79 Stroke
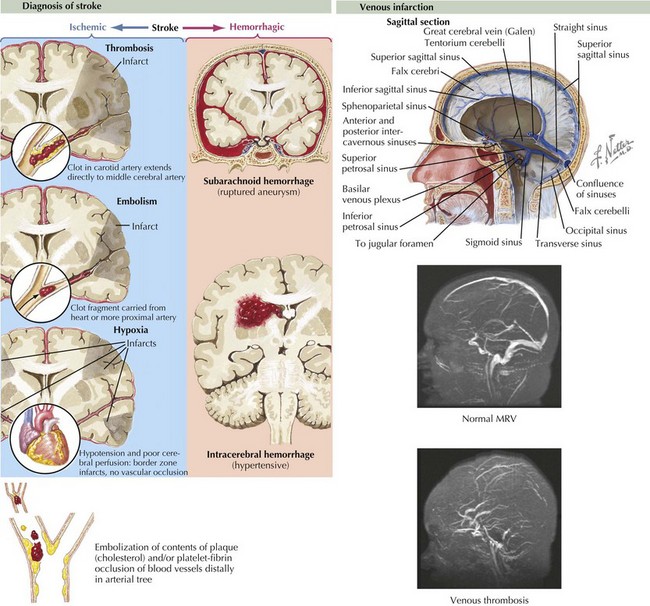
Stroke refers to acute vascular events involving the brain or brainstem. Childhood stroke occurs at a rate approximating that of childhood brain tumors. It is among the top 10 causes of death in children and is a significant cause of morbidity among survivors. Pediatric stroke can be subdivided into perinatal stroke, occurring from 28 weeks of gestation to 1 month of age, and childhood stroke, occurring from 1 month to 18 years. Important subtypes of stroke include arterial ischemic stroke (AIS), watershed infarction, intracerebral hemorrhage (ICH), and cerebral sinus venous thrombosis (CSVT). AIS is usually defined as an acute neurologic deficit of any duration consistent with focal brain ischemia conforming to an arterial distribution. Transient ischemic attacks (TIAs) are defined as focal deficits in a vascular territory lasting less than 24 hours, with some authors including the caveat that there must be no magnetic resonance imaging (MRI) evidence of infarction (Figure 79-1).
Etiology and Pathogenesis
Unlike adults, in whom hypertension, diabetes, and atherosclerosis predominate as risk factors for ischemic stroke, children presenting with AIS have much more varied etiologies. Approximately 50% of children presenting with stroke have an obvious underlying cause at the time of presentation, with arteriopathy, congenital heart disease, and sickle cell anemia representing some of the most common causes. In an additional 20% to 40%, an underlying cause can be found with further investigation. Somewhere between 10% and 30% are cryptogenic. Factors associated with pediatric AIS include inherited or acquired prothrombotic states, cardiac disease, arteriopathies or vasculopathies, trauma, and infections (Box 79-1). In older teenagers, traditional risk factors for adults, such as hypertension, diabetes, high cholesterol, and smoking, may also play a role. Cocaine or sympathomimetic medications are additional risk factors. Watershed strokes can be seen after cardiac arrest or other causes of shock, near drowning, and cardiac surgery.
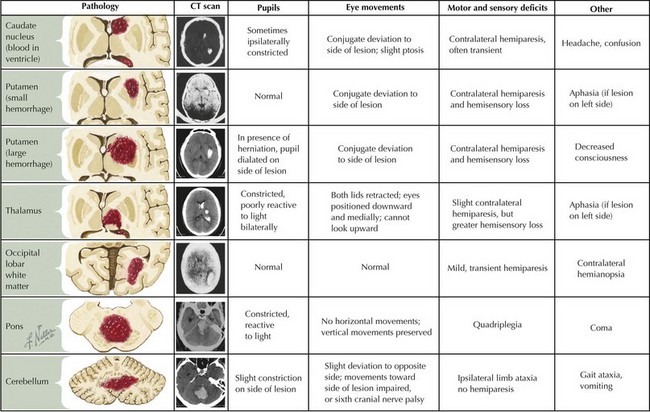
ICH in children is also etiologically distinct from ICH in adults. Unlike adults, in whom hypertension and amyloid angiopathy are the most common causes, childhood ICH is most commonly caused by ruptured vascular malformations (e.g., arteriovenous malformations, cavernomas, and aneurysms), hematologic abnormalities, and brain tumors (Box 79-2 and Figure 79-2).
Clinical Presentation
Intraparenchymal hemorrhage classically presents with focal neurologic signs or seizures accompanied by severe headache, emesis, and loss of consciousness but may be impossible to distinguish from AIS based on clinical features alone because presentations vary (see Figure 79-2). Children with CSVT may present acutely with headaches, emesis, seizures, altered mental status, or focal neurologic signs but can also present more insidiously with symptoms of increased ICP such as chronic headache, blurry vision, or diplopia secondary to sixth cranial nerve palsies.
