18 Strabismus
Strabismus is present when the foveas of both eyes are not simultaneously aligned on the object of regard; for distance fixation this means that the visual axes are not parallel. Strabismus is classified by the direction of the deviation: if the visual axes converge there is esodeviation; if they diverge there is exodeviation. If the visual axes differ in vertical direction there is hyperdeviation or hypodeviation, depending on whether the eye described is higher or lower than its fellow. Strabismus may be manifest—a ‘tropia’—if the deviation is present with both eyes open or latent—a ‘phoria’—when the deviation is demonstrable only with the eyes dissociated and binocular visual reflexes disrupted. Strabismus is concomitant when the angle of deviation remains constant (or nearly so) irrespective of the position of gaze or the eye that fixates or incomitant when the angle of deviation varies with the direction of gaze and fixing eye. Incomitant deviations are generally associated with ocular muscle paresis or mechanical restriction of rotation of the globe.
ANATOMY
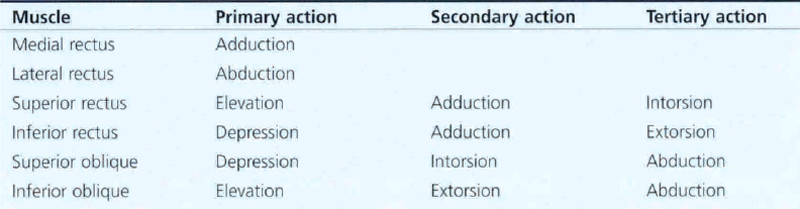
Table 18.1 shows the primary, secondary and tertiary actions of the extraocular muscles. Torsional movements are described as intorsional or extorsional in relation to the midline.

Fig. 18.1 The extraocular muscles consist of two layers: a ‘global layer’ adjacent to the globe, and the ‘orbital’ layer peripheral to this. (Left) Histology of the orbit of a 17-month-old infant stained with Masson trichrome shows the extraocular muscles suspended in blue orbital connective tissue. The orbital layer is more blue and the global layer more red. (Right) Higher magnification of the lateral rectus muscle shows collagen fibrils interspersed in the outer orbital layer; these fibrils are contiguous with the collagenous muscle pulleys of the orbit, explaining the layer’s bluish histological hue.
From Demer JL, Oh SY, Poukens V. Invest Ophthalmol Vis Sci 2000; 41: 1280–1290.

Fig. 18.2 Recent research has shown that the extraocular muscles are attached by connective tissue to the orbital septa just posterior to their insertion on the globe producing a dynamic pulley system that is believed to play an important role in positioning the globe by interacting with the actions of the orbital extraocular muscle layer. For example, on adduction, the medial rectus pulley is displaced posteriorly and the lateral rectus pulley anteriorly.
Reprinted with permission from Denser J F, Oh S Y, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci 2000; 41:1280–1290. ©2000 Association for Research in Vision and Ophthalmology.
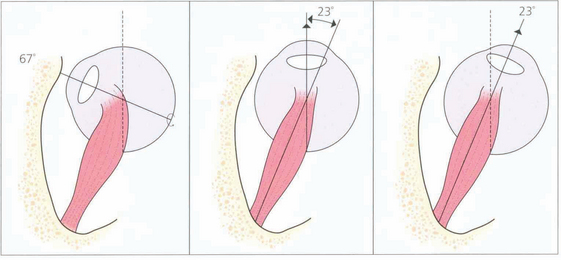
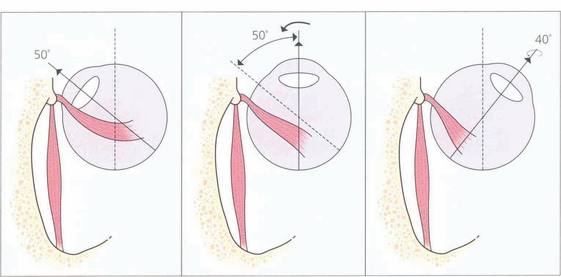
Fig. 18.3 The action of the two horizontal recti is the simplest to understand as they work around a common vertical axis. The action of the vertical recti is more complex because they attach medial to the vertical axis of the globe. As a result they produce pure elevation and depression only when the eye is abducted by 23°. Either side of that angle some torsion is also produced: in a more adducted eye the superior rectus produces more intorsion and the inferior rectus more extorsion. In addition, the vertical recti have a horizontal component to their action.
VISUAL ACUITY AND AMBLYOPIA
Measurement of acuity (see Ch. 1) is critical in the assessment and treatment of amblyopia and strabismus. It should be measured both binocularly and uniocularly, with and without correcting glasses and with and without associated head posture, particularly if nystagmus is present. Testing acuity in children demands time and patience; the method depends on the child’s development (Table 18.2).
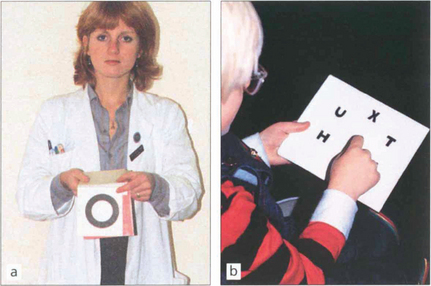
Fig. 18.5 The Sheridan–Gardiner test requires recognition rather than identification of letters and can be performed by cooperative children by the age of 2–3 years. The examiner has a flip-over book of the various Snellen sizes and the child identifies the letter. The main problem with this test is that acuity is overestimated because of the crowding phenomenon when acuity is better for single letters rather than rows.
BINOCULAR SINGLE VISION (BSV)
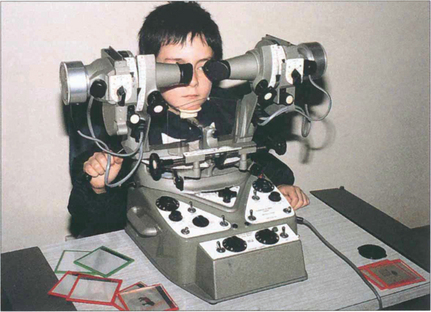
Fig. 18.6 The synoptophore can be used to assess the objective and subjective angle of horizontal, vertical and torsional strabismus. Each eye is dissociated and views an object that can be moved into each of the nine positions of gaze to test simultaneous macular perception, fusion, stereopsis and suppression. The objective angle of strabismus is measured by making the patient fixate each picture in turn and moving the tubes until there is no movement of the eye on fixation. This can also be measured by means of the prism cover test. The subjective angle is measured by letting the patient move the tubes so that he can perceive, for example, a bird in the cage. With normal retinal correspondence the subjective and objective angles are the same. With abnormal retinal correspondence the objective angle is the angle of deviation but the subjective angle is 0°.
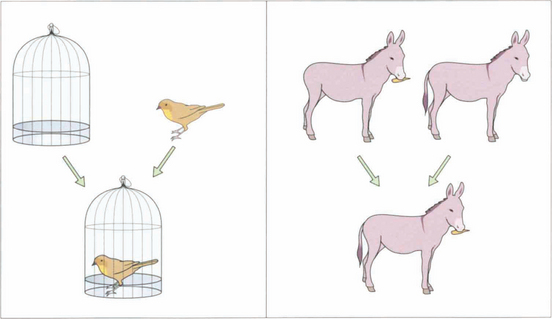
Fig. 18.7 To test simultaneous macular perception, different slides are presented to each eye (e.g. a bird and a cage). The patient is then asked to superimpose the images; for example, to put the bird into the cage. If the patient has no BSV, this will be impossible: the bird will jump from one side of the cage to the other (across the patient’s suppression scotoma). Sensory fusion is tested by getting the patient to superimpose two images that are similar but have uniocular controls. For example, one eye sees a donkey with no tail and a carrot, the other sees a donkey with a tail and no carrot. The tubes are converged and diverged until either the patient gets diplopia or one of the uniocular controls disappears. A normal person has a broad horizontal fusional range of 30Δ eso to 15Δ exo and a narrow vertical range of 3° (Panum’s fusional area). Diplopia is experienced outside these limits. Motor fusion is the ability to use both eyes to fuse similar images through a range of vergences (i.e. convergence and divergence, fusional convergence will control an exophoria and divergence an esophoria. It is usually tested with prisms but can also be tested on the synoptophore.
