14 Stroke
Introduction
What follows is an overview of stroke that offers a clinician’s perspective of management. No message is more important than the need for early presentation. The previously accepted three-hour time window has been expanded to four-and-a-half hours in which to safely offer intravenous tissue-type plasminogen activator (tPA),1 but sooner is still better.
Atherosclerosis
‘Brain attacks’ and ‘heart attacks’ have a common pathophysiology. An embolus of thrombus dislodges from the surface of an atherosclerotic plaque and becomes lodged downstream to occlude blood flow and hence nutrition distal to the blockage. This accounts for ischaemic strokes, comprising 85% of all strokes (only 15% have haemorrhagic aetiology).2
Atherosclerosis increases with age, hypertension, hyperlipidemia, diabetes and cigarette smoking.2 It follows that medical management must address these factors. The thrombus that is dislodged from the plaque was formed consequent to prothrombotic effects involving tissue factor. Activated factor VII with platelet recruitment results in formation of platelet-rich fibrin thrombus, which embolised. Thus antiplatelet agents are important in preventing thrombotic emboli and hence strokes.
Cardiac causes
While there has been an attempt to classify AF into acute and chronic,3 this concept has less relevance to the neurologist/stroke-ologist, whose most important take-home-message concerning AF is the need to prevent emboli. Hence, one must recognise the need for anticoagulation. The actual treatment of AF is supervised by the cardiologist, but anticoagulation with Coumadin® is a high priority for stroke prevention. More recently a new class of anticoagulation drug has emerged.4 It is still too early to be certain about the place of dabigatran, but it appears destined to replace Coumadin® as the drug of choice for patients with AF. This remains a question for consultants until there is wider experience.
There are other cardiac causes of emboli, such as micotic emboli, with growths particularly on rheumatic valves. Unless this is appreciated with an index of suspicion for bacterial endocarditis, it may be overlooked. Mercifully this is rare but echocardiography is mandatory in the stroke evaluation. Transoesophageal echocardiogram is preferable, especially in the young stroke patient. Another cause detected by echocardiogram is patent foramen ovale (PFO), which may be under-diagnosed in stroke patients. One study reported in excess of 15% in over 55-year-old cryptogenic stroke patients having PFO with atrial septal aneurysm.5 The question of closure of PFOs in stroke patients or those with transient ischaemic attacks (TIAs) is a topic of some debate, which is not yet fully resolved. Evidence suggests an association of PFO with hypercoagulation, especially factor V leiden and prothrombin G20210A genetic mutations.6 Often hypercoagulable states travel together to evoke symptoms, such as dehydration or antiphospholipid antibodies in association with prothrombin G20210A mutations. The take-home-message is that the stroke patient deserves a detailed assessment of their hypercoagulable profile. This should have been done while in hospital, but the general practitioner can check to make sure.
Cardiac consideration is not restricted to stroke prevention but also offers a window to predict post-stroke mortality. Conventional heart rate variability measures were not of prognostic value, but abnormal long-term heart rate dynamics do predict post-stroke mortality. They may have value in risk stratification in stroke.7
Carotid artery disease
The North American Symptomatic Carotid Endarterectomy Trial (NASCET)8 found that stroke was reduced by 17% where carotid stenosis exceeded 70%, hence becoming the benchmark for ordering endarterectomy. Some advocate surgical intervention with stenosis as low as 60%, even in asymptomatic patients.9 Personal preference favours the higher figure.
