81 Sudden Cardiac Death
Implantable Cardioverter-Defibrillators
Since its initial development in the 1970s1 and its introduction to clinical practice in the 1980s,2 the implantable cardioverter-defibrillator (ICD) has revolutionized the management of patients with or at risk for life-threatening ventricular arrhythmias. Large randomized controlled trials3–13 have shown that these devices prevent death from ventricular tachycardia (VT) or ventricular fibrillation (VF). Device-based treatment of recurrent VT or VF is the initial treatment of choice for many patients who have experienced or are at high risk for experiencing these rhythm disturbances.14 Device complexity makes a detailed understanding of ICD technology challenging for practitioners, but a general understanding of these devices and associated clinical problems is increasingly important because of their widespread use.
 Epidemiology of Sudden Cardiac Death
Epidemiology of Sudden Cardiac Death
Sudden cardiac death, arbitrarily defined as death from a cardiac cause occurring within 1 hour of cardiovascular symptom onset or without preceding symptoms,15 is a major public health problem responsible for approximately 450,000 deaths annually in North America alone.16 Out-of-hospital cardiac arrest carries a dismal prognosis, with reported rates of survival to hospital admission of 5% to 10% and minimal improvement in survival rates over the past several decades.17 This poor outcome occurs despite public health efforts to improve public recognition of cardiac symptoms and shorten the time to therapy by means of bystander cardiopulmonary resuscitation (CPR) and better access to emergency medical services.18 Among patients who survive to hospital admission, mortality and morbidity remain exceedingly high,19,20 highlighting the need for preventive efforts.
A significant proportion of sudden cardiac deaths are due to a treatable arrhythmia such as VT or VF,18,21 with the remainder being due to asystole or pulseless electrical activity (PEA). In autopsy studies, a majority of sudden cardiac death victims have pathologically apparent structural heart disease, particularly coronary atherosclerosis.22 In many cases, recent unstable coronary disease can be demonstrated by pathologic evidence of recent plaque rupture, with or without thrombosis.23 In cases in which cardiac monitoring was in place at the time of death, arrhythmia is commonly present.24
A significant proportion of sudden cardiac death occurs in patients without previously identified cardiac disease.19,25 Currently there is no feasible means of screening the population at large to identify all individuals who are at risk for this catastrophic event. Prediction and prevention strategies have therefore focused on identifying patients with clinical characteristics that place them at particularly high risk for sudden cardiac death.26,27 From the public health perspective, the most important conditions that predispose to a high risk of sudden cardiac death include cardiovascular risk factors, coronary artery disease, and left ventricular (LV) dysfunction of ischemic etiology and a variety of hereditary conditions that are listed in Box 81-1.
Approximately 50% of deaths in patients with heart failure are sudden.27,28 The majority of these are due to ventricular tachyarrhythmias.24 However, asystole and PEA are more common modes of sudden unexpected death in patients with end-stage heart failure.29 Among the factors that predict sudden cardiac death, severity of LV systolic dysfunction and age are by far the strongest predictors.30–32 Trials of ICD therapy have largely focused on patients with LV dysfunction, coronary disease, and spontaneous or inducible ventricular arrhythmias.33
 Prevention of Tachyarrhythmic Sudden Cardiac Death: Non-Device Therapy
Prevention of Tachyarrhythmic Sudden Cardiac Death: Non-Device Therapy
Previously, antiarrhythmic drugs were the cornerstone of treatment and prevention of recurrent VT and VF. However, it is recognized that these drugs are intrinsically hazardous, given their arrhythmogenicity and other adverse effects.34–39 Currently, antiarrhythmic drugs retain a primary role in patients with other conditions for which these agents are indicated (e.g., concurrent atrial fibrillation) or to decrease the frequency of ICD shocks. In this instance, D–L sotalol, dofetilide, or amiodarone are most often utilized.
Although class IC antiarrhythmic drugs, including encainide, flecainide, and moricizine, are effective at suppressing ventricular ectopy, they have been shown to significantly increase mortality in the landmark Cardiac Arrhythmia Suppression Trials.34,36 D-Sotalol, a pure class III antiarrhythmic agent, was evaluated in a randomized controlled trial and, similar to class IC agents, was found to increase mortality.40 The L-isomer that confers the beta-blocking effect may attenuate this hazard.41 Dofetilide, a class III agent, has been shown to be safe in patients with symptomatic heart failure and LV dysfunction when initiated in the hospital.42 In contrast, dronedarone, a newer antiarrhythmic agent, was found to increase mortality in patients with advanced heart failure.43 Thus, its role in the management of arrhythmias in patients with heart failure is unclear. Newer antiarrhythmic agents including azimilide, celivarone, and vernakalant are under investigation.
Amiodarone is the only available empirical choice for arrhythmia prevention in patients with heart failure or LV dysfunction. Several trials have shown decreased risk of death among patients treated with amiodarone after myocardial infarction (MI).35,44 Among patients at risk for arrhythmic death, a meta-analysis of controlled trials showed a reduction in total, cardiac, and sudden cardiac deaths with amiodarone therapy.45 In patients with heart failure, emperic amiodarone does not increase the risk of death (in contrast to class IC agents).10,37
Guided approaches to antiarrhythmic drug choice have also been evaluated.46 This can be done noninvasively using serial ambulatory cardiac monitoring to assess the response to specific drug choices, or invasively using serial programmed electrical stimulation to evaluate the drug effect on inducibility of VT or VF. Both approaches have been evaluated and can predict response to medical treatment reasonably well.47–49
The high recurrence rates of VT/VF and medication-related adverse events limit both empirical and guided therapies.38,50,51 For example, although amiodarone is the most effective antiarrhythmic drug for preventing the recurrence of VT and VF, a substantial proportion of patients (up to 20%) treated with amiodarone are unable to continue therapy in the long term owing to cumulative side effects, recurrent arrhythmia prompting a change in therapy, or death.38,52
Medications other than antiarrhythmic drugs have also been evaluated. Beta-blockers clearly reduce the risk of death among patients with recent MI53,54 and LV dysfunction,55–57 and it appears that approximately 50% of this decreased risk is due to reductions in sudden death.53 Beta-blockers have been shown to suppress ventricular arrhythmias among patients at elevated risk58,59 and may reduce death when used as primary antiarrhythmic therapy.60 Use of HMG-CoA reductase inhibitors (“statins”) has been associated with a lower risk of sudden death compared with nonuse in several studies.61–63 However, there are no large randomized controlled trials to confirm this finding. Trials of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with heart failure and coronary disease have shown reductions in the risk of sudden cardiac death in these populations.64 Omega-3 fatty acids (“fish oils”) appear to reduce the risk of sudden cardiac death in epidemiologic studies41,65 and in prospective randomized trials.66,67 A report68 has raised methodological concerns on one of these prospective trials.67 A recent randomized trial designed to look at the effect of highly purified omega-3 fatty acids on secondary prevention of sudden cardiac death after MI showed no benefit,69 possibly related to a low event rate in both groups. Aldosterone inhibition (spironolactone, eplerenone) has also been shown to be useful in preventing sudden death in patients with heart failure and after MI. While more widespread use of automated external defibrillator (AED) therapy was hoped to have a significant benefit in the prevention of sudden death, the Home AED Trial (HAT) failed to show a survival benefit of an AED in addition to CPR versus CPR alone among a large group of patients with a history of prior MI.70
Catheter ablation and surgery are often effective in preventing recurrent VT in patients who are difficult to treat by other means. Both techniques attempt to destroy or “ablate” involved myocardial tissue to interrupt reentrant VT circuits, thus preventing the development of sustained arrhythmias. In the past, VT surgery was considered a primary form of therapy in experienced centers, as it could offer a cure to patients with few other therapeutic options.71–74 Currently, VT surgery has a limited role owing to very high operative morbidity and mortality and improved nonsurgical approaches. Catheter ablation is a technique using intracardiac catheters to induce VT, map the pathologic circuits or substrate, and ablate small areas of involved myocardial tissue with radiofrequency energy.75,76 Ablation may carry a lower procedural risk than open surgical approaches, but a substantial number of patients have recurrent ventricular arrhythmias.74,77 Thus, it is presently not a replacement for ICD therapy. VT related to ischemic heart disease may be difficult to manage with catheter ablative procedures,77,78 owing to multiple pathologic intracardiac circuits. Like antiarrhythmic drugs, VT ablation is used as an adjunct to decrease the frequency of ICD therapy rather than a means to prevent sudden death.79
Revascularization is of primary importance in patients with coronary artery disease and malignant ventricular arrhythmias. One study evaluated the role of ICD in patients undergoing coronary artery bypass grafting (CABG) and showed no benefit in this population.80 Other studies have demonstrated an association between CABG and decreased risk of sudden death.11,81,82 Two randomized trials of ICD therapy early following MI found no difference in mortality with usual medical care versus an ICD9,11 (see Clinical Trials).
Lifestyle factors have been associated with lower risks of sudden death. Tobacco avoidance, exercise, moderate alcohol consumption,83 and a diet rich in fish65 have all been shown to be protective, and lifestyle modification programs may prevent sudden death.84,85
 Implantable Cardioverter-Defibrillator Therapy
Implantable Cardioverter-Defibrillator Therapy
Device Basics
The pulse generators of early devices were large (approximately 250 cm3) and required surgical implantation in the abdomen. Leads were large (150 to 180 cm2) epicardial pads placed via a thoracotomy. Separate epicardial screw-in sensing leads were also required. Implantation was associated with significant perioperative morbidity and mortality. Rhythm analysis was rudimentary and relatively insensitive. Only medium- or high-energy shock therapy was available, and data storage capacity was limited to information regarding the number of shocks. When intracardiac electrogram storage and analysis became available, it was apparent that inappropriate shocks, predominantly for atrial fibrillation, were common.86,87
The initial primary purpose of the ICD was to detect VT and VF and terminate these arrhythmias with effective defibrillation. Reports of early experiences suggested a substantially lower annual mortality among ICD recipients versus similar historical comparative groups.88 Recent refinements in ICD technology have improved the safety and tolerability of the devices substantially, but effective defibrillation remains the crucial lifesaving feature.