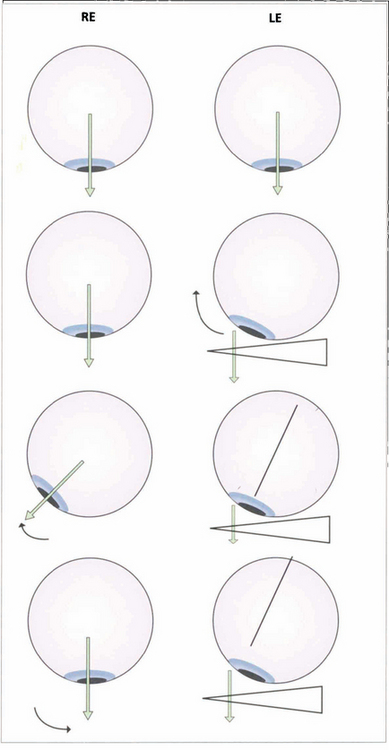
Fig. 18.8 Fusion can be tested with the 20D prism test in small children who cannot cooperate with the synoptophore. The diagram shows a 20D base-out prism being placed in front of the left eye so that the image is moved away from the fovea as the patient fixates a small target. The left eye adducts to maintain fixation. According to Hering’s law, the right eye will abduct but the child then appreciates diplopia and, to maintain BSV, makes an adducting movement of the right eye to overcome the effect of the prism.
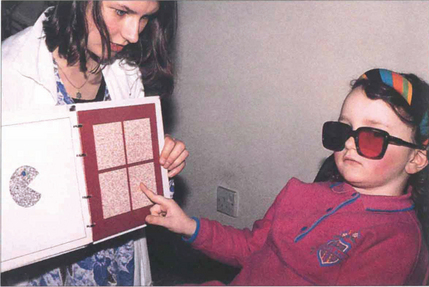
Fig. 18.9 There are a number of tests for stereopsis that rely on polarization or red-green goggles. The TNO test is a popular one in which the patient views an apparently random pattern of red and green dots through red and green glasses. The plates contain a stereo image and with stereopsis a geometrical shape is seen instead of the random pattern. This test has the advantage that it does not involve dissociation of the eyes and still works in colour-blind patients. Stereopsis is measured in seconds of arc; a person with normal vision can fuse more than 60 seconds of arc disparity.
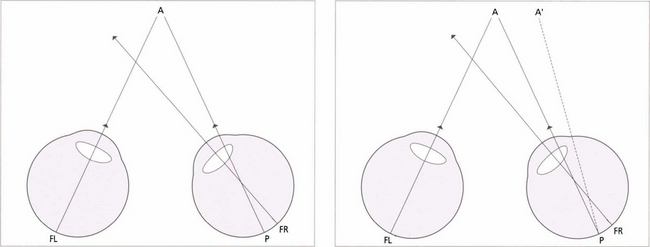
Fig. 18.10 Children with small-angle constant strabismus may develop an abnormal form of BSV known as abnormal retinal correspondence. (Left) This diagram of a small-angled constant right convergent strabismus shows an example of abnormal retinal correspondence: the fovea of the left eye (FL) corresponds to the pseudo-fovea (P) in the right eye, with the fovea of the right eye (FR) being suppressed when strabismus is of early onset. These patients tend to have some BSV despite their strabismus but there is usually mild amblyopia in the deviating eye and worse than 120 seconds of arc of stereopsis. (Right) In a late-onset strabismus the fovea of the right eye (FR) is not suppressed. The image falls at a point P in the right eye, it does not correspond to the fovea of the left eye (FL) and projects to A’. This results in diplopia.
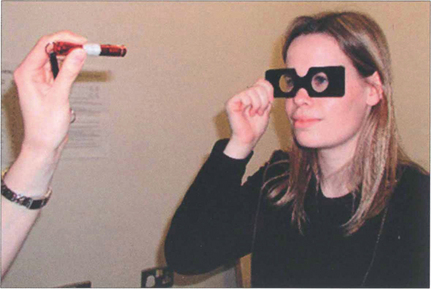
Fig. 18.11 Retinal correspondence is tested with Bagolini glasses which have micro-striations that produce perpendicular streaks of light perpendicular to each other in each eye when viewing a spotlight. A cross of light is seen with binocular vision; in the absence of strabismus this indicates normal retinal correspondence. When a cross of light is seen in the presence of strabismus this indicates abnormal retinal correspondence. If only one streak of light is seen this is consistent with suppression. If the patient sees two lights and two lines, diplopia is present. Worth lights, seen through red and green goggles, work on a similar principle.
AMBLYOPIA
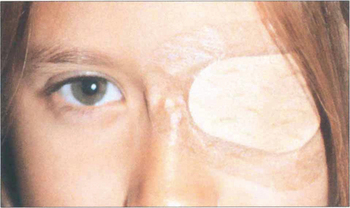
Fig. 18.12 The age at which treatment starts is critical to success: the younger the child, the speedier the response and the better the prognosis for visual improvement. Treatment usually has to be completed by 6–7 years of age, when the visual system matures. The mainstay of treatment is the correction of any refractive error followed by occlusion of the eye with better visual acuity to stimulate the amblyopic eye. The duration of occlusion and the length of treatment depend on the child’s age and response to treatment. Visual acuity must be monitored carefully as there is a real risk of making the good eye amblyopic (occlusion amblyopia). Atropine occlusion is a reasonable alternative for mild and moderate amblyopia.
DIAGNOSIS AND EVALUATION OF STRABISMUS
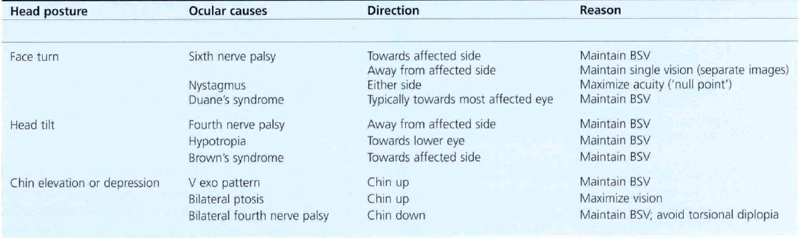
Strabismus is sometimes associated with other signs, the most common of which is an abnormal head posture. When examining for abnormal head posture it is important that the patient fixates on a distant target. A cover test should be performed with and without the head posture. Not all abnormal head postures are adopted for ocular reasons (e.g. torticollis, vestibular disease, habitual), but those that are usually help the patient to achieve a limited area of binocular vision (Table 18.3). Occasionally a head posture will be used to separate images or to fixate with a better seeing eye.
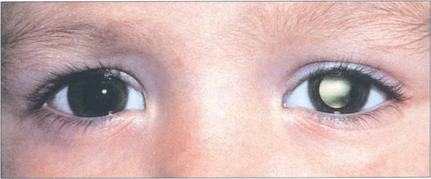
Fig. 18.13 It is extremely important to remember that strabismus is also commonly the presenting feature of structural eye disease. Most infantile strabismus is convergent. It is therefore important to beware of a constant exotropia in an infant—this may signify structural brain or ocular abnormalities. The most serious example of this is shown by this child with strabismus and leucocoria from a retinoblastoma. Exotropia is relatively common in children with hydrocephalus, intellectual impairment and inherited syndromes. Children presenting with an exotropia before the age of 1 year require both ophthalmic and neurological examination.
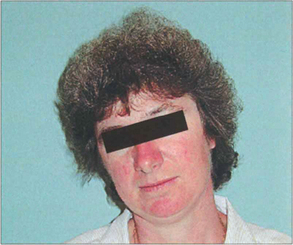
Fig. 18.14 Longstanding abnormal head postures of many years may cause secondary skeletal abnormalities such as scoliosis or facial asymmetry. This woman has relative maxillary hypoplasia associated with a congenital left fourth nerve palsy.
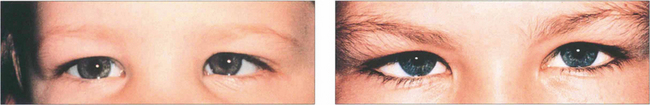
Fig. 18.15 Strabismus must be distinguished from pseudo-strabismus by demonstrating a manifest deviation on cover testing. Epicanthus is by far the commonest cause of misdiagnosed convergent strabismus. (a) A false impression of convergence is given, particularly on adduction, because the sclera is more covered medially. It should not be forgotten, however, that strabismus can coexist with epicanthus, as demonstrated in (b). Other causes of pseudo-strabismus include a wide interpupillary distance, facial asymmetry, enophthalmos and ptosis.
