19 Subcutaneous Implantable Cardioverter-Defibrillators
In the almost four decades since Michel Mirowski implanted the first automatic defibrillator, these devices have transitioned from controversial to a routine facet of patient care.1,2 At that pivotal moment in 1972, Dr. Mirowski was under tremendous scrutiny and heavy skepticism.3,4 The original device that he implanted was rudimentary by present standards, with a crude, probability-density detection algorithm for sensing ventricular fibrillation (VF) and the requirement of thoracotomy for placement of an epicardial electrode system. Nevertheless, this device performed reasonably well, despite its simple imperative of shocking VF using a coil in the superior vena cava and a patch over the cardiac apex. We remember Dr. Mirowski arguing passionately at the scientific meetings, when more complex devices were introduced, that his original device, a shock-only defibrillator, was all that was needed. Although no clinician currently would argue that a shock-only device is sufficient in the care of patients with diverse diseases, Mirowski’s passionate adherence to that premise should not be summarily dismissed in an age when the basic technology of an implanted defibrillator has become subservient to many, less critical functions. This chapter revisits Dr. Mirowski’s vision after almost 40 years, with some technologic twists along the way.
 Background
Background
Successive waves of technologic advances have created the modern, sophisticated device comprising a modest-sized, electrically active generator (i.e., active can) implanted pectorally, utilizing defibrillation-efficient biphasic waveforms, one to three transvenously inserted leads, and antibradycardia/antitachycardia and biventricular pacing capability, as well as programmable detection algorithms and defibrillation energy strengths. The success of this technology is mirrored by the estimated 200,000 devices implanted worldwide in 2009. Despite these remarkable achievements, implantable cardioverter-defibrillators (ICDs) still generate controversy, particularly in the United States, where both physicians and the public are questioning ICD reliability and safety. This peaked in 2009 with the withdrawal of the Fidelis lead from the market amid extensive and often critical publicity.5–11 Further complaints over lead reliability continue, together with concerns over engineering reliability.6,11
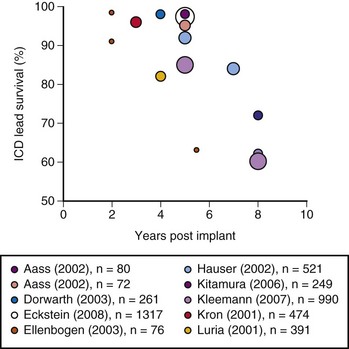
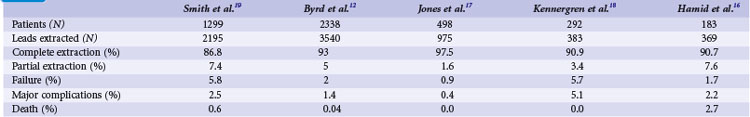
The growing number of lead failures has exposed the Achilles heel of ICDs: the need to attach an electrode directly to the heart via the vascular system. The complicated path that the lead must follow, the hostile environment of the body, and the requirement that leads be supple combine to threaten lead integrity. Moreover, the necessary adherence of the electrode tip to the myocardium and potential attachment of the body of the lead to the veins traversed result in an appreciable risk of injury and death if lead extraction becomes necessary. Reported ICD lead “survival” rates vary from 85% to 98% at 5 years and 60% to 72% at 8 years; Figure 19-1 plots data from 10 studies.10 Table 19-1 summarizes the reported incidence of complications when lead extraction is judged necessary, with mortality ranging from zero to 2.7%, the latter figure relating to associated overwhelming sepsis.12–19 These concerns, along with the well-known complications of routine transvenous lead insertion (perforation, tamponade, pneumothorax, hemothorax, sepsis, endocarditis), indicated the need to develop an ICD that would not require transvenous leads.
