Chapter 50 Stroke Syndromes
The past 20 years has witnessed a transformation in the therapeutic approach to the rehabilitation of those with stroke, spurred by a growing literature on motor recovery after focal brain injury.181 It is now evident both clinically and scientifically that improvement in motor control after stroke is training dependent, responding best to repetitive practice mixed with continuous modification of the program to keep training tasks challenging to the patient.246 When this approach is focused on sensorimotor retraining in the hemiplegic limb it is called task-oriented therapy. Newer research is now leading beyond just therapeutic exercise, adding novel interventions such as pharmacology, new modalities, and robotics as potentially enhancing the results of motor retraining.
Understanding stroke and the rehabilitation of patients who sustain stroke is important, not only because stroke is a common diagnosis among patients in rehabilitation programs, but also because it provides an opportunity to learn about the functioning of the central nervous system, as well as the application of rehabilitation principles in general. This chapter reviews the mechanisms and clinical features of stroke; the preventive, diagnostic, and acute management techniques; and the principles and practices of stroke rehabilitation assessment and intervention that enable rehabilitation providers to assist the patient in achieving the ultimate goal of maximizing quality of life. Special emphasis is placed on new and recent developments in stroke rehabilitation. In light of the many challenges to both providing and investigating stroke rehabilitation, the recent developments in stroke care and research are striking. An important recurring theme during both acute management and rehabilitation care, which has been consistent over time, is the centrality of an attitude that replaces therapeutic nihilism with optimism and aggressiveness.26
Definitions
Stroke or Cerebrovascular Accident?
Ancient writers of history, science, and poetry used the word apoplexia, meaning a sudden strike of paralysis, dumbness, or fainting from which the victim frequently failed to recover. Such a stroke of illness, whether delivered by the gods or disease, was a spontaneous event of the same character as a “stroke of genius,” a “stroke of luck,” or a “stroke of misfortune.” The word stroke then connotes the presence of strong external forces causing the disease that would render treatment useless. The more modern term cerebrovascular accident (CVA) merely perpetuates this nihilistic view of stroke care.19 Clinicians today have retained the name stroke because of the sudden and surprising nature of symptomatic cerebrovascular disease. We recognize, however, that stroke is associated with known risk factors, and that both acute medical care and rehabilitation can reduce mortality and disability.
Epidemiology of Stroke
Stroke is a neurologic syndrome caused by a heterogeneous group of vascular etiologies requiring different management.56 The causes can be grossly categorized as hemorrhagic or ischemic. Intracranial hemorrhage accounts for 15% of all strokes and can be further divided into intracerebral (10%) and subarachnoid (5%) hemorrhage. Subarachnoid hemorrhages (SAHs) typically result from aneurysmal rupture of a cerebral artery with blood loss into the space surrounding the brain. Rupture of weakened vessels within brain parenchyma as a result of hypertension, arteriovenous malformation (AVM), or tumor causes intracerebral hemorrhage (ICH).
Stroke Incidence, Mortality, Prevalence, and Survival
Data from several population-based study cohorts estimate that the yearly incidence of stroke in the United States is 795,000, which comprise 600,000 new strokes and 185,000 recurrent strokes.9 Stroke continues to result in significant morbidity, mortality, and disability, particularly among people older than 65 years.
Stroke is primarily a disease of older individuals, but 28% of strokes occur in persons younger than 65 years. Children have an annual incidence of 2.7 strokes per 100,000. The primary cause of ischemic stroke in adults is atherosclerosis, whereas in children the causes include cerebrovascular anomalies, congenital heart disease, carotid dissection, sickle cell disease, inherited disorders of coagulation, and previous infection with varicella zoster.278 Hemorrhagic strokes in children can occur as a result of moyamoya disease and hemophilia.
Stroke was the primary cause of death in 143,579 persons in 2005, and it remains the third leading cause of death in the United States; it is exceeded only by cardiovascular disease and cancer.9 A well-documented reduction in annual stroke mortality, however, has taken place within the United States in the past century.203 A sharp decline was noted in the annual stroke deaths for both men and women that began in the 1970s, and this continued well into the 1980s before the slope flattened in the early 1990s.245,290 Approximately 200,000 fewer fatal strokes occurred in this period than would have been predicted from data of the previous decade.245 It can be argued that the improved detection and treatment of hypertension that began in the 1960s, and escalated in 1973 with introduction of the National High Blood Pressure Education and Control Program, are directly responsible for the steep decline in stroke mortality.245,290
Stroke survivors, many of whom require rehabilitation services, presently number nearly 6.5 million in the U.S. population. Although the mortality from stroke has declined in the United States, hospitalizations for stroke increased by 18.6% between 1988 and 1997.117 As our population ages, the incidence and prevalence of stroke will continue to increase. Stroke rehabilitation will have an important role in reducing the burden of long-term stroke care on society.
Stroke Risk Factors
Hypertension remains the most important public health concern today because it is the leading risk factor for two of the top three causes of death in the United States: coronary heart disease and stroke. Hypertension is treatable, and its control has the potential for widespread reduction in death and disability in the United States. The combination of stroke and heart disease is not unusual and can have a significant impact on medical care and rehabilitation.361 A major but often neglected part of physiatric care for stroke survivors and their families is stroke and coronary heart disease prevention and risk factor reduction.
Modifiable Risk Factors
Hypertension
Prevalence of hypertension within the U.S. adult population is 35%. Defined as a systolic pressure greater than 165 mm Hg or a diastolic pressure greater than 95 mm Hg, hypertension increases the relative risk of stroke by a factor of 6. Among stroke survivors, 67% have chronic hypertension.171 Several metaanalyses of randomized trials of antihypertensive medications have demonstrated that a 10- to 12-mm Hg reduction of systolic and a 5- to 6-mm Hg reduction of diastolic pressure are associated with a 35% reduction in stroke risk in both hypertensive and normotensive subjects.76,280 It should be noted that no threshold diastolic value was found below which further pressure reduction lacked an additional effect on stroke risk. Consequently, reductions in diastolic blood pressure below traditionally normotensive values contributed to further risk reduction in these studies.
The Hypertension Detection and Follow-up Program was the first major study to demonstrate a reduction in stroke incidence with antihypertensive treatment. This was a population-based randomized clinical trial with a 5-year follow-up period involving 11,000 hypertensive persons who were either provided with a stepped care antihypertensive program or referred for traditional care. A 1.9% incidence of stroke among patients on stepped care treatment was observed compared with 2.9% on a referred care program, equaling a 35% reduction in stroke incidence and a 44% reduction in fatal strokes.212 Isolated systolic hypertension is more common among individuals older than 60 years and is an independent risk factor for stroke and cardiovascular disease.231 The Systolic Hypertension in the Elderly Program383 randomized more than 4700 subjects aged 60 years and older with systolic pressures greater than 160 mm Hg and diastolic pressure less than 90 mm Hg to antihypertensive treatment or placebo. During the 5-year study period, subjects treated with antihypertensive medication had an average reduction in systolic blood pressure of 17 mm Hg and a 36% reduction in the incidence of stroke compared with control subjects.
