Chapter 19 Sudden Cardiac Arrest and Sudden Cardiac Death
Please go to expertconsult.com for supplemental chapter material.
Clinical Aspects of Cardiac Arrest
Key Point
The absence of pulses in an unresponsive individual is the major diagnostic sign of cardiac arrest.
When cardiac arrest is recognized, cardiopulmonary resuscitation (CPR) efforts must be started without delay. The latest (2010) recommendations for the general public in the initial basic life support treatment of a witnessed cardiac arrest involve an approach of effective, continuous chest compressions without interruption for mouth-to-mouth resuscitation (so-called hands-only resuscitation). These new recommendations (Box 19-1) are designed to improve the practice of resuscitation in untrained bystanders; they apply to adults, children, and infants but exclude newborns.
BOX 19-1 Cardiopulmonary Resuscitation (CPR) Guidelines
Bystanders Trained in CPR
2. Deliver 30 forceful, manual chest compressions of the sternum, at a rate of 100 compressions per minute and at a depth of approximately 2 inches.
3. Open the airway with a head tilt and chin lift.
4. Perform CPR with a 30:2 sternal compression to breath ratio.
5. Continue until paramedics arrive or the victim becomes responsive.
Basic ECG Patterns in Cardiac Arrest
The three basic ECG patterns seen with cardiac arrest were mentioned in earlier chapters. Cardiac arrest may be associated with the ECG patterns listed in Box 19-2.
BOX 19-2 Three Basic ECG Patterns with Cardiac Arrest
• Ventricular tachyarrhythmia, including ventricular fibrillation (VF) or a sustained type of pulseless ventricular tachycardia (VT)
• Ventricular asystole or a brady-asystolic rhythm with an extremely slow rate
• Pulseless electrical activity (PEA), also referred to as electromechanical dissociation (EMD)
The ECG patterns seen in cardiac arrest are briefly reviewed in the following sections, with emphasis placed on their clinical implications (Figs. 19-1 to 19-6).

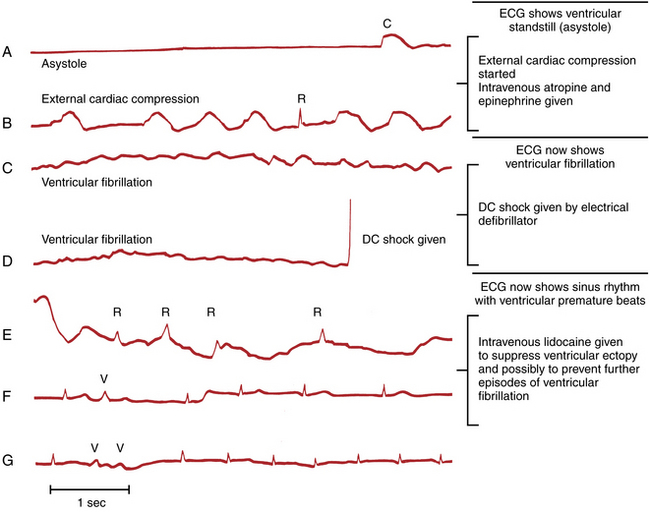
Figure 19-3 Complete ventricular standstill (asystole) producing a straight-line pattern during cardiac arrest.
Ventricular Tachyarrhythmia (Ventricular Fibrillation or Pulseless VT)
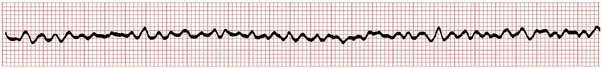
With ventricular fibrillation (VF) the ventricles do not contract but instead twitch rapidly in a completely ineffective way. No cardiac output occurs, and the patient loses consciousness within seconds. The characteristic ECG pattern, with its unmistakable fast oscillatory waves, is illustrated in Figure 19-1.
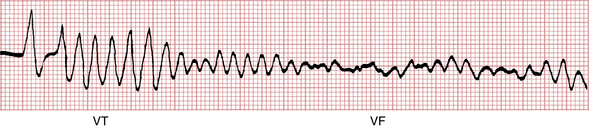
VF may appear spontaneously, as noted in Chapter 14, but is often preceded by another ventricular arrhythmia (usually ventricular tachycardia [VT] or premature ventricular beats). Figure 19-2 shows a run of VT degenerating into VF during cardiac arrest.
