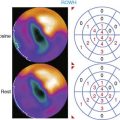
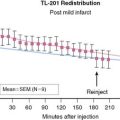
Chapter 29 Stress Myocardial Perfusion Imaging in Patients with Diabetes Mellitus
INTRODUCTION
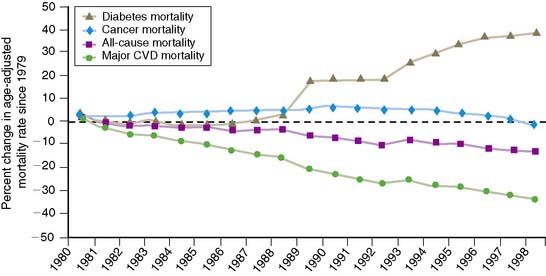
In 2005, the Centers for Disease Control and Prevention (CDC) estimated that there were approximately 21 million people with diabetes mellitus in the United States: 15 million with diagnosed diabetes mellitus and 6 million with undiagnosed diabetes. Greater than 95% of these individuals have type 2 diabetes.1 The number of patients with diabetes mellitus increases every year by 5%, adding approximately 1.5 million new cases annually. Compared to the nondiabetic population, patients with diabetes mellitus have an increased risk of developing cardiovascular disease and an increased risk for death from myocardial infarction or congestive heart failure. Although in the general population, the overall age-adjusted mortality from cardiovascular diseases decreased by 30% between 1990 and 2000, the mortality rate in patients with diabetes mellitus has increased by approximately the same amount (Fig. 29-1). Importantly, 80% of the mortality among diabetics can be attributed to cardiovascular causes. Because of these alarming statistics, the American Diabetes Association; the National Heart, Lung and Blood Institute; the Juvenile Diabetes Foundation International; the National Institute of Diabetes and Digestive and Kidney Diseases; and the American Heart Association issued a joint statement in 1999 indicating the importance of diabetes mellitus as a major risk factor for cardiovascular disease.2
EARLY DETECTION OF CORONARY ARTERY DISEASE IN DIABETES MELLITUS
To maximize the effect of appropriate treatment of cardiovascular disease, it is important that diabetic patients at risk of developing or who have already developed coronary artery disease (CAD) are identified as early as possible. A major hurdle toward this goal is that CAD in patients with diabetes is frequently silent; when clinically manifest, it is often in an advanced stage. Twenty-five percent of patients with diabetes mellitus in the Framingham Study had electrocardiographic evidence of prior unrecognized infarction, and half of these individuals were asymptomatic.3–5 Less than a third of diabetic patients with exercise-induced ischemia on myocardial perfusion imaging (MPI) had angina during exertion, compared to more than two-thirds of nondiabetic patients. In 1998, the American Diabetes Association published a consensus statement on diagnostic testing for CAD in people with diabetes mellitus.6 It was recommended that asymptomatic diabetic patients with two or more risk factors for CAD in addition to diabetes mellitus should have exercise stress testing. The consensus statement further recommended exercise electrocardiography (ECG) as the first diagnostic test. These recommendations were based on the clinical judgment of a panel of experts rather than on published scientific data in truly asymptomatic patients. At the time of the consensus statement, it was evident that a substantial gap existed in the knowledge about the prevalence of silent ischemia, how to detect preclinical CAD, and how to manage asymptomatic patients with diabetes in whom silent ischemia was demonstrated. In subsequent years, a number of studies have demonstrated unequivocally that the presence or absence of traditional risk factors for CAD in patients with diabetes are not helpful for predicting silent myocardial ischemia. Therefore, other clinical and diagnostic algorithms need to be explored for identifying diabetic patients at increased cardiovascular risk.