More recently, the role of angiotensin-converting enzyme inhibitors in the prevention of stroke has been appreciated. The Heart Outcomes Prevention Study demonstrated that ramipril provides a 32% relative reduction in stroke occurrence in patients with a history of myocardial infarction, stroke, peripheral vascular disease, or other risk factors.196 The Perindopril Protection Against Recurrent Stroke Study randomized patients with stroke or TIA with or without hypertension to perindopril versus placebo, finding a 28% relative risk reduction with antihypertensive treatment. The combination of a diuretic with the angiotensin-converting enzyme inhibitor improved blood pressure reduction and provided better risk reduction.339 A recent large clinical trial assessing the benefit of telmisartan (an angiotensin II receptor agonist) failed to reduce secondary stroke.471 Only a modest reduction in pressures was observed in this study, however, and the follow-up period was limited to 2.5 years. The benefit of antihypertensives in stroke reduction improves with greater pressure reduction and time.
Smoking
Cigarette smoking is an important risk factor for cardiovascular disease, but its negative influence on stroke was questioned for many years. Community-based data from the Framingham Study have confirmed that smoking is independently associated with an increased risk of atherothrombotic stroke in both men and women. The relative risk of stroke for heavy smokers (>40 cigarettes/day) is twice that of light smokers (<10 cigarettes/day). Cessation of smoking reverses risk to that of nonsmokers within 5 years after quitting.457 Smoking is also a significant risk factor for SAH and ICH in both men and women.255,256,370
Hypercholesterolemia.
The role of elevated serum cholesterol has not been epidemiologically linked to increased stroke incidence per se, but its strong influence on the development of coronary artery disease and atherosclerosis359 indicates that hypercholesterolemia is at least an indirect risk factor for stroke. Indeed, an association between carotid artery atherosclerosis and increased serum cholesterol levels has been noted.316,372 The use of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors or statins can reduce the risk of stroke,51,428 but their role in prevention can have as much to do with their ability to stabilize atherosclerotic plaques and reduce inflammation as their ability to reduce serum cholesterol285,400 In a metaanalysis that included 90,000 subjects there was a significant reduction in stroke risk (21%).8 This study showed that a 10% reduction in low-density lipoprotein (LDL) will reduce risk for stroke by 15.6% and carotid intima media thickness by 0.73%. There also remains a role for dietary reduction of cholesterol and saturated fatty acids in the prevention of stroke. Current targets for patients with coronary heart disease and stroke are an LDL less than 100 mg/dL and total cholesterol less than 200 mg/dL. High-density lipoprotein (HDL) levels more than 60 mg/dL are desirable.3
Diabetes Mellitus and Other Risk Factors.
Diabetes mellitus increases the relative risk of ischemic stroke to 3 to 6 times that of the general population. This risk can be partly attributed to the higher prevalence of hypertension and heart disease among persons with diabetes, but even after controlling for these factors, diabetes independently doubles stroke risk.1,21,232 The prevalence of diabetes among stroke survivors is 20%.1,171,296
Whether obesity is a risk factor for stroke has been challenged. Hypertension and diabetes mellitus are more common in the obese and are strong influences for stroke risk. Weight loss has a positive influence on blood pressure and diabetic control, and probably has a risk-reducing effect on stroke and cardiovascular disease. Although obesity can indirectly increase stroke risk, its independence as a risk factor remains questionable.
The metabolic syndrome is a cluster of interrelated metabolic risk factors for atherosclerotic disease. These include high waist circumference, increased blood pressure, low HDL level, elevated serum triglyceride, and elevated fasting glucose. A recent data analysis from the Atherosclerosis Risk in Communities Study showed a step-wise increase in stroke risk with an increased number of metabolic syndrome components, such that the presence of all five components resulted in a nearly fivefold increase in stroke risk.356
Elevated plasma levels of homocysteine have been associated with increased risk of stroke and carotid artery disease.381 Hyperhomocysteinemia can result from inherited enzyme deficiencies or acquired deficiencies of required enzyme cofactors such as folate, vitamin B12, or vitamin B6. The hyperviscosity that can occur with hyperhomocysteinemia can lead to hypercoagulability or enhanced atherogenesis by microvascular damage from traumatic shearing forces against vessel walls. Patients with acute stroke who have elevated plasma homocysteine are at risk for recurrent stroke, and supplementation with folate, vitamin B12, and vitamin B6 is advised.37
Stroke Pathophysiology
Ischemic Stroke
Thrombosis
The entire pathophysiology of infarction from cerebral thrombosis remains controversial, but it is strongly associated with atherosclerotic cerebrovascular disease. Atherosclerotic plaque formation occurs frequently at major vascular branching sites, including the common carotid and vertebrobasilar arteries. Atherosclerosis is an inflammatory disease that often develops in the presence of chronic hypertension, beginning with increased permeability of vascular intima followed by leukocyte adhesion and infiltration. Monocyte and T-cell accumulation produce lipid-laden foam cells within the vessel wall, and fatty streaks appear on the endothelial surface. Eventually, smooth muscle cell migration, continued inflammatory activity, and the formation of a fibrous cap compromise blood flow, leading to turbulence. Rupture of the fibrous cap can rapidly promote initial thrombus formation by stimulating platelet aggregation and activation of the extrinsic pathway of the coagulation system. The loosely attached thrombus, or “white clot,” that forms is composed of platelet cells and fresh fibrin.358
It is unclear whether symptoms of transient ischemic attack (TIA) are caused by transient thrombotic occlusion of major cerebral arteries or by microemboli that break away from a thrombus, but both phenomena might be important. In either case, these events must resolve, usually in a few minutes, to be considered TIA. TIA is no longer defined based on time (i.e., events lasting <24 hours). The American Stroke Association recently redefined TIA as “a brief episode of neurologic dysfunction caused by focal brain or retinal ischemia, with clinical symptoms typically lasting less than an hour, and without evidence of acute infarction” as determined by cranial imaging.108 This means that any transient neurologic event that is associated with an acute infarction on imaging is considered a stroke rather than TIA, regardless how long the event lasts. Symptoms of transient monocular blindness, or amaurosis fugax, are probably due to microemboli from the internal carotid artery that cause a branch occlusion of the ipsilateral ophthalmic and retinal arteries.330 Other intracranial branch occlusions can similarly result from microemboli arising in the extracranial vessels, leading to injury or infarction in focal regions.95
In contrast, a large arterial thrombus can occlude a major extracranial artery, producing a low-flow state that causes ischemic injury to neural tissue supplied by the most distal arterial branches.30 The volume of damage that results from such hemodynamic compromise can be large, but it depends on the length of time the vessel is occluded, the rate of flow through the occluded site, and the effectiveness of the collateral circulation. Fibrinolytic enzymes are released that control acute thrombus formation, potentially dissolving the clot within minutes to hours. Recanalization might fail or be delayed, however, permitting the arterial thrombus to completely or partially occlude blood flow. Collateral circulation can support the compromised cortical zone, but it can be less effective in elderly persons or in those with diffuse atherosclerotic disease or diabetes.