Current devices are much smaller, allowing subpectoral or subcutaneous implantation. Using nonthoracotomy lead systems, implantation methods are identical to permanent pacemaker implantation. Local anesthetic with mild sedation is used for implantation; heavy sedation or a brief general anesthetic is needed to test defibrillation thresholds. Operative mortality for nonthoracotomy systems is less than 0.5%.89 The risk of defibrillator-threshold or safety-margin testing is estimated to be less than 0.05% for death or stroke and less than 0.2% for necessitating prolonged resuscitation, based on a large series of registry data.90 This risk is higher in patients with severe LV dysfunction where even a brief induction of VF can have persistent and detrimental efftects.90,91 Obesity, cachexia, limited vascular access, pulmonary hypertension, anticoagulation, bleeding disorders, and vascular or cardiac anomalies may increase the technical challenge of implantation. Tricuspid valve prosthesis or significant tricuspid valvular disease may preclude use of endocardial lead systems. Features of contemporary ICD systems are listed in Table 81-1.
TABLE 81-1 Features of Current Implantable Cardioverter-Defibrillators
| Size | 30-45 cm3 |
| Weight | 70-100 g |
| Batteries | Low-resistance lithium or silver vanadium for charging defibrillation capacitor; separate battery for pacing functions |
| Leads | Steroid-eluting, silicone- or polyurethane-coated, 4-9F (1.3–3 mm) caliber, depending on type; ports for ventricular, atrial, left ventricular (coronary sinus), and superior vena cava leads |
| Output, charge | 30-39 J (delivered), 750-800 V |
| Battery life | 3-8 yr, depending on manufacturer, device, and use |
| Arrhythmia detection | Rate-based; enhanced ventricular tachycardia detection features vary by device and manufacturer |
| Arrhythmia management | Defibrillation with biphasic waveform, low-energy cardioversion, antitachycardia pacing (ATP) features; atrial therapies, including ATP and cardioversion; bradycardic ventricular and dual chamber pacing; biventricular pacing |
| Storage capabilities | Device and lead identification, implantation date, physician contact; arrhythmia event data, including date and time, onset, heart rate, therapies delivered, shock counters, rate histograms, electrograms, marker channel; pacemaker functions, including pacing thresholds, lead impedances, R-wave and P-wave amplitude, percent pacing, heart failure diagnostic information |
| Programmable functions | Pacing parameters, tachyarrhythmic therapies, tiered therapy algorithms; many other refined programmed functions vary by manufacturer |
Complications Related to Transvenous Icd Placement
Although placement of a transvenous ICD system is routine in many centers, complications related to system placement do occur. Common procedural complications are summarized in Box 81-2.
Therapeutic Functions
Bradycardia and Pacing
Patients with significant heart failure commonly have symptomatic bradycardia due to conduction disturbances, inadequate chronotropic responses, and medications that induce bradycardia.29 Moreover, postcardioversion and postshock bradycardia is common among ICD patients. To meet these needs, all current ICDs have pacing capabilities. ICD systems are available with ventricular, dual-chamber, or biventricular pacing modalities.
Although patients who receive an ICD may have an indication for single or dual-chamber pacing, there are concerns about the potential adverse effects of right ventricular pacing. One major trial showed that atrioventricular sequential pacing at a rate of 70 beats per minute was associated with higher rates of heart failure, hospitalization, or death when compared with backup ventricular pacing at 40 beats per minute.92 This effect was ascribed to the untoward hemodynamic effects of right ventricular pacing. Other studies have supported this finding.93 Furthermore, pacing can precipitate ventricular tachyarrhythmias in some patients.94 Thus, the pacemaker backup rate should be turned down to the lowest acceptable rate in patients with LV dysfunction.
Biventricular pacing, or resynchronization therapy, is a pacing modality incorporated in some devices. The intent of biventricular pacing is not to treat bradycardia per se. Instead, it coordinates synchronous left and right ventricular contraction.95 In the presence of left bundle branch block or right ventricular pacing, the interventricular septum moves rightward during systole. This decreases the contribution of septal contraction to LV output, leading to less efficient LV systolic function. Biventricular pacing coordinates left and right ventricular contraction to minimize this effect. The left ventricle is approached through the venous system (coronary sinus) using specially designed leads to allow epicardial LV pacing.
Several studies evaluated biventricular pacing in patients with advanced symptomatic heart failure (NYHA III-IV) and significant intraventricular conduction delay (QRS duration ≥ 120 milliseconds).13,96–98 Results show improvements in symptoms, exercise tolerance, and quality of life99 among a significant proportion of these selected patients. A survival benefit has also been demonstrated (Table 81-2).13,98,100 More recent studies looking at less severe heart failure (NYHA I-II) have demonstrated a decrease in symptomatic heart failure episodes and favorable LV remodeling without a survival benefit.101,102 Another trial in less symptomatic patients (RAFT) will be reported later this year.103 Heart failure patients with QRS durations less than 120 milliseconds have not been shown to benefit from cardiac resynchronization therapy (CRT),104 but studies addressing methods other than QRS duration are ongoing (EchoCRT).
Tachyarrhythmia Detection
The primary method of detecting sustained VT is assessment of ventricular rate and duration of the tachycardia. Therapy is delivered for persistent heart rates exceeding a cutoff that is manually programmed. Different algorithms can be programmed for different rates (Figure 81-1). The major limitation of an exclusively rate-based rhythm analysis is that tachycardias other than VT (e.g., supraventricular tachycardia [SVT]) cannot be distinguished by rate alone.
Enhanced arrhythmia detection features in current dual-chamber systems enable sensitive and specific detection of VT and VF, decreasing the occurrence of inappropriate therapies.105–110 Onset criteria allow the distinction between sinus tachycardia, which generally has a gradual onset, and VT, which is abrupt. Rate stability criteria distinguish irregular atrial fibrillation from VT. Devices also use the intracardiac electrogram to identify VT. Analysis of QRS morphology during tachycardia compared with a sinus rhythm template is a feature found in many single and dual-chamber devices. Dual-chamber devices use atrial lead sensing to evaluate the relationship between ventricular and atrial activity to distinguish supraventricular tachycardia from VT.109 Judicious use of these features is highly sensitive for VT and specific for discrimination of SVT. Another method used to limit ICD shocks is to increase the number of intervals to detect before the device treats the arrhythmia. This prevents unnecessary therapies for arrhythmias that would otherwise have self-terminated, but with the tradeoff of an increased likelihood of syncope from the delay in administration of therapy.111,112 Trials assessing the utility of delayed detection are ongoing.113 Combining multiple algorithms to withhold unnecessary ICD shocks (SVT, noise, and more frequent use of antitachycardia pacing [ATP]) also holds promise.
Tachyarrhythmic Therapies: Tiered Therapy Algorithms
Using the methods outlined previously, the ICD detects arrhythmias and administers therapies as programmed. In contrast to early devices, current ICDs can deliver therapies other than defibrillation, including lower-energy cardioversion and ATP. Some devices also have atrial antitachycardia and cardioversion features, whose clinical benefit remains to be proven.114,115 A tiered therapy algorithm (see Figure 81-1) uses different “zones” of detection to preferentially administer ATP or shocks depending on the rapidity of the detected rhythm.
High-energy defibrillation is the primary and most important function of the ICD. It is highly effective for VF or very rapid VT. Other therapies are intended to abort hemodynamically tolerated VT to obviate a painful high-energy shock. Typically, tachycardias above 200 beats per minute are promptly treated with high-voltage shocks. If the ICD detects a ventricular rhythm in the “VF zone,” the battery charges the capacitor, which then discharges, or “shocks,” if a second rhythm analysis confirms ongoing VF. Current is transmitted between the right ventricular lead and either the device itself (“active” or “hot” can) or other electrodes or coils.116 The current passes through ventricular myocardium and depolarizes a proportion of myocytes with 27 to 35 J of energy, depending on the manufacturer and configuration. This depolarized mass of myocardium interrupts the fibrillating electrical wavefronts and terminates VF. After each therapy, the device reinstates a diagnostic algorithm to detect ongoing VT/VF. If the arrhythmia persists, the capacitor recharges, discharges, and continues this cycle of behavior until another rhythm is detected or the therapies are exhausted (e.g., 4–6 consecutive high-energy shocks for a single episode).
The major limitation of high-energy shocks is the associated discomfort experienced if the patient remains conscious during the arrhythmia. Many patients report that shocks are painful and are associated with fear, embarrassment, or other unpleasant emotions.117 Quality of life is significantly impaired in patients who receive ICD ≥ 5 shocks, from either the shock itself or the health condition necessitating the shock.118,119 It is important to prevent ICD shocks, given that both appropriate and inappropriate shocks have been associated with an increased risk of death.120 However, it is unclear whether the shock itself is responsible for the increased risk of death, or changes in the underlying condition both increase the occurrence of arrhythmias and the risk of death.
Low-energy cardioversion is an established method of terminating hemodynamically tolerated VT, with a success rate greater than 80%.121,122 When the device detects a rhythm in the VT zone, it charges the capacitor and delivers a lower-energy shock synchronized to the R wave (see Figure 81-1). Energy outputs of 0.1 to 5 J can terminate some VT events. Patient discomfort increases substantially with increased output, particularly above 0.5 to 1 J. Above 5 to 10 J, no benefit is gained with low-energy cardioversion versus defibrillation in terms of patient comfort, although avoidance of high-energy output may prevent long-term device dysfunction123,124 and prolong battery life. The other major risks of low-energy cardioversion are acceleration of the tachycardia rate, which occurs in up to 10% of cases, and delay of definitive therapy.122 Less commonly, cardioversion can cause the rhythm to degenerate to polymorphic VT or VF, necessitating defibrillation. ATP is generally favored over shocks to limit the problem.
ATP, when effective, is ideal therapy for terminating hemodynamically tolerated VT. ATP is painless, although awareness of palpitations can occur. ATP is usually the initial therapy attempted for episodes of VT, because success rates are similar to those obtained with low-energy cardioversion; up to 90% of VTs can be terminated with pacing.125–127
ATP is more complex than defibrillation or cardioversion. The principle is to deliver pacing stimulation to the ventricle to gain control over the reentrant circuit that is perpetuating the tachycardia (overdrive suppression). If pacing is effective in entering the VT circuit, when pacing is terminated, the patient’s native or paced control over ventricular depolarization is restored. In order to enter the circuit, pacing must occur in the excitatory gap when the ventricle is not refractory to stimulation, and the device must pace at a rate faster than the VT rate. Rates with a cycle length between 70% and 90% of the VT cycle length (i.e., approximately 10% to 40% faster) are most effective in terminating the tachycardia.125,127 ATP techniques intended to improve entry into the circuit and termination of the tachycardia have been developed. Manufacturers do not share a standard nomenclature to describe ATP algorithms, but each method employs several comparatively simple principles. Burst pacing delivers a series of several beats at a fixed cycle length. Ramp pacing progressively shortens cycle length (i.e., accelerates). Adaptive therapy modes allow pacing at differing rates, depending on the VT rate. Scanning allows the device to introduce pacing at varying points in the VT cycle. In the setting of VT, the device delivers several different ATP protocols in an attempt to terminate the tachycardia.
