81. Sturge-Weber Syndrome
Definition
Sturge-Weber syndrome (SWS) is a congenital disorder characterized by leptomeningeal angiomas as well as cutaneous angiomas along distributions of the trigeminal nerve (usually only on one side), in particular the ophthalmic and maxillary tracts. The cutaneous angioma is known as the port-wine stain or port-wine angioma. Sturge-Weber syndrome is also called encephalotrigeminal angiomatosis and Sturge-Kalischer-Weber syndrome.
Incidence
The incidence of SWS is estimated to be 1:50,000.
Etiology
The etiology of SWS is not clear. The angiomas appear to develop as the result of a somatic mutation that alters the structure, function, and innervation of blood vessels, expressed as seen in the extracellular matrix and vasoactive molecules. SWS is seen in people of all races and both genders.
Signs and Symptoms
• Attention deficit hyperactivity disorder
• Buphthalmos (congenital glaucoma)
• Choroidal angioma
• Glaucoma
• Headache
• Hemianopsia
• Hemiparesis
• Heterochromia of the iris
• Macrocephaly
• Mental retardation
• Port wine stain or angioma
• Seizures
• Soft-tissue hypertrophy
• Stroke
• Tomato-catsup colored fundus of the eye
• Vision loss
• Weakness
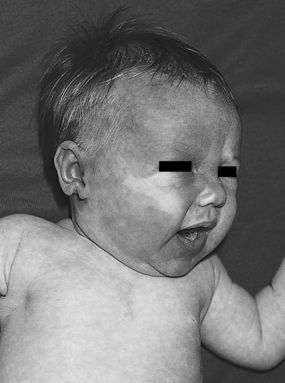 |
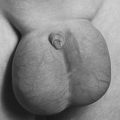
| Sturge-Weber Syndrome. Port wine stain on infant who subsequently developed seizures with Sturge-Weber syndrome. |
Medical Management
The primary medical concerns for care of the patient with SWS include seizure control, headache relief (both symptomatic and prophylactic), reduction of intraocular pressure, and removal of the port-wine stain. Seizure control is typically achieved with administration of anticonvulsant medications. Seizures associated with SWS are generally focal and, as a result, frequently respond well to anticonvulsant therapy.
Gaining control of the patient’s intraocular pressure is achieved with standard glaucoma medications used to lower the intraocular pressure either by reducing aqueous fluid production or by enhancing/promoting the outflow of aqueous fluid.
Headaches are generally recurrent and are treated with acetaminophen or ibuprofen for analgesia. Prophylactic measures may include more proactive/preventive medications, such as gabapentin, valproate, or amitriptyline. Stroke-like episodes may be treated with aspirin in doses from 3 to 5 mg/kg/day. However, because of the risk for Reye syndrome, aspirin use in children must be undertaken with caution.
Port-wine stain is evident at birth and must be closely evaluated within the first week of life, as well as differentiated from hemangioma. Treatment of port-wine stain is accomplished with laser therapy. The number of laser treatments required depends on the extensiveness of the area affected by the port-wine stain. The treatments should be initiated as soon as possible.
Surgical intervention for the patient with SWS is generally reserved for those with refractory seizures, unresponsive or poorly controlled glaucoma, or for specific problems (most notably, scoliosis). In some instances, the seizures of a patient with SWS become refractory to pharmacologic interventions, and the patient may deteriorate to a state of status epilepticus. Surgical interventions for medically refractory seizures include focal cortical resection, corpuscallosotomy, vagal nerve stimulation, and hemispherectomy. Some investigators advocate early surgical intervention to prevent refractory seizures, developmental delay, or hemiparesis. Others advocate exploratory craniotomy and possible lobectomy on confirmation of the diagnosis of SWS because of evidence that links early-onset seizures with developmental delay and/or mental retardation.