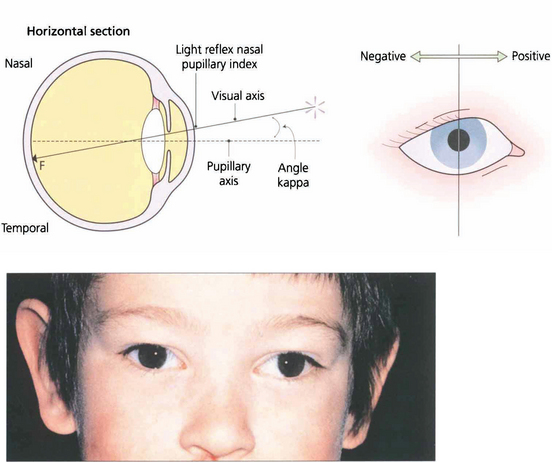
Fig. 18.16 Angle kappa is the angle between the visual axis and the line perpendicular to the cornea. It occurs because the fovea does not lie centrally on the anatomical axis but is usually slightly temporal to it; this accounts for the slightly nasal displacement of the corneal light reflex in a normal eye (normally about 4°). A large positive angle kappa, as in this child, gives an impression of divergent strabismus and a negative angle (which is unusual) denotes apparent convergence. The only way to distinguish between apparent and real strabismus is by a cover test. Angle kappa is also of considerable importance in corneal refracture surgery, which if centred on the pupillary axis can lead to post treatment aberrations and patient dissatisfaction.
THE COVER TEST
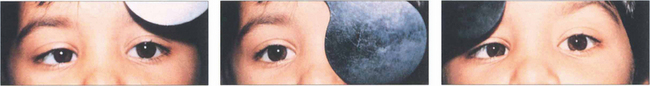
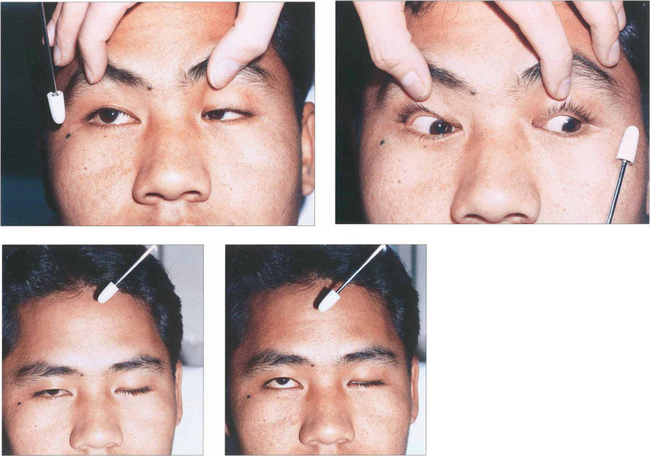
Fig. 18.17 This illustrates a cover–uncover test on a child with right esotropia. The fixating left eye is covered forcing the right eye to take up fixation (an interesting accommodative target should be used—a light is insufficient). On removing the cover the right eye does not hold fixation and fixation is preferentially taken up by the left eye again. It can be inferred that the left eye has better acuity than the right eye. The speed and accuracy of refixation gives information about the difference in acuity (faster with better acuity) and also shows the direction and degree of strabismus.
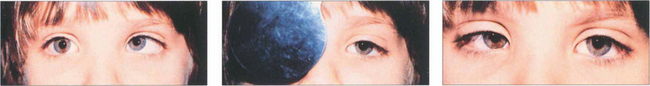
Fig. 18.18 In this child fixation changes from the right to left eye following cover and persists on uncover. This is called alternation; it signifies equal or nearly equal acuity in the two eyes.
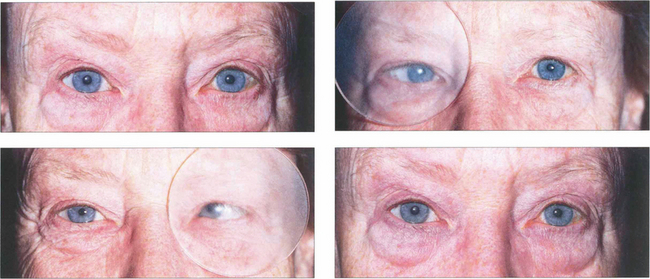
Fig. 18.19 With latent strabismus (phoria) the eyes are straight but tend to deviate if binocular reflexes are interrupted. An alternate cover test detects latent strabismus. In this patient with latent esophoria the right eye becomes convergent when occluded by the Spielman occluder (this is a frosted occluder that is sufficiently opaque to blur vision but allows eye position to be seen through the occluder). When the cover is rapidly transferred to the left eye the right eye refixates and the left becomes convergent. The cover must be moved from one eye to the other without allowing for refixation during the test; several passages are usually needed to dissociate the eyes fully and to reveal the full magnitude of the deviation. Removal of the cover restores binocular vision and allows the rate of binocular recovery and degree of control over the latent strabismus to be assessed. A cover–uncover test of each eye in turn should be performed initially to elicit a manifest strabismus followed by the alternate cover test. Cover tests should be performed for near and distant fixation, with and without an associated head posture, and with and without spectacles.
MEASUREMENT OF STRABISMUS
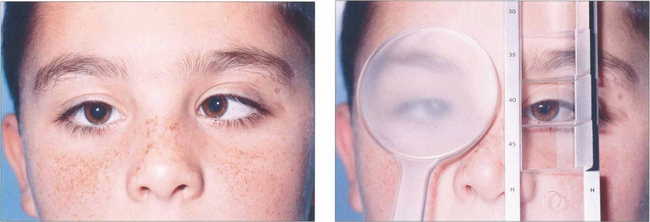
Fig. 18.20 In the prism cover test, a prism bar is used to increase the strength of the prism until there is no recovery movement on alternate cover testing. This is equal to the deviation, expressed in prism dioptres (1 prism dioptre (Δ)= ½° of angle). The test should be performed at 33 cm and 6 m and sometimes at 60 m (for intermittent exodeviations). If spectacles are normally worn the test should be performed both with and without them.
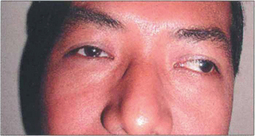
Fig. 18.21 Some patients because of age, disability or very poor vision in one eye, may not reliably fixate for a prism cover test. Under these circumstances the angle of strabismus can be estimated by noting the light reflex from a fixation light on the strabismic eye when fixating with the fellow eye. This is termed the Hirschberg test; 1 mm displacement of the light reflex equates to 15Δ or 7° of strabismus; if the light reflex is at the limbus this equates to about 70Δ of deviation.
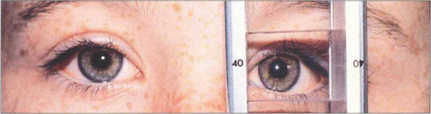
Fig. 18.22 The light reflex may be centralized on the deviated eye by prisms placed before the fixing eye. This is known as the Krimsky test. Its disadvantage is that it measures only the manifest component of the strabismus and does not take into account any latent element, thereby tending to underestimate the angle.
EXAMINATION OF EYE MOVEMENTS
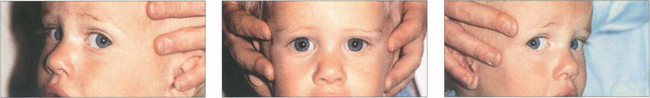
Fig. 18.24 Doll’s head movements are induced by the brainstem vestibular–ocular reflexes (see Ch. 19) and are demonstrated by turning the patient’s head manually while the patient looks at the examiner’s face.
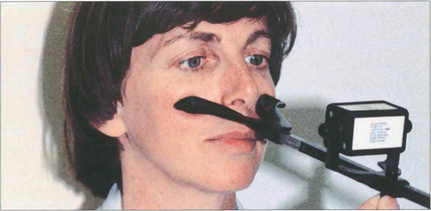
Fig. 18.25 Vergences are assessed by asking the patient to fix a target that is moved towards and away from the patient. The range of convergence and divergence may be measured on the synoptophore, with prisms or the RAF near-point rule (accommodometer), as shown in this photograph. The near-point of convergence is the distance at which diplopia is first appreciated; the near-point of accommodation is when the text becomes blurred.