Atrial therapies incorporated in some devices include ATP and cardioversion. Their effectiveness in preventing and terminating atrial arrhythmias has been demonstrated,114,128,129 but the clinical value of this approach remains controversial. It is very uncommon to implant a device to treat atrial arrhythmias solely, but this is occasionally done in highly symptomatic patients who are intolerant of medical therapy.
 Clinical Trials
Clinical Trials
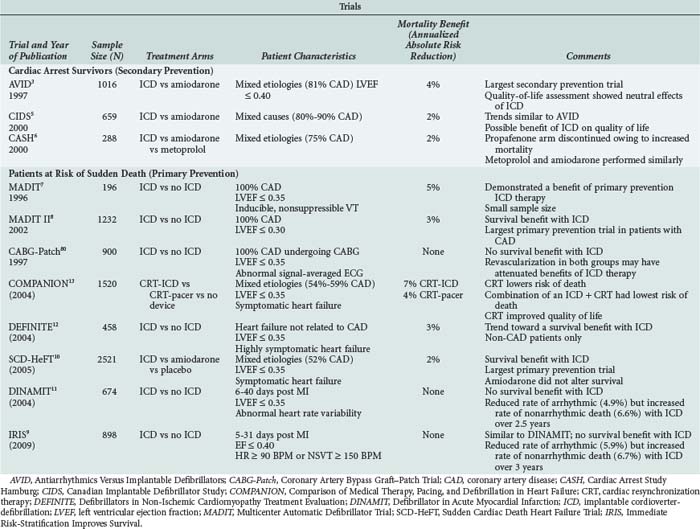
Many large (N > 100) randomized controlled trials assessing the efficacy of ICD therapy have been completed (see Table 81-2).3–1380 Three large trials assessed the role of ICD therapy as secondary prevention of sudden cardiac death among patients with ischemic LV dysfunction and sustained, hemodynamically significant ventricular arrhythmias.3,5,6 The largest of these trials (Antiarrhythmics versus Implantable Defibrillators [AVID]) randomized 1016 patients with symptomatic VT or VF and LV dysfunction (LV ejection fraction < 0.40) to therapy with ICD versus antiarrhythmic drugs (82.4% amiodarone).3 This study was stopped before completion of enrollment because of a statistically significant survival benefit (11.3% absolute risk reduction at 3 years) of the ICD. The Canadian Implantable Defibrillator Study (CIDS)5 and the Cardiac Arrest Study Hamburg (CASH)6 demonstrated trends toward decreased mortality, but these findings were not statistically significant. Meta-analysis of these three randomized trials supported data consistency, with a significant relative reduction in mortality risk of 28% (95% confidence interval [CI] 13%–40%).130
Several primary prevention trials assessed the role of ICD therapy among patients at risk for but without clinically sustained VT or VF.4,7,8,80 Although inclusion criteria varied, enrollment in these trials focused on patients with LV dysfunction. Similar to the secondary prevention trials, results of the primary prevention trials were consistent. Mortality reductions in the primary and secondary prevention trials have demonstrated similar results (see Table 81-2). From these studies it is clear ICD therapy reduces annual mortality by 2% to 7% in most patient groups. These studies also indicate that patients with both ischemic and nonischemic etiologies of LV dysfunction benefit from ICD therapy and that amiodarone has a limited role in the prevention of sudden death in patients with heart failure.
All but three of the primary prevention trials demonstrated a mortality benefit from ICD therapy. As previously discussed, routine aggressive coronary artery revascularization was likely responsible for the lack of benefit from routine ICD therapy in the CABG-Patch Trial.80 This inference is supported by a lower than anticipated mortality rate in that trial and the fact that the ICD resulted in a significantly lower rate of arrhythmic death.82 ICD therapy also did not reduce the risk of death in DINAMIT or IRIS (see Table 81-2). Similar to CABG-Patch, the proportion of arrhythmic deaths to the total deaths in these trials was also lower than anticipated.9,11 The lack of benefit from ICD therapy in these three studies illustrates that when considering a patient for an ICD, careful thought must be given to the long-term risk of arrhythmic death and the competing modes of death. ICDs have less impact with reduced rates of arrhythmic death.
A marked increase in the number of ICDs is occurring because of these trials. It is worth emphasizing that ICD therapy is costly,131,132 and the magnitude of benefit is sensitive to baseline risk.133 Studies to date have assessed ICD therapy in relatively high-risk populations, but even within these populations, risk appears to vary substantially. For example, in AVID, no benefit was observed among the subgroup of patients with an LV ejection fraction greater than 0.35.30 Whether ICD therapy is appropriate in lower-risk high-risk patients, particularly those with relatively preserved LV ejection fraction, remains to be determined. Further studies will aid in determining whether ICD therapy in such patients provides no benefit, small but costly benefit, small but clinically important benefit, or harm.
 Device-Related Issues Among Patients in Intensive Care
Device-Related Issues Among Patients in Intensive Care
Lead Failure
Lead failure due to dislodgment, fracture, or insulation breach occurs in 5% to 10% of patients, and lead replacement is usually required.134–136 Risk of lead failure is higher with a subclavian route compared with a cephalic vein approach, owing to the compressive effects of the clavicle and first rib on the subclavian vein.135 Lead failure is also more likely in younger patients, as well as certain specific leads that have been subject to manufacture advisory.137 Presenting complaints include inappropriate shocks, syncope or presyncope from device failure to deliver therapy, or proarrhythmia. Increased defibrillation thresholds can occur in the absence of lead defects, dislodgment, or change in physiologic conditions from ischemia, electrolyte abnormalities, or antiarrhythmic medications. This is thought to be due to myocardial fibrosis at the point of contact of the defibrillation lead. Frequent shocks appear to exacerbate this response. Steroid-eluting leads attenuate the inflammatory-fibrotic myocardial response and the associated increase in thresholds.
Infection
Infections involving ICDs have been reported to occur in 1% to 16% of patients.138–140 This is a devastating complication carrying substantial morbidity and reported mortality as high as 10%.141,142 Staphylococcus epidermidis and Staphylococcus aureus cause the majority of infections, although any pathogenic bacteria or fungus can theoretically seed the device. Infection in the first several months following implantation usually results from bacterial contamination with skin colonizers introduced during or immediately after the implantation procedure.143 Late device infections (>1 year after implantation) are equally common144 and usually implicate primary sources of bacteremia other than the ICD.145–147
Diagnosis of device infection is often challenging. Clinical suspicion must be high in patients with an implanted device who present with fever, weight loss, fatigue, systemic inflammation, or pulmonary embolism.141,148 All ICD or pacemaker patients with fever of uncertain cause should undergo careful examination of the generator pocket site for signs of inflammation, and blood cultures should be performed. In patients with proven bacteremia or fungemia, transthoracic and transesophageal echocardiography may be helpful.149 The presence of S. aureus bacteremia—given its association with device endocarditis (54%-72%)—should be approached with the presumption that the device is infected and warrants transesophageal echocardiography (TEE) to help guide duration of antibiotic therapy.150
Treatment of confirmed ICD system infection requires extraction of all device components, a prolonged (e.g., 2-6 weeks) intensive antibiotic course, and reimplantation.144 The optimal duration of antibiotic therapy is uncertain, and individualized timing of reimplantation is important in patients at high risk for life-threatening arrhythmias or those who are pacemaker dependent. When infection is suspected but unconfirmed, a trial of prolonged antibiotic therapy and close clinical vigilance for relapse may obviate system extraction. The risk of occult lead infection among patients with staphylococcal bacteremia is high,149,151 and consideration should be given to extraction,151 especially if relapse of infection occurs.
Peri-implantation antistaphylococcal antibiotic prophylaxis for pacemakers and ICDs is reccomended.143,152,153 Endocarditis prophylaxis for subsequent invasive procedures, especially in the first 6 months post implant in patients with ICDs or pacemakers who have no other indications, remains controversial and is not universally recommended.14
Arrhythmias and Antiarrhythmic Drugs
ICD patients often receive additional antiarrhythmic therapy to prevent device-provided therapies.154 These antiarrhythmics may decrease the frequency of VT and VF and thus decrease the need for defibrillation therapies, avoiding patient discomfort. Moreover, most antiarrhythmics will increase the tachycardia cycle length and make the arrhythmia more hemodynamically stable. A handful of drugs have been studied in the prevention of ICD shocks:
Using antiarrhythmic drugs is a double-edged sword. Despite their effectiveness, they each have their own known side-effect profile. Most will decrease the tachycardia cycle length. This makes the VT more hemodynamically stable and more amenable to termination with antitachycardia pacing. However, one has to consider the programmed tachycardia detection interval of the ICD to ensure that the VT is within its treatment range. This may also mean that a tachycardia that once caused syncope will now be treated while the patient is fully aware and conscious. Although it seems somewhat intuitive to consider reprogramming the ICD when managing VT/VF with antiarrhythmics, this process can easily be overlooked when the reason for initiating these drugs is to treat SVT, such as atrial fibrillation. Another important point to consider is the effects of these drugs on pacing and defibrillation threshold. Class I drugs, except propafenone,163 and chronic amiodarone use164 have this effect, which may be clinically important in patients whose defibrillation threshold is close to the maximum output of the device. In a substudy of OPTIC,165 defibrillation threshold was increased by 1.29 J with amiodarone and beta-blocker, compared to a decrease of 0.89 J with sotalol and a decrease of 1.67 J with beta-blockers alone. In most patients, this variation is well within their defibrillator safety margin. However, if amiodarone therapy is initiated, consideration should be given to follow-up testing of device function in patients with high thresholds at baseline.166 Another unintended effect of these drugs is that they may increase pacemaker dependence and result in increased right ventricular pacing which may have detrimental effects concerning LV function. In a monitored hospital setting, these issues are less important, but consultation with an electrophysiologist or a cardiologist familiar with the patient’s device and its programming should be obtained with regard to introduction of antiarrhythmic drugs for long-term use.