The treatment of VF was described in Chapter 16. The patient should be immediately defibrillated, given a direct current electric shock (360 joules) to the heart by means of paddles or pads placed on the chest wall (usually in an anterior-posterior position).
VT and VF are the only “shockable” sudden cardiac arrest rhythms. An example of successful defibrillation is presented in Figure 19-6D.
Ventricular Asystole and Brady-Asystolic Rhythms
The normal pacemaker of the heart is the sinus node, which is located in the right atrium. Failure of the sinus node to function (sinus arrest) leads to ventricular standstill (asystole) if no other subsidiary pacemaker (e.g., in the atria, atrioventricular [AV] junction, or ventricles) takes over. In such cases the ECG records a straight-line pattern (see Fig. 19-3), indicating asystole. Whenever you encounter a straight-line pattern, you need to confirm this finding in at least two leads (as seen in most conventional telemetry systems) and check to see that all electrodes are connected to the patient. Electrodes often become disconnected during a cardiac arrest, leading to the mistaken diagnosis of asystole. Very low amplitude VF (so-called “fine VF”) may also mimic a straight-line pattern. Increasing the gain on the monitor may reveal this “hidden” VF pattern.
The treatment of asystole also requires continued external cardiac compression; however, unlike VT or VF, defibrillation is not appropriate, nor effective. Sometimes spontaneous cardiac electrical activity resumes. Drugs such as vasopressin or epinephrine may help support the circulation or stimulate cardiac electrical activity. Patients with refractory ventricular standstill require a temporary pacemaker, inserted into the right ventricle through the internal jugular or femoral veins. Noninvasive, transcutaneous pacing uses special electrodes that are pasted on the chest wall. However, transcutaneous pacing may only be effective with bradycardia, not frank asystole, and it may be quite painful in conscious patients.
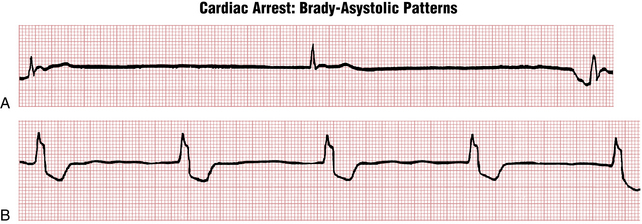
Not uncommonly with ventricular standstill, you also see occasional QRS complexes appearing at infrequent intervals against the background of the basic straight-line rhythm. These are escape beats and represent the attempt of intrinsic cardiac pacemakers to restart the heart’s beating (see Chapter 14). Examples of escape rhythms with underlying ventricular standstill are shown in Figure 19-4. In some cases the escape beats are narrow, indicating their origin from either the atria or the AV junction (see Fig. 19-4A). In others they come from a lower focus in the ventricles, producing a slow idioventricular rhythm with wide QRS complexes (see Fig. 19-4B). The term brady-asystolic pattern is used to describe this type of cardiac arrest ECG.
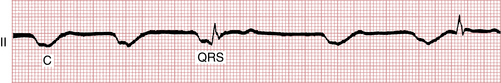
Escape beats should not be confused with artifacts produced by external cardiac compression. Artifacts are large, wide deflections that occur with each compression (see Fig. 19-5). Their size varies with the strength of the compression, and their direction varies with the lead in which they appear (i.e., usually negative in leads II, III, and aVF, positive in leads aVR and aVL).
Pulseless Electrical Activity (Electromechanical Dissociation)
PEA with a physiologic rate can arise in a number of settings. When assessing a patient with PEA, you must consider potentially reversible causes first. Box 19-3 presents an adaptation of the classic “5 Hs and the 5 Ts” that may lead to PEA.
In summary, the main ECG patterns seen with cardiac arrest are a sustained ventricular tachyarrhythmia or VF, ventricular asystole (including brady-asystolic patterns), and PEA. During the course of resuscitating any patient, you may see two or even all three of these ECG patterns at different times during the arrest. Figure 19-6 shows the “ECG history” of a cardiac arrest.
Clinical Causes of Cardiac Arrest
Cardiac arrest may occur in numerous settings. It can be due to any type of organic heart disease. For example, a patient with an acute or prior MI (Box 19-4) may have cardiac arrest for at least five reasons.