CONCOMITANT STRABISMUS
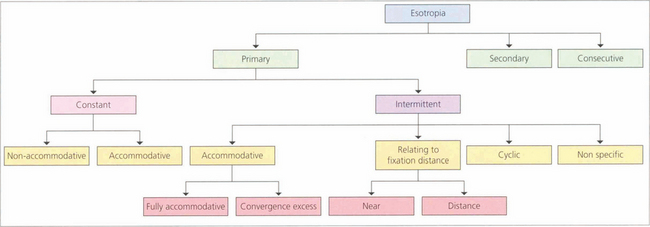
ESOTROPIA
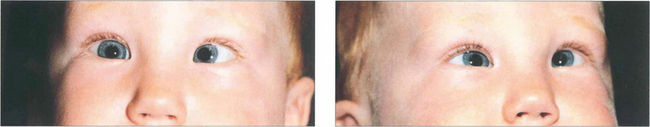
Fig. 18.27 Infantile esotropia (previously called ‘congenital’, although rarely present from birth) is a large-angle alternating esotropia presenting before 6 months of age. Infants usually cross-fixate: the left eye fixates objects to the right and right eye fixes to the left so that amblyopia is rare. Care must be taken not to confuse infantile esotropia with bilateral sixth nerve palsies; abduction may appear limited, but patching one eye or testing doll’s head movements should show that abduction is actually intact. Infantile esotropia may be associated with dissociated vertical deviation and latent nystagmus that develops later (at age 2–3 years). In latent nystagmus covering either eye induces a manifest nystagmus with the fast phase towards the side of the uncovered eye; visual acuity also drops so that binocular visual acuity is characteristically much better than uniocular acuity.
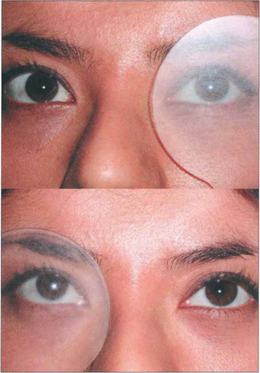
Fig. 18.28 Dissociated vertical divergence (DVD) is a phenomenon of unknown cause in which the occluded eye deviates upwards and outwards, often with excyclotorsion of the globe. On removing the cover the eye makes a recovery movement. The condition is usually bilateral but may be asymmetrical: it is usually more marked when the eye with poorer acuity is occluded. Common associations are infantile strabismus (usually esotropia) and latent nystagmus. The left eye of this patient with DVD elevates under the semi-opaque Spielmann occluder (this is very useful as both the occluded and the nonoccluded eye can be observed simultaneously). On moving the cover to the right eye the left eye drifts downward to take up fixation while the right eye elevates and extorts under the cover.
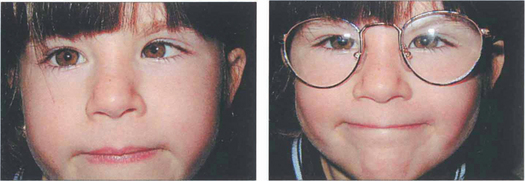
Fig. 18.29 A hyperopic patient needs to accommodate to see distant objects clearly. This girl is straight on distant fixation but breaks down to a left esotropia for near vision (left). Spectacles with the full hyperopic correction abolish the strabismus (right), which is therefore a fully accommodative esotropia that will be controlled by spectacles.
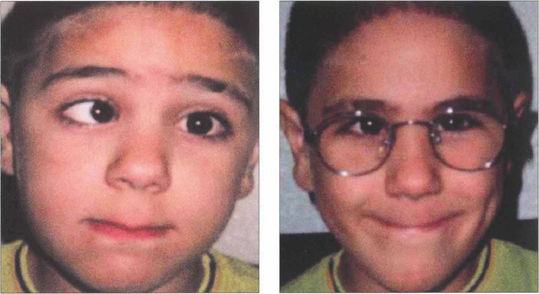
Fig. 18.30 Strabismus that is only partially corrected with the full hyperopic correction is known as partially accommodative strabismus. It is important to make sure that the full cycloplegic correction is prescribed before labelling a strabismus as partially accommodative. In these cases the residual angle is assessed: if it is small, cosmesis is often good and abnormal retinal correspondence may develop; if the residual angle is large and cosmetically unsatisfactory surgery may be required. Surgery to correct the full angle of strabismus without taking into account the hyperopic spectacle correction will result in exotropia of the patient still needs to wear their glasses.
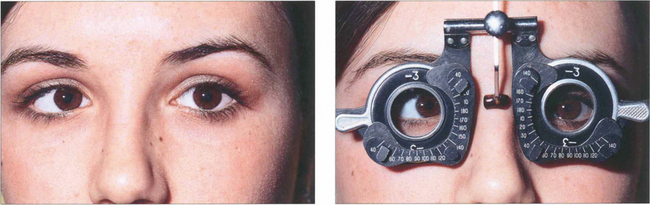
Fig. 18.31 Convergence excess esotropia occurs when accommodative effort results in esotropia or increased esotropia (i.e. the deviation is greater at near than at distance). Convergence during accommodation can be measured by the AC:A ratio, the amount of convergence induced by each dioptre of accommodation (expressed in prism dioptres per dioptre). A commonly used method to measure this is the gradient method. A prism cover test is performed and the deviation is measured at 6 m with and without –3.0 d lenses. These are assumed to induce 3 d of accommodation. The ratio is calculated by dividing the change in deviation by the 3 d of accommodation. The normal range is 2–4:1; a high ratio indicates excessive convergence for a given amount of accommodation. This is of particular importance in planning surgery.
EXOTROPIA
Fig. 18.32 Exotropia, like esotropia, can be primary or secondary to poor vision or other ocular problems. Consecutive exotropia usually arises in patients who have had previous esotropia with inadequate binocular vision, that allows the eye to drift out over time. Patients with a consecutive exotropia presenting for cosmetic strabismus surgery must be assessed carefully using prisms and, if necessary, botulinum toxin to exclude loss of suppression in primary gaze as surgery may result in constant diplopia. Intermittent exotropias are relatively common and many people, particularly myopes, have a divergence excess exophoria that tends to manifest with tiredness or when unwell. Myopic correction often helps.
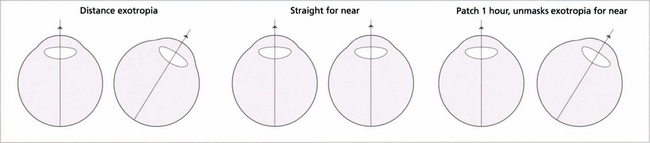
Fig. 18.33 This patient has a distance exotropia. A cover test indicates that the eyes are straight for near but not for distance. The question is whether the lack of near deviation is due to its control by using the accommodation reflex. Clinically, ‘simulated’ and true distance exotropias look identical; they can be distinguished by measuring the AC:A ratio or by occluding one eye for at least 1 hour to eliminate fusion. A simulated distance exotropia will then decompensate for near when accommodation that has been controlling the near deviation is relieved (high AC:A ratio) or when fusional stimulation is prevented. In true distance exotropia, which is less common, the angle is unchanged despite interrupted accommodation and fusion and the AC:A ratio is normal. Differentiation of true from simulated exotropia is extremely important when planning treatment.
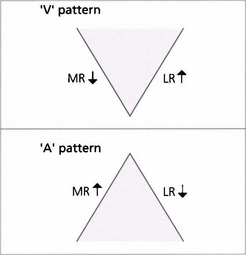
A AND V PATTERNS
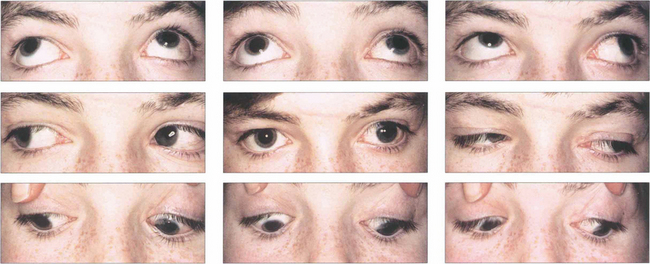
Fig. 18.34 This girl has a manifest exotropia that increases markedly on upgaze in a V pattern. In addition, on horizontal gaze the adducting eye elevates compared with the abducting eye, signifying inferior oblique overaction. Such a pattern could lead to a ‘chin up’ head posture to maintain single vision. Bilateral inferior oblique muscle weakening procedures may be required in addition to horizontal rectus surgery to correct the exotropia.