Stenting is a viable alternative for cardiac vasculopathy and is becoming a respected alternative to carotid endarterectomy.10 Its exact place remains undefined with growing popularity. Furlan11 reviewed the SAPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy) trial, which showed that carotid artery stenting was safer than carotid endarterectomy in patients at high surgical risk, because of lower risk of myocardial infarction within 30 days after carotid stenting when compared to surgery. Furlan11 reviewed the EVA-35 Study (Endarterectomy versus Angioplasty in patients with Symptomatic Severe Carotid Stenosis in the same issue of The New England Journal of Medicine), which concluded that stenting was more risky than endarterectomy for 30-day incidence of stroke or death. Carotid endarterectomy is also considered a safer option than is stenting in the elderly.12
Hyperlipidemia
The meta-analysis of 90 000 patients from studies assessing the benefits of statins in stroke prevention demonstrated that the reduced risk of stroke was particularly reflected in the lowering of low-density lipoprotein cholesterol levels.13 Statins have become a basic component of stroke management.
The recent SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial of 4731 stroke or TIA patients concluded that 80 mg of atorvastatin per day reduced the overall incidence of stroke and cardiovascular events. It was associated with an increased incidence of haemorrhagic stroke.14 Personal preference favours starting with a lower dosage of statin and titrating to effect by monitoring cholesterol profile.
Transient ischaemic attacks (TIAs)
Perhaps the best way to consider TIAs is to equate them with mini strokes that resolve within a day. There is an even more worrying attitude to TIAs than there is to stroke. Because it has resolved by day’s end and very often much sooner, the attitude to TIA is complacent. A recent study confirmed that hospital admissions of TIA patients to a specialised stroke unit has beneficial effect on short-term outcome,15 and is preferable to home management and nihilism. Reliance on the ABCD2 score does not necessarily increase protection by the theoretical admission of TIAs at greater risk16,17 (see Table 14.1).
TABLE 14.1 ABCD2 scores
| Characteristic | Points |
|---|---|
| A—Age ≥ 60 years | 1 |
| B—Blood pressure > 140/90 mmHg at presentation | 1 |
| C—Clinical features:• unilateral• speech impairment without weakness | 21 |
| D—Duration:• > 60 minutes• 10–59 minutes | 21 |
| D—Diabetes | 1 |
| 2 × Day stroke risk:• low score (< 4) = 1.0%• moderate score (4–5) = 4.1%• high score (6–7) = 8.1% |
This reinforces that stroke and TIA should not be taken lightly—they demand referral to a hospital as a matter of urgency. TIA is a warning of an impending more significant ‘brain attack’ and should be treated with respect. TIA mimics, including syncope, seizure, migraine, vertigo and its causes, encephalopathy of non-vascular origin, multiple sclerosis or even transient global amnesia, may confound the picture. It remains far safer, where doubt exists, to have these assessed in hospital rather than expose the patient to risk of stroke. Many of the mimics are themselves worthy of admission, and there should be no shame in referring them to hospital for assessment.18
Diagnosis of stroke
Correct diagnosis pre-empts appropriate treatment and, while an established stroke is easily diagnosed, the section on TIA highlights the scope of differential diagnoses. The pathophysiology and anatomical site defines the stroke’s expression, be it motor or sensory deficit, loss of eloquent functions of speech, number manipulation, comprehension, orientation, consciousness or more subtle effects determined by smaller lesions.
The mnemonic ‘FAST’ remains a valuable tool for the general practitioner (see Table 14.2) and may be the start of the diagnostic approaches. The Cincinnati Pre-hospital Stroke Scale (CPSS) was developed to help recognise strokes. It has since been modified to produce the ‘Sudden Symptoms’ and ‘FAST’ mnemonic to assist with early stroke recognition (see Table 14.2). Kleindorder et al19 found that ‘Suddens’ would fail to detect 0.1% of strokes and ‘FAST’ would miss 11.1%.