Evaluation
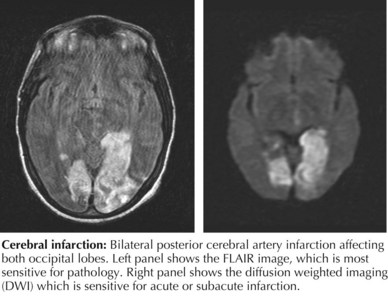
The initial evaluation of a child with suspected stroke should focus on confirming the diagnosis and ruling out common stroke mimics. The initial history and physical examination should assess the child’s ability to maintain a natural airway and adequate perfusion. Initial testing should include a complete blood count, electrolytes, blood glucose, prothrombin time, international normalized ratio, partial thromboplastin time, toxicology screen, and electrocardiography (ECG). Brain imaging should be obtained as soon as is possible. In most cases, unless MRI can be performed immediately, head computed tomography (HCT) should be performed first. HCT is fast and widely available and allows for the quick assessment of hemorrhage or mass lesions. In AIS, HCT results may be normal early in symptom evolution, but subtle findings of AIS, such as hyperdense middle cerebral artery (MCA) sign or blurring of the gray-white matter border can sometimes be seen. However, MRI is usually required to confirm the diagnosis of AIS. The most sensitive MRI sequence for detecting acute ischemic stroke is diffusion-weighted imaging with apparent diffusion coefficient; abnormalities on these sequences usually last 7 to 10 days from the acute stroke (Figure 79-3).
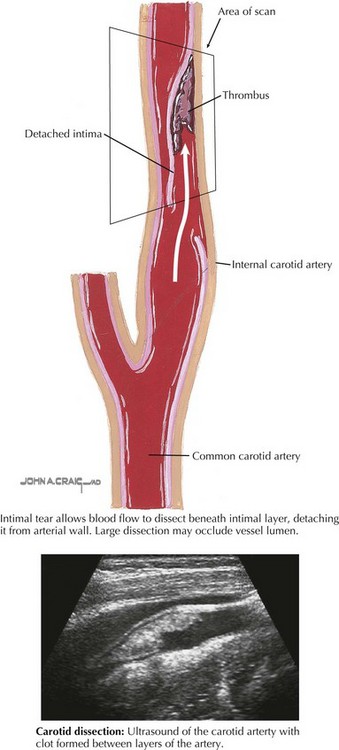
Additional testing should attempt to identify the etiology of the stroke (Box 79-3). Imaging of the vessels of the head and neck with MR angiography (MRA) or computed tomography angiography (CTA) should be performed to look for arterial dissection (Figure 79-4) or other arteriopathy. In certain cases, conventional catheter angiography may be necessary.
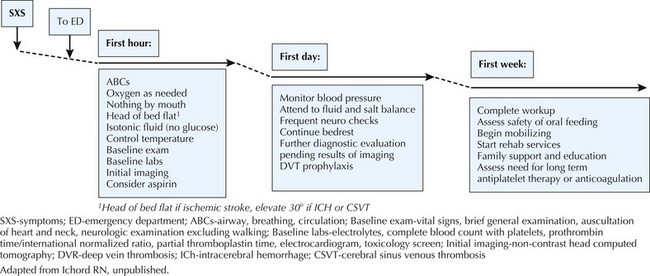
Management
An overview of initial therapy is summarized in Figure 79-5. In the acute setting, the child should lie supine with the head of the bed flat and isotonic intravenous fluids given to maximize perfusion. Hypertension is an adaptive response to ischemia, and thus outside of extreme hypertension, blood pressure should not be aggressively reduced. In most cases, aspirin should be given after hemorrhage has been excluded with neuroimaging; however, in many centers, anticoagulation with a heparinoid is started after hemorrhage is excluded. In certain cases of AIS such as arterial dissection, cardioembolism, or a known prothrombotic disorder, anticoagulation should strongly be considered (see Figure 79-5). Fever and seizures should be treated to minimize additional metabolic demands, and normoglycemia should be maintained. After the neonatal period, dextrose-free fluids are preferred to minimize the risk of hyperglycemia.
Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5-11.
Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the international pediatric stroke study. Circulation. 2009;119:1417-1423.
deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417-423.
Elbers J, Benseler SM. Central nervous system vasculitis in children. Curr Opin Rheumatol. 2008;20:47-54.
Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology. 2001;57:1155-1160.
Fullerton HJ, Wu YW, Zhao S, et al. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189-194.
Hutchison JS, Ichord R, Guerguerian AM, et al. Cerebrovascular disorders. Semin Pediatr Neurol. 2004;11:139-146.
Jordan LC. Stroke in childhood. Neurologist. 2006;12:94-102.
Jordan LC, Hillis AE. Hemorrhagic stroke in children. Pediatr Neurol. 2007;36:73-80.
Lynch JK. Cerebrovascular disorders in children. Curr Neurol Neurosci Rep. 2004;4:129-138.
Lynch JK, Han CJ. Pediatric stroke: what do we know and what do we need to know? Semin Neurol. 2005;25:410-423.
Kirton A, deVeber G. Therapeutic approaches and advances in pediatric stroke. NeuroRx. 2006;3:133-142.
Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644-2691.
Shellhaas RA, Smith SE, O’Toole E, et al. Mimics of childhood stroke: characteristics of a prospective cohort. Pediatrics. 2006;118:704-709.
Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226-1237.
Zimmer JA, Garg BP, Williams LS, et al. Age-related variation in presenting signs of childhood arterial ischemic stroke. Pediatr Neurol. 2007;37:171-175.