 Early Investigation Experience with Subcutaneous Defibrillation
Early Investigation Experience with Subcutaneous Defibrillation
In August 2001 a collaborative research group explored the index question of how much energy was needed for subcutaneous defibrillation. With appropriate ethics committee approval and patient consent, the first test of subcutaneous defibrillation was performed in humans at Green Lane Hospital in Auckland, New Zealand.20 The original test was conducted in a 56-year-old man undergoing transvenous ICD implantation because of a recent cardiac arrest. A small incision was made over the low lateral chest wall to permit tunneling of an anteriorly and posteriorly situated subcutaneous oval disk electrode of approximately 5 cm2 in surface area. A standard transvenous right ventricular lead was used to induce ventricular fibrillation, which was terminated, to the team’s satisfaction, with the first shock, chosen at a strength of 70 joules using a biphasic waveform with a 50% tilt and 100-µF capacitance. This shock resulted in success at an energy level that was much less than was then known possible with transthoracic defibrillation, using two large surface pad electrodes. At that time, the lower limit of average defibrillation threshold efficacy was presumably 100 to 115 J. Clearly, avoiding the resistive effect of the skin was surprisingly beneficial. As discussed later, the energy requirements proved to be even more remarkable.
Although defibrillation with our initial configuration was successful, the posterior space proved too difficult to access and therefore was subsequently abandoned. Attempting to access a posterior subcutaneous space from an anterior approach with a supine patient clearly was impractical, regardless of how low the defibrillation energy would prove to be. Four more patients were tested using various anterior/anterolateral configurations in the latter half of 2001, and defibrillation was successful in all. Each of these pilot explorations led to a more definitive and sequential approach to vector and electrode selection. Our original intention had been to develop a curved generator, resembling a flattened banana, which would mold to the natural contour of the intercostal space (Fig. 19-2). In fact, early prototypes with this shape were tested, but unfortunately it proved too difficult to accommodate the necessary components of the generator to this design and was reluctantly abandoned. Subsequent studies undertaken with additional help (Johannes Sperzel, Jorg Neuzner, and Stefan Spritzer in Germany; Andrew Grace at Papworth Hospital in the United Kingdom; Andrei Ardashev at Burdenko Hospital in Moscow, as well as at Green Lane; Jon Hunt of Cameron Health) allowed rapid progress in identifying a practical lead system.
 Optimal Defibrillation Configuration
Optimal Defibrillation Configuration
Having demonstrated proof of concept, the research aim was then to evaluate systematically which of many electrode configurations would be most effective, as well as most practical from a surgical perspective. Although multiple systems were examined and abandoned, four viable configurations were eventually chosen for detailed study. In this next phase of development, these four defibrillation systems compared the utility of retaining the conventional pectoral generator site versus a novel, left lateral placement of the generator. In addition, a variety of electrode shapes, lengths, and positions were examined. The specific four combinations chosen were as follows (Fig. 19-3):
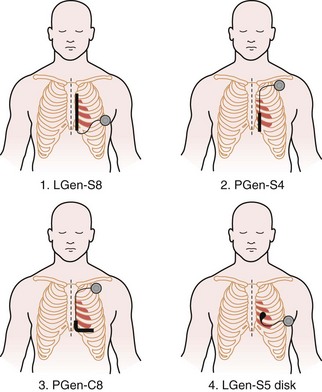
Figure 19-3 Four lead systems acutely tested for the “optimal” system.
(Modified from Bardy GH, Smith WM, Hood MA, et al: An entirely subcutaneous implantable cardioverter-defibrillator, N Engl J Med 363:36-44, 2010.)
Defibrillation testing of these temporary lead configurations was completed immediately before permanent transvenous ICD implantation in a total of 78 patients in a number of centers, most at Green Lane and Papworth Hospitals. These data have been published previously.21 A Latin Square design was used for testing with analysis of variance (ANOVA). A step-up/step-down protocol was used to determine defibrillation thresholds (DFTs) using a 50%-tilt biphasic waveform. An initial test shock of 40 J was delivered after 10 seconds of induced VF, incrementing to a maximum of 80 J or decrementing to a minimum of 10 J. Bardy et al.21 fully describe the DFT testing protocol. Unsuccessful shocks were followed by prompt, transthoracic rescue defibrillation.
For the four configurations just described, the mean DFT (±SD) was as follows, respectively (Fig 19-4):
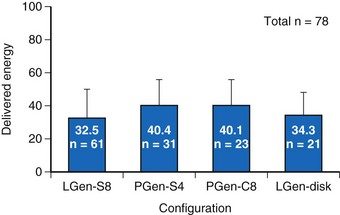
Figure 19-4 Delivered defibrillation threshold energies.