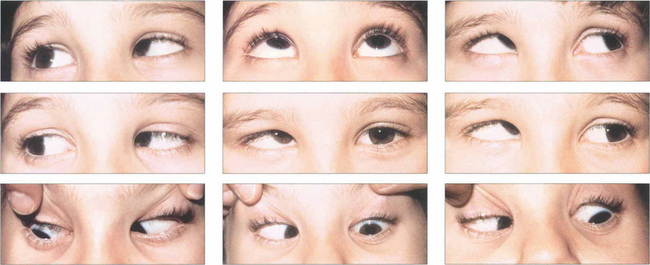
Fig. 18.35 This child has a marked V esotropia with right hypertropia in the primary position. This is probably the commonest type of A or V deviation. On upgaze the eyes are almost straight but the esotropia increases in downgaze. On horizontal gaze both inferior oblique muscles overact, the right more than the left. Such a pattern would lead to a ‘chin down’ head posture to maintain single vision. Even where there is asymmetry it is advisable to weaken both overacting inferior obliques at the time of surgery.
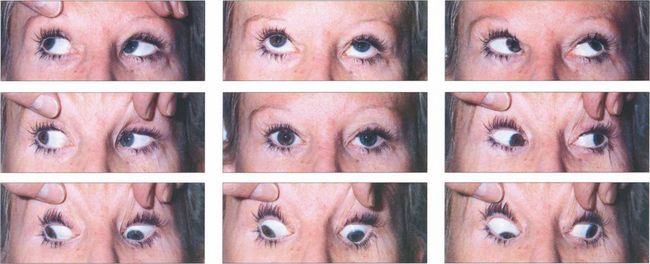
Fig. 18.36 A patterns are less common than V patterns. This patient has an A-pattern exotropia following previous surgery. She is divergent on depression but straight in the primary position, so there is no abnormal head posture. She has overaction of both superior oblique muscles, more on the right than the left.
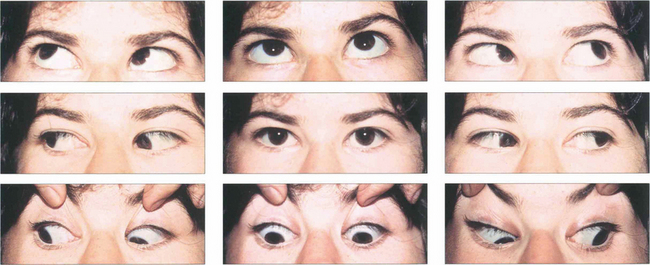
Fig. 18.37 This patient’s eyes are straight in primary gaze and marginally divergent in downgaze with esotropia on upgaze. There is bilateral superior oblique overaction, best seen by the downdrift of the adducting eye in both right and left depression. The adducting eye is also slightly hypotropic on right and left elevation (underaction of the inferior obliques).
INCOMITANT STRABISMUS
Table 18.5 lists common causes of incomitant strabismus classified by site of lesion.
Table 18.5 Common causes of incomitant strabismus classified by site of lesion
| Site of lesion | Examples | Common causes |
|---|---|---|
| Internuclear | Internuclear ophthalmoplegia | Demyelination, rnicrovascular, tumour |
| Cranial nerve palsies | Third nerve palsy | Aneurysm (with pupil involvement), diabetes or rnicrovascular (without pupil involvement), trauma (often with skull fracture), congenital |
| Fourth nerve palsy | Congenital, rnicrovascular, trauma (deceleration injury), | |
| Sixth nerve palsy | Raised intracranial pressure, inflammation (e.g. arteritis), trauma, tumour, microvascular, demyelinatton | |
| Multiple cranial nerve palsies | Orbital apex lesions, cavernous sinus lesions, meningitis, intrinsic brainstem lesions | |
| Neuromuscular junction | Myasthenia gravis | Can mimic almost any ocular motility problem (but does not effect pupillary action) |
| Muscular | Thyroid eye disease, progressive external ophthalmoplegia, orbital myositis, | |
| Orbital | Trauma | Orbital blow-out fracture |
| Fibrosis | Thyroid eye disease, scarring from previous surgery (e.g. retinal detachment surgery) | |
| ’Musculofascial’ syndromes | Brown’s syndrome, Duane’s syndrome |
DIPLOPIA
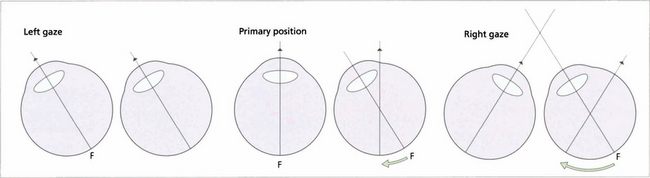
Fig. 18.39 This diagram illustrates an incomitant strabismus from a right sixth nerve palsy. On left gaze the ocular axes are aligned and there is no diplopia. When looking straight ahead the right eye becomes slightly esotropic; the left fovea is stimulated as normal but the target stimulates a nasal retinal point in the right eye resulting in diplopia. The angle between the two eyes increases on right gaze and the images separate more. On cover testing the more peripheral image ‘belongs’ to the eye with the underacting muscle. Diplopia is always maximal in the direction of action of the affected muscle. In this position, the image from the eye with the muscle palsy will always be more peripheral because retinal projection is at its most disparate; it will also be less distinct as the retinal area being stimulated is less sensitive than the fovea. For this reason patients occasionally cope with diplopia by adopting a head posture which produces maximal separation of the images.
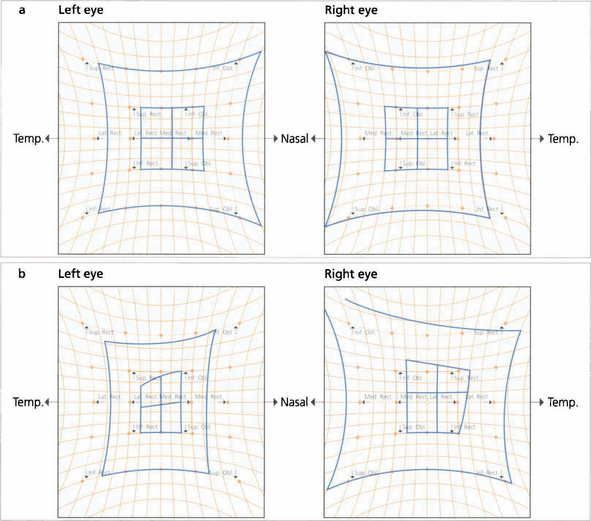
Fig. 18.40 These Hess charts show a left sixth nerve palsy secondary to a cavernous sinus aneurysm (the smaller squares belong to the affected eye; one small square is equivalent to 5°). Six months later the sixth nerve palsy has increased and there is now limitation of the medial and superior recti, suggesting early involvement of the third nerve.
Fig. 18.41 Another simple and useful method of monitoring a patient’s progress is to plot the field of binocular single vision with the head fixed using an arc perimeter.
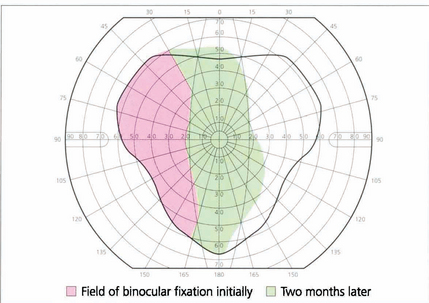
Fig. 18.42 Serial plotting of the field of binocular vision shows improvement in the area of single vision in a patient with right lateral rectus palsy after 2 months. Serial fields complement the diagnostic information of a Hess chart as they give some idea of the patient’s functional visual field.
MECHANICAL (RESTRICTIVE) STRABISMUS
Brown’s syndrome
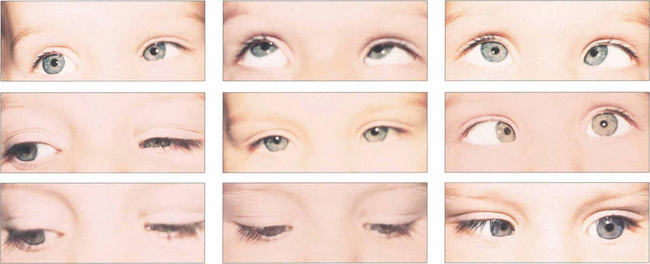
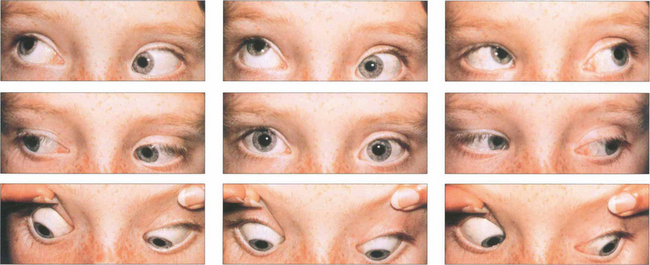
Fig. 18.43 Patients may present with a compensatory face turn to the affected side or with an esotropia with suppression and amblyopia. Ocular movements in a left Duane’s syndrome demonstrate limitation of abduction with widening of the palpebral aperture. Left adduction is associated with narrowing of the palpebral aperture and retraction of the globe. Surgery is rarely of value but is sometimes done for abnormal head postures or large up- and down-shoots. Eye movements always remain abnormal and patient expectations must be realistic.