Stress Modality
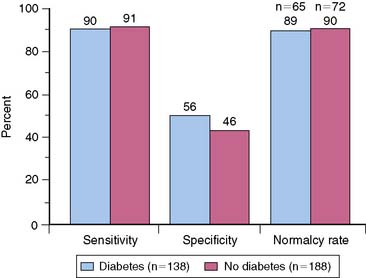
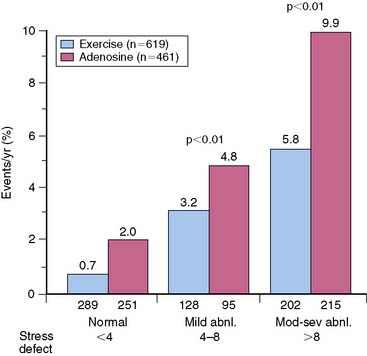
In the general population, the preferred stress modality for detecting myocardial ischemia is physical exercise on a treadmill or bicycle. However, many diabetic patients have diminished exercise ability because of obesity, deconditioning, peripheral neuropathy, or peripheral vascular disease. Several studies have observed that about a third to half of diabetic patients were unable to achieve adequate exercise levels for diagnostic testing.7 Therefore, pharmacologic stress may be the more appropriate stress modality in many patients with diabetes. Stress MPI has been shown to have comparable sensitivity and specificity in nondiabetics and diabetic patients for detecting CAD (Fig. 29-2).8 Moreover, the incremental prognostic value of pharmacologic MPI was similar to that using physical exercise (Fig. 29-3).9,10
Prevalence of Silent Myocardial Ischemia
Screening for CAD is a controversial issue in clinical cardiology. To avoid an unacceptably large number of false-positive test results, the prevalence of the disease for which screening is to be performed must be at least intermediate. The true prevalence of silent myocardial ischemia in asymptomatic patients with diabetes was until recently unknown because most estimates were based on retrospective database analyses. The results of the prospective Detection of Ischemia in Asymptomatic Diabetics (DIAD) study made very clear that indeed most previous studies were significantly biased owing to patient selection. Retrospective database analyses may not be accurate because patients were most likely referred for stress testing to investigate symptoms suspected to be due to CAD. The available published information consisted of three types of analyses: retrospective analysis of large, combined nuclear cardiology databases, retrospective analysis of single laboratory databases, and prospective epidemiologic studies. In two large retrospective database studies, the prevalence of abnormal myocardial perfusion images ranged from 41% to 58%.8,11,12 Detailed information on patients’ symptoms was not available, and it was unclear how many patients were truly asymptomatic. In other retrospective analyses of apparently asymptomatic patients with diabetes, 26% to 59% had abnormal stress myocardial perfusion images.13–16 These patients were referred for risk assessment before general surgery or because of the presence of multiple risk factors for CAD. Several prospective studies have been performed in Europe in asymptomatic patients with diabetes. The observed prevalence of ischemia in these studies varied as well. Whereas Janand-Delenne and colleagues17 reported a prevalence of silent myocardial ischemia of 18.4%, Gazzaruso and colleagues18 and the Italian MiSAD group19 reported a prevalence of 8.6% and 6.4%, respectively. In the latter studies, characteristically, exercise electrocardiography was the first stress test, and MPI was only performed if exercise electrocardiography was positive or equivocal. Because of the known low sensitivity of exercise electrocardiography, a substantial number of patients may have been missed in these studies.
The prospective multicenter DIAD study20 published in 2004 shed a different light on the practical problem of silent myocardial ischemia in asymptomatic patients with diabetes. It is important to consider the entry criteria for the DIAD study. All patients enrolled in the study had type 2 diabetes mellitus, were 50 to 75 years of age, had no symptoms suggesting CAD (including a negative Rose questionnaire for angina or chest discomfort), had a normal resting electrocardiogram, and had no prior cardiac stress testing. In addition to carefully ruling out symptoms of angina and anginal equivalents, the requirement of a normal rest ECG is an important characteristic of this study. Well over 1100 patients were recruited and randomized to either screening for CAD with adenosine technetium 99mTc sestamibi gated single-photon emission computed tomography (SPECT) imaging or no screening. The prevalence of silent myocardial ischemia, defined as regional myocardial perfusion abnormalities on imaging or ischemic ECG changes during adenosine infusion, was lower in the DIAD study than previously suggested in the literature. Although 22% of asymptomatic subjects had abnormal screening test results, in only 6% of participants were the myocardial perfusion abnormalities severe.20 Thus, 1 out of every 5 asymptomatic patients with diabetes may have silent CAD, and 1 of 16 may have markedly abnormal screening results. In a recent French study, similar to the DIAD study in design, age appeared to be an important factor for the occurrence of silent ischemia. Whereas asymptomatic patients with diabetes younger than 60 years had a prevalence of about 30%, patients older than 60 years had a prevalence of 43%.21
Endothelial Dysfunction
Many patients with diabetes mellitus have evidence for endothelial dysfunction.22,23 Persistent hyperglycemia may be in part responsible for impaired endothelial-dependent vasodilation. Oxidative stress and hyperproduction of superoxide are common in diabetes and might cause impaired endothelial nitric oxide synthesis. In addition, the insulin-resistant state itself may blunt nitric oxide–mediated vasodilation. Impaired regional coronary flow reserve has been observed in patients with diabetes mellitus, in particular in conjunction with cardiac autonomic dysfunction. The presence of microvascular endothelial dysfunction is of relevance for MPI. Regional myocardial perfusion abnormalities may occur in the absence of significant epicardial CAD.24 Whether such myocardial perfusion abnormalities have the same prognostic implications as those associated with epicardial CAD is as yet unclear. Emmett et al.25 reported that transient ischemic left ventricular dilation (TID) on SPECT imaging was strongly associated with diabetes mellitus and left ventricular hypertrophy, rather than with the amount of ischemia or severity of CAD. Thus, microvascular disease and endothelial dysfunction may play an important role in the etiology of TID.