Ischemic injury from a cerebrovascular thrombus probably results in simultaneous distal branch occlusions from microemboli and compromise of blood flow proximally. The neurologic outcome of cerebral thrombi varies widely and can include brief TIAs, minor strokes without functional compromise, or major strokes resulting in significant impairment and functional disability.
Embolism
Beyond the microemboli produced by cerebrovascular thrombi, the majority of embolic strokes have a cardiac origin. Thrombus formation within the cardiac chambers is generally caused by structural or mechanical changes within the heart. Atrial fibrillation is a significant risk factor for embolic stroke as a result of poor atrial motility and outflow, with stasis of blood and atrial thrombus formation. Atrial fibrillation is often caused by rheumatic valvular disease or coronary artery disease, but it can be idiopathic. Mural thrombus within the left ventricle after myocardial infarction, in the presence of cardiomyopathy or after cardiac surgery, is the other major cause of embolic stroke.55,63 Mechanical heart valves universally cause cerebral emboli if anticoagulation is insufficient. Infectious endocarditis can lead to septic emboli.
Lacunes
Lacunar infarcts are small, circumscribed lesions that measure less than 1.5 cm in diameter and are located in subcortical regions of the basal ganglia, internal capsule, pons, and cerebellum.295 The area of a lacune (meaning “little lake”) roughly corresponds to the vascular territory supplied by one of the deep perforating branches from the circle of Willis or major cerebral arteries. Lacunar strokes are strongly associated with hypertension and pathologically associated with microvascular changes that often develop in the presence of chronic hypertension. Histologic changes such as arteriolar thickening and evenly distributed deposition of eosinophilic material, called lipohyalinosis and fibrinoid necrosis, are commonly seen in the subcortical perforating arteries of hypertensive persons who have had lacunar strokes. Microatheromas within deep perforating arteries are also important causes of lacunar infarction. In addition to hypertension, diabetes mellitus is associated with lacunar stroke as a result of chronic microvascular changes.
Hemorrhagic Stroke
Intracerebral Hemorrhage
Nearly one half of all ICHs occur within the putamen and the cerebral white matter.144 Sudden hemorrhage into brain parenchyma is related to both acute elevations in blood pressure and chronic hypertension. Microvascular changes associated with hypertensive hemorrhages include lipohyalinosis and Charcot–Bouchard aneurysms.129 The latter are not true aneurysms of the vessel wall but are pockets of extravasated blood or “pseudoaneurysms,” a sign of previous microscopic ruptures within the vascular wall. The bleeding typically lasts no more than 1 to 2 hours, corresponding to the usual time course of acute symptom development. Late neurologic decline is related to posthemorrhagic edema or rebleeding.
Cerebral amyloid angiopathy is unusual but is gaining recognition as an important cause of ICH in the elderly population.121 Lobar hemorrhages located near the cortex that occur in patients older than 55 years who have some premorbid history of mild dementia are characteristic of this disease, but in the absence of tissue staining for Congo red amyloid deposits within the adventitia of cerebral vessels, diagnostic uncertainty remains. Other notable causes of ICH include the use of anticoagulants, intracranial tumor, and vasculitis.
Subarachnoid Hemorrhage
SAH, or bleeding that occurs within the dural space around the brain and fills the basal cisterns, is most commonly caused by rupture of a saccular aneurysm or an AVM. Saccular aneurysms develop from a congenital defect in an arterial wall followed by progressive degeneration of the adventitia, which causes ballooning or outpouching of the vessel. The risk of bleeding from unruptured aneurysms is speculative but appears greatest for aneurysms greater than 10 mm in diameter.415
Saccular aneurysms often rupture during the fifth or sixth decade of life. When a rupture occurs, the extravasation of blood into the subarachnoid space is irritating to the dura and results in a severe headache often described as “the worst in my life.” Because of a sudden decrease in cerebral perfusion pressure, acute loss of consciousness is frequent. Focal neurologic changes or coma can ensue. As many as one third of patients with aneurysmal hemorrhage will die immediately. Patients who present with coma, stupor, or severe hemiplegia have the worst prognosis for proximate survival. The risk of rebleeding from an unoperated aneurysm is as high as 30% within the first month after hemorrhage and declines thereafter. The risk for long-term rebleeding remains 3% per year.456
Saccular aneurysms are most often found in the anterior region of the circle of Willis, particularly near branches of the anterior communicating, internal carotid, and middle cerebral arteries. They can also be found at the junction of almost any branch site within the cerebral circulation. Early surgical and endovascular management using modern neurosurgical clipping techniques and coiling is as safe as late surgery, and it significantly reduces risk of rebleeding.
AVMs present with hemorrhage earlier in life than do aneurysms, often in the second or third decade. Although they cause nearly 9% of all SAHs, vascular malformations are also important causes of ICH and intraventricular hemorrhage.326 An AVM is a congenital structure consisting of a tangled web of vascular tissue that contains multiple arteriovenous fistulas, which permit arterial to venous shunting of blood. They can be located anywhere in the central nervous system and can grow large, displacing normal neural structures, usually without disruption of function. Seizure, migraine, and hemorrhage are typical presenting symptoms.
The incidence of lifetime hemorrhage with AVM is 40% to 50%,297 with a rebleeding rate of 4% per year and a mortality rate of 1% per year.319 Treatment options include endovascular embolization, surgical resection, and radiotherapy (gamma knife).
Hydrocephalus
Normal pressure hydrocephalus is very common after SAH and often develops during rehabilitation care. The pathophysiologic cause is a functional disruption of CSF resorption as a result of fibrosis of the arachnoid granulations from subarachnoid blood.205 The classic symptoms of subcortical dementia, incontinence, and gait disorder are clues to the presence of hydrocephalus, but the physiatrist should also have a high level of suspicion when a patient with recent hemorrhage is not making expected functional gains in a rehabilitation program. That suspicion is often confirmed when the patient makes a remarkable recovery after shunting.
The Anatomic Basis of Stroke Syndromes
Motor Control and Strength
Recovery
With hemiplegia, weakness and poor control of voluntary movement are present initially, associated with reduced resting muscle tone. As voluntary movement returns, nonfunctional mass flexion and extension of the limbs are first noted (Table 50-1). Synergy patterns, or mass contraction of multiple muscle groups, are seen.432 Later, movement patterns can be independent of synergy.377
| Upper Limb | Lower Limb | |
| Flexor synergy | Shoulder retraction | Hip flexion |
| Shoulder abduction | Hip abduction | |
| Shoulder external rotation | Hip external rotation | |
| Elbow flexion | Knee flexion | |
| Forearm supination | Ankle eversion | |
| Wrist flexion | Dorsiflexion | |
| Finger flexion | Toe extension | |
| Extensor synergy | Shoulder protraction | Hip extension |
| Shoulder adduction | Hip adduction | |
| Elbow extension | Knee extension | |
| Forearm pronation | Ankle inversion | |
| Wrist extension | Plantar flexion | |
| Finger flexion | Toe flexion |
Motor Coordination and Balance
Anatomy
Anterior to the precentral gyrus within the frontal lobe is the premotor area, which is important in motor planning. Multiple fiber tracts from this region descend via the anterior limb of the internal capsule to the basal ganglia and the cerebellum, with input from the vestibular, visual, and somatosensory systems. Injury to either the efferent or the afferent systems (or both) can cause poor static and dynamic balance as well as movement disorders such as ataxia, chorea, hemiballismus, and tremors.