Catheter Ablation to Reduce Icd Therapies
Aside from the issues with antiarrhythmics already discussed, these drugs are limited by their efficacy, patient compliance, and side-effect profiles. VT catheter ablation is an attractive option in some patients as a means to decrease ICD therapies. The efficacy of this approach has been demonstrated in two single-center trials.76,167 Multicenter trials dealing predominantly with an ischemic heart disease population demonstrated a success rate of 41% for all inducible VTs, with a recurrence of sustained VT at 1 year of 56% in one trial,168 compared to an another reporting a success rate of 49% for elimination of all inducible VTs with a recurrence of 47% at 6 months.169 Although the absolute success rate is not fantastic, in both trials there was a substantial reduction in the frequency of VT documented in patients who had an ICD. There was, however, an increase in the frequency of VT in 20% of patients with an ICD in one of the studies.169 Procedure-related deaths were 2.7% and 3% in these studies. There are no large multicenter trials for ICD patients with nonischemic VT, but this may be effective in some.
Cardiac Arrest and Direct-Current Cardioversion
Cardiopulmonary resuscitation and external direct-current cardioversion involve particular issues for patients with ICDs.170,171 In principle, given the dire circumstances surrounding cardiac arrest, the presence of an ICD should not be a distraction to the resuscitation process. Potential for device-related problems should be recognized. Cardiac compressions theoretically increase the risk of lead dislodgment, leading to asystole in pacemaker-dependent patients. Elective external cardioversion or emergent defibrillation exposes the device to potentially damaging high voltage.172 Contemporary devices have incorporated elements that shunt energy away from the pulse generator. As a result, a circuit can develop, causing thermal damage at the lead/tissue interface and raise pacing and defibrillation thresholds.173 Inadvertent reprogramming has been reported as well. Transient elevations in thresholds are common; however, failure to capture following cardiac arrest or cardioversion should prompt immediate assessment for lead dislodgment or potentially permanent lead failure. Direct-current cardioversion-defibrillation paddles should be placed as far from the pulse generator as possible in an anteroposterior position, and the lowest effective energy should be used.170,171 Potential for electromagnetic interference from external defibrillation should be recognized, and applying a magnet over the ICD should be undertaken to disable the device.
For elective cardioversion, there are several special considerations.170 Thought should be given to attempting programmed cardioversion through the device rather than externally. If external cardioversion is necessary, a device programmer should be available in the room for immediate assessment of abnormal device function. Given the potential for a transient increase in capture threshold, the practitioner should be prepared to externally pace if necessary. Pacing and sensing thresholds should be checked immediately after a successful cardioversion and then again in 24 hours if feasible. The local device clinic should be contacted before attempting elective cardioversion, if possible, to ensure that immediate assistance is available and to identify any device peculiarities in advance.
Evaluation of the Icd Recipient After A Shock
Many ICD patients experience a shock within 2 years of implantation,174 and most isolated appropriate device therapies do not require a change in treatment, although addition or increase of a beta-blocker, amiodarone, or sotalol may be considered. Symptoms and patient-perceived device behavior before the shock should be assessed. The presence of presyncope, syncope, or palpitations suggests that the shock was due to arrhythmia. It is important to identify precipitants of arrhythmia such as exercise, angina, noncompliance with medications, or symptoms of worsening heart failure. Unstable myocardial ischemia and electrolyte disturbances should be excluded and treated. Diagnosis of ischemic events after a shock is challenging, because pacing, antitachycardia pacing, and shocks can cause nonspecific abnormalities of the ST segments,175 and cardiac markers are often transiently elevated.176
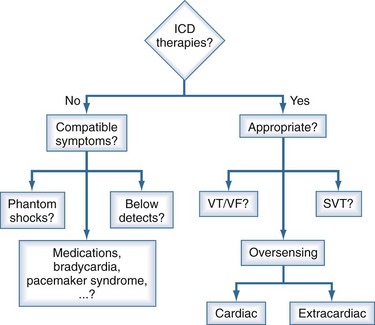
In addition to baseline clinical parameters, the initial assessment of a patient after a shock includes device interrogation. Patients’ memory of the sequence of events can be inaccurate, and interrogation provides information about the heart rate and rhythm before therapy initiation, therapy attempts, rhythm response to therapy, and definitive therapy, including number of shocks. Such information is crucial for evaluating the appropriateness of the shock and possible precipitating events to allow tailored programming of the device. An approach to the management of a patient who has received ICD therapies is provided in Figure 81-2.
Multiple Shocks and Electrical Storm
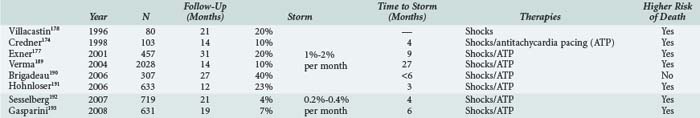
Multiple repetitive shocks can occur in 10% to 20% of ICD patients.174,177,178 When these occur, it is crucial to rapidly determine whether such therapies are appropriate. Frequent shocks are often highly psychologically distressing179 and can result in a syndrome similar to posttraumatic stress disorder.180 Sedation with benzodiazepines improves patient comfort and may decrease catecholamine-dependent arrhythmias.181 If the shocks are inappropriate, tachyarrhythmia detection should be disabled by magnet application. Urgent device reprogramming and therapy directed at the underlying condition (e.g., atrial tachyarrhythmias) is required. More than three episodes of VT/VF occurring within 24 hours is labeled an electrical storm. This ominous entity has been shown to predict an increased risk of non-sudden death in the next several months.177 The incidence of electrical storm is approximately 1% to 2% per month in non-CRT recipients and less than 0.5% per month in CRT recipients (Table 81-3). The mean time to development of electrical storm is quite variable but is usually in the initial 6 months after ICD implantation.
| Myocardial ischemia/infarction | 4-14% |
| Electrolyte/metabolic abnormality | 4-10% |
| Worsening heart failure | 9-19% |
| No clear cause | 57-87% |
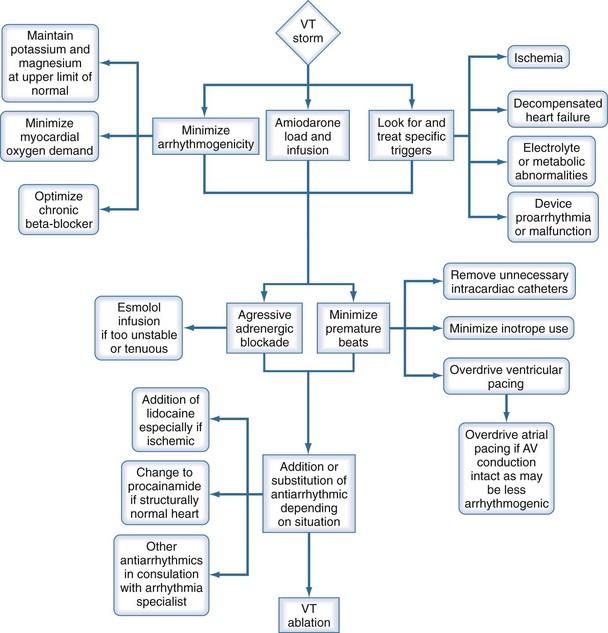
Recurrent VT or VF is most appropriately treated with beta-blockade alone182 or in combination with intravenous amiodarone.174 Sedation with benzodiazepines may be beneficial. It is essential that potential precipitants for the electrical storm be sought. These include myocardial ischemia, electrolyte abnormalities, and a worsening in LV function/decompensated heart failure. Despite careful evaluation for such precipitants, a clear cause for the electrical storm event is not found in over half of patients (Table 81-4). Nonetheless, a careful search for these precipitants is necessary, since they are often amenable to intervention and will reduce the likelihood of recurrent VT/VF.174 A stepwise approach recommended for management of this condition is provided in Figure 81-3. After initial therapeutic maneuvers are performed, there may be a role in suppressing or limiting premature beats that may be triggering the arrhythmia. Transient overdrive pacing in this circumstance may be of some benefit. If AV conduction is intact, overdrive pacing from the atria might be less proarrhythmic than ventricular pacing. If amiodarone is ineffective, other antiarrhythmics may be used, depending on LV function. A recent single-center case series demonstrated the usefulness of VT ablation, where electrical storm was suppressed in 89% of patients after 1 to 3 procedures.183
Electromagnetic Interference
Several environmental and medical sources of electromagnetic interference can affect device functioning (Table 81-5).170,184 Noise (electromagnetic interference) can be interpreted as rapid cardiac activity. Noise reversion algorithms on pacemakers prevent prolonged inhibition of pacing by activating an asynchronous pacing mode when prolonged noise is detected; however, asynchronous pacing can have adverse hemodynamic effects and can initiate ventricular arrhythmias. In ICDs, noise will be treated as VT/VF and if prolonged enough will result in therapies being delivered. In a pacemaker-dependent patient with an ICD, noise will result in inhibition of pacing until therapies are delivered, resulting in syncope which will mimic an appropriate shock.
| Source | Potential Problems | Preventive Measures |
|---|---|---|
| Imaging techniques (MRI)170 | Device motion Diathermy (lead heating) Oversensing Reprogramming |
MRI generally contraindicated. If unavoidable, program to asynchronous pacing mode and disable tachyarrhythmia therapies; resuscitation team must be available during imaging. |
| Surgical procedures involving electrosurgical (electrocautery) techniques170,171,194 | Oversensing Spurious tachyarrhythmia therapies |
Use alternative cutting and hemostatic techniques. Use bipolar electrocautery if working within 15 cm of the device and/or leads. Preoperative reprogramming (decrease sensitivity, asynchronous pacing, or noise reversion mode). Provide internal or external alternative pacing system for pacemaker-dependent patients. Peripheral monitoring (e.g., pulse oximeter). Place ground pad on leg to direct current away from pulse generator. Use brief bursts with pauses of at least 10 sec; use lowest power output possible and do not use near pulse generator. Assess and reprogram device immediately after procedure. |
| Muscle and nerve stimulators (including spinal, peripheral, and transcutaneous) | Oversensing | Test stimulator functioning, and interrogate device’s sensed activity and response before use. |
| Radiotherapy | Cumulative dose-dependent pulse generator damage Prolonged charge time Battery depletion |
Minimize dose. Shield device. Check device functioning after sessions. |
| Temporary intracardiac foreign bodies (including pulmonary artery catheters, temporary pacemakers, and instruments used in percutaneous coronary interventions) | Lead dislodgment | Avoid these manipulations with recently implanted devices. Use fluoroscopy or echocardiographic guidance if necessary. |
| Environmental (including cellular telephones, security systems [retail and airport], electrical equipment [including household appliances])195 | Usually not problematic in an inpatient setting Possible interference with device sensing functions |
Observe for unusual device behavior (rapid pacing, pacing inhibition, shocks) during use of electrical equipment near patient. Awareness of potential for interaction. |
| Other medical procedures (e.g., radiofrequency ablation, percutaneous coronary interventions, extracorporeal shock wave lithotripsy)170 | Several case reports of interaction with devices | Device interrogation following exposure. |
ATP, antitachycardia pacing; MRI, magnetic resonance imaging.