‘Blow-out’ fractures
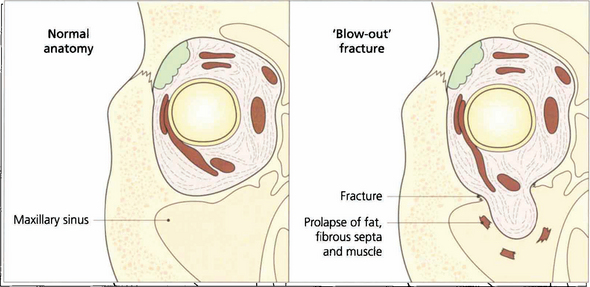
Fig. 18.45 The normal anatomy of the orbital walls can be compared to that following a ‘blow-out’ fracture. Pressure from a direct blow to the eye is transmitted through the orbital contents to the walls which fracture at their thinnest place. Orbital fat, fibrous septa and even extraocular muscle may become trapped in the fracture, restricting rotation of the globe. Intraocular damage is common (see Chs 8, 11, 12 and 14).
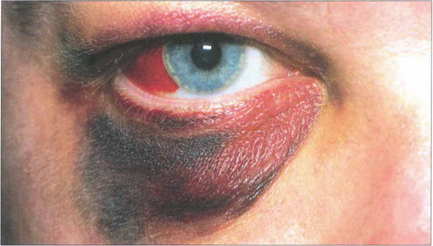
Fig. 18.46 Lid and subconjunctival haematomas with no posterior limit following blunt injury to the orbit suggest the possibility of a blow-out fracture. In this patient blood has accumulated forward from an orbital floor fracture site. Air may enter the lids from the surrounding paranasal sinuses, especially on blowing the nose, to cause crepitus on palpatation. In blow-out fractures of the orbital floor the infraorbital nerve may be contused and cause numbness of the cheek and incisor area of the upper jaw. Intraocular damage should be excluded in every patient.
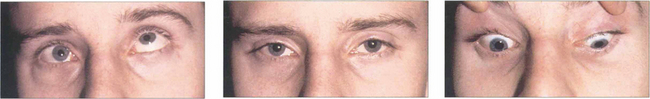
Fig. 18.47 This patient has a blow-out fracture of the right orbital floor. The right eye is slightly enophthalmic in the primary position. It is restricted in its excursion in both attempted elevation and depressions. The cause is usually not incarceration of the inferior rectus muscle in the fracture site but trapping of the fibrous orbital septa connected to the inferior rectus. The patient has vertical diplopia with a small central area of binocular vision.
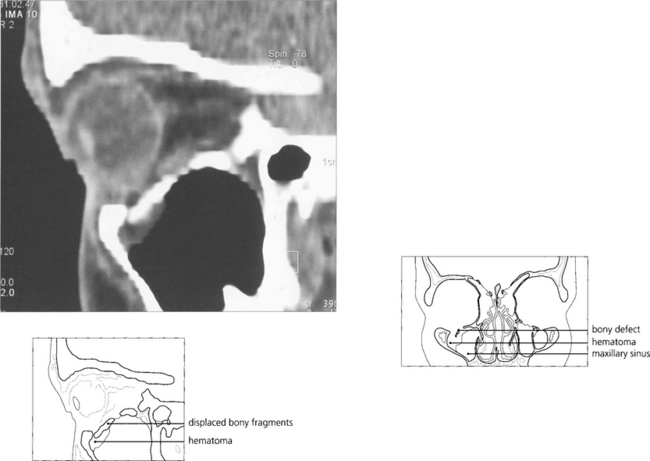
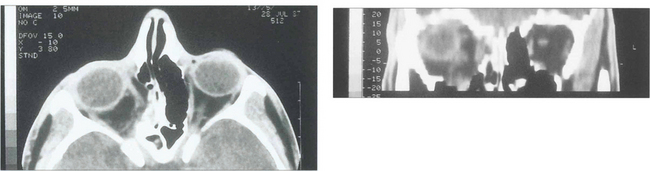
Fig. 18.48 Coronal computed tomography with bony windows is the investigation of choice to demonstrate the fracture and extent of soft tissue displacement.
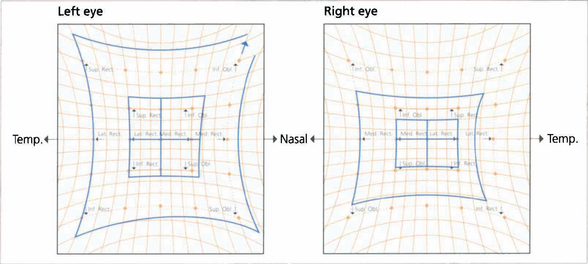
Fig. 18.49 Hess charts demonstrate the restriction of movement. Their use is a good way of demonstrating whether or not ocular motility is recovering.
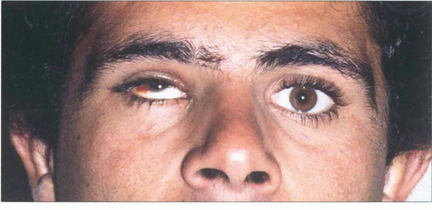
Fig. 18.50 This patient, a Spanish bullfighter, was hit in the right eye by the horn. He has gross enophthalmos, as can be seen from asymmetry of the palpebral apertures. There was gross restriction of horizontal movement. In most patients medial wall fractures are less symptomatic because of the greater ability to fuse horizontal rather than vertical disparity and the ease of compensatory head movement.
CRANIAL NERVE PALSIES
THIRD NERVE PALSY
Table 18.6 lists features of third nerve palsy. Bilateral third nerve palsy is rare and is usually seen with pituitary apoplexy or uncal herniation from raised intracranial pressure and coning.
| Localization | Diagnostic features |
|---|---|
| Nuclear | Bilateral ptosis, contralateral superior rectus palsy ± mydriatic pupil |
| Fascicular | Contralateral hemiplegia ± contralateral ataxia (superior cerebellar peduncle) |
| Subarachnoid space | All muscles ± pupil sparing |
| Cavernous sinus | Associated fourth, fith and sixth nerve palsies, Horner’s syndrome, aberrant regeneration, bilateral |
| Apex, orbital | Superior or inferior divisions ± proptosis |
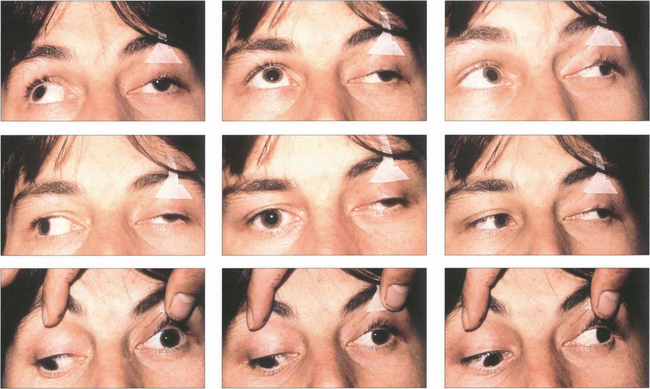
Fig. 18.52 Ocular motility in a patient with a left third nerve palsy. In the primary position the globe is divergent from the unopposed action of the lateral rectus. There is absent adduction, elevation and depression of the left eye.
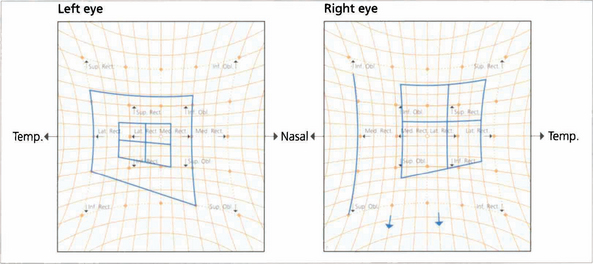
Fig. 18.53 The Hess chart of a patient with a partial left third nerve palsy shows underaction of the affected muscles and corresponding overaction of the contralateral synergists (owing to Hering’s law).