ABNORMAL MYOCARDIAL PERFUSION IMAGING IN PATIENTS WITH SUSPECTED OR KNOWN CORONARY ARTERY DISEASE (See Chapter 16)
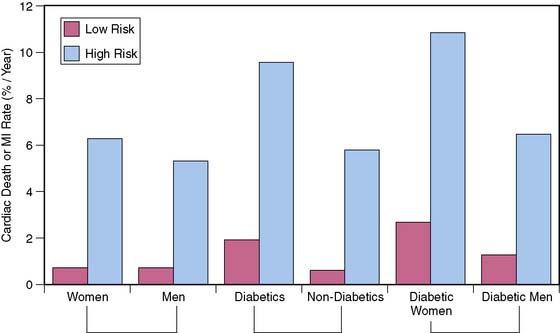
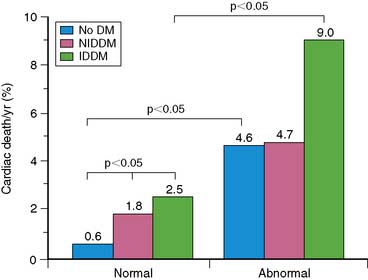
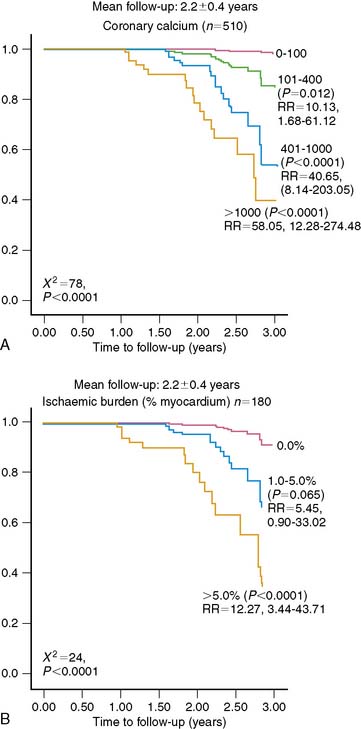
Observations in selected patient populations suggest that SPECT myocardial perfusion abnormalities in patients with diabetes mellitus carry a significantly worse prognosis than similar abnormalities in patients without diabetes mellitus. The most extensive retrospective five-center database analysis published thus far involved 4755 patients, of whom 929 had diabetes mellitus.11 More patients with diabetes were unable to exercise adequately and had vasodilator stress. Abnormal SPECT MPI was an independent predictor of cardiac death and nonfatal myocardial infarction in both nondiabetic patients and diabetic patients. However, in spite of higher revascularization rate, patients with diabetes had an almost two times higher cardiac event rate than nondiabetic patients. Unadjusted cardiac survival rate was lower for diabetic patients but became comparable to that in nondiabetic patients after adjustment for pretest clinical risk and severity of MPI abnormalities. An important observation was that diabetic women had a significantly worse outcome for any given extent of myocardial ischemia on SPECT imaging (Fig. 29-4).11,26 Another study that evaluated specifically the value of adenosine stress SPECT MPI in women confirmed the preceding observations.10 Although men and women without diabetes mellitus had similar outcomes for any given MPI abnormality, women with diabetes mellitus had significantly higher cardiac mortality. Furthermore, insulin-dependent patients were at higher risk than non-insulin-dependent diabetic patients (Fig. 29-5).10
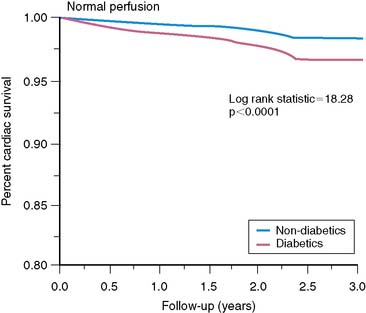
Not unexpectedly, many asymptomatic patients with diabetes mellitus who are screened have entirely normal stress myocardial perfusion images. In the general population, patients with normal exercise myocardial perfusion images have an excellent prognosis with a low hard cardiac event rate of less than 1% per year.27 However, in patients with diabetes mellitus, this expectation appeared invalid. In a retrospective database analysis, Giri and colleagues11 observed that even though the prognosis in patients with diabetes mellitus and normal stress perfusion images was significantly better than that of patients with diabetes and perfusion abnormalities, their prognosis was worse compared to patients without diabetes mellitus (Fig. 29-6). Outcome was even worse in insulin-dependent patients with normal perfusion images (see Fig. 29-4).10 Thus, the “warranty period” of normal stress perfusion images in patients with diabetes mellitus is apparently limited. Hachamovitch and colleagues28 subsequently demonstrated that the prognostic value of normal stress perfusion images must be viewed in the clinical context and is less favorable in the elderly, patients with known CAD, those who require pharmacologic testing, and, in particular those with diabetes.