BOX 19-4 Causes of Cardiac Arrest in Coronary Artery Disease
• Acute myocardial ischemia and increased ventricular electrical instability, precipitating ventricular fibrillation (VF) or polymorphic ventricular tachycardia (VT) leading to VF
• Damage to the specialized conduction system resulting in high-degree atrioventricular (AV) block
• Sinus node dysfunction leading to marked sinus bradycardia or even asystole
• Pulseless electrical activity (PEA) related to extensive myocardial injury
• Rupture of the infarcted ventricular wall, leading to pericardial tamponade
• Chronic myocardial infarction (MI) with ventricular scarring, leading to monomorphic VT and then VF
An electric shock (including a lightning strike) may produce cardiac arrest in the normal heart. Cardiac arrest may also occur during surgical procedures, particularly in patients with underlying heart disease.
Drugs such as epinephrine can produce VF. Quinidine, disopyramide, procainamide, ibutilide, dofetilide, and related “antiarrhythmic” drugs may lead to long QT(U) syndrome with torsades de pointes (see Chapter 16). Digitalis toxicity can also lead to fatal ventricular arrhythmias (see Chapter 18). Other cardiac drugs may also precipitate sustained ventricular tachyarrhythmias through their so-called proarrhythmic effects (see Chapter 16). The recreational use of cocaine may also induce fatal arrhythmias.
Sudden Cardiac Death/Arrest
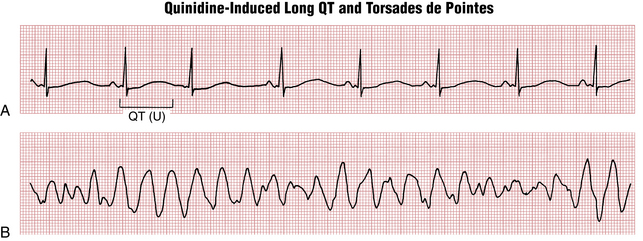
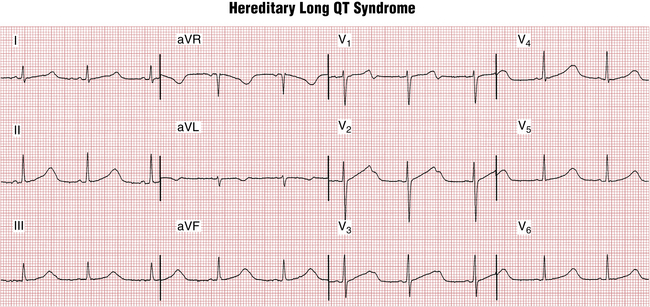
QT prolongation, a marker of risk for torsades de pointes type of VT, was discussed in Chapter 17. QT prolongation syndromes may be divided into acquired and hereditary (congenital) subsets. The major acquired causes include drugs, electrolyte abnormalities, and bradyarrhythmias, especially high-degree AV blocks. Figure 19-7 shows an example of marked QT prolongation due to quinidine that was followed by torsades de pointes and cardiac arrest. Hereditary long QT syndromes (Fig. 19-8) are due to a number of different abnormalities of cardiac ion channel function (“channelopathies”). A detailed list of factors causing long QT syndrome and risk of torsades is given in Chapter 24.
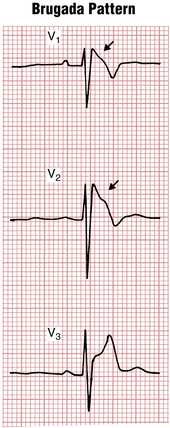
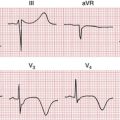
The Brugada syndrome refers to the association of a characteristic ECG pattern with risk of ventricular tachyarrhythmias. The Brugada pattern consists of unusual ST segment elevations in the right chest leads (V1-V3) with a QRS pattern somewhat resembling a right bundle branch block (Fig. 19-9). The basis of the Brugada pattern and associated arrhythmias is a topic of active study. Abnormal repolarization of right ventricular muscle related to sodium channel dysfunction appears to play an important role.
As noted, “recreational” drugs, such as cocaine, or cardiac antiarrhythmic agents, such as flecainide and quinidine, may induce lethal arrhythmias. A small subset of individuals sustains sudden cardiac arrest without having any demonstrable structural or currently identifiable electrophysiologic abnormality.
The identification and management of patients at high risk for sudden death are central areas of investigation in cardiology today. The important role of implantable cardioverter-defibrillator (ICD) devices in preventing sudden death in carefully selected high-risk patients is discussed in Chapter 21.