Magnetic Resonance Imaging
The functioning of ICDs can be adversely affected by magnetic resonance imaging (MRI) techniques and can create artifacts that limit image quality (see Table 81-5). There are several major potential risks of exposure to clinically relevant magnetic field strengths (0.2-3 T).170,185 Magnetic force induces significant device torque, which can cause motion of the pulse generator, resulting in local pain, tissue damage, or device dislodgment.186,187 Electromagnetic interference can precipitate rapid pacing or inadvertent therapies or interfere with sensing functions, leading to therapy inhibition. ICDs are more sensitive to inhibition of pacing than pacemakers are. Diathermy of the lead (heating) is well described, but its clinical significance is not known. Theoretically, heating of the lead tip can cause local tissue damage, myocardial perforation, or scar and increase sensing and pacing thresholds.185 In addition to the risk to the patient, the presence of any foreign body with ferromagnetic properties can create imaging artifacts, limiting the diagnostic value of MRI scanning in the area of the pulse generator or leads.
The absolute risk of adverse events in routine clinical situations is unknown, because there are no large-scale studies. With current technology, the presence of an ICD or implanted pacemaker is considered a contraindication to MRI. In the rare case in which a patient is foreseen to require an implantable device but also requires an MRI, implantation may be deferred if the potential diagnostic benefit of MRI in the near future outweighs the risk of delaying device implantation. In situations in which the diagnostic value of MRI is considered essential to the care of an ICD patient, scanning should be considered only after appropriately planning for the risks; a team prepared to address potentially life-threatening complications must be present to attend to the patient. Based on a few case series, MRI of extrathoracic regions can be undertaken with minimal risk as long as the proper precautions are taken.188 “MRI-safe” ICDs and pacemakers represent a potential solution to this dilemma in some patients, and much progress has been made in their development over the last 5 years.
Surgery
With careful planning, most if not all surgical procedures can be safely performed in ICD patients. ICD patients have a high burden of cardiovascular morbidity, and perioperative cardiac events (ischemia, heart failure, arrhythmias) are relatively common. Adherence to established guidelines for perioperative assessment,171 appropriate consultation, and anticipation of potential complications may reduce complications. The greatest risks related to the device itself are malfunction due to electromagnetic interference, arrhythmia precipitation, and changes in defibrillation, pacing, and sensing thresholds due to anesthetic agents or metabolic changes.171 Strategies to prevent complications from electromagnetic interference are listed in Table 81-5.
Key Points
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:e1-62. Available at: http://content.onlinejacc.org/cgi/content/full/51/21/2085
Poole JE, Johnson GW, Hellkamp AS, Anderson J, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009-1017.
Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303:322-324.
Pinski SL, Trohman RG. Interference in implanted cardiac devices. Pacing Clin Electrophysiol. 2002;25:1367-1381. (Part I) and 25:1496-1509 (Part II)
1 Mirowski M, Mower MM, Langer A, Heilman MS, Schreibman J. A chronically implanted system for automatic defibrillation in active conscious dogs. Experimental model for treatment of sudden death from ventricular fibrillation. Circulation. 1978 Jul;58(1):90-94.
2 Mirowski M, Reid PR, Mower MM, Watkins L, Gott VL, Schauble JF, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980 Aug 7;303(6):322-324.
3 A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997 Nov 27;337(22):1576-1583.
4 Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999 Dec 16;341(25):1882-1890.
5 Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, et al. Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000 Mar 21;101(11):1297-1302.
6 Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000 Aug 15;102(7):748-754.
7 Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996 Dec 26;335(26):1933-1940.
8 Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002 Mar 21;346(12):877-883.
9 Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009 Oct 8;361(15):1427-1436.
10 Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005 Jan 20;352(3):225-237.
11 Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004 Dec 9;351(24):2481-2488.
12 Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004 May 20;350(21):2151-2158.
13 Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004 May 20;350(21):2140-2150.
14 Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAMIII, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008 May 27;51(21):e1-e62.
15 Goldstein S. The necessity of a uniform definition of sudden coronary death: witnessed death within 1 hour of the onset of acute symptoms. Am Heart J. 1982 Jan;103(1):156-159.
16 Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001 Oct 30;104(18):2158-2163.
17 Rea TD, Eisenberg MS, Becker LJ, Murray JA, Hearne T. Temporal trends in sudden cardiac arrest: a 25-year emergency medical services perspective. Circulation. 2003 Jun 10;107(22):2780-2785.
18 Herlitz J, Andersson E, Bang A, Engdahl J, Holmberg M, lindqvist J, et al. Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Goteborg. Eur Heart J. 2000 Aug;21(15):1251-1258.
19 de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, et al. Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997 Nov 15;30(6):1500-1505.
20 Herlitz J, Eek M, Holmberg M, Engdahl J, Holmberg S. Characteristics and outcome among patients having out of hospital cardiac arrest at home compared with elsewhere. Heart. 2002 Dec;88(6):579-582.
21 Stratton SJ, Niemann JT. Outcome from out-of-hospital cardiac arrest caused by nonventricular arrhythmias: contribution of successful resuscitation to overall survivorship supports the current practice of initiating out-of-hospital ACLS. Ann Emerg Med. 1998 Oct;32(4):448-453.
22 Kannel WB, Thomas HEJr. Sudden coronary death: the Framingham Study. Ann N Y Acad Sci. 1982;382:3-21.
23 Davies MJ, Bland JM, Hangartner JR, Angelini A, Thomas AC. Factors influencing the presence or absence of acute coronary artery thrombi in sudden ischaemic death. Eur Heart J. 1989 Mar;10(3):203-208.
24 Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989 Jan;117(1):151-159.
25 Myerburg RJ, Mitrani R, Interian AJr, Castellanos A. Interpretation of outcomes of antiarrhythmic clinical trials: design features and population impact. Circulation. 1998 Apr 21;97(15):1514-1521.
26 Cannom DS, Prystowsky EN. Management of ventricular arrhythmias: detection, drugs, and devices. JAMA. 1999 Jan 13;281(2):172-179.
27 Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998 Nov 24;98(21):2334-2351.
28 Estes NA3rd, Weinstock J, Wang PJ, Homoud MK, Link MS. Use of antiarrhythmics and implantable cardioverter-defibrillators in congestive heart failure. Am J Cardiol. 2003 Mar 20;91(6A):45D-52D.
29 Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989 Dec;80(6):1675-1680.
30 Domanski MJ, Sakseena S, Epstein AE, Hallstrom AP, Brodsky MA, Kim S, et al. Relative effectiveness of the implantable cardioverter-defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. AVID Investigators. Antiarrhythmics Versus Implantable Defibrillators. J Am Coll Cardiol. 1999 Oct;34(4):1090-1095.
31 Exner DV, Sheldon RS, Pinski SL, Kron J, Hallstrom A. Do baseline characteristics accurately discriminate between patients likely versus unlikely to benefit from implantable defibrillator therapy? Evaluation of the Canadian implantable defibrillator study implantable cardioverter defibrillatory efficacy score in the antiarrhythmics versus implantable defibrillators trial. Am Heart J. 2001 Jan;141(1):99-104.
32 Sheldon R, Connolly S, Krahn A, Roberts R, Gent M, Gardner M. Identification of patients most likely to benefit from implantable cardioverter-defibrillator therapy: the Canadian Implantable Defibrillator Study. Circulation. 2000 Apr 11;101(14):1660-1664.
33 Ezekowitz JA, Armstrong PW, McAlister FA. Implantable cardioverter defibrillators in primary and secondary prevention: a systematic review of randomized, controlled trials. Ann Intern Med. 2003 Mar 18;138(6):445-452.
34 Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. The Cardiac Arrhythmia Suppression Trial II Investigators. N Engl J Med. 1992 Jul 23;327(4):227-233.
35 Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet. 1997 Mar 8;349(9053):675-682.
36 Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991 Mar 21;324(12):781-788.
37 Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995 Jul 13;333(2):77-82.
38 Vorperian VR, Havighurst TC, Miller S, January CT. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol.. 1997 Sep;30(3):791-798.
39 Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002 Dec 5;347(23):1825-1833.
40 Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral D-Sotalol. Lancet. 1996 Jul 6;348(9019):7-12.
41 Julian DG, Prescott RJ, Jackson FS, Szekely P. Controlled trial of sotalol for one year after myocardial infarction. Lancet. 1982 May 22;1(8282):1142-1147.
42 Kober L, Bloch Thomsen PE, Moller M, Torp-Pedersen C, Carlsen J, Sandoe E, et al. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000 Dec 16;356(9247):2052-2058.
43 Kober L, Torp-Pedersen C, McMurray JJV, Gotzsche O, Levy S, Crijns H, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358(25):2678-2687. 2008 June 19
44 Julian DG, Camm AJ, Frangin G, Janse MJ, Munoz A, Schwartz PJ, et al. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet. 1997 Mar 8;349(9053):667-674.
45 Sim I, McDonald KM, Lavori PW, Norbutas CM, Hlatky MA. Quantitative overview of randomized trials of amiodarone to prevent sudden cardiac death. Circulation. 1997 Nov 4;96(9):2823-2829.
46 Mitchell LB. Clinical trials of antiarrhythmic drugs in patients with sustained ventricular tachyarrhythmias. Curr Opin Cardiol. 1997 Jan;12(1):33-40.
47 Graboys TB, Lown B, Podrid PJ, DeSilva R. Long-term survival of patients with malignant ventricular arrhythmia treated with antiarrhythmic drugs. Am J Cardiol. 1982 Sep;50(3):437-443.
48 Mason JW. A comparison of electrophysiologic testing with Holter monitoring to predict antiarrhythmic-drug efficacy for ventricular tachyarrhythmias. Electrophysiologic Study versus Electrocardiographic Monitoring Investigators. N Engl J Med. 1993 Aug 12;329(7):445-451.
49 Wilber DJ, Garan H, Finkelstein D, Kelly E, Newell J, McGovern B, et al. Out-of-hospital cardiac arrest. Use of electrophysiologic testing in the prediction of long-term outcome. N Engl J Med. 1988 Jan 7;318(1):19-24.