Defibrillation threshold data (mean ± SD) for four practical lead/electrode configurations (see Fig. 19-3) in acute human trials (total of 78 patients).
(Modified from Bardy GH, Smith WM, Hood MA, et al: An entirely subcutaneous implantable cardioverter-defibrillator, N Engl J Med 363:36-44, 2010.)
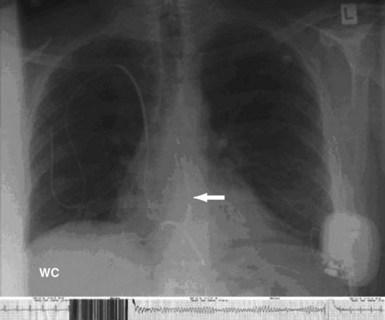
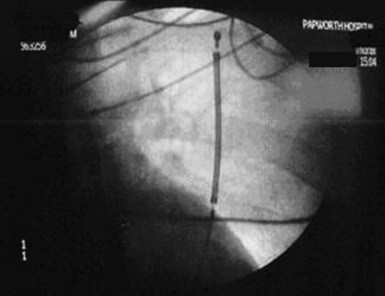
For the direct, Paired Transvenous Defibrillation Efficacy Trial a total of 49 patients were enrolled between April 2004 and June 2005. Importantly, the subcutaneous electrode was placed using only anatomic landmarks, and its position documented by fluoroscopy only after testing was complete. A typical electrode position is shown in Figure 19-5. After both systems were in place and both pockets closed, DFTs were compared in a randomized and interleaved manner, using the same protocol previously described.21 The mean DFT for transvenous and subcutaneous ICD systems, respectively, was 11.1 ± 8.5 J (95% CI: 8.6-13.5) and 36.6 ± 19.8 J (95% CI: 31.1-42.5), P < .001. Only one patient failed to defibrillate with the subcutaneous system, and subsequent screening showed that the electrode was grossly malpositioned laterally, with the coil electrode outside the left lateral margin of the cardiac silhouette (Fig. 19-6). As part of this study, it should also be noted that one patient also failed to defibrillate with the transvenous system, although with appropriate electrode location.
 Other Investigator Approaches to Subcutaneous Defibrillation
Other Investigator Approaches to Subcutaneous Defibrillation
In 2005, Burke et al.22 evaluated the defibrillation energy requirement of a hybrid left chest anterior cutaneous patch to a pectoral subcutaneous emulator canister. With this can-to-patch shocking vector, they reported 78% success in nine patients starting with 70 J and 91% success in a further 11 patients starting with 50 J. Seven of nine patients in this second group (78%) were successfully defibrillated with a 30-J shock. Further efforts to develop a subcutaneous ICD system seemed warranted.
In 2008, Lieberman et al.23 reported an 81% success rate in 32 patients using a step-down protocol beginning at 35 J. Although our group had abandoned a posterior approach, their defibrillation configuration consisted of an electrically active generator emulator with a surface area of 60 cm2 implanted in a low pectoral position, together with a 25-cm coil electrode tunneled around the back of the left thorax between the sixth and tenth intercostal spaces, with the lead tip as close to the spine as possible. Postshock pacing by the subcutaneous electrode was not attempted. No complications were reported.
Isolated case studies in the pediatric population also demonstrated the feasibility of shocking the heart between an abdominally sited generator and a subcutaneous array, or an electrode variably positioned toward the left axilla.24–28 Each of these patient-specific adaptations, however, still required some form of transvenous or epicardial sensing electrodes. Also, these were primarily adapted to developments in patient clinical status, surgical limitations or lead failures, and the clinically limited options to repair the previously implanted intrathoracic defibrillation lead system.