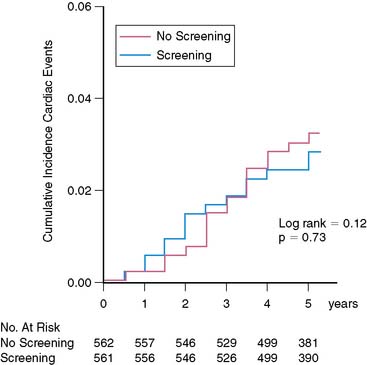
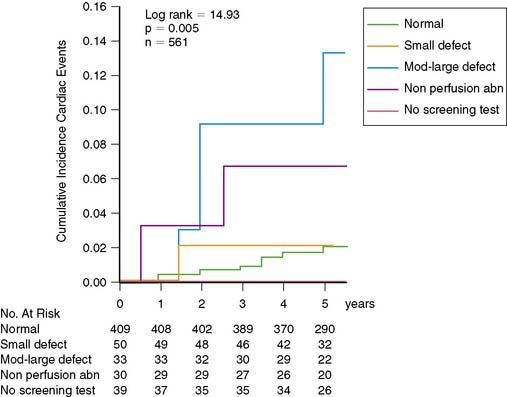
In the recently completed DIAD study, 1123 patients without symptoms or clinical signs of CAD were followed prospectively for 5 years.29 Unexpectedly, the outcome in these patients was considerably more favorable than anticipated. This illustrates (again) that retrospective database analyses may have significant limitations. The cumulative 5-year hard cardiac event rate (cardiac death or nonfatal myocardial infarction) in DIAD was only 2.9%, or less than 1% per year.29 Moreover, cardiac event rate was not different in patients randomized to screening or no screening (Fig. 29-7). A relatively small and equal number of patients in both groups had coronary revascularization, so this does not explain the favorable outcome. In the DIAD study, the only intervention consisted of systematic screening with adenosine stress SPECT imaging in one group of the participants. The other group had no intervention but regular follow-up. Screened participants with normal stress images or small myocardial perfusion defects had a very low cardiac event rate (0.4% per year), whereas participants with moderate to large myocardial perfusion defects had a significantly higher cardiac event rate (2.4% per year) (Fig. 29-8). Subsequent clinical management in both groups was entirely at the discretion of the patients’ medical providers, and one can argue therefore that DIAD reflects “real world” contemporary clinical care. Importantly, during the course of the study, the majority of DIAD patients were aggressively treated with antiischemic medications (statins, angiotensin-converting enzyme [ACE] inhibitors, aspirin). Thus it appears that patients with type 2 diabetes mellitus without symptoms or signs of CAD fare very well on contemporary and aggressive primary cardiac preventive treatment.29
CLINICAL PREDICTORS OF SILENT MYOCARDIAL ISCHEMIA
Because of the relatively low prevalence of silent ischemia in asymptomatic patients with diabetes, and because of the large number of asymptomatic diabetic patients potentially to be screened, investigators have tried to derive simple and inexpensive clinical markers that would identify a subgroup of patients at higher risk. A number of predictors for the presence of silent CAD have been suggested in various studies: retinal vasculopathy,17 micro/macro albuminuria,30 C-reactive protein, hemoglobin A1c, duration of diabetes, body mass index (BMI), peripheral neuropathy, lipoprotein (a),18 traditional risk factors for CAD,2,17 peripheral arterial disease, rest electrocardiogram,19 and cardiac autonomic dysfunction.20,31–35 Many of these variables either have too high a prevalence in the diabetic population to be practically useful, or their statistical association with ischemia has been inconsistent in various published studies. In recent years, a number of studies confirmed that conventional risk factors for CAD were not predictive of stress-inducible ischemia.20,36 The DIAD study showed that male gender, duration of diabetes, and the presence of cardiac autonomic dysfunction were strong predictors of inducible ischemia.20 Although the statistical association of cardiac autonomic dysfunction and myocardial ischemia was strong, the positive predictive value was low.