TABLE 14.2 ’Sudden Symptoms’ and ‘FAST’ mnemonic for stroke detection
| Sudden symptoms | FAST |
|---|---|
| Stroke patients may have sudden onset of: |
One cannot over emphasise the need for early intervention should tPA for thrombolysis be appropriate. Rapid referral to a hospital capable of providing such intervention is mandatory. Before tPA can be given, within the critical four-and-a-half hour window, there must be cerebral imaging to exclude intracerebral haemorrhage, as haemorrhage and tPA are incompatible. Use of tPA is becoming more widely available and it may assist the general practitioner to understand some of the inclusion or exclusion criteria for its use (see Table 14.3). Early referral is enhanced by appreciating the process necessary to be completed by administration of tPA. Intra-arterial thrombolysis is also a valid form of intervention but it is only available in hospitals with access to interventional radiology, which restricts it to tertiary referral hospitals. This too is important for the family doctor to appreciate, as it may determine which in a selection of hospitals should be the preferred place of referral. The availability of a stroke unit is also an important deciding factor as over the last fifteen years they have been shown to improve prognosis.20
TABLE 14.3 Criteria for use of tPA for acute stroke
| Inclusion | Exclusion |
|---|---|
| Ischaemic stroke causing definable neurological deficit | Resolution or clearing or improving of neurological deficits |
| CT imaging excluding haemorrhage | CT evidence of widespread or large infarction (e.g. hypodensity > 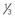 cerebral hemisphere) cerebral hemisphere) |
| Presentation and CT imaging available within 4.5 hours | INR > 1.7 |
| Informed consent | Given heparin in previous 48 hours with prolonged PTT |
| Platelets < 100 E9/L | |
| Hypertension:
or needing aggressive BP intervention |
|
| Previous stroke or head injury within 3 months | |
| Major surgery within 2 weeks | |
| Symptoms suggestive of subarachnoid haemorrhage | |
| GI or UT bleed within 3 weeks |
Lifestyle issues
Family physicians play an absolutely fundamental and pivotal role in stroke management. As has already been demonstrated, stroke and lifestyle issues are inseparable. Risk factors, such as hypertension, AF, hyperlipidemia, diabetes, cigarette smoking, excessive consumption of alcohol, dieting indiscretions, past history of stroke or vasculopathy, lack of exercise and overall disrespect of personal health, plus non-compliance with existing advice to curtail these activities and to take medications, are just as important as sophisticated intra-hospital intervention (see Table 14.4).
| Non-modifiable | Modifiable |
|---|---|
| Age | Prior stroke or TIA resulting in secondary stroke prevention—compliance |
| Gender | Hypertension |
| Race or ethnicity | Diabetes |
| Family history | Hyperlipidaemia |
| Atrial fibrillation (AF) | |
| Homocysteinaemia | |
| Carotid stenosis | |
| Smoking | |
| Excess alcohol | |
| Obesity | |
| Lack of exercise |
Patient assessment
Should the timeframe permit, non-contrast enhanced CT will allow exclusion of intracerebral bleed. Should this show a very large stroke then risk of haemorrhagic transformation also causes exclusion (see Table 14.3).
Medical treatment of stroke
a Antiplatelet agents
There have been a multitude of trials designed to ascertain which antiplatelet agent or combination of agents is superior. Each has had the mandatory anagram such as: ESPS 1 and 2—European Stroke Prevention Study (J Neurol Sci 1996, 143:1–13); CAPRIE— Clopidogrel v Aspirin in Patients at Risk of Ischaemic Events (Lancet 1996; 348:1329–1339); CURE—Clopidogrel in Unstable Angina to Prevent Recurrent Episodes (N Engl J Med 2001, 345:494–502); MATCH—Management of Athero-Thrombosis with Clopidogrel in High-risk Patients (Lancet 2004, 364:331–337); CHARISMA—Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilisation, Management and Avoidance (N Engl J Med 2006, 354:1706–1717) or ESPRIT—European/Australasian Stroke Prevention in Reversible Ischaemia Trial (Lancet 2006, 367:1665–1673).