Tolerability and Comfort of Lateral Generator Site
To help answer these concerns, Smith and Hood29 conducted a small study in 2007 of seven patients, in whom a mock generator, with sensing capability only, was left in situ after the completion of acute testing, together with the subcutaneous electrode. The patients also received a standard transvenous ICD, and over the next 6 to 12 months, were asked to compare the pectoral and lateral implant sites for any inconvenience or concerns. Only one patient reported any discomfort with the lateral implant site. Completing a questionnaire before explant, three patients preferred either the subcutaneous left lateral thoracic site or the standard infraclavicular ICD location. One cited no difference. Serial recordings from the subcutaneous electrode over the duration of the implant maintained the quality of sensed myocardial electrograms. These data suggest that generator size, although larger to accommodate the need for higher energies, and device conformation were not concerns with respect to the left lateral thoracic location, and presented no more of a comfort issue than infraclavicular devices.
Sensing with a Subcutaneous Lead
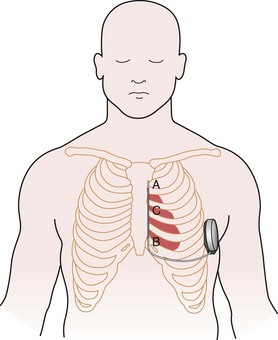
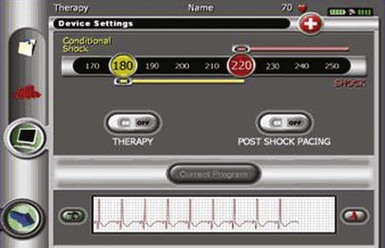
The subcutaneous electrode has two sensing electrodes, one positioned distally adjacent to the manubrium-sternal junction and one positioned in the vicinity of the xiphoid (Fig. 19-7). Electrograms may be detected either between the two electrodes or between either sense electrode and the pulse generator. The S-ICD system automatically selects the vector optimal for rhythm detection and avoidance of double QRS counting or T-wave oversensing. The need for treatment is predicated by feature analysis of signals followed by rate detection. Also, a conditional discrimination zone can be programmed at 170 to 240 beats per minute (bpm) to identify and exclude supraventricular tachycardia (SVT). Extensive testing of these sensing algorithms in the studies conducted to date and in-vitro showed an excellent capability to correctly identify VF and rapid ventricular tachycardia (VT).30 The capability exists to screen patients by comparing the QRS/T-wave amplitudes from the surface electrocardiogram at chest wall locations with a simple ruler, to confirm myocardial sensing will be adequate for a subcutaneous device. Figure 19-8 shows the programmer screen with the parameters available for programming.
 Prelude to First Use of Stand-Alone Subcutaneous Defibrillator
Prelude to First Use of Stand-Alone Subcutaneous Defibrillator
The time seemed appropriate for definitive human implants. The first permanent implant of a subcutaneous-only ICD was in Auckland, New Zealand, on July 28, 2008, by the authors, Gust Bardy, Margaret Hood, and Warren Smith. A schematic of the S-ICD system in place is shown in Figure 19-7. Thereafter, implants were also performed at Christchurch Hospital (by Ian Crozier and Iain Melton), resulting in a total of six patients with class I indications for ICD treatment implanted with a subcutaneous system in New Zealand for the initial pilot experience. Patients were excluded if they required antibradycardia pacing or were known to have VT slower than 170 bpm or monomorphic VT, known to be reliably terminated with ATP. The primary endpoint was two successful conversions from VF with 65 J in a maximum of four attempts. An example of a successful conversion is shown in Figure 19-5. All patients underwent implantation as planned, and after 33 months’ follow-up, the system has been well tolerated without complication. The full detail of this experience has previously been reported.21
Since this initial experience, the S-ICD has undergone successful completion of a European regulatory trial, the details of which are reported earlier.21 Of 55 patients, 52 satisfied implant criteria of two successive VF conversions at 65 J; 12 episodes of spontaneous VT were treated in three patients in that early experience, with only one death, in an 85-year-old man with end-stage renal failure who asked that his S-ICD be turned off in the weeks before his death from kidney disease. The subcutaneous defibrillator is currently released for clinical use in Europe. A U.S. Food and Drug Administration (FDA) trial is also currently underway, with the experience to date proving that S-ICD therapy has a growing role to play in prevention of sudden cardiac death.