Spasticity
Spasticity is a velocity-dependent increase in resistance to muscle stretch that develops after an upper motor neuron injury within the central nervous system.258 When severe, spasticity can cause reduced flexibility, posture, and functional mobility, as well as joint pain, contracture, and difficulty with positioning for comfort and hygiene. In stroke, an increase occurs in both tonic and phasic reflexes. Loss of upper motor neuron control causes disinhibited α- and γ-motor neuron activity and heightened sensitivity to class 1a and 2 muscle spindle afferents.167 Consequently, both monosynaptic and multisynaptic spinal reflexes become hyperactive.
Spasticity develops shortly after completed stroke and is initially manifested as an increased phasic response to tendon tap and a slight catch with passive ranging. Later, ranging can become difficult, and the patient might show tonic positioning in flexion or extension. As voluntary motor activity returns, a reduction in tone and reflex response is often noted, but if recovery is incomplete, spasticity usually remains (see Chapter 30).
Language and Communication
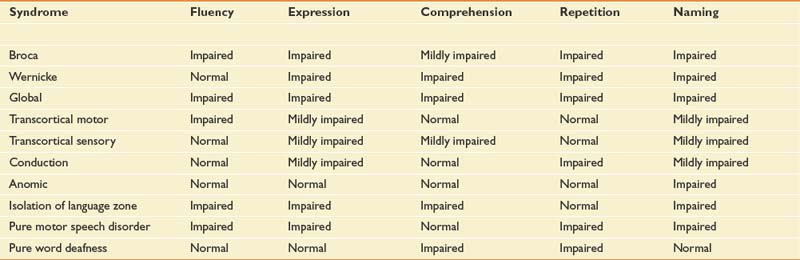
Aphasia is an impairment of language, but typical lesions that cause aphasia affect comprehension and the use of symbolic material for the purpose of communication and meaning (see also Chapter 3). Testing of language should include an examination of oral expression, verbal comprehension, naming, reading, writing, and repeating. The classic aphasia syndromes are listed in Table 50-2. Although this anatomic classification of aphasia is useful for functionally describing communication problems after stroke and for determining prognosis, it is not very useful for guiding therapy. A newer trend is used to describe aphasia, a psycholinguistic approach that is presently being worked out in the aphasiology literature.54
Although language is considered a function of the left or dominant hemisphere, some elements of communication such as prosody have nondominant hemisphere control. Prosody is the rhythmic pattern and vocal intonation of speech that add emphasis and emotional content to language. Some clinical and pathologic evidence exists that prosody might have similar anatomic topography as verbal language in the nondominant hemisphere.357
Anatomy
Patients with Broca-type aphasia have lesions near the frontal operculum, anterior to the precentral gyrus. This location has been aptly named Broca’s area and is considered a premotor association area because it is adjacent to the oral motor area of the primary motor cortex. The Broca-type aphasia, however, is a primary language deficit with mildly compromised comprehension and impaired oral expression. It cannot be considered a purely motor impairment. The topographic location for the Wernicke type of aphasia is Wernicke’s area, which is found in the posterior superior portion of the first temporal gyrus near the primary auditory cortex. It is considered an auditory association area. Lesions near but not involving Broca’s or Wernicke’s area are associated with transcortical motor and sensory aphasias, respectively.4
Ross357 has described aprosodia that is associated with lesions of the frontal operculum of the right or nondominant hemisphere. Patients who have aprosody speak at an even tempo with flat intonation when asked to express a sentence with an emotional tone. Despite this, they are able to hear and comprehend the emotional content of language. In contrast, patients with a lesion in the temporoparietal region have an affective agnosia, in that they are unable to recognize the emotional prosody of spoken language. Despite the agnosia, these patients express prosody without difficulty.
Conduction aphasia with severely impaired repetition of language is associated with a lesion of the arcuate fasciculus, which is a bundle of fibers that pass from the temporal to the frontal lobe.154 Disorders of reading (alexia) and writing (agraphia) are associated with disconnection of the primary language area from the primary visual cortices, which correlates to lesions in the angular gyrus at the junction of the occipital and temporoparietal lobes.141
Apraxia
Disorders of skilled movement in the absence of motor, sensory, or cognitive impairment are called apraxia. Patients with apraxia often have difficulty performing simple functional activities such as using a spoon or a comb, or they will perform them in a clumsy manner. It is often difficult to test for apraxia in the presence of a language deficit because the examiner must be assured that the patient understands the command. Patients with apraxia, however, can have difficulty waving goodbye or using a gesture for hitchhiking when asked to demonstrate these maneuvers. Apraxia is most commonly seen in left hemisphere strokes and affects the left nonhemiplegic limb. Geshwind155 attributes apraxia in this situation to a disconnection of the right cortical motor association area from the left hemisphere as a result of an injury of the anterior callosal fibers. Under these circumstances, the right brain cannot know what the left brain wants to do!
Neglect Syndrome
Heilman et al.188 define hemispatial neglect as a failure to report, respond, or orient to novel or meaningful stimuli presented to the side opposite a brain lesion. It is important to exclude visual, somatosensory, or motor impairments that would explain the lack of response before attributing it to neglect. Hemispatial neglect significantly contributes to disability after stroke because it has a negative impact on sitting balance, visual perception, wheelchair mobility, safety awareness, skin and joint protection, and fall risk. Patients with neglect have difficulty completing hygiene and self-care on the affected side, fail to eat food items in the neglected visual space, and frequently run into objects and walls. Neglect is a disorder of visual and spatial attention, and is associated with temporoparietal strokes and lesions of the frontal eye fields, cingulate gyrus, thalamus, and reticular formation.197
Dysphagia
Dysphagia is common after stroke, occurring in 30% to 65% of patients with unilateral or bilateral hemispheric and brain stem infarctions.∗ Risk for aspiration pneumonia is strongly associated with a delayed initiation of pharyngeal swallow and reduced pharyngeal transit times frequently seen on videofluoroscopic swallow evaluation.218,219 Other neurologic factors that influence risk for aspiration after stroke include reduced labial and lingual mobility and sensation, unilateral neglect, pooling of pharyngeal residue within the vallecula and pyriform sinuses, and cricopharyngeal dysmotility. Laryngeal elevation during swallow normally declines with age and can have a negative influence on aspiration risk after stroke (see also Chapter 27).