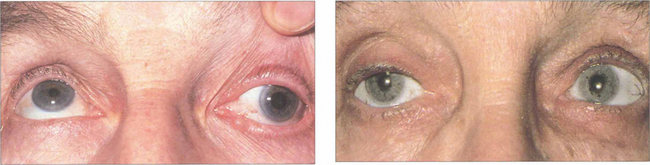
Fig. 18.54 Careful pupillary examination is mandatory in all patients. Pupillary dilatation with an isolated left third nerve palsy (left) indicates compression by a posterior communicating aneurysm and a substantial risk of subarachnoid haemorrhage (see Ch. 19). Emergency magnetic resonance angiography is required. An isolated left third nerve palsy in an elderly patient with pupil sparing (right) is due to microvascular ischaemia (hypertension, diabetes, atherosclerosis); these patients do not require neuroimaging. Such palsies recover spontaneously over 3–4 months. Both types of palsy can be painful.
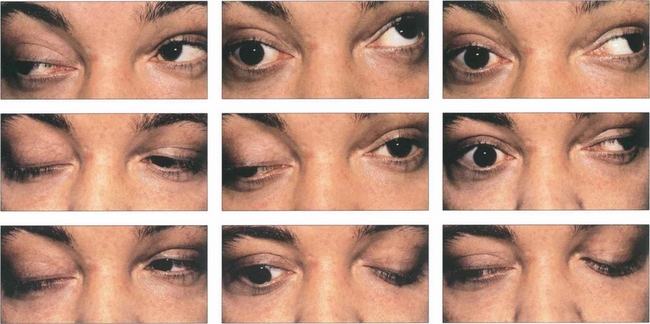
Fig. 18.55 In some cases of third nerve palsy, particularly those due to trauma or chronic compression from aneurysms or meningiomas and in some congenital cases, recovery of the palsy may lead to ‘aberrant regeneration’. Aberrant regeneration is never seen with microvascular lesions. In the absence of a history of these conditions (primary aberrant regeneration), aberrant regeneration is a feature of slowly compressive cavernous sinus lesions. Typically there is constriction of the pupil on looking into the direction of gaze of the affected muscle or lid elevation on downgaze (the pseudo-von Graefe sign) or adduction. This patient with a right third nerve palsy shows lid elevation on attempted left medial gaze and downgaze.
FOURTH NERVE PALSIES
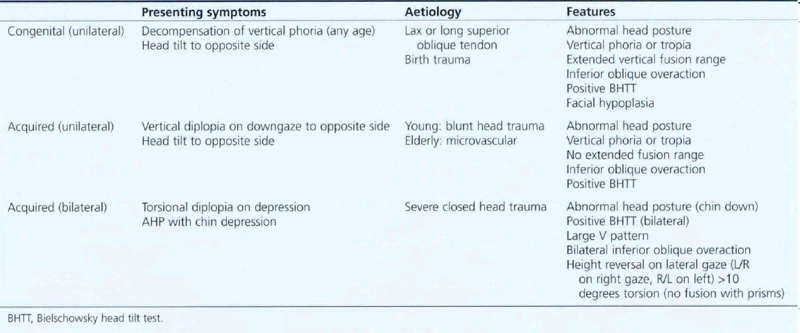
Fourth nerve palsies may be congenital or acquired and unilateral or bilateral; it is important to think of these as different entities as they have different presenting symptoms, causes and clinical features (Table 18.7).
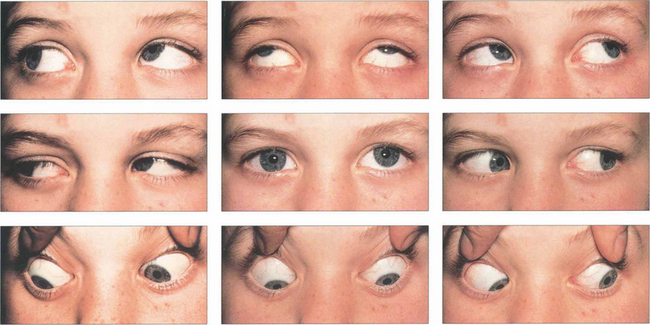
Fig. 18.56 This patient has a left fourth nerve palsy. There is underaction of the left superior oblique on right depression and associated overaction of the left inferior oblique on right gaze due to unopposed action.
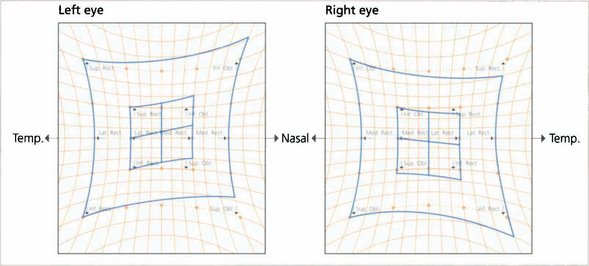
Fig. 18.57 The Hess chart shows underaction of the left superior oblique, overaction of the left inferior oblique (unopposed ipsilateral antagonist), overaction of the right inferior rectus (contralateral synergist) and underaction of the right superior rectus (contralateral antagonist), indicating a longstanding palsy with fully developed ocular motility sequelae.
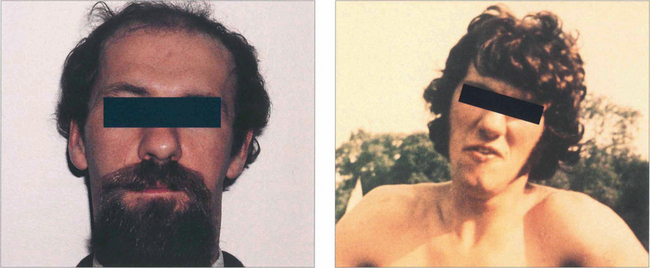
Fig. 18.58 Clues to a longstanding congenital aetiology are an increased vertical fusion range and old photographs showing a longstanding abnormal head posture, as in this patient. Congenital palsies frequently decompensate to a manifest tropia. Patients have an associated head posture tilting away from the side of the palsy; chin depression and a face turn to the opposite side are less consistent. Hypoplasia of the face ipsilateral to the tilt may be present.
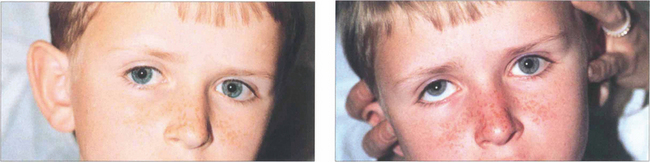
Fig. 18.59 In the Bielschowsky head-tilt test the patient is asked to fixate on a target at 3 metres and the head is tilted to each side (Table 18.8). This stimulates intorsion of the inferior eye. If the superior oblique is weak, the superior rectus (the other intorter of the globe) is unopposed and will elevate the eye to cause up-shoot of the affected eye, seen as increased vertical separation of diplopia or increased hypertropia on cover testing. Here, a positive finding on a head-tilt test to the right confirms a right superior oblique palsy. Note that the patient has an abnormal head tilt to the left in the primary position to compensate for this.
Table 18.8 Diagram of mechanism of Bielschowsky test in the presence of right superior oblique palsy
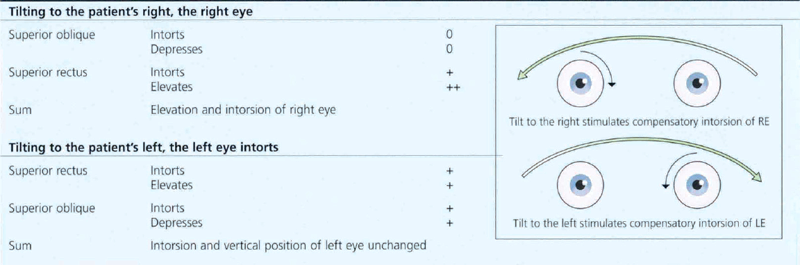
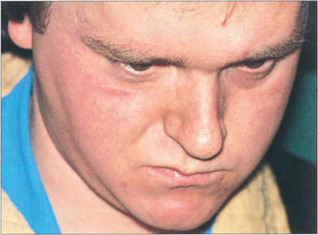
Fig. 18.60 Bilateral fourth nerve palsies are almost invariably due to severe closed head injuries in which the nerve rootlets are ruptured by contusion as they decussate superiorly over the midbrain; such patients usually have other serious neurological damage. Patients have difficulty in reading and close work as the unopposed action of the inferior recti on downgaze causes excyclotorsion of the globes. Patients may obtain some benefit from a chin-down posture to reduce ocular depression. Symptoms are often severe in the absence of gross ocular deviation and the condition is frequently misdiagnosed as due to loss of BSV from head injury or compensation seeking. Asymmetry is common and it is advisable to suspect bilateral damage in apparently unilateral cases. Torsion needs to be measured on the synoptophore. This patient has to maintain a marked chin-down posture to be able to read following a severe head injury; note the tracheostomy scar.