 Overview of Experience to Date
Overview of Experience to Date
As potential limitations, one must be cognizant that there will be a few patients in whom body habitus may render myocardial sensing marginal. Fortunately, this is readily identifiable before implant with the use of a special surface electrocardiographic (ECG) template that mimics the S-ICD sensing algorithm and easily identifies those few patients who might have difficulty with subcutaneous sensing. There are several more definitive exclusions to use of the S-ICD. The absence of a chronic pacing capability presently excludes patients requiring antibradycardia pacing, and a transvenous device is more appropriate for patients with relatively slow VT or who demonstrated reliable antitachycardia termination of VT. There is no substantive evidence to demonstrate that empiric ATP is superior to shock-only therapy in patients not demonstrating monomorphic VT before implant. Indeed, the only randomized trial to date shows increased mortality and shock rates with ATP. This is consistent with recent observations showing that a majority of primary prevention patients have polymorphic nonsustained VT, which would easily be accelerated into VF with empiric ATP during charging.31,32 Consequently, we remain conservative in this domain.
One of the less obvious advantages of the S-ICD will be its role in the preservation of the intravascular system for later use, especially in younger patients. The 30-year-old patient with hypertrophic cardiomyopathy at increased risk for sudden death would be fortunate not to need one or more lead extractions in his lifetime. For such patients, the subcutaneous ICD has the potential to confer a long-term morbidity and mortality benefit separate from that intrinsic to its treatment of ventricular tachyarrhythmias. In other patients, being able to defer the possible need for transvenous access by starting with the subcutaneous system is likely to be a clinically useful strategy. Children are another group likely to benefit substantially. Present technology in children requiring ICDs is associated with a disturbing frequency of serious complications, which reflects their smaller veins and more rapid growth rates24,25 (see Chapter 18). Also, we are currently exploring the value of the S-ICD in women because it is unlikely to interfere with mammography; neither the lead nor the generator is within the breast tissue.
 Conclusion
Conclusion
The advent of ICD technology began in 1961 with Zacuoto and de Boissoudy33 using automated antiarrhythmia therapy through temporary transvenous catheters in hospitalized patients. Subsequently, Schuder et al.34 and then Mirowski et al.1 developed a fully implantable defibrillator for animal use by 1970. Prototype human implants by Mirowski et al.2 in 1980 were followed by the advances of biphasic waveforms, transvenous leads, and active generator technology. Where an entirely subcutaneous ICD fits in this history, and whether or not it proves a significant advance, awaits further evidence.
1 Mirowski M, Mower MM, Staewen WS, et al. Standby automatic defibrillator: an approach to prevention of sudden coronary death. Arch Intern Med. 1970;126:158-161.
2 Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303:322-324.
3 Lown B, Axelrod P. Implanted standby defibrillators. Circulation. 1972;46:637-639.
4 Lown B, Axelrod P. Implanted standby defibrillators: the authors’ reply. Circulation. 1973;47:1136.
5 Burton T. Medtronic says wires may have had role in 13 deaths, Sprint Fidelis. http://online.wsj.com/article/SB123697437188922919.html, March 14, 2009.
6 Faulknier BA, Traub DM, Aktas MK, et al. Time-dependent risk of Fidelis lead failure. Am J Cardiol. 2010;105:95-99.
7 Feder BJ. Patients warned as maker halts sale of heart implant. New York Times. http://www.nytimes.com/2007/10/15/business/15device.html?_r=1&oref=slogin, Oct 15, 2007.
8 Feder BJ. Defibrillators are lifesaver, but risks give pause. New York Times. http://www.nytimes.com/2008/09/13/business/13defib.html?_r=1&oref=slogin, Sept 13, 2008.
9 U.S. Food and Drug Administration. Statement on Medtronic’s voluntary market suspension of their Sprint Fidelis defibrillator leads. http://www.fda.gov/bbs/topics/NEWS/2007/NEW01724.html, Oct 15, 2007.
10 Maisel WH, Kramer DB. Implantable cardioverter-defibrillator lead performance. Circulation. 2008;117:2721-2723.
11 Morrison BT, Rea RF, Hodge DO, et al. Risk factors for implantable defibrillator lead fracture in a recalled and a nonrecalled lead. J Cardiovasc Electrophysiol. 2010;21:671-677.
12 Byrd CL, Wilkoff BL, Love CJ, et al. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994-1996. U.S. Lead Extraction Database, MED Institute. Pacing Clin Electrophysiol. 1999;22:1348-1357.