Uninhibited Bladder and Bowel
Bladder and bowel incontinence are frequent consequences of stroke. Because the pontine micturition center is typically preserved, reflex voiding usually shows normal synchronous internal sphincter relaxation with detrusor contraction. Postvoid residual volumes are generally low in the absence of prostatic hypertrophy or other forms of bladder outlet obstruction. Incontinence is caused by a lack of voluntary inhibition to void from upper motor neuron injury and results in urgency of urination. In alert individuals, awareness of the need to void is unaffected, but immobility, unilateral neglect, and communication deficits often impair a patient’s ability to use equipment or call for assistance when the need arises. Although most stroke survivors with diabetes have uninhibited voiding, some might have a hypotonic bladder from a parasympathetic autonomic neuropathy to the detrusor muscle. Special care should be taken to check postvoid residual volumes in these patients. Bowel incontinence results from uninhibited reflex rectal emptying by the same mechanism as the uninhibited bladder (see also Chapter 28).
Clinical Stroke Syndromes
Anatomic localization of lesions within the central nervous system predicts physical or cognitive impairment and disability. Understanding the clinical syndromes associated with defined cerebrovascular lesions in ischemic stroke can be a valuable tool to the physiatrist leading the rehabilitation team.179
Middle Cerebral Artery Syndromes
Middle Cerebral Artery Stroke
Main Stem
The impairments after occlusion of the MCA main stem (M1 segment) are listed in Box 50-1. The hemiplegia in a main stem stroke is complete, affecting the upper and lower limbs and lower portions of the face equally. This results primarily from ischemia from within the deep lenticulostriate circulation to the posterior limb of the internal capsule through which the descending fibers of the primary motor cortex pass. Sensory deficits can be significant because the ascending sensory fibers are injured as well, but deep pain sensation can be intact. Although the MCA perforators supply only the upper half of the visual radiations, complete hemianopsia is frequently described. Dysphagia and uninhibited voiding are commonly found, even in unilateral strokes.
Upper Division
MCA upper division strokes are listed in Box 50-2. The clinical presentation is very similar to that of a main stem infarction, but hemiplegia and language comprehension deficits are usually not as severe. Because the M1 segment of the MCA is spared, the vascular supply to the internal capsule is preserved, and ischemia is limited to the inferolateral portion of the primary motor cortex. As a result, motor strength and control are better in the lower limb than in the hand and face. A classic Broca-type aphasia is typical in a dominant hemisphere stroke, and aprosodia without affective agnosia is found in nondominant hemisphere stroke.
Lower Division.
Branch obstruction of the MCA lower division is much less common than upper division stroke and is usually caused by an embolic event. Motor and sensory function is generally intact. Despite this, patients with stroke of the MCA lower division can have significant functional disability from impaired language and vision, and poor awareness of deficits. Box 50-3 lists the impairments associated with lower division strokes.
Anterior Cerebral Artery Syndromes
Anatomy
The ACA supplies the interhemispheric cortical surface of the frontal and parietal lobes. The A1 segment branches medially from the internal carotid bifurcation to the anterior communicating artery. Turning superiorly, the artery passes over the optic nerve, along the rostrum of the corpus callosum (A2), and around the genu (A3); it passes posteriorly to the coronal suture (A4) and terminates at the parietal lobe (A5). The ACA divides during its course into two major branches, the pericallosal and callosomarginal branches, which provide smaller branches to the cortical surface. The recurrent artery of Heubner is a branch from the A1 or proximal A2 segment that deeply perforates to supply important structures such as the head of the caudate, the anterior limb of the internal capsule, the anterior putamen and globus pallidus, and the hypothalamus.
Anterior Cerebral Artery Stroke
The disorders resulting from an ACA infarction are listed in Box 50-4. The hemiplegia in ACA strokes shows weakness of the shoulder and foot with relative sparing of the forearm, hand, and face because the focus of ischemia is over the paracentral lobule of the interhemispheric cortex. Unilateral foot drop can be a long-term impairment requiring orthotic management. A left upper limb apraxia to verbal commands can also result from infarction of the anterior corpus callosum, which disconnects the right hemisphere prefrontal area from the left hemisphere language area.
Posterior Cerebral Artery Syndromes
Posterior Cerebral Artery Stroke
The syndrome of PCA infarction is listed in Box 50-5. The blood supply of the thalamus is provided by the perforating arteries of the PCA. Infarcts in the region can cause hemisensory deficits, including hypoesthesia, dysesthesia, and occasionally hyperesthesia or pain. Thalamic pain syndrome was first described by Dejerine and Roussy in 1906,93 but nearly any disruption of sensory afferent fibers within the central nervous system can cause a central poststroke pain syndrome. Visual disturbances result from injury to the lateral geniculate, temporal, and occipital visual radiations and the calcarine cortex of the occipital lobe. In addition, damage to visual association areas can cause dyschromatopsia, or altered color discrimination. A disorder of reading without impaired writing (alexia without agraphia) associated with a right visual field deficit results from infarction of the left occipital cortex and posterior corpus callosum, disconnecting the intact right visual cortex from the primary language area of the left hemisphere. Impaired memory can result from infarction of the temporal lobe and the hippocampal gyri.
Vertebrobasilar Syndromes
Anatomy
The major arterial branches supplying the brain stem and cerebellum are the posterior inferior cerebellar artery (PICA), the anterior inferior cerebellar artery (AICA), and the superior cerebellar artery (SCA). The PICA originates from the distal vertebral artery and wraps dorsally around the medulla, whereas the AICA is a branch of the basilar artery circling around the pons. Both supply the inferior lobe of the cerebellum. The superior lobe of the cerebellum receives its blood supply from the SCA branching from the basilar artery at the level of the midbrain. Throughout the course of the basilar artery and its major tributaries, small, deep, perforating arteries branch to supply the brain stem. These branches include paramedian penetrators supplying the medial and basal portions of the brain stem, and the short and long circumferential arteries supporting the lateral brain stem.
Brain Stem Stroke Syndromes
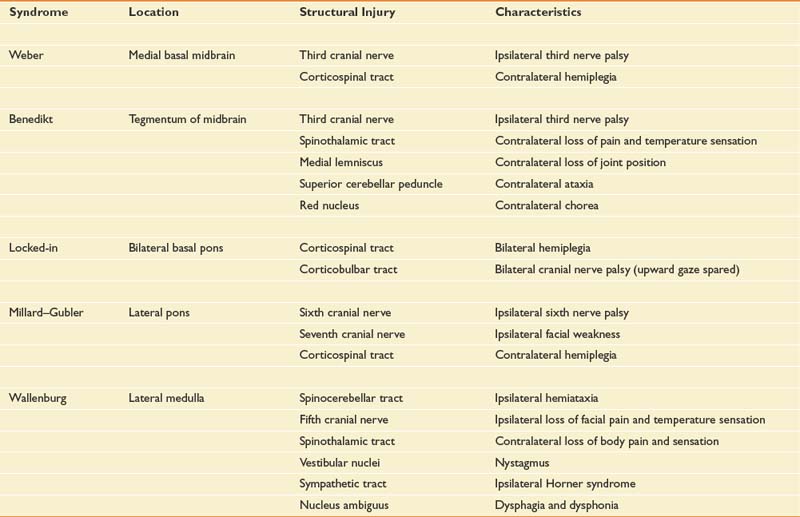
The brain stem is a complex structure containing cranial nerves, bulbar nuclei, and tracts. The bulbar nuclei form afferent and efferent cranial nerves that innervate the ipsilateral side of the body, whereas the ascending and descending bulbar and spinal tracts innervate contralaterally. Consequently, unilateral brain stem strokes often cause loss of cranial nerve function ipsilaterally and sensorimotor dysfunction contralaterally.156 Cerebellar strokes result in ipsilateral ataxia, whereas brain stem strokes can cause ipsilateral, contralateral, or bilateral limb ataxia. The common brain stem syndromes are listed in Table 50-3, along with their anatomic correlates.
