Chapter 47 Stereotactic Radiosurgery of Vascular Malformations
• Regardless of modality, radiosurgical devices achieve the desired characteristics of small fields, fast-dose falloff, and highly accurate targeting through the use of two basic principles: superposition of beams and stereotactic targeting.
• The goal of radiosurgery for arteriovenous malformations is to deliver a high absorbed radiation dose to the nidus, while largely sparing the surrounding normal brain tissue. Endothelial damage followed by subendothelial and intimal-medial proliferation of smooth muscle cells and subsequent cellular degeneration and hyaline transformation eventually obliterate the nidus.
• The reported obliteration rate following radiosurgery ranges between 60% and 90%. The outcomes of radiosurgery for arteriovenous malformations are dose and volume dependent. The role of preradiosurgical embolization remains to be fully defined.
• Complications associated with radiosurgery for arteriovenous malformations include symptomatic radiation-induced brain damage in 8.7% (permanent in 1.8%), late cyst formation in 1.6%, and radiation-induced tumor within 10 years following radiosurgery in 0.7%.
• Radiosurgery has a smaller role for the treatment of dural arteriovenous fistulas owing to its delayed effects. For patients with cavernous malformations, radiosurgery should be used primarily in compelling cases of repeated hemorrhages from a lesion located in an inoperable site.
Arteriovenous Malformations
Management
Three management modalities are employed alone or in combination for the management of cerebral AVMs: microsurgery, embolization, and radiosurgery. Several factors should be weighed when choosing the most appropriate choice of treatment. Microsurgery should be used in superficially located nidi because it eliminates the risk of hemorrhage immediately upon a complete total extirpation. Embolization is most commonly used to reduce the size of large AVMs, making them amenable to subsequent microsurgery or radiosurgery. However, new liquid embolization materials (e.g., Onyx) have increased the chance of obliteration solely by embolization.1,2 Radiosurgery is usually reserved for small to moderate-sized AVMs located in deep or critical areas of the brain.
Role of Radiosurgery
History
The first case of AVM treated with radiotherapy was reported by Magnus.3 In a patient he operated on with an AVM at the motor cortex, he did not attempt surgical removal because of the high possibility of neurological deficits. After decompressive craniectomy, he treated the patient with radium therapy and reported that the patient was seizure-free 2 years after radiotherapy. No imaging or histological studies were available. Cushing and Bailey reported the first successful surgery on an AVM.4 Cushing explored a vascular tumor and felt that the lesion could not be attacked without fatal hemorrhage. The patient was treated with radiotherapy. He reexplored the lesion 3 years later and described that the tangle of pulsating vessels previously encountered was largely thrombosed and transformed into a multitude of small bloodless shreds which could be easily separated from the adjacent normal cortex.
There was intense interest in the use of radiation for AVMs following Cushing’s discovery, but the initial results were not encouraging. Although some studies5,6 with small numbers of cases provided evidence of the possible utility of radiation in the treatment of AVMs, most did not provide imaging or histological proof of the efficacy of radiotherapy. This led to an almost unanimous consensus in the assessment of radiation as being worthless in the management of AVMs.
With the introduction of Gamma Knife, the potential value of irradiation in vascular malformations was reassessed. Contributing factors included an increasing body of evidence that the cells constituting the vessel wall were responsive to ionizing radiation. Long-term angiographic follow-up of a small series of AVMs treated with fractionated conventional radiation by Johnson in the 1950s revealed that the AVMs were obliterated in 45% of cases.7 In 1970, the first radiosurgical treatment for an AVM was performed by Ladislau Steiner and associates at Karolinska Institute in Stockholm. The patient refused surgery, and given the patient’s renal insufficiency, the risk of surgery was considered too high. Although the intention was to deliver focused radiation to the nidus, because only small collimators were available, the feeding arteries were targeted and 25 Gy was given as the prescription dose to the 50% isodose line. On angiography 19 months after the treatment, the feeding vessels were obliterated, and the malformation no longer filled. Subsequently, larger collimators were available and could cover the AVM nidi, and more patients were treated successfully. Today more than 50,000 AVM patients have been treated with Gamma Knife radiosurgery, which has been proved to be a safe and effective treatment alternative for AVMs.
Modality
The goal of radiosurgery for AVMs is to deliver a high absorbed radiation dose to the AVM nidus, typically in a single session, while largely sparing the surrounding brain significant dose and thereby minimizing undesirable effects from the treatment. As the number of modalities for delivering radiosurgery have increased over time, so have the numbers that have been applied to the treatment of AVMs. Reports in the literature exist for AVM treatment with various radiosurgical modalities including the Gamma Knife, isocentrically mounted linear accelerators,8–11 and robotic linear accelerators (CyberKnife).12 Regardless of modality, radiosurgical devices achieve the desired characteristics of small fields, fast-dose falloff, and highly accurate targeting through the use of two basic principles: superposition of beams and stereotactic targeting.
The details of beam superposition vary by modality. In the case of the Gamma Knife, 192 (or 201, depending on the model of the unit) individual beams are precisely aimed at a focal point, or isocenter, to achieve this effect. The beams are collimated through individual beam channels. In older units, this was achieved with a combination of internal primary and secondary collimation and external “helmet”-based final collimation. In the case of the newer Perfexion model Gamma Knife, the collimator assembly is housed entirely within the main body of the unit.13
For early linear accelerators adapted for radiosurgery, finely collimated beams were achieved through the use of circular collimator “cones” that could be attached to the accessory tray of the accelerator.14 Many currently available accelerators are equipped with micro-multileaf collimators