The anatomic substrate of cardiac autonomic dysfunction has been visualized by positron emission tomography with C-11 hydroxyephedrine. In patients with diabetes, myocardial uptake of hydroxyephedrine often was heterogeneous, consistent with regional sympathetic denervation, and was associated with diminished coronary flow reserve due to endothelial dysfunction.37,38 In addition, increased in vitro platelet activation39 has been reported in conjunction with cardiac autonomic dysfunction in patients with type 1 diabetes mellitus, potentially explaining the association with impaired myocardial perfusion. It is as yet unclear whether there is a causal relationship between the presence of cardiac autonomic dysfunction and coronary vasculopathy or whether it is merely an epiphenomenon. Valensi et al.40 observed in a large multicenter study that about 25% of patients with type 1 and type 2 diabetes have evidence of cardiac autonomic dysfunction. Although clinical testing for cardiac autonomic dysfunction has recently become easier, more standardized, and more reproducible by the development of automated portable devices, it is not a routine test in most outpatient clinics. Another relatively simple method to recognize cardiac autonomic dysfunction may be to assess heart rate response to vasodilator stress testing with adenosine. The normal increase of heart rate that may be observed in response to coronary vasodilation is significantly blunted in patients with diabetes, compared to nondiabetic patients.41 However, in individual patients, the threshold of an abnormal response associated with increased cardiac risk is not yet defined.
MULTIMODALITY APPROACH FOR DETECTION OF CAD IN DIABETES MELLITUS (See Chapter 20)
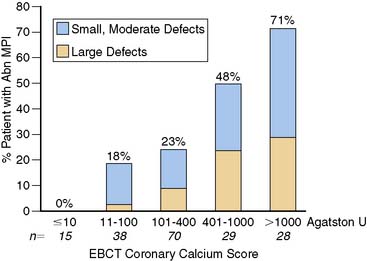
Another potentially attractive and perhaps cost-effective first step in the screening of asymptomatic patients with diabetes may be the assessment of coronary artery calcium by x-ray computed tomography.42–44 The degree of calcification, expressed as the coronary artery calcium (CAC) score, is a measure of atherosclerotic plaque burden.45 In the general population, patients with high levels of coronary artery calcium have a high incidence of abnormal stress myocardial perfusion images, whereas in the absence of significant calcium deposits, this incidence is low.46 In selected populations, high CAC scores have been shown to be associated with higher future cardiac event rates.47–49 In a study of self-referred low- to intermediate-risk asymptomatic patients, calcium scoring appeared to be an independent predictor for hard and soft cardiac events, in addition to conventional risk factors.49 A number of recent studies explored the prognostic value of coronary artery calcium in the diabetic population.50–54 In a retrospective analysis of a largely self-referred population of asymptomatic subjects, subjects with metabolic syndrome or diabetes had a significantly higher likelihood of having significant coronary artery calcium on electron beam computed tomography (EBCT) compared to subjects with neither.52 In comparison to nondiabetic patients, patients with CAC scores above 100 Agatston units (Au) and metabolic syndrome or diabetes had a higher likelihood of inducible ischemia.53 Anand et al.54 prospectively studied asymptomatic patients with diabetes mellitus using a stepwise diagnostic approach: After initial EBCT coronary calcium scoring, patients with a calcium score over 100 Au had stress MPI. With increasing calcium burden, the number of patients with abnormal SPECT increased substantially; 60% of patients with CAC scores above 400 Au had abnormal stress SPECT (Fig. 29-9). In addition, significant coronary artery calcification (>400 Au) was associated with adverse outcome in these asymptomatic patients with diabetes (Fig. 29-10). The recent PREDICT study, a prospective cohort study of 589 patients with type 2 diabetes with no history of cardiovascular disease, confirmed that coronary artery calcium was a significant predictor of combined hard and soft cardiac events.55
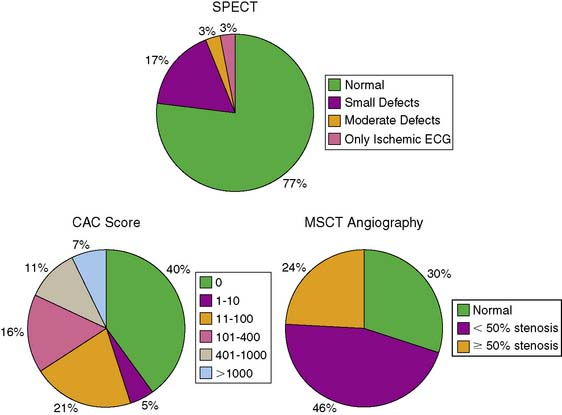
A recent study by Scholte et al.56 explored prospectively the interrelationship of SPECT stress myocardial perfusion abnormalities, significant coronary artery calcium, and significant coronary artery stenoses on noninvasive CT angiography in the same asymptomatic patients with diabetes mellitus. Anatomic evidence of CAD (coronary calcium and luminal stenoses) occurred more frequently than functional evidence of CAD by stress SPECT imaging. Clinically significant manifestations of CAD by any of the three technologies occurred in about one-quarter to one-fifth of patients, either alone or in combination (Fig. 29-11). At the present time, the comparative prognostic significance of these different manifestations of CAD is still unclear and needs to be clarified by long-term follow-up.
