Chapter 1 Structure and function of the skin
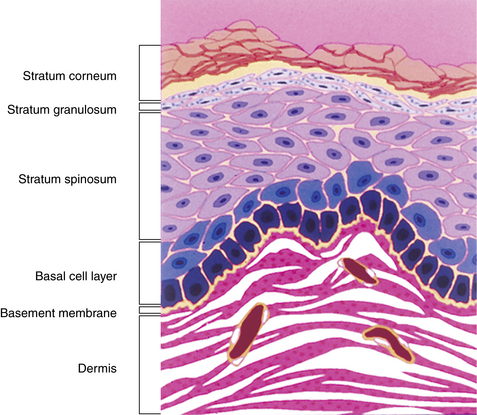
The three layers above the basal cell layer are histologically distinct and demonstrate differentiation of the keratinocytes as they move toward the skin surface and become “cornified.” Just above the basal cell layer is the spiny cell layer (stratum spinosum), so called because of a high concentration of desmosomes and keratin filaments that give the cells a characteristic “spiny” appearance (Fig. 1-3A). Above the spiny layer is the granular cell layer (stratum granulosum). In this layer, keratohyalin granules are formed and bind to the keratin filaments (tonofilaments) to form large electron-dense masses within the cytoplasm that give this layer its “granular” appearance.
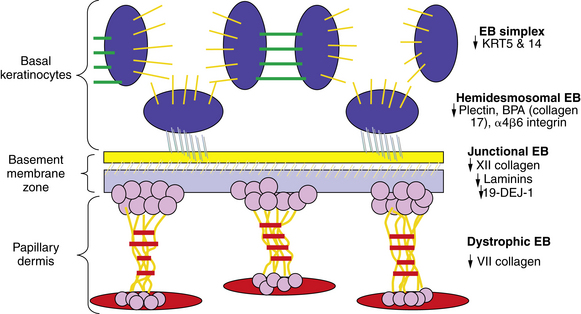
The skin diseases associated with antibodies and damage to the desmosomes and their characteristics are listed in Table 1-1.
Table 1-1. Diseases Associated with Antibodies and Damage to Desmosomes
| DISEASE INVOLVED | CLINICAL APPEARANCE AND LOCATION | MAIN DERMOSOMAL MOLECULES |
|---|---|---|
| Pemphigus vulgaris | Oral and diffuse superficial flaccid blisters with ulcers | Desmogleins 1 and 3 (Dsg1 and 3) and plakoglobin |
| Pemphigus foliaceus | Diffuse superficial blisters and crusting | Desmoglein 1 (Dsg1) |
| Pemphigus vegetans | Vegetating, weeping lesions in the intertriginous areas | Desmoglein 3 (Dsg3) |
| Pemphigus erythematosus | Butterfly eruption with blistering in malar areas | Desmoglein 3 (Dsg3) |
| Paraneoplastic pemphigus | Diffuse erythema multiforme-like painful eruption | Desmoplakins 1 and 2 (Dsg1 and 2), BP antigen 1 (BP230), plectin, desmogleins 1 and 3 (Dsg1 and 3), envoplakin, periplakin |
| IgA pemphigus | Pustular or vesiculopustular eruption | Desmogleins 1 and 3 (Dsg1 and 3), desmocollin 1 |
In the DEB group, separation occurs below the BMZ in the dermal layer, and a decreased amount or absence of type VII collagen has been noted. As a rule, the deeper in the skin the separation occurs, the more severe the clinical picture with increased scarring and loss of function. DEB patients typically have severe deforming scars and decreased life span (see Fig. 1-4).
A partial list of the skin diseases associated with antibodies and damage to the basement membrane structures and dermis are listed in Table 1-2.
Table 1-2. Skin Diseases Associated with Antibodies and Damage to Basement Membrane Structures and Dermis
| DISEASE | CLINICAL APPEARANCE AND LOCATION | BASEMENT MEMBRANE MOLECULE INVOLVED |
|---|---|---|
| Bullous pemphigoid | Tense blisters diffusely | BP antigens 1 (BP230) and 2 (BP180) |
| Pemphigoid (herpes) gestationis | Urticarial blisters with pruritus in late pregnancy | BP antigen 2 (BP180 or collagen XVII antigen) |
| Epidermolysis bullosa acquisita | Friable skin and blistering knees, elbows, and sites of increased pressure | Type VII dermal collagen (anchoring fibril antigen) |
| Bullous lupus erythematosus | Blistering face and trunk with flairs of systemic lupus erythematosus (SLE) | Type VII dermal collagen and lamins 5 and 6 |
| Linear IgA bullous disease (LIBD) | Tense vesicles in annular and target-like patterns on the trunk | BP antigen 2 (BP180 or collagen XVII antigen) and 97 kDa |
Key Points: Anatomy and Function of Skin
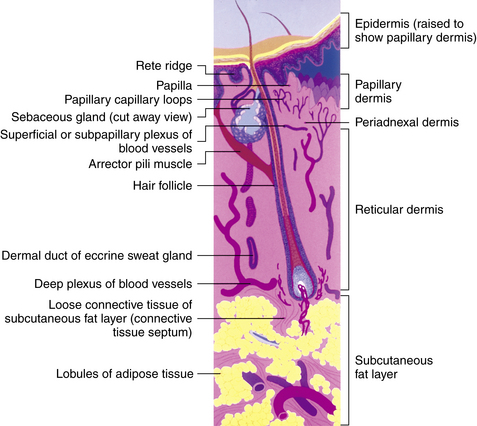
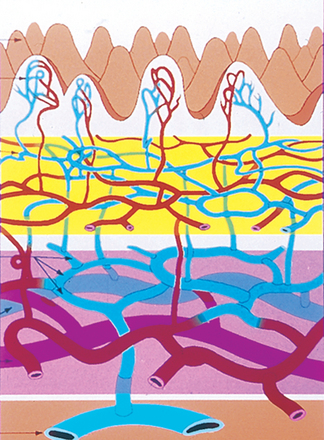
The deeper zone is the reticular dermis, which comprises the bulk of the dermis. It is less vascular than the papillary dermis and demonstrates thick, well-organized collagen bundles.
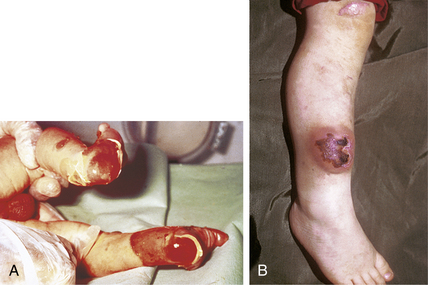
Congenital abnormalities in the various collagens in the dermis, especially types I and III, are found in several of the Ehlers-Danlos syndromes. The cutaneous manifestations of these syndromes are hyperextensibility of the skin, easy bruising, and poor healing with resultant wide scar formation (Fig. 1-5B).
1. Bergstresser PR, Costner MI. Anatomy and physiology. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. London: Mosby; 2003:25-38.
2. Chan LS. Human skin basement membrane in health and in autoimmune diseases. Front Biosci. 1997;2:d343-d352.
3. McGrath JA, Eady RAJ, Pope FM. Anatomy and organization of the human skin. In: Burns S, Breathnach SM, Cox N, Griffiths C, editors. Rook’s textbook of dermatology. ed 7. Malden, MA: Blackwell; 2004:3.1-3.84.