As already stated, Coumadin® is preferred when the emboli are of cardiac origin, at least until we fully appreciate the exact position of dabigatran4 in our treatment algorithm.
b Statins
Statins, such as pravastatin21 and atorvastatin,22,23 have long been advocated to reduce strokes. As already discussed, doses of 80 mg of atorvastatin may be advocated14 but personal preference is to start low at 10–20 mg nocte and titrate to LDL level. Statins also help stabilise vessel wall integrity, so enhancing stroke prevention. They have proven to be safe and effective agents in stroke prevention.24
c Antihypertensives
The HOPE (Heart Outcome Prevention Evaluation) study was designed to look at antihypertensives in management of cardiovascular disease using ramipril.25 It was followed by closer examination of use of ramipril in stroke prevention.26 The combined studies entrenched the use of angiotensin converting enzyme inhibitors (ACE inhibitors), particularly ramipril (building up to 10 mg per day), in stroke management. Use of ACE inhibitors was further reinforced with PROGRESS (Perindopril Protection Against Recurrent Stroke Study), which showed that perindopril was also effective in stroke prevention.27
Following the recognition of the benefits of ACE inhibitors in stroke management, use of angiotensin II receptor blockade was also explored28 and hypertension management has become fundamental for stroke.29 While various pharmaceutical companies espouse the virtue of their particular antihypertensive agent, the most important take-home-message is to tackle hypertension as a major focus in stroke prevention.
d Other medications
While antiplatelet agents, statins and antihypertensives are the cornerstone of stroke therapy, there has been a move to consider other neuro-protective agents. One of these agents has been the use of the semi-synthetic second-generation derivative of the tetracycline, minocycline. There is evidence suggesting better outcomes when treating acute stroke with minocycline rather than placebo.30
1 Amaro S, Canovas D, Castellanos M, Gallego J, Marti-Febregas J, Segura T, Chamorro A. The URICO–ICTUS study: a phase 3 study of combined treatment with uric acid and rtPA administered intravenously in acute ischaemic stroke patients within the first 4.5 h of onset of symptoms. International J Stroke. 2010;5(4):325-328.
2 Levi CR. Atherothrombosis, antiplatelet therapy and ischaemic stroke prevention. Medicine Today. 2007 Jan:4-10.
3 American College of Cardiology–American Heart Association–European Society of Cardiology. Pocket guidelines: guidelines for the management of patients with atrial fibrillation. ACA & AHA: Bethesda; 2002.
4 Connolly SJ, Ezekoqitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Darius H, Diener HC, Joyner CD, Wallentin L, the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. New England J Medicine. 2009;361:1139-1151.
5 Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. New England J Medicine. 2007;357(22):2262-2268.
6 Botto N, Spadoni I, Giusti S, Ait-Ali L, Sicari R, Andreassi MG. Prothrombotic mutations as risk factors for cryptogenic ischemic cerebrovascular events in young subjects with patent foramen ovale. Stroke. 2007;38(7):2070-2073.
7 Makikallio AM, Makikallio TH, Korpelainen JJ, Sotaniemi KA, Huikwu HV, Myallyla VV. Heart rate dynamics predict post-stroke mortality. Neurology. 2004;62:1822-1826.
8 North American Symptomatic Carotid Endarterectomy Trial (NASCET). Investigators clinical alert: benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. Stroke. 1991;22:816-817.
9 Hobson RWII, Weiss DG, Fields WC, Goldstone J, Moore WS, Towne JB. Efficacy of carotid endarterectomy for asymptomatic carotid artery stenosis. The Veteran Affairs Co-operative Study Group. New England J Medicine. 1993;328:221-227.
10 Featherstone RL, Brown MM, Coward LJ. International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovas Dis. 2004;18(1):69-74.
11 Furlan AJ. Carotid-artery stenting—case open or closed? New England J Medicine. 2006;355(16):1726-1729.
12 Rockman CB, Jacobowitz GR, Adelman MA, Lamparellow PJ, Gagne PJ, Landis R, Riles TS. The benefits of carotid endarterectomy in the octogenarian: a challenge to the results of carotid angioplasty and stenting. Ann Vascul Surgery. 2003;17(1):9-14.