Cerebellar strokes are common and can cause life-threatening obstruction of the fourth ventricle and hydrocephalus if cerebellar edema develops. Such strokes occur with PICA, AICA, or SCA occlusion. PICA and AICA strokes are generally caused by arterial thrombosis of the vertebrobasilar system, but SCA strokes are more commonly cardioembolic.154
Lacunar Strokes
Lacunar strokes are located within the deep cerebral white matter, basal ganglia, thalamus, and pons, and result from occlusion of single, small, perforating arteries.295 Strokes are common and present with a wide variety of neurologic and functional deficits. The most common syndromes are listed in Table 50-4.
| Syndrome | Anatomic Sites |
|---|---|
| Pure motor stroke | Posterior limb internal capsule |
| Basis pontis | |
| Pyramids | |
| Pure sensory stroke | Thalamus |
| Thalamocortical projections | |
| Sensory–motor stroke | Junction of internal capsule and thalamus |
| Dysarthria, clumsy hand | Anterior limb internal capsule |
| Pons | |
| Ataxic hemiparesis | Corona radiata |
| Internal capsule | |
| Pons | |
| Cerebellum | |
| Hemiballismus | Head of caudate |
| Thalamus | |
| Subthalamic nucleus |
Acute Stroke Management
A basic pathophysiologic understanding of cerebral ischemia clarifies the actions necessary to protect the brain during acute stroke. Cerebral tissue necrosis occurs when CBF, normally under tight autoregulation, is compromised from either arterial thrombosis or embolism. Normal cerebral autoregulation maintains a cerebral perfusion rate of 50 mL/100 g cerebral tissue/min, which remains constant regardless of acute changes in systemic mean arterial pressure. During cerebrovascular compromise, normal neural activity can be sustained with a CBF as low as 20 mL/100 g/min, but a rate below 10 mL/100 g/min results in cellular death. Within the CBF range between 10 and 20 mL/100 g/min, basic cellular functions are supported but the sodium–potassium pump fails, rendering the neural cells “electrically silent.”388 In an acute stroke, these surviving but inactive neural cells are located at the rim of the ischemic injury, where collateral circulation provides the minimal tissue perfusion needed. This rim has been called the ischemic penumbra, after the partial shadow surrounding a solar eclipse. Improved blood flow to the ischemic penumbra can theoretically restore normal neurologic function. The longer the ischemic period before reperfusion, however, the less likely is the ischemic penumbra to survive. Recent acute stroke management protocols have focused on vascular reperfusion to maximally save the ischemic penumbra. From the standpoint of public education regarding stroke, the National Stroke Association has emphasized that to reduce neural impairment acute stroke management should be implemented within the first 6 hours of the event.
A number of treatments are available and are used more frequently for acute ischemic stroke. The most commonly used treatment is intravenous recombinant tissue plasminogen activator (rt-PA), a thrombolytic agent that was approved for use in the United States in 1996. Because rt-PA can be only safely given within 4.5 hours of stroke onset, considerable effort has been taken to educate the public about the signs and symptoms of stroke and the need to seek immediate treatment. More and more hospitals are also developing clinical programs to manage acute ischemic stroke. The Joint Commission is currently in the process of certifying acute stroke centers throughout the United States that meet basic criteria for the rapid identification and treatment of acute stroke.5
The efficacy of intravenous rt-PA is well supported when given within 3 hours after onset of stroke symptoms, reducing the absolute risk of being dead or dependent by 16%.448 Better outcomes are achieved in patients with mild to moderate neurologic impairment, in persons younger than 75 years, and when the drug is administered within 90 minutes of onset.2 In the definitive U.S. trial, there was a 12% absolute increase in patients with little or no disability 3 months after treatment. The major risk of giving intravenous rt-PA is intracranial bleeding, which can be fatal. Symptomatic brain hemorrhage occurred in 6.4% of patients treated with rt-PA and in only 0.6% of the placebo group. Yet 3 months after treatment, mortality was similar in both groups.309 Strict adherence to the Food and Drug Administration–approved intravenous rt-PA protocol can maximally reduce serious hemorrhagic complications. Recently the European Cooperative Acute Stroke Study demonstrated acceptable safety with intravenous rt-PA between 3 and 4.5 hours after onset of symptoms.176 Based on this study and others the American Heart Association expanded the time window for treatment of acute stroke to 4.5 hours with additional restrictions for patients treated between 3 and 4.5 hours of onset.96
In addition to intravenous rt-PA, many experienced centers are now providing intraarterial rt-PA to patients with large vessel occlusions of the MCA or basilar artery who present within 6 hours of onset. Although there are no intraarterial thrombolytics approved for treatment of acute stroke, there are data to support the use of this technique in selected patients.143 Endovascular techniques are also used to mechanically snare, disrupt, and retrieve a thrombus from major cerebral arteries.277
Emergency Medical Management
Initial medical care for a patient with acute stroke requires careful and frequent neurologic monitoring to prevent and manage medical complications that compromise cerebral tissue perfusion. If the patient is obtunded, concern for airway protection is critical to maintain oxygenation, and an endotracheal tube should be placed, with ventilatory support if necessary. Cerebral edema and acute hydrocephalus can develop rapidly (particularly after ICH), requiring placement of an external ventriculostomy device to relieve intracranial pressure. In cases of brain stem compression and hydrocephalus from cerebellar infarction or hemorrhage, surgical decompression of the posterior fossa can be lifesaving.442
Blood pressure is often acutely elevated during stroke, usually as a response to cerebral injury, and often decreases spontaneously over the following week.332 Clinicians should resist the temptation to rapidly correct elevated blood pressure because often it is a necessary compensatory response to impaired autoregulation after acute ischemic brain injury. A higher mean arterial pressure is needed to maintain cerebral perfusion pressure to the ischemic area, whereas a rapid decrease in blood pressure can potentially enlarge an ischemic infarct. This is especially true in individuals with chronic hypertension, whose brains are accustomed to a higher perfusion pressure.190,409 Although there are no definitive recommendations, acute hypertension after stroke is usually treated only if it is symptomatic, if there is evidence of end-organ injury, if the systolic pressure increases above 220 mm Hg, or if the diastolic pressure increases above 120 mm Hg.2 When treated, elevated blood pressure should be lowered gently, and it is usually best to allow the systolic pressure to remain above 150 mm Hg and the diastolic pressure above 90 mm Hg. Agents that do not increase intracranial pressure and provide a predictable blood pressure response with a short-term antihypertensive effect are preferred for the acute management of blood pressure after stroke. Both intravenous labetalol and nicardipine meet these criteria and have been recommended for stroke managment.130,379 Intravenous nicardipine might provide smoother blood pressure control than labetalol in the emergency setting.271
Hyperglycemia in response to acute physiologic stress occurs in both diabetic and nondiabetic patients during stroke and is associated with increased levels of serum cortisol. Frequent monitoring and control of serum glucose levels during acute stroke have been recommended. Animal studies reveal that a high glucose concentration within partially perfused ischemic tissue provides the substrate for anaerobic cellular metabolism and lactic acid production.92,305 The accumulation of lactic acid is cytotoxic and can lead to further tissue injury.92,389 Careful use of insulin and strict glucose control are potentially neuroprotective.