13 Corrado A, Gasparini G, Raviele A. Lead malfunctions in implantable cardioverter-defibrillators: where are we and where should we go? Europace. 2009;11:276-277.
14 Duray GZ, Schmitt J, Cicek-Hartvig S, et al. Complications leading to surgical revision in implantable cardioverter-defibrillator patients: comparison of patients with single-chamber, dual-chamber, and biventricular devices. Europace. 2009;11:297-302.
15 Eckstein J, Koller MT, Zabel M, et al. Necessity for surgical revision of defibrillator leads implanted long-term: causes and management. Circulation. 2008;117:2727-2733.
16 Hamid S, Arujuna A, Ginks M, et al. Pacemaker and defibrillator lead extraction: predictors of mortality during follow-up. PACE. 2009;33:209-216.
17 Jones SO, Eckart RE, Albert CM, Epstein LM. Large, single-center, single-operator experience with transvenous lead extraction: outcomes and changing indications. Heart Rhythm. 2008;5:520-525.
18 Kennergren C, Bucknall CA, Butter C, et al. Laser-assisted lead extraction: the European experience. Europace. 2007;9:651-656.
19 Smith HJ, Fearnot NE, Byrd CL, et al. Five-years experience with intravascular lead extraction. U.S. Lead Extraction Database. Pacing Clin Electrophysiol. 1994;17:2016-2020.
20 Bardy GH, Cappato R, Smith WM, et al. The totally subcutaneous ICD system (the S-ICD) [abstract]. Pacing Clin Electrophysiol. 2002;24:II-578.
21 Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36-44.
22 Burke MC, Coman JA, Cates AW, et al. Defibrillation energy requirements using a left anterior chest cutaneous to subcutaneous shocking vector: implications for a total subcutaneous implantable defibrillator. Heart Rhythm. 2005;2:1332-1338.
23 Lieberman R, Havel WJ, Rashba E, et al. Acute defibrillation performance of a novel, non-transvenous shock pathway in adult ICD indicated patients. Heart Rhythm. 2008;5:28-34.
24 Alexander ME, Cecchin F, Walsh EP, et al. Implications of implantable cardioverter-defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004;15:72-76.
25 Friedman RA, Garson AJr. Implantable defibrillators in children: from whence to shock. J Cardiovasc Electrophysiol. 2001;12:361-362.
26 Gradaus R, Hammel D, Kotthoff S, Bocker D. Nonthoracotomy implantable cardioverter-defibrillator placement in children: use of subcutaneous array leads and abdominally placed implantable cardioverter-defibrillators in children. J Cardiovasc Electrophysiol. 2001;12:356-360.
27 Jolley M, Stinstra J, Pieper S, et al. A computer modeling tool for comparing novel ICD electrode orientations in children and adults. Heart Rhythm. 2008;5:565-572.
28 Nanthakumar K. Trying to make “sense” of extra thoracic implantable defibrillator. Heart Rhythm. 2008;5:35-36.
29 Smith WM, Hood MA. Comfort/tolerability test results for a subcutaneous ICD compared to a standard transvenous lead [abstract]. Heart Rhythm. 4, 2007. S210-PO3-30
30 Gold MR, Theuns DA, Knight BP, et al. Comparison of arrhythmia discrimination by subcutaneous versus dual-chamber ICD systems: primary results From START [abstract]. Circulation. 2009;120:S649.
31 Chen J, Poole JE, Johnson GW, et al. Implications of rapid non-sustained ventricular tachycardia in ICD heart failure patients in the Sudden Cardiac Death in Heart Failure Trial [abstract]. Heart Rhythm. 2010;7:S54-AB26-5.
32 Wathen MS, Degroot PJ, Sweeney MO, et al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591-2596.
33 Zacouto F, de Boissoudy P. On an electronic device allowing the determination of the mechanism of cardiac syncope and starting corresponding reanimation in mammals. CR Seances Soc Biol Fil. 1961;155:1257-1260.
34 Schuder JC, Stoeckle H, Gold JH, et al. Experimental ventricular defibrillation with an automatic and completely implanted system. Trans Am Soc Artif Intern Organs. 1970;16:207-212.