Cranial Surgery Indications in Sturge-Weber Syndrome
• Cognitive function deterioration
• Early (age) onset of seizures
• Extensive leptomeningeal angioma
• Focal seizures, subsequently generalized
• Headaches/mild trauma associated with transient motor deficits
• Hemiparesis development
• Increasing duration of postictal deficit
• Increasing focal or diffuse atrophy
• Increasing frequency and duration of seizures
• Medically refractory seizures
• Permanent motor deficits
• Progressive atrophy or calcifications
• Progressive neurologic damage
Indications of Sturge-Weber Syndrome Progression
• Deterioration of cognitive function
• Development of hemiparesis
• Focal seizures initially progress in frequency, secondarily to general, seizures
• Increased duration of transient postictal deficits
• Increased focal or diffuse atrophy
• Increased frequency of seizures (initially) despite anticonvulsant/antiepileptic drugs
• Increased progression of calcifications
When pharmacologic therapy is unsuccessful in controlling intraocular pressure, surgical intervention may be necessary. Trabeculectomy promotes the outflow of aqueous fluid from the anterior chamber by reopening the anatomical outflow tract. Goniotomy accomplishes the same goal intraocularly rather than extraocularly. A Molteno valve, which is analogous to a shunt, may be inserted to promote outflow of anterior chamber aqueous fluid, while the production of aqueous fluid may be reduced by employing cyclodestructive procedures that use cryogenic applications or laser treatments.
Complications
• Blindness
• Buphthalmos (congenital glaucoma)
• Cognitive dysfunction deterioration
• Glaucoma
• Hemianopsia
• Hemiparesis
• Hydrocephalus
• Increased intracranial pressure
• Refractory seizures
• Status epilepticus
• Stroke
Anesthesia Implications
The patient with SWS most often presents to the operating room for treatment of the port-wine stain, refractory seizures, uncontrolled glaucoma, or ocular angioma. The patient should be evaluated preoperatively for untoward intracranial developments, such as increased intracranial pressure (ICP) and/or hydrocephalus. Further evaluation is required with regard to the patient’s potential neurologic pathology and delineation of any associated symptoms. If the surgery is not directed toward regaining control of seizure activity or eliminating seizures, the patient’s anticonvulsant therapy should be optimized before surgery whenever possible.
The patient presenting with signs and symptoms of hydrocephalus and/or ICP should be hyperventilated to begin reducing the diameter of cerebral blood vessels, thus reducing the intracranial pressure. Additional measures to help “relax” the brain include inducing diuresis with an osmotic agent, such as mannitol, and use of sufentanil for analgesia.
The anesthetist must keep in mind the close association of glaucoma with SWS. Topical β-blocking agents used to decrease intraocular pressure can produce systemic effects, including bradycardia and asthma exacerbation. Cholinesterase inhibitors (echothiophate) may decrease plasma cholinesterase, thus prolonging the effects of succinylcholine. Long-term use of acetazolamide may result in sodium and bicarbonate diuresis as well as metabolic acidosis. Where possible, glaucoma medications should be continued perioperatively, with the exception of echothiophate, which should be discontinued 2 to 3 weeks before surgery. Discontinuation of echothiophate can result in exacerbation of glaucoma. The anesthetist should avoid succinylcholine in the patient with SWS, whether echothiophate has been discontinued or not, because of its tendency to elevate intraocular pressure.
The patient with SWS, particularly one being treated with acetazolamide, should have a basic metabolic panel completed preoperatively to assess the sodium concentration and determine the possible need for arterial blood gas analysis to measure the bicarbonate concentration. Direct laryngoscopy for intubation should be accomplished as quickly as possible because of the associated elevation in intraocular pressure. Laryngeal mask airway use does not produce such intraocular pressure elevation and may be a good choice for appropriate surgical procedures. The anesthetist may wish to consider extubation while the patient is relatively deeply anesthetized (so-called “deep extubation”) to minimize the coughing, straining, and/or bucking on the endotracheal tube, which, in turn, elevates the intraocular pressure.