Stroke I
Stroke refers to damage to the brain caused by abnormalities of blood supply. This presents with a rapidly developing focal neurological deficit, which may lead to coma or death. If this deficit lasts less than 24 h it is referred to as a transient ischaemic attack (TIA; p. 70).
Pathogenesis
Cerebral infarction accounts for 80% of strokes, 15% are primary intracerebral haemorrhages and 5% are due to subarachnoid haemorrhages (p. 72).
Cerebral infarction
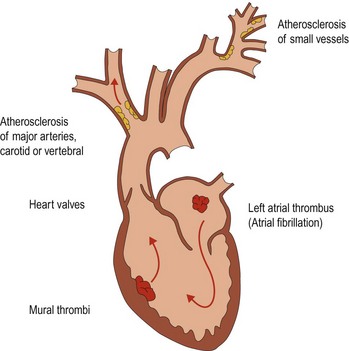
Cerebral infarction results from an interruption in blood supply to an area of the brain. This can be due to (Fig. 1):
Atheroma
The risk factors for atheroma, and other disease resulting from it, are given in Table 1. The most important risk factor is hypertension, with the risk increasing with the blood pressure.
Table 1 Risk factors for atheroma and relative risk of stroke
| Risk factors for atheroma | Relative risk of stroke |
|---|---|
| Hypertension | 5 |
| Diabetes | 2 |
| Smoking | 3 |
| Family history | |
| Cholesterol | |
| Excess alcohol intake | 1–4 |
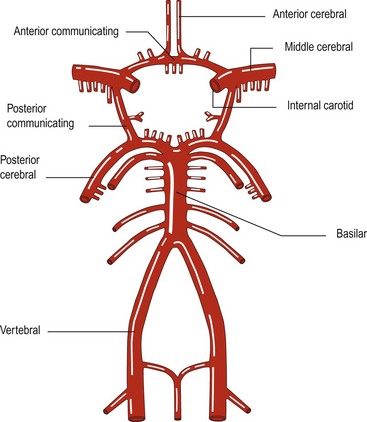
Atheroma commonly arises at the junctions of arteries, for example the carotid bifurcation and the point where the two vertebral arteries join to form the basilar artery (Fig. 2).
The build up of atheroma may lead to narrowing of the arteries. This is usually a gradual process and anastomotic channels can develop, either around the circle of Willis (Fig. 2) or with enlargement of meningeal anastomoses. Stenosis can develop and proceed to occlusion of a vessel without resulting in cerebral ischaemia. However, the atheromatous plaque can lead to cerebral ischaemia in several ways:
Intracerebral haemorrhage
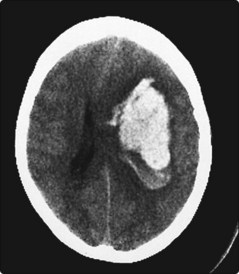
Intracerebral haemorrhage is usually due to hypertension (primary intracerebral haemorrhage). This occurs when small penetrating arteries within the brain rupture at sites of weakness, as a result of lipohyalinosis and microaneurysms (Charcot–Bouchard aneurysms). Intracerebral haemorrhages occur most frequently in the basal ganglia (50%; Fig. 3), lobar white matter (20%), pons (10%) and cerebellum (10%).
Ruptured saccular aneurysms produce subarachnoid haemorrhage (p. 72). However, sometimes the direction of rupture of an aneurysm can result in most of the haemorrhage being intracerebral rather than subarachnoid. Arteriovenous malformations can rupture and produce intracerebral haemorrhage. Patients with bleeding disorders, particularly related to drugs (anticoagulants and thrombolytics such as streptokinase), can develop intracerebral haemorrhages.
Development of an ischaemic stroke
Areas of necrosis will then swell, so-called cytotoxic oedema, which may interfere with the perfusion of areas in the penumbra. This swelling increases over 5 days and then gradually clears. If large areas of brain are affected (e.g. a middle cerebral artery occlusion; p. 66), this may produce a significant mass effect causing a further deterioration due to cerebral herniation. This is also seen in intracerebral haemorrhage where the haematoma increases the mass effect.