Anatomic Neuroimaging
MRI is more sensitive than computed tomography (CT) in demonstrating the changes of acute stroke in the first 48 hours.49 Edema within an infarcted zone can appear as early as 2 to 4 hours after stroke onset and is best seen on T2-weighted images. Infarcts near the cortex or periventricular region are better seen on FLAIR images because the bright CSF signal normally seen on T2 weighting is suppressed and does not obscure subtle changes in these regions. T1-weighted imaging is less sensitive for acute cerebral infarct but can show early effacement of gyri and occasionally a vascular thrombus. MRI is more sensitive than CT for lacunar strokes after the first 24 hours, and MRI is the test of choice for imaging the posterior fossa, where bone artifact is not a problem.288 Diffusion-weighted MRI (DW) techniques are superior to conventional MRI for detecting early ischemic changes, and whole brain scans can now be performed rapidly. DW images show high signal in areas that have a diffusion failure as occurs in acute ischemia. Because DW images have T2 contrast, areas of chronic injury (fluid-filled lesions) that have increased diffusion also show high signal. Removal of T2 contrast using exponential techniques provides images that show high signal only in the areas of acute ischemic injury. In contrast, images using the apparent diffusion coefficient (ADC) technique show low signal in areas of acute ischemia and high signal in chronic lesions. In addition to DW, ADC, and exponential images, gadolinium contrast-enhanced perfusion weighted (PW) imaging can also be performed. PW images can highlight a mismatch between the ischemic core and the perfusion deficit in acute injury. The role of perfusion and diffusion MRI in clinical decision making, especially in the selection of patients for thrombolytic therapy, is currently being debated. Newer scanning techniques with multidimensional CT imaging, such as CT angiography and CT perfusion scanning, show promise for improving acute stroke diagnosis.416
MRI is nearly equivalent to CT for the detection of ICH in the acute setting. Cranial CT remains the test of choice for examination of hemorrhage because it is less costly than MRI. With subacute or chronic hemorrhagic stroke, MRI can differentiate methemoglobin from soft tissue and is better than CT for the detection of late hemorrhages.38
Magnetic resonance angiography is a noninvasive option for examining extracranial and intracranial cerebral vessels. Using the two-dimensional “time of flight” technique, extracranial vessels can be visualized, often revealing the presence of carotid or vertebrobasilar atherosclerotic disease. The three-dimensional time of flight technique produces excellent images of the circle of Willis and the cerebral artery stems.140 Magnetic resonance angiography is indicated as a screening test for extracranial and intracranial atherosclerotic disease, and when combined with conventional duplex ultrasound can often obviate the need for conventional angiography.208 Magnetic resonance angiography is the test of choice for detecting carotid dissection.
Other Diagnostic Tests
Arterial duplex scanning combines either standard or color Doppler imaging with two-dimensional ultrasound and is a useful screening tool for carotid atherosclerosis. In practical use, a negative carotid duplex scan excludes the need for carotid endarterectomy, but it does not rule out the presence of significant intracranial atherosclerosis. Similarly a duplex scan that is positive for critical stenosis extracranially cannot exclude the presence of an arterial occlusion in the distal internal carotid artery, which is a contraindication to surgical treatment. Transcranial Doppler imaging can measure flow characteristics of the intracranial vessels, but it lacks imaging capability. It is most useful when serial measurements of CBF are needed, such as in the monitoring of cerebral vasospasm after SAH.209 Although it is invasive and not without complications, conventional contrast-enhanced cerebral angiography is the most accurate method of detecting and anatomically defining intracranial cerebrovascular disease when surgical treatment is considered. Contrast-enhanced angiography is the method of choice for detecting and defining the anatomy of cerebral aneurysms and AVMs.
Prevention of Recurrent Stroke
Antiplatelet Therapy
An Oxford-based group called the Antithrombotic Trialists’ Collaboration has published a metaanalysis of pooled results from 145 trials of various antiplatelet agents for the prevention of vascular events, including nonfatal myocardial infarction, nonfatal stroke, and vascular death.14 As a group, these studies include more than 70,000 subjects with risk factors for vascular disease who were randomized to receive various forms of antiplatelet therapy or placebo over 2 to 6 years. Antiplatelet agents were found to reduce the risk of nonfatal stroke by 25% in men and women. Subgroups of hypertensive and diabetic subjects also benefited from treatment. Among patients with previous stroke or TIA, the use of long-term antiplatelet therapy resulted in 36 fewer nonfatal strokes per 1000 patients, with only one or two additional major intracranial hemorrhages. Although this study supports the benefit of antiplatelet agents in the reduction of vascular disease in general, the use of and indications for these agents in stroke prophylaxis should be based on specific trials having clearly stated endpoints.
Aspirin is the most frequently prescribed antiplatelet agent for secondary stroke and cardiovascular disease prevention. By irreversibly inhibiting cyclooxygenase-dependent platelet aggregation, aspirin achieves a significant antiplatelet effect at fairly low serum concentrations.281 Two large randomized controlled trials have compared aspirin with placebo in the prevention of death or recurrent infarct in nearly 40,000 patients hospitalized with acute ischemic stroke. In combined analysis, the International Stroke Trial214 and the Chinese Acute Stroke Trial62 showed that aspirin (160 to 300 mg/day) administered early after acute stroke resulted in 9 fewer deaths and recurrent strokes per 1000 patients during the first few weeks, without significant complications. These studies justify the routine use of aspirin in patients with acute ischemic stroke.
The combined use of aspirin with extended-release dipyridamole was studied in the European Stroke Prevention Study 2.99 This study randomized 6602 patients to receive aspirin (50 mg), extended-release dipyridamole (400 mg), or the combination daily for the long-term prevention of secondary stroke after stroke or TIA. Aspirin was found to reduce recurrent stroke by 18%, dipyridamole by 16%, and combined aspirin and dipyridamole by 37%, suggesting an additive effect. The most common side effect of combined aspirin and dipyridamole is headache, which often limits its use.