TREATMENT OF SILENT MYOCARDIAL ISCHEMIA IN PATIENTS WITH DIABETES
Once silent myocardial ischemia has been detected in asymptomatic patients with diabetes, a dilemma presents itself: how to treat these patients. Is outcome in these asymptomatic patients sufficiently poor to justify major interventions such as revascularization? A retrospective database study suggested a significantly worse outcome in diabetic patients with evidence of inducible ischemia, particularly if the presenting symptom was shortness of breath.14 However, preliminary follow-up data of the DIAD study suggest that the overall 5-year prognosis of initially asymptomatic patients is very favorable, even in patients with mild inducible ischemia.29 Patients with severe myocardial perfusion abnormalities and ischemia ECG during adenosine infusion have a significantly poorer prognosis, however (see Fig. 29-8).29 The ongoing BARI-2D trial can be expected to provide new insights in how to treat these patients.57 The only data available at present are those from a nonrandomized retrospective study that evaluated outcome after revascularization of asymptomatic patients with diabetes with inducible silent ischemia on SPECT imaging.58 Only patients who had severe stress myocardial perfusion abnormalities had improved outcome after coronary bypass surgery, but not after coronary angioplasty. Patients who had mild inducible myocardial ischemia had no difference in outcome when treated medically or when treated by revascularization.
Interestingly, repeat stress SPECT imaging after 3 years in the DIAD study showed unexpected resolution of inducible myocardial ischemia in a significant number of patients. This appeared to be most likely due to optimized medical treatment with aspirin, statins, and ACE inhibitors.59
For patients with diabetes mellitus, the stakes are too high to wait for the first obvious clinical manifestation of CAD. When symptoms occur, it may already be too late for many patients because of the advanced stage of CAD. Screening of selected subgroups of asymptomatic patients with diabetes is probably justified. However, as the results of the DIAD study suggested,29 careful clinical observation and appropriately timed stress radionuclide MPI, alone or in conjunction with other diagnostic tests, can play important roles in early detection of CAD in patients with diabetes mellitus.
1. Centers for Disease Control and Prevention mortality database, Available at: www.cdc.gov
2. Diabetes mellitus: A major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; the National Heart, Lung, and Blood Institute; the Juvenile Diabetes Foundation International; the National Institute of Diabetes and Digestive and Kidney Diseases; and the American Heart Association. Circulation. 1999;100:1132-1133.
3. Nesto R.W. Screening for asymptomatic coronary artery disease in diabetes [editorial; comment]. Diabetes Care. 1999;22(9):1393-1395.
4. Boland L.L., Folsom A.R., Sorlie P.D., et al. Occurrence of unrecognized myocardial infarction in subjects aged 45 to 65 years (The ARIC Study). Am J Cardiol. 2002;90:927-931.
5. Naka M., Hiramatsu K., Aizawa T., et al. Silent myocardial ischemia in patients with non-insulin-dependent diabetes mellitus as judged by treadmill exercise testing and coronary angiography. Am Heart J. 1992;123:46-52.
6. ADA consensus statement American Diabetes Association. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes. Diabetes Care. 1998;21:1551-1559.
7. Vanzetto G., Halimi S. Hammoud, et al: Prediction of cardiovascular events in clinically selected high-risk NIDDM patients. Prognostic value of exercise stress test and thallium-201 single-photon emission computed tomography. Diabetes Care. 1999;22:19-26.
8. Kang X., Berman D.S., Lewin H., et al. Comparative ability of myocardial perfusion single-photon emission computed tomography to detect coronary artery disease in patients with and without diabetes mellitus. Am Heart J. 1999;137:949-957.
9. Kang X., Berman D.S., Lewin H.C., et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography in patients with diabetes mellitus. Am Heart J. 1999;138:1025-1032.
10. Berman D.S., Kang X., Hayes S.W., et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared to men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol. 2003;41:1125-1133.
11. Giri S., Shaw L.J., Murthy D.R., et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002;105:32-40.
12. Rajagopalan N., Miller T.D., Hodge D.O., Frye R.L., Gibbons R.J. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol. 2005;45:43-49.
13. DeLorenzo A., Lima R., Siqueira-Filho A.G., et al. Prevalence and prognostic value of perfusion defects detected by stress technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography in asymptomatic patients with diabetes mellitus and no known coronary artery disease. Am J Cardiol. 2002;90:827-832.