50 Podrid PJ, Lampert S, Graboys TB, Blatt CM, Lown B. Aggravation of arrhythmia by antiarrhythmic drugs–incidence and predictors. Am J Cardiol. 1987 Apr 30;59(11):38E-44E.
51 Ravid S, Podrid PJ, Lampert S, Lown B. Congestive heart failure induced by six of the newer antiarrhythmic drugs. J Am Coll Cardiol. 1989 Nov 1;14(5):1326-1330.
52 Goldschlager N, Epstein AE, Naccarelli GV, Olshansky B, Singh B, Collard HR, et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007 Sep;4(9):1250-1259.
53 Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999 Jun 26;318(7200):1730-1737.
54 Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction. An overview of results from randomized controlled trials. JAMA. 1993 Oct 6;270(13):1589-1595.
55 Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999 Jun 12;353(9169):2001-2007.
56 The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999 Jan 2;353(9146):9-13.
57 Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996 May 23;334(21):1349-1355.
58 Cice G, Tagliamonte E, Ferrara L, Iacono A. Efficacy of carvedilol on complex ventricular arrhythmias in dilated cardiomyopathy: double-blind, randomized, placebo-controlled study. Eur Heart J. 2000 Aug;21(15):1259-1264.
59 Steinbeck G, Andresen D, Bach P, Haberl R, Oeff M, Hoffmann E, et al. A comparison of electrophysiologically guided antiarrhythmic drug therapy with beta-blocker therapy in patients with symptomatic, sustained ventricular tachyarrhythmias. N Engl J Med. 1992 Oct 1;327(14):987-992.
60 Exner DV, Reiffel JA, Epstein AE, Ledingham R, Reiter MJ, Yao Q, et al. Beta-blocker use and survival in patients with ventricular fibrillation or symptomatic ventricular tachycardia: the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. J Am Coll Cardiol. 1999 Aug;34(2):325-333.
61 De Sutter J, Tavernier R, De Buyzere M, Jordaens L, De Backer G. Lipid lowering drugs and recurrences of life-threatening ventricular arrhythmias in high-risk patients. J Am Coll Cardiol. 2000 Sep;36(3):766-772.
62 Mitchell LB, Powell JL, Gillis AM, Kehl V, Hallstrom AP. Are lipid-lowering drugs also antiarrhythmic drugs? An analysis of the Antiarrhythmics versus Implantable Defibrillators (AVID) trial. J Am Coll Cardiol. 2003 Jul 2;42(1):81-87.
63 Coleman CI, Kluger J, Bhavnani S, Clyne C, Yarlagadda R, Guertin D, et al. Association between statin use and mortality in patients with implantable cardioverter-defibrillators and left ventricular systolic dysfunction. Heart Rhythm. 2008 Apr;5(4):507-510.
64 Latini R, Tognoni G, Maggioni AP, Baigent C, Braunwald E, Chen ZM, et al. Clinical effects of early angiotensin-converting enzyme inhibitor treatment for acute myocardial infarction are similar in the presence and absence of aspirin: systematic overview of individual data from 96,712 randomized patients. Angiotensin-converting Enzyme Inhibitor Myocardial Infarction Collaborative Group. J Am Coll Cardiol. 2000 Jun;35(7):1801-1807.
65 Albert CM, Hennekens CH, O’Donnell CJ, Ajani UA, Carey VJ, Willett WC, et al. Fish consumption and risk of sudden cardiac death. JAMA. 1998 Jan 7;279(1):23-28.
66 Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002 Apr 23;105(16):1897-1903.
67 Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival–4. Cardiovasc Drugs Ther. 1997 Jul;11(3):485-491.
68 White C. Suspected research fraud: difficulties of getting at the truth. BMJ. 2005 Jul 30;331(7511):281-288.
69 Senges J, Schiele R, Schneider S, Gohlke H, Gottwik M, Steinbeck G, et al. Randomized trial of omega-3 fatty acids on top of modern therapy after acute myocardial infarction: the OMEGA-trial. 59th Annual Scientific Session of the American College of Cardiolgy 2009; 2009; Orlando; 2009.
70 Bardy GH, Lee KL, Mark DB, Poole JE, Toff WD, Tonkin AM, et al. Home use of automated external defibrillators for sudden cardiac arrest. N Engl J Med. 2008 Apr 24;358(17):1793-1804.
71 Fieguth HG, Trappe HJ, Wahlers T, Siclari F, Frank G, Borst HG. Surgical interventions in ischemic ventricular tachyarrhythmias–endocardial resection or implanted cardioverter/defibrillator. Eur J Cardiothorac Surg. 1994;8(8):400-403.
72 Frapier JM, Hubaut JJ, Pasquie JL, Chaptal PA. Large encircling cryoablation without mapping for ventricular tachycardia after anterior myocardial infarction: long-term outcome. J Thorac Cardiovasc Surg. 1998 Oct;116(4):578-583.
73 Glick DB, Ferguson TBJr. Surgery for cardiac arrhythmias. Curr Opin Cardiol. 1994 Mar;9(2):222-230.
74 Trappe HJ, Pfitzner P, Figuth HG, Wenzlaff P, Kielblock B, Klein H. Nonpharmacological therapy of ventricular tachyarrhythmias: observations in 554 patients. Pacing Clin Electrophysiol. 1994 Nov;17(11 Pt 2):2172-2177.
75 Morady F, Harvey M, Kalbfleisch SJ, el-Atassi R, Calkins H, Langberg JJ. Radiofrequency catheter ablation of ventricular tachycardia in patients with coronary artery disease. Circulation. 1993 Feb;87(2):363-372.
76 Soejima K, Suzuki M, Maisel WH, Brunckhorst CB, Delacretaz E, Blier L, et al. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001 Aug 7;104(6):664-669.
77 Stevenson WG, Friedman PL, Kocovic D, Sager PT, Saxon LA, Pavri B. Radiofrequency catheter ablation of ventricular tachycardia after myocardial infarction. Circulation. 1998 Jul 28;98(4):308-314.
78 Scheinman MM, Huang S. The 1998 NASPE prospective catheter ablation registry. Pacing Clin Electrophysiol. 2000 Jun;23(6):1020-1028.
79 Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007 Dec 27;357(26):2657-2665.
80 Bigger JTJr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997 Nov 27;337(22):1569-1575.
81 Cook JR, Rizo-Patron C, Curtis AB, Gillis AM, Bigger JTJr, Kutalek SP, et al. Effect of surgical revascularization in patients with coronary artery disease and ventricular tachycardia or fibrillation in the Antiarrhythmics Versus Implantable Defibrillators (AVID) Registry. Am Heart J. 2002 May;143(5):821-826.
82 Veenhuyzen GD, Singh SN, McAreavey D, Shelton BJ, Exner DV. Prior coronary artery bypass surgery and risk of death among patients with ischemic left ventricular dysfunction. Circulation. 2001 Sep 25;104(13):1489-1493.
83 Albert CM, Manson JE, Cook NR, Ajani UA, Gaziano JM, Hennekens CH. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999 Aug 31;100(9):944-950.
84 Hamalainen H, Luurila OJ, Kallio V, Knuts LR. Reduction in sudden deaths and coronary mortality in myocardial infarction patients after rehabilitation. 15 year follow-up study. Eur Heart J. 1995 Dec;16(12):1839-1844.
85 Hamalainen H, Luurila OJ, Kallio V, Knuts LR, Arstila M, Hakkila J. Long-term reduction in sudden deaths after a multifactorial intervention programme in patients with myocardial infarction: 10-year results of a controlled investigation. Eur Heart J. 1989 Jan;10(1):55-62.
86 Grimm W, Flores BF, Marchlinski FE. Electrocardiographically documented unnecessary, spontaneous shocks in 241 patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 1992 Nov;15(11 Pt 1):1667-1673.
87 Nanthakumar K, Paquette M, Newman D, Deno DC, Malden L, Gunderson B, et al. Inappropriate therapy from atrial fibrillation and sinus tachycardia in automated implantable cardioverter defibrillators. Am Heart J. 2000 May;139(5):797-803.
88 Mirowski M, Reid PR, Winkle RA, Mower MM, Watkins LJr, Stinson EB, et al. Mortality in patients with implanted automatic defibrillators. Ann Intern Med. 1983 May;98(5 Pt 1):585-588.
89 Bardy GH, Yee R, Jung W. Multicenter experience with a pectoral unipolar implantable cardioverter-defibrillator. Active Can Investigators. J Am Coll Cardiol. 1996 Aug;28(2):400-410.
90 Birnie D, Tung S, Simpson C, Crystal E, Exner D, Ayala Paredes FA, et al. Complications associated with defibrillation threshold testing: the Canadian experience. Heart Rhythm. 2008 Mar;5(3):387-390.
91 Swerdlow CD. Implantation of cardioverter defibrillators without induction of ventricular fibrillation. Circulation. 2001 May 1;103(17):2159-2164.
92 Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002 Dec 25;288(24):3115-3123.
93 Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003 Aug 20;42(4):614-623.
94 Himmrich E, Przibille O, Zellerhoff C, Liebrich A, Rosocha S, Andreas K, et al. Proarrhythmic effect of pacemaker stimulation in patients with implanted cardioverter-defibrillators. Circulation. 2003 Jul 15;108(2):192-197.
95 Boehmer JP. Device therapy for heart failure. Am J Cardiol. 2003 Mar 20;91(6A):53D-59D.
96 Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002 Jun 13;346(24):1845-1853.
97 Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001 Mar 22;344(12):873-880.
98 Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005 Apr 14;352(15):1539-1549.
99 Linde C, Braunschweig F, Gadler F, Bailleul C, Daubert JC. Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the Multisite Stimulation in Cardiomyopathy study (MUSTIC). Am J Cardiol. 2003 May 1;91(9):1090-1095.
100 Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003 May 28;289(20):2685-2694.
101 Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008 Dec 2;52(23):1834-1843.
102 Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009 Oct 1;361(14):1329-1338.
103 Tang AS, Wells GA, Arnold M, Connolly S, Hohnloser S, Nichol G, et al. Resynchronization/defibrillation for ambulatory heart failure trial: rationale and trial design. Curr Opin Cardiol. 2009 Jan;24(1):1-8.
104 Beshai JF, Grimm RA, Nagueh SF, Baker JH2nd, Beau SL, Greenberg SM, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007 Dec 13;357(24):2461-2471.