13 Amarenco P, Labreuche J, Lavallee P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35(12):2902-2909.
14 Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. New England J Medicine. 2006;355:549-559.
15 Kehdi EE, Cordato DJ, Thomas PR, Beran RG, Cappelen-Smith C, Griffith NC, Hanna IY, McDougall AJ, Worthington JM, Hodgkinson SJ. Outcomes of TIA patients following admission to hospital or discharge from ED. Medical J Aust. 2008;189(1):9-12.
16 Johnston SC, Rothwell PM, Nguyen-Hunyh MN, Giles MF, Elkins JS, Bernstein AL, Sidney S. Validation and refinement scores to predict very early stroke risk after transient ischemic attack. Lancet. 2007;369:283-292.
17 Ghia D, Thomas P, Cordato D, Epstein D, Beran RG, Cappelen-Smith C, Griffith N, Hanna IY, McDougall AJ, Hodgkinson SJ, Worthington JM. Low positive predictive value of the ABCD2 score in emergency department transient ischemic attack (TIA) diagnoses: The South Western Sydney TIA (SWS-TIA) Study. Int Med J (in press).
18 Ghia D, Beran RG, Cappelen-Smith C, Griffith NC, Hanna IY, McDougall AJ, Worthington JM, Hodgkinson SJ. The frequency of TIA mimic and stroke discharge diagnosis in patients admitted to Liverpool Hospital, Sydney, from Jan 2003 to Dec 2007 with an emergency department diagnosis of TIA. Int Med J. 2008;38:A93.
19 Kleindorfer DO, Miller R, Moomaw CJ, Alwell K, Broderick JP, Khoury J, Woo D, Flaherty ML, Zakaria T, Kissela BM. Designing a message for public education regarding stroke: does FAST capture enough stroke? Stroke. 2007;38(10):2864-2868.
20 Kalra L. The influence of stroke unit rehabilitation on functional recovery from stroke. Stroke. 1994;25:821-825.
21 Plehn JF, Davis BR, Sacks FM, Rouleau JL, Pfeffer MA, Bernstein V, Cuddy TE, Move LA, Piller LB, Rutherford J, Simpson LM, Braunwald E. Reduction of stroke incidence after myocardial infarction with pravastatin: the Cholesterol and Recurrent Events (CARE) study. Circulation. 1999;99(2):216-223.
22 Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brian E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT–LLA): a multicenter, randomised controlled trial. Lancet. 2003;361(9364):1149-1158.
23 Gaspardone A, Arca M. Atorvastatin: its clinical role in cerebrovascular prevention. Drugs. 2007;67(Suppl 1):55-62.
24 Wiviott SD, Cannon CP. The safety and efficacy of achieving very low LDL-cholesterol concentrations with high dose statin therapy. Curr Op Lipidol. 2006;17(6):626-630.
25 The HOPE Study Investigators. The HOPE (Heart Outcomes Prevention Evaluation) study: the design of a large, simple, randomised trial of an angiotensin-converting enzyme inhibitor (ramipril) and vitamin E in patients at high risk of cardiovascular events. Can J Cardiol. 1996;12:127-137.
26 Bosch J, Yusuf S, Pogue J, Sleight P, Donn E, Rangoonwala B, Davies R, Ostergren J, Probstfield J on behalf of the HOPE Investigators. Use of ramipril in preventing stroke: double-blind randomised trial. British Medical J. 2002;324:1-5.
27 PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure lowering regime among G105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033-1041.
28 Schrader J, Luders S, Kulschewski A, Berger J, Zidek W, Treib J, Einhaupl K, Diener HC, Dominiak P. The ACCESS Study: evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke. 2003;34:1699-1703.
29 Lampl Y, Boaz M, Gilad R, Lorbergoym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minomycine treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404-1410.
30 Hess DC, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke minocycline. R Neurol. Disease. 2010;7(Suppl. 1):S7-13.