1727.
9. Watson S.R., Halmagyi G.M., Colebatch J.G. Vestibular hypersensitivity to sound (Tullio phenomenon): Structural and functional assessment. Neurology. 2000;54:722-728.
10. Halmagyi G.M., Aw S.T., McGarvie L.A., et al. Superior semicircular canal dehiscence simulating otosclerosis. J Laryngol Otol. 2003;117:553-557.
11. Cremer P.D., Minor L.B., Carey J.P., Della Santina C.C. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000;55:1833-1841.
12. Minor L.B., Cremer P.D., Carey J.P., et al. Symptoms and signs in superior canal dehiscence syndrome. Ann N Y Acad Sci. 2001;942:259-273.
13. Krombach G.A., DiMartino E., Schmitz-Rode T., et al. Posterior semicircular canal dehiscence: A morphologic cause of vertigo similar to superior semicircular canal dehiscence. Eur Radiol. 2003;13:1444-1450.
14. Sheehy J.L., Brackmann D.E. Cholesteatoma surgery: Management of the labyrinthine fistula—a report of 97 cases. Laryngoscope. 1979;89:78-87.
15. Merchant S.N., Rosowski J.J. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29:282-289.
16. Mikulec A.A., McKenna M.J., Ramsey M.J., et al. Superior semicircular canal dehiscence presenting as conductive hearing loss without vertigo. Otol Neurotol. 2004;25:121-129.
17. Songer J.E., Rosowski J.J. A mechano-acoustic model of the effect of superior canal dehiscence on hearing in chinchilla. J Acoust Soc Am. 2007;122:943-951.
18. Welgampola M.S., Colebatch J.G. Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology. 2005;28(7):920-926.
19. Welgampola M.S., Myrie O.A., Minor L.B., Carey J.P. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70:464-472.
20. Zhou G., Gopen Q., Poe D.S. Clinical and diagnostic characterization of canal dehiscence syndrome: A great otologic mimicker. Otol Neurotol. 2007;28(7):920-926.
21. Crane B.T., Minor L.B., Carey J.P. Three-dimensional computed tomography of superior canal dehiscence syndrome. Otol Neurotol. 2008;29(5):699-705.
22. Sheykholeslami K., Schmerber S., Habiby Kermany M., Kaga K. Vestibular-evoked myogenic potentials in three patients with large vestibular aqueduct. Hear Res. 2004;190:161-168.
23. Belden C.J., Weg N., Minor L.B., Zinreich S.J. CT evaluation of bone dehiscence of the superior semicircular canal as a cause of sound- and/or pressure-induced vertigo. Radiology. 2003;226:337-343.
24. Williamson R.A., Vrabec J.T., Coker N.J., Sandlin M. Coronal computed tomography prevalence of superior semicircular canal dehiscence. Otolaryngol Head Neck Surg. 2003;129:481-489.
25. Minor L.B. Meniere’s disease and migraine. Arch Otolaryngol Head Neck Surg. 2005;131:460.
26. Muchnik C., Hildesheimer M., Rubinstein M., Arenberg I.K. Low frequency air-bone gap in Meniere’s disease without middle ear pathology: A preliminary report. Am J Otol. 1989;10:1-4.
27. Poe D.S. Diagnosis and management of the patulous eustachian tube. Otol Neurotol. 2007;28:668-677.
28. Minor L.B. Labyrinthine fistulae: Pathobiology and management. Curr Opin Otolaryngol Head Neck Surg. 2003;11:340-346.
29. Hakuba N., Hato N., Shinomori Y., et al. Labyrinthine fistula as a late complication of middle ear surgery using the canal wall down technique. Otol Neurotol. 2002;23:832-835.
30. Friedland D.R., Wackym P.A. A critical appraisal of spontaneous perilymphatic fistulas of the inner ear. Am J Otol. 1999;20:261-276.
31. Eggers S.D. Migraine-related vertigo: Diagnosis and treatment. Curr Pain Headache Rep. 2007;11:217-226.
32. Tepper S.J. A pivotal moment in 50 years of headache history: The first American migraine study. Headache. 2008;48:730-731.
33. Kayan A., Hood J.D. Neuro-otological manifestations of migraine. Brain. 1984;107(Pt 4):1123-1142.
34. Jacobson G.P., Newman C.W. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424-427.
35. Shaia W.T., Zappia J.J., Bojrab D.I., et al. Success of posterior semicircular canal occlusion and application of the dizziness handicap inventory. Otolaryngol Head Neck Surg. 2006;134:424-430.
36. Tufarelli D., Meli A., Labini F.S., et al. Balance impairment after acoustic neuroma surgery. Otol Neurotol. 2007;28:814-821.
37. Humphriss R.L., Baguley D.M., Moffat D.A. Change in dizziness handicap after vestibular schwannoma excision. Otol Neurotol. 2003;24:661-665.
38. Badke M.B., Pyle G.M., Shea T., Miedaner J. Outcomes in vestibular ablative procedures. Otol Neurotol. 2002;23:504-509.
39. Crane B.T., Minor L.B., Carey J.P. Superior canal dehiscence plugging reduces dizziness handicap. Laryngoscope. 2008;118(10):1809-1813.
40. O’Reilly R.C., Elford B., Slater R. Effectiveness of the particle repositioning maneuver in subtypes of benign paroxysmal positional vertigo. Laryngoscope. 2000;110:1385-1388.
41. Perez N., Martin E., Garcia-Tapia R. Dizziness: relating the severity of vertigo to the degree of handicap by measuring vestibular impairment. Otolaryngol Head Neck Surg. 2003;128:372-381.
42. Limb C.J., Carey J.P., Srireddy S., Minor L.B. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006;27:969-980.
43. Wilkinson E.P., Liu G.C., Friedman R.A. Correction of progressive hearing loss in superior canal dehiscence syndrome. Laryngoscope. 2008;118:10-13.
44. Sanna M., Taibah A., Russo A., et al. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol. 2004;25:379-386.
45. Oghalai J.S., Buxbaum J.L., Pitts L.H., Jackler R.K. The effect of age on acoustic neuroma surgery outcomes. Otol Neurotol. 2003;24:473-477.
46. Agrawal S.K., Parnes L.S. Transmastoid superior semicircular canal occlusion. Otol Neurotol. 2008;29:363-367.
47. Crovetto M., Areitio E., Elexpuru J., Aguayo F. Transmastoid approach for resurfacing of superior semicircular canal dehiscence. Auris Nasus Larynx. 2008;35:247-249.
48. Brantberg K., Bergenius J., Mendel L., et al. Symptoms, findings and treatment in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol. 2001;121:68-75.
49. Kirtane M.V., Sharma A., Satwalekar D. Transmastoid repair of superior semicircular canal dehiscence. J Laryngol Otol. 2009;123(3):356-358.
50. Carey J.P., Migliaccio A.A., Minor L.B. Semicircular canal function before and after surgery for superior canal dehiscence. Otol Neurotol. 2007;28:356-364.