14. Zellweger M.J., Hachamovitch R., Kang X., Hayes S.W., Friedman J.D., Germano G., Pfisterer M.E., Berman D.S. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25:543-550.
15. Prior J.O., Monbaron D., Koehli M., Calcagni M.L., Ruiz J., Bischof Delaloye A. Prevalence of asymptomatic and silent stress-induced perfusion defects in diabetic patients with suspected coronary artery disease referred for myocardial perfusion scintigraphy. Eur J Nucl Med Mol Imaging. 2005;32:60-69.
16. Miller T.D., Rajagopalan N., Hodge D.O., Frye R.L., Gibbons R.J. Yield of stress single-photon emission computed tomography in asymptomatic patients with diabetes. Am Heart J. 2004;147:890-896.
17. Janand-Delenne B., Savin B., Habib G., et al. Silent myocardial ischemia in patients with diabetes. Diabetes Care. 1999;22:1396-1400.
18. Gazzaruso C., De Amici E., Garzaniti A., et al. Assessment of asymptomatic coronary artery disease in apparently uncomplicated type 2 diabetic patients. Diabetes Care. 2002;25:1418-1424.
19. Milan Study on Atherosclerosis and Diabetes Group. Prevalence of unrecognized silent myocardial ischemia and its association with atherosclerotic factors in noninsulin-dependent diabetes mellitus. Am J Cardiol. 1997;79:134-139.
20. Wackers FJTh, Young L.H., Inzucchi S.E., et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects-The DIAD study. Diabetes Care. 2004;27:1954-1961.
21. Valensi P., Paries J., Brulport-Cerisier V., et al. Predictive value of silent myocardial ischemia for cardiac events in diabetic patients. Diabetes Care. 2005;28:2722-2727.
22. Williams S.B., Cusco J.A., Roddy M.A., et al. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567-574.
23. Hogykian R.V., Galecki A.T., Pitt B., et al. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes. J Clin Endocrinol Metab. 1998;83:1946-1952.
24. Hasdai D., Gibbons R.J., Holmes D.R., et al. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390-3395.
25. Emmett L., Van Gaal W.J., Magee M., Bass S., Ali O., Freedman B., Van der Wall H., Kritharides L. Prospective evaluation of the impact of diabetes and left ventricular hypertrophy on the relationship between ischemia and transient ischemic dilation of the left ventricle on single-day adenosine Tc-99m myocardial perfusion imaging. J Nucl Cardiol. 2008;15:638-643.
26. Shaw L.J., Iskandrian A.E. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11:171-185.
27. ASNC statementBateman T. Clinical relevance of a normal myocardial perfusion scintigraphy study. J Nucl Cardiol. 1996;4:172-173.
28. Hachamovitch R., Hayes S., Friedman J.D., et al. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans. What is the warranty period of a normal scan. J Am Coll Cardiol. 2003;41:1329-1340.
29. Young L.H., Wackers FJTh, Chyun D.A., et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: The DIAD study: A randomized controlled clinical trial. JAMA. 2009;301:1547-1555.
30. Rutter M.K., McComb J.M., Brady S., et al. Silent myocardial ischemia and microalbuminuria in asymptomatic subjects with non-insulin-dependent diabetes mellitus. Am J Cardiol. 1999;83:27-31.
31. Langer A., Freeman M.R., Josse R.G., et al. Detection of silent myocardial ischemia in diabetes mellitus. Am J Cardiol. 1992;67:1073-1078.
32. Ziegler D. Cardiovascular autonomic neuropathy: clinical manifestations and measurement. Diabetes Rev. 1999;7:342.
33. Seshadri N., Acharya N., Lauer M. Association of diabetes mellitus with abnormal heart rate recovery in patients without known coronary artery disease. Am J Cardiol. 2003;91:108-111.
34. Lee K., Jang H., Kim Y., et al. Prognostic value of cardiac autonomic neuropathy independent and incremental to perfusion defects in patients with diabetes and suspected coronary artery disease. Am J Cardiol. 2003;92:1458-1461.
35. Marchant B., Umachandran V., Stevenson R., et al. Silent myocardial ischemia: Role of subclinical neuropathy in patients with and without diabetes. J Am Coll Cardiol. 1993;22:1433-1437.
36. Scognamiglio R., Negut C., Ramondo A., Tiengo A., Avogaro A. Detection of coronary artery disease in asymptomatic patients with type 3 diabetes mellitus. J Am Coll Cardiol. 2006;47:65-71.