105 Bardy GH, Troutman C, Poole JE, Kudenchuk PJ, Dolack GL, Johnson G, et al. Clinical experience with a tiered-therapy, multiprogrammable antiarrhythmia device. Circulation. 1992 May;85(5):1689-1698.
106 Brugada J, Mont L, Figueiredo M, Valentino M, Matas M, Navarro-Lopez F. Enhanced detection criteria in implantable defibrillators. J Cardiovasc Electrophysiol. 1998 Mar;9(3):261-268.
107 Hurwitz JL, Hook BG, Flores BT, Marchlinski FE. Importance of abortive shock capability with electrogram storage in cardioverter-defibrillator devices. J Am Coll Cardiol. 1993 Mar 15;21(4):895-900.
108 Swerdlow CD, Chen PS, Kass RM, Allard JR, Peter CT. Discrimination of ventricular tachycardia from sinus tachycardia and atrial fibrillation in a tiered-therapy cardioverter-defibrillator. J Am Coll Cardiol. 1994 May;23(6):1342-1355.
109 Swerdlow CD, Schsls W, Dijkman B, Jung W, Sheth NV, Olson WH, et al. Detection of atrial fibrillation and flutter by a dual-chamber implantable cardioverter-defibrillator. For the Worldwide Jewel AF Investigators. Circulation. 2000 Feb 29;101(8):878-885.
110 Wilkoff BL, Kuhlkamp V, Volosin K, Ellenbogen K, Waldecker B, Kacet S, et al. Critical analysis of dual-chamber implantable cardioverter-defibrillator arrhythmia detection : results and technical considerations. Circulation. 2001 Jan 23;103(3):381-386.
111 Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, et al. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008 Aug 12;52(7):541-550.
112 Wilkoff BL, Ousdigian KT, Sterns LD, Wang ZJ, Wilson RD, Morgan JM. A comparison of empiric to physician-tailored programming of implantable cardioverter-defibrillators: results from the prospective randomized multicenter EMPIRIC trial. J Am Coll Cardiol. 2006 Jul 18;48(2):330-339.
113 Schwab JO, Gasparini M, Lunati M, Proclemer A, Kaup B, Santi E, et al. Avoid delivering therapies for nonsustained fast ventricular tachyarrhythmia in patients with implantable cardioverter/defibrillator: the ADVANCE III Trial. J Cardiovasc Electrophysiol. 2009 Jun;20(6):663-666.
114 Adler SW2nd, Wolpert C, Warman EN, Musley SK, Koehler JL, Euler DE. Efficacy of pacing therapies for treating atrial tachyarrhythmias in patients with ventricular arrhythmias receiving a dual-chamber implantable cardioverter defibrillator. Circulation. 2001 Aug 21;104(8):887-892.
115 Jung W, Wolpert C, Esmailzadeh B, Spehl S, Herwig S, Schumacher B, et al. Specific considerations with the automatic implantable atrial defibrillator. J Cardiovasc Electrophysiol. 1998 Aug;9(8 Suppl):S193-S201.
116 Haffajee C, Martin D, Bhandari A, Bardy GH, DeSouza C, Kuehlkamp V, et al. A multicenter, randomized trial comparing an active can implantable defibrillator with a passive can system. Jewel Active Can Investigators. Pacing Clin Electrophysiol. 1997 Jan;20(1 Pt 2):215-219.
117 Ahmad M, Bloomstein L, Roelke M, Bernstein AD, Parsonnet V. Patients’ attitudes toward implanted defibrillator shocks. Pacing Clin Electrophysiol. 2000 Jun;23(6):934-938.
118 McCready MJ, Exner DV. Quality of life and psychological impact of implantable cardioverter defibrillators: focus on randomized controlled trial data. Card Electrophysiol Rev. 2003 Jan;7(1):63-70.
119 Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002 Feb 5;105(5):589-594.
120 Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008 Sep 4;359(10):1009-1017.
121 Saksena S, Chandran P, Shah Y, Boccadamo R, Pantopoulos D, Rothbart ST. Comparative efficacy of transvenous cardioversion and pacing in patients with sustained ventricular tachycardia: a prospective, randomized, crossover study. Circulation. 1985 Jul;72(1):153-160.
122 Bardy GH, Poole JE, Kudenchuk PJ, Dolack GL, Kelso D, Mitchell R. A prospective randomized repeat-crossover comparison of antitachycardia pacing with low-energy cardioversion. Circulation. 1993 Jun;87(6):1889-1896.
123 Hauser RG, Hayes DL, Almquist AK, Epstein AE, Parsonnet V, Tyers GF, et al. Unexpected ICD pulse generator failure due to electronic circuit damage caused by electrical overstress. Pacing Clin Electrophysiol. 2001 Jul;24(7):1046-1054.
124 Natale A, Sra J, Axtell K, Akhtar M, Newby K, Kent V, et al. Undetected ventricular fibrillation in transvenous implantable cardioverter-defibrillators. Prospective comparison of different lead system-device combinations. Circulation. 1996 Jan 1;93(1):91-98.
125 Clinical outcome of patients with malignant ventricular tachyarrhythmias and a multiprogrammable implantable cardioverter-defibrillator implanted with or without thoracotomy: an international multicenter study. PCD Investigator Group. J Am Coll Cardiol. 1994 Jun;23(7):1521-1530.
126 Nasir NJr, Pacifico A, Doyle TK, Earle NR, Hardage ML, Henry PD. Spontaneous ventricular tachycardia treated by antitachycardia pacing. Cadence Investigators. Am J Cardiol. 1997 Mar 15;79(6):820-822.
127 Wathen MS, Sweeney MO, DeGroot PJ, Stark AJ, Koehler JL, Chisner MB, et al. Shock reduction using antitachycardia pacing for spontaneous rapid ventricular tachycardia in patients with coronary artery disease. Circulation. 2001 Aug 14;104(7):796-801.
128 Friedman PA, Dijkman B, Warman EN, Xia HA, Mehra R, Stanton MS, et al. Atrial therapies reduce atrial arrhythmia burden in defibrillator patients. Circulation. 2001 Aug 28;104(9):1023-1028.
129 Wellens HJ, Lau CP, Luderitz B, Akhtar M, Waldo AL, Camm AJ, et al. Atrioverter: an implantable device for the treatment of atrial fibrillation. Circulation. 1998 Oct 20;98(16):1651-1656.
130 Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg . Canadian Implantable Defibrillator Study. Eur Heart J. 2000 Dec;21(24):2071-2078.
131 Larsen G, Hallstrom A, McAnulty J, Pinski S, Olarte A, Sullivan S, et al. Cost-effectiveness of the implantable cardioverter-defibrillator versus antiarrhythmic drugs in survivors of serious ventricular tachyarrhythmias: results of the Antiarrhythmics Versus Implantable Defibrillators (AVID) economic analysis substudy. Circulation. 2002 Apr 30;105(17):2049-2057.
132 Stanton MS, Bell GK. Economic outcomes of implantable cardioverter-defibrillators. Circulation. 2000 Mar 7;101(9):1067-1074.
133 Sheldon R, O’Brien BJ, Blackhouse G, Goeree R, Mitchell B, Klein G, et al. Effect of clinical risk stratification on cost-effectiveness of the implantable cardioverter-defibrillator: the Canadian implantable defibrillator study. Circulation. 2001 Oct 2;104(14):1622-1626.
134 Ellenbogen KA, Wood MA, Shepard RK, Clemo HF, Vaughn T, Holloman K, et al. Detection and management of an implantable cardioverter defibrillator lead failure: incidence and clinical implications. J Am Coll Cardiol. 2003 Jan 1;41(1):73-80.
135 Kron J, Herre J, Renfroe EG, Rizo-Patron C, Raitt M, Halperin B, et al. Lead- and device-related complications in the antiarrhythmics versus implantable defibrillators trial. Am Heart J. 2001 Jan;141(1):92-98.
136 Rosenqvist M, Beyer T, Block M, den Dulk K, Minten J, Lindemans F. Adverse events with transvenous implantable cardioverter-defibrillators: a prospective multicenter study. European 7219 Jewel ICD investigators. Circulation. 1998 Aug 18;98(7):663-670.
137 Morrison TB, Rea RF, Hodge DO, Crusan D, Koestler C, Asirvatham SJ, et al. Risk factors for implantable defibrillator lead fracture in a recalled and a nonrecalled lead. J Cardiovasc Electrophysiol. 2010;21:671-677.
138 Kearney RA, Eisen HJ, Wolf JE. Nonvalvular infections of the cardiovascular system. Ann Intern Med. 1994 Aug 1;121(3):219-230.
139 Lai KK, Fontecchio SA. Infections associated with implantable cardioverter defibrillators placed transvenously and via thoracotomies: epidemiology, infection control, and management. Clin Infect Dis. 1998 Aug;27(2):265-269.
140 Smith PN, Vidaillet HJ, Hayes JJ, Wethington PJ, Stahl L, Hull M, et al. Infections with nonthoracotomy implantable cardioverter defibrillators: can these be prevented? Endotak Lead Clinical Investigators. Pacing Clin Electrophysiol. 1998 Jan;21(1 Pt 1):42-55.
141 Klug D, Lacroix D, Savoye C, Goullard L, Grandmougin D, Hennequin JL, et al. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation. 1997 Apr 15;95(8):2098-2107.
142 Samuels LE, Samuels FL, Kaufman MS, Morris RJ, Brockman SK. Management of infected implantable cardiac defibrillators. Ann Thorac Surg. 1997 Dec;64(6):1702-1706.
143 Da Costa A, Kirkorian G, Cucherat M, Delahaye F, Chevalier P, Cerisier A, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998 May 12;97(18):1796-1801.
144 Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000 Oct 17;133(8):604-608.
145 Arber N, Pras E, Copperman Y, Schapiro JM, Meiner V, Lossos IS, et al. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine (Baltimore). 1994 Nov;73(6):299-305.
146 O’Nunain S, Perez I, Roelke M, Osswald S, McGovern BA, Brooks DR, et al. The treatment of patients with infected implantable cardioverter-defibrillator systems. J Thorac Cardiovasc Surg. 1997 Jan;113(1):121-129.
147 Trappe HJ, Pfitzner P, Klein H, Wenzlaff P. Infections after cardioverter-defibrillator implantation: observations in 335 patients over 10 years. Br Heart J. 1995 Jan;73(1):20-24.