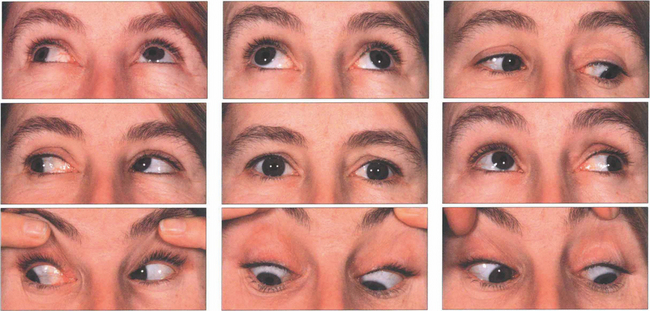
Fig. 18.61 Bilateral fourth nerve palsies. Note mild underaction of both superior obliques in downgaze worse on the right than left. There is some evidence of inferior oblique overactions, which can produce an alternating hypertropia on right and left gaze. The patient had 8° excyclotorsion in the left eye and 12° in the right eye. This can be corrected by laterally advancing the anterior fibres of the superior oblique in each eye (the Harado–Ito procedure).
By courtesy of Mr J Anderson.
SIXTH NERVE PALSY
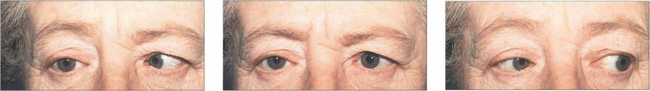
Fig. 18.62 This patient has a right sixth nerve palsy. Isolated sixth nerve palsies are common. In elderly patients they are often due to microvascular ischaemia from hypertension, diabetes or arteriosclerosis; such patients require vascular investigation. The palsy usually resolves over 3 months; in other patients and where the palsy does not resolve spontaneously, magnetic resonance imaging is required. Temporal arteritis must be considered in any patient aged over 60 years and vasculitis in younger patients. Isolated palsies can also be seen with multiple sclerosis and following viral illness in children.
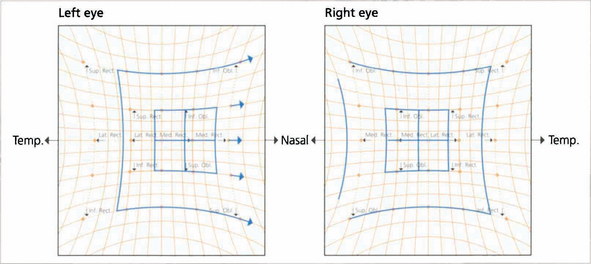
Fig. 18.63 The Hess chart shows limitation of the left lateral rectus and overaction of the ipilateral medial rectus. The eyes are convergent. With longstanding palsy the deviation becomes more concomitant with associated three-muscle sequelae.
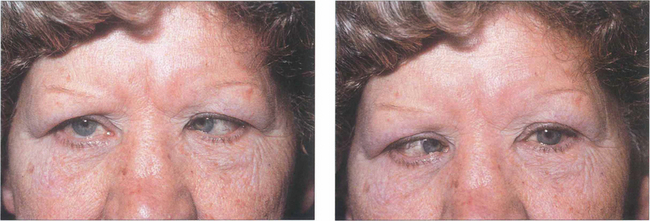
Fig. 18.64 Convergence spasm can simulate unilateral or bilateral sixth nerve palsy. The aetiology is nonorganic. Excessive convergence limits the ability to abduct, diplopia is usually spasmodic (maintaining excessive convergence requires a lot of effort) and the clue to diagnosis lies in noting pupillary miosis with the attacks.
COMBINED THIRD, FOURTH AND SIXTH NERVE PALSIES
Space occupying lesions involving the cavernous sinus are likely to produce palsies of more than one oculomotor nerve owing to the close proximity of the nerves. These lesions may present as a progressive isolated sixth or partial third nerve palsy with progressive involvement of other nerves. Cavernous sinus lesions are often painful. Patients may also have involvement of the first and second divisions of the fifth nerve, Horner’s syndrome or field loss from expansion up into the chiasm. Common causes are invasive nasopharyngeal carcinoma, metastases, meningiomas and aneurysms of the internal carotid artery. Primary aberrant regeneration of the third nerve is pathognomic of a slowly expanding cavernous sinus lesion (see Fig. 18.55). Lesions at the orbital apex will also cause oculomotor palsies with the addition of optic nerve signs; it may be difficult to differentiate the neurological effects from the mechanical effects of the lesion (see Ch. 20).
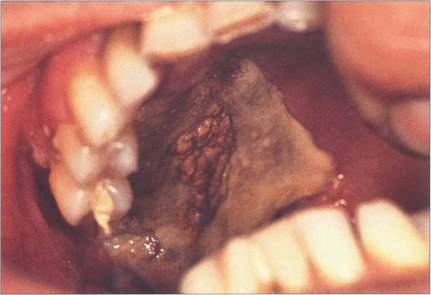
Fig. 18.65 Mucor mycosis is a rare cause of a cavernous sinus syndrome, seen most commonly in diabetics with ketoacidosis and rapidly progressive ocular motor palsies from obliterative invasion of the arterial supply by the fungus. This patient has necrosis of the hard palate.
By courtesy of Mr W A Green.
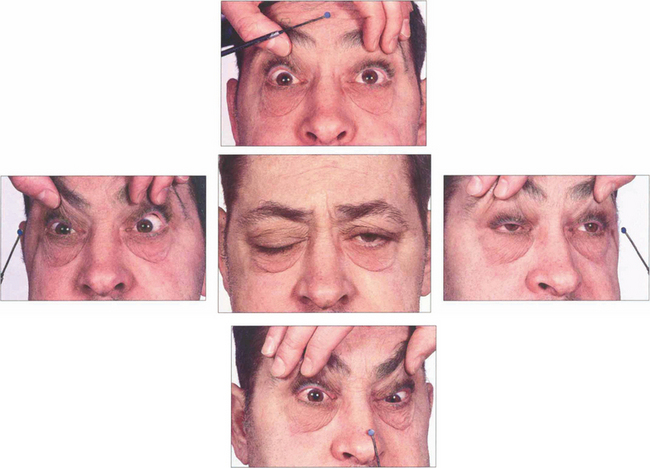
Fig. 18.66 This patient had a nasopharyngeal carcinoma invading the base of the skull and cavernous sinus. He has bilateral sixth nerve palsies and bilateral third nerve palsies, more marked on the right than the left. There is a right fourth nerve palsy but the left fourth nerve is intact allowing some depression and intorsion of the left eye.
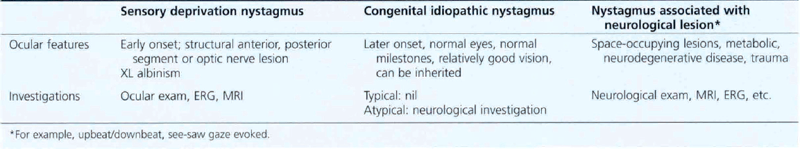
CONGENITAL NYSTAGMUS
Congenital nystagmus is present by 6 months of age. It has a pendular horizontal waveform in primary gaze that becomes jerky in eccentric gaze. Amplitude is the same in both eyes, it can have a rotatory (but not vertical) component and patients do not get oscillopsia. Various factors (Table 18.9) affect the nystagmus: it may have a ‘null point’ where amplitude is diminished which can be exploited by assuming a certain head posture (usually a face turn) to improve acuity when concentrating; it is dampened by convergence so that patients may tend to hold objects close to them. This improves reading vision and children can almost always cope with normal schooling. They may sometimes develop a variable esotropia (nystagmus blockage syndrome) using convergence to suppress the nystagmus. Head shaking may improve vision (spasmus nutans); and optokinetic stimuli characteristically cause an ‘inverted’ OKN with beats in the direction of the stripe.
Table 18.9 Features of congenital nystagmus
There are various types of congenital nystagmus (Table 18.10). In infantile esotropia latent nystagmus is seen on occlusion of one eye with the fast phase beating away from the occluded eye. This can become manifest if there is sensory deprivation of one eye for some reason.