Clopidogrel is a nonaspirin antiplatelet agent that prevents platelet aggregation for the life of the cell by directly inhibiting adenosine diphosphate (ADP)-induced platelet aggregation, without affecting prostaglandin metabolism. It lacks antipyretic, antiinflammatory, and analgesic effects, and it does not affect the integrity of the gastric mucosa.58 In patients with a history of previous stroke, clopidogrel provides a nonstatistically significant 7.3% relative risk reduction for recurrent stroke, myocardial infarction, or other vascular events, making it an effective alternative to aspirin for patients with aspirin allergy or intolerance. The combination of aspirin (75 to 325 mg daily) and clopidogrel reduces the risk of recurrent myocardial infarction by 23% over aspirin alone in patients with a history of acute coronary syndrome without ST elevation. The combined use of aspirin and clopidogrel significantly increased the risk of major bleeding over aspirin use (3.7 vs. 2.7), which was an acceptable risk given the cardioprotective effect of combined treatment.80 In contrast, the risks for bleeding with combined aspirin (325 mg) and clopidogrel (75 mg) daily outweighed the small and nonstatistically significant reduction (6.4%) in recurrent stroke, myocardial infarction, or vascular death over clopidogrel alone in patients with a history of recent ischemic stroke or TIA and other risk factors.100 Clopidogrel has also been compared with the combination of aspirin plus extended-release dipyridamole in a large randomized trial that included more than 20,000 patients with noncardioembolic ischemic stroke. This study demonstrated that the risk of recurrent stroke or major hemorrhagic event was similar with either medication. Because of better efficacy for prevention of cardiovascular events with clopidogrel, patients with comorbid cardiac disease might benefit from clopidogrel for stroke prevention over the combination therapy.369
The relative efficacy of warfarin for prevention of recurrent stroke in patients with a history of noncardioembolic stroke was investigated in the Warfarin and Aspirin for the Prevention of Recurrent Ischemic Stroke study.298 This study demonstrated equivalent efficacy for either warfarin (international normalized ratio goal 1.4 to 2.8) or aspirin (325 mg daily) for secondary stroke prevention. No difference was seen in the rate of major bleeding between groups, but warfarin was associated with a higher rate of minor bleeding. The ongoing Warfarin Aspirin Stroke Intracranial Disease trial will test whether warfarin or aspirin is superior for recurrent stroke prevention in patients with symptomatic carotid stenosis.
Anticoagulation and Antiplatelet Therapy in Atrial Fibrillation
Atrial fibrillation is commonly found among the elderly population and is present in 15% of persons older than 75 years. Individuals with nonvalvular atrial fibrillation have 5 times the relative risk for cardioembolic stroke, and those with rheumatic heart disease have a seventeenfold increase.458 Other clinical factors, such as a history of TIA, stroke, hypertension, recent congestive heart failure, and electrocardiographic evidence of left ventricular dysfunction, are additional predictors of stroke when associated with atrial fibrillation. Clinical trials have supported the use of aspirin to prevent primary cardioembolic stroke in nonvalvular atrial fibrillation. In the Copenhagen AFA-SAK Study,331 a 75-mg/day dose of aspirin reduced embolic stroke risk by 15% compared with placebo. The U.S.-sponsored Stroke Prevention in Atrial Fibrillation (SPAF) Study410 measured a 42% reduction in stroke risk using 325 mg of aspirin daily. In the SPAF Study, however, aspirin was not clearly effective in men, and it was ineffective in women older than 75 years. The AFA-SAK and SPAF studies, as well as two additional placebo-controlled trials,33,115 have tested the use of warfarin anticoagulation for primary stroke prevention in nonvalvular atrial fibrillation. Warfarin reduces relative stroke risk by 58% to 86% over that in control subjects.
Although warfarin proved more effective than aspirin for stroke prevention in atrial fibrillation, a second phase of the SPAF trial (SPAF II)412 compared warfarin with aspirin with the special intention of determining which medication provided superior stroke prevention for individuals older than 75 years with nonvalvular atrial fibrillation. This study concluded that care must be taken when considering anticoagulation in patients older than 75 years, as the risk of intracranial hemorrhage is higher in elderly persons, even without the use of warfarin. The results of the SPAF III trial demonstrated that low-intensity, fixed-dose warfarin plus aspirin is inferior to adjusted-dose warfarin for stroke prevention in high-risk patients with nonvalvular atrial fibrillation.410
Statin Therapy
As mentioned earlier, HMG-CoA reductase inhibitors or statins used for cholesterol-lowering effect might also have antiinflammatory effects on vascular intima and lead to plaque reduction.51,285,400 As a consequence they have become standard medications to use after ischemic stroke in patients with atherothrombotic disease and with LDL cholesterol levels more than 100 mg/dL. Indeed high-dose statins, such as atorvastatin, 80 mg daily, have been shown to effectively reduce the absolute risk of recurrent stroke by 2.2% in patients with stroke or TIA whose LDL cholesterol ranges from 100 to 199 mg/dL.428 Recurrent stroke prevention with high-dose statin therapy is effective regardless of stroke severity or subtype of ischemic injury (large vs. small vessel injury).7,164 When high-dose statin therapy is initiated in acute care, screening for elevation of hepatic enzymes during rehabilitation is prudent. Although a slight elevation of the hepatic enzymes can be tolerated, significant elevation will require decreasing the statin dose or discontinuation.
Statins are also occasionally used for up to 2 weeks after SAH to prevent vasospasm; however, their efficacy has been questioned.238,390
Surgical Management of Carotid Artery Disease
Data from the North American Symptomatic Carotid Endarterectomy Trial20,312 revealed a 17% absolute reduction in stroke incidence with carotid endarterectomy over a 2-year follow-up period in patients with critical stenosis of 70% to 99%. This represents a relative risk reduction of 65% for surgically treated patients. Perioperative risk of disabling stroke or death was 2.0% by 90 days after surgery. Patients with symptomatic carotid stenosis of 50% to 69% had a more modest absolute risk reduction of 6.5% with carotid endarterectomy, representing a relative risk reduction of 29% and suggesting that surgical treatment for moderate-grade stenosis be reserved only for selected cases. No advantage was seen for surgery with symptomatic stenosis of less than 50%.
Carotid endarterectomy for asymptomatic carotid artery stenosis also results in reduced stroke occurrence and death compared with aspirin alone in centers with less than 3% perioperative mortality. Results from two large clinical trials demonstrated a significant 5.4% to 5.9% absolute reduction in stroke for surgically treated patients with greater than 60% stenosis after a 5-year follow-up period, representing a greater than 50% reduction in relative risk of stroke.114,302 The relative benefits of surgical treatment for asymptomatic carotid stenosis is rather marginal, suggesting that carotid endarterectomy should probably be reserved for patients who are otherwise medically stable, who have more than 80% stenosis, and who are expected to live 5 years or longer, and that it is performed only in centers with a less than 3% perioperative complication rate.102