148 Cacoub P, Leprince P, Nataf P, Hausfater P, Dorent R, Wechsler B, et al. Pacemaker infective endocarditis. Am J Cardiol. 1998 Aug 15;82(4):480-484.
149 Chamis AL, Peterson GE, Cabell CH, Corey GR, Sorrentino RA, Greenfield RA, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001 Aug 28;104(9):1029-1033.
150 Paulin FL, Gula LJ, Yee R, Skanes AC, Klein GJ, Krahn AD. Management of infections involving implanted cardiac electrophysiologic devices. Curr Treat Options Cardiovasc Med. 2008 Sep;10(5):380-387.
151 Camus C, Leport C, Raffi F, Michelet C, Cartier F, Vilde JL. Sustained bacteremia in 26 patients with a permanent endocardial pacemaker: assessment of wire removal. Clin Infect Dis. 1993 Jul;17(1):46-55.
152 Bertaglia E, Zerbo F, Zardo S, Barzan D, Zoppo F, Pascotto P. Antibiotic prophylaxis with a single dose of cefazolin during pacemaker implantation: incidence of long-term infective complications. Pacing Clin Electrophysiol. 2006 Jan;29(1):29-33.
153 Baddour LM, Bettmann MA, Bolger AF, Epstein AE, Ferrieri P, Gerber MA, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003 Oct 21;108(16):2015-2031.
154 Steinberg JS, Martins J, Sadanandan S, Goldner B, Menchavez E, Domanski M, et al. Antiarrhythmic drug use in the implantable defibrillator arm of the Antiarrhythmics Versus Implantable Defibrillators (AVID) Study. Am Heart J. 2001 Sep;142(3):520-529.
155 Kuhlkamp V, Mewis C, Mermi J, Bosch RF, Seipel L. Suppression of sustained ventricular tachyarrhythmias: a comparison of d,l-sotalol with no antiarrhythmic drug treatment. J Am Coll Cardiol. 1999 Jan;33(1):46-52.
156 Pacifico A, Hohnloser SH, Williams JH, Tao B, Saksena S, Henry PD, et al. Prevention of implantable-defibrillator shocks by treatment with sotalol. d,l-Sotalol Implantable Cardioverter-Defibrillator Study Group. N Engl J Med. 1999 Jun 17;340(24):1855-1862.
157 Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES, et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA. 2006 Jan 11;295(2):165-171.
158 Kettering K, Mewis C, Dornberger V, Vonthein R, Bosch RF, Kuhlkamp V. Efficacy of metoprolol and sotalol in the prevention of recurrences of sustained ventricular tachyarrhythmias in patients with an implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2002 Nov;25(11):1571-1576.
159 Seidl K, Hauer B, Schwick NG, Zahn R, Senges J. Comparison of metoprolol and sotalol in preventing ventricular tachyarrhythmias after the implantation of a cardioverter/defibrillator. Am J Cardiol. 1998 Sep 15;82(6):744-748.
160 Singer I, Al-Khalidi H, Niazi I, Tchou P, Simmons T, Henthorn R, et al. Azimilide decreases recurrent ventricular tachyarrhythmias in patients with implantable cardioverter defibrillators. J Am Coll Cardiol. 2004 Jan 7;43(1):39-43.
161 Dorian P, Borggrefe M, Al-Khalidi HR, Hohnloser SH, Brum JM, Tatla DS, et al. Placebo-controlled, randomized clinical trial of azimilide for prevention of ventricular tachyarrhythmias in patients with an implantable cardioverter defibrillator. Circulation. 2004 Dec 14;110(24):3646-3654.
162 Mazur A, Anderson ME, Bonney S, Roden DM. Pause-dependent polymorphic ventricular tachycardia during long-term treatment with dofetilide: a placebo-controlled, implantable cardioverter-defibrillator-based evaluation. J Am Coll Cardiol. 2001 Mar 15;37(4):1100-1105.
163 Stevens SK, Haffajee CI, Naccarelli GV, Schwartz KM, Luceri RM, Packer DL, et al. Effects of oral propafenone on defibrillation and pacing thresholds in patients receiving implantable cardioverter-defibrillators. Propafenone Defibrillation Threshold Investigators. J Am Coll Cardiol. 1996 Aug;28(2):418-422.
164 Zhou L, Chen BP, Kluger J, Fan C, Chow MS. Effects of amiodarone and its active metabolite desethylamiodarone on the ventricular defibrillation threshold. J Am Coll Cardiol. 1998 Jun;31(7):1672-1678.
165 Hohnloser SH, Dorian P, Roberts R, Gent M, Israel CW, Fain E, et al. Effect of amiodarone and sotalol on ventricular defibrillation threshold: the optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation. 2006 Jul 11;114(2):104-109.
166 Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh B. Practical guidelines for clinicians who treat patients with amiodarone. Practice Guidelines Subcommittee, North American Society of Pacing and Electrophysiology. Arch Intern Med. 2000 Jun 26;160(12):1741-1748.
167 Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000 Mar 21;101(11):1288-1296.
168 Calkins H, Epstein A, Packer D, Arria AM, Hummel J, Gilligan DM, et al. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J Am Coll Cardiol. 2000 Jun;35(7):1905-1914.
169 Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert T, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008 Dec 16;118(25):2773-2782.
170 Pinski SL, Trohman RG. Interference in implanted cardiac devices, part II. Pacing Clin Electrophysiol. 2002 Oct;25(10):1496-1509.
171 Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol. 2007 Oct 23;50(17):e159-e241.
172 Levine PA, Barold SS, Fletcher RD, Talbot P. Adverse acute and chronic effects of electrical defibrillation and cardioversion on implanted unipolar cardiac pacing systems. J Am Coll Cardiol. 1983 Jun;1(6):1413-1422.
173 Das G, Staffanson DB. Selective dysfunction of ventricular electrode-endocardial junction following DC cardioversion in a patient with a dual chamber pacemaker. Pacing Clin Electrophysiol. 1997 Feb;20(2 Pt 1):364-365.
174 Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol. 1998 Dec;32(7):1909-1915.
175 Gurevitz O, Lipchenca I, Yaacoby E, Segal E, Perel A, Eldar M, et al. ST-segment deviation following implantable cardioverter defibrillator shocks: incidence, timing, and clinical significance. Pacing Clin Electrophysiol. 2002 Oct;25(10):1429-1432.
176 Schluter T, Baum H, Plewan A, Neumeier D. Effects of implantable cardioverter defibrillator implantation and shock application on biochemical markers of myocardial damage. Clin Chem. 2001 Mar;47(3):459-463.
177 Exner DV, Pinski SL, Wyse DG, Renfroe EG, Follmann D, Gold M, et al. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation. 2001 Apr 24;103(16):2066-2071.
178 Villacastin J, Almendral J, Arenal A, Albertos J, Ormaetxe J, Peinado R, et al. Incidence and clinical significance of multiple consecutive, appropriate, high-energy discharges in patients with implanted cardioverter-defibrillators. Circulation. 1996 Feb 15;93(4):753-762.
179 Dunbar SB, Warner CD, Purcell JA. Internal cardioverter defibrillator device discharge: experiences of patients and family members. Heart Lung. 1993 Nov-Dec;22(6):494-501.
180 Hamner M, Hunt N, Gee J, Garrell R, Monroe R. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics. 1999 Jan-Feb;40(1):82-85.
181 Lampert R, Jain D, Burg MM, Batsford WP, McPherson CA. Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Circulation. 2000 Jan 18;101(2):158-164.
182 Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM. Treating electrical storm : sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation. 2000 Aug 15;102(7):742-747.
183 Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008 Jan 29;117(4):462-469.
184 Pinski SL, Trohman RG. Interference in implanted cardiac devices, Part I. Pacing Clin Electrophysiol. 2002 Sep;25(9):1367-1381.
185 Anfinsen OG, Berntsen RF, Aass H, Kongsgaard E, Amlie JP. Implantable cardioverter defibrillator dysfunction during and after magnetic resonance imaging. Pacing Clin Electrophysiol. 2002 Sep;25(9):1400-1402.
186 Luechinger R, Duru F, Scheidegger MB, Boesiger P, Candinas R. Force and torque effects of a 1.5-Tesla MRI scanner on cardiac pacemakers and ICDs. Pacing Clin Electrophysiol. 2001 Feb;24(2):199-205.
187 Shellock FG, Tkach JA, Ruggieri PM, Cardiac MasarykTJ. pacemakers, ICDs, and loop recorder: evaluation of translational attraction using conventional (“long-bore”) and “short-bore” 1.5- and 3.0-Tesla MR systems. J Cardiovasc Magn Reson. 2003;5(2):387-397.
188 Gotte MJ, Russel IK, de Roest GJ, Germans T, Veldkamp RF, Knaapen P, et al. Magnetic resonance imaging, pacemakers and implantable cardioverter-defibrillators: current situation and clinical perspective. Neth Heart J. 2010 Jan;18(1):31-37.
189 Verma A, Kilicaslan F, Marrouche NF, Minor S, Khan M, Wazni O, et al. Prevalence, predictors, and mortality significance of the causative arrhythmia in patients with electrical storm. J Cardiovasc Electrophysiol. 2004 Nov;15(11):1265-1270.
190 Brigadeau F, Kouakam C, Klug D, Marquie C, Duhamel A, Mizon-Gerard F, et al. Clinical predictors and prognostic significance of electrical storm in patients with implantable cardioverter defibrillators. Eur Heart J. 2006 Mar;27(6):700-707.
191 Hohnloser SH, Al-Khalidi HR, Pratt CM, Brum JM, Tatla DS, Tchou P, et al. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J. 2006 Dec;27(24):3027-3032.
192 Sesselberg HW, Moss AJ, McNitt S, Zareba W, Daubert JP, Andrews ML, et al. Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: a MADIT-II substudy. Heart Rhythm. 2007 Nov;4(11):1395-1402.
193 Gasparini M, Lunati M, Landolina M, Santini M, Padeletti L, Perego G, et al. Electrical storm in patients with biventricular implantable cardioverter defibrillator: incidence, predictors, and prognostic implications. Am Heart J. 2008 Nov;156(5):847-854.
194 Pinski SL. Emergencies related to implantable cardioverter-defibrillators. Crit Care Med. 2000 Oct;28(10 Suppl):N174-N180.
195 Goldschlager N, Epstein A, Friedman P, Gang E, Krol R, Olshansky B. Environmental and drug effects on patients with pacemakers and implantable cardioverter/defibrillators: a practical guide to patient treatment. Arch Intern Med. 2001 Mar 12;161(5):649-655.