37. Allman K.C., Stevens M.J., Wieland D.M., et al. Noninvasive assessment of cardiac diabetic neuropathy by carbon-11 hydroxyephedrine and positron emission tomography. J Am Coll Cardiol. 1993;22:1425-1432.
38. Stevens M.J., Dayanikli F., Raffel D.M., et al. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J Am Coll Cardiol. 1998;31:1575-1584.
39. Rauch U., Ziegler D., Piolot R., et al. Platelet activation in diabetic cardiovascular autonomic neuropathy. Diabetes Med. 1999;16:848-852.
40. Valensi P., Paries J., Attali J.R., and the French Group for Research and Study of Diabetes Neuropathy. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications: The French multicenter study. Metabolism. 2003;52:815-820.
41. Bravo P.E., Hage F.G., Woodham R.M., Heo J., Iskandrian A.E. Heart rate response to adenosine in patients with diabetes mellitus and normal myocardial perfusion imaging. Am J Cardiol. 2008;102:1103-1106.
42. Agatston A.S., Janowitz W.R., Hildner F.J., et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827-832.
43. Achenbach S., Moshage W., Ropers D., et al. Value of electron-beam computed tomography for the noninvasive detection of high-grade coronary artery stenoses and occlusions. N Engl J Med. 1998;339:1964-1971.
44. Budoff M.J., Diamond G.A., Raggi P., et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation. 2002;105:1791-1796.
45. Rumberger J., Simons D.B., Fitzpatrick L.A., et al. Coronary artery calcium areas by electron-beam computed tomography and coronary atherosclerotic plaque area: A histopathologic correlative study. Circulation. 1995;92:2157-2162.
46. Berman D.S., Wong N.D., Gransar H., et al. Relationship between stress myocardial perfusion SPECT and the extent of subclinical atherosclerosis assessed by CT coronary calcium. Circulation. 2003;108(Suppl 4):634.
47. Arad Y., Spadaro L.A., Goodman K., et al. Predictive value of electron beam computed tomography of the coronary arteries: 19-month follow-up of 1173 asymptomatic subjects. J Am Coll Cardiol. 2000;36:1253-1260.
48. Raggi P., Callister T.Q., Cooil B., et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation. 2000;101:850-855.
49. Kondos G.T., Hoff J.A., Sevrukov A., et al. Electron-beam tomography coronary artery calcium and cardiac events: A 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571-2576.
50. Starkman H.S., Cable G., Hala V., et al. Delineation of prevalence and risk factors for early coronary artery disease by electron beam computed tomography in young adults with type 1 diabetes. Diabetes Care. 2003;26:433-436.
51. Wolfe M.L., Gefter N.I., Warren G., et al. Coronary artery calcification at electron beam computed tomography is increased in asymptomatic type 2 diabetics independent of traditional risk factors. J Cardiovasc Risk. 2002;9:369-376.
52. Wong N.D., Sciammarella M.G., Polk D., et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41:1547-1553.
53. Wong N.D., Rozanski A., Gransar H., Miranda-Peats R., Kang X., Hayes S., Shaw L., Friedman J., Polk D., Berman D.S. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible ischemia among patients with subclinical atherosclerosis. Diabetes Care. 2005;28:1445-1450.
54. Anand D.V., Lim E., Hopkins D., Corder R., Shaw L.J., Sharp P., Lipkin D., Lahiri A. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27:713-721.
55. Elkeles R.S., Godsland I.F., Feher M.D., Rubens M.B., Roughton M., Nugara F., Humphries S.E., Richmond W., Flather M.D. for the PREDICT study group. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29:2244-2251.
56. Scholte A.J.H.A., Schuijf J.D., Kharagjitsingh A.V., Dibbets-Schneider P., Stokkel M.P., Jukema J.W., vander Wall E.E., Bax J.J., Wackers F.J.T.H. Different manifestations of coronary artery disease by stress SPECT myocardial perfusion imaging, coronary calcium scoring, and multislice CT coronary angiography in asymptomatic patients with type 2 diabetes mellitus. J Nucl Cardiol. 2008;15:503-509.
57. Sobel B.E., Frye R., Detre K.M. Burgeoning dilemmas in the management of diabetes and cardiovascular disease. Rationale for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2003;107:636-642.
58. Sorajja P., Chareonthaitawee P., Rajagopalan N., Miller T.D., Frye R.L., Hodge D.O., Gibbons R.J. Improved survival in asymptomatic diabetic patients with high-risk SPECT imaging treated with coronary bypass grafting. Circulation. 2005;112(Suppl I):I-311–I-316.
59. Wackers FJTh, Chyun D.A., Young L.H., et al. Resolution of asymptomatic myocardial ischemia in patients with type 2 diabetes in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study. Diabetes Care. 2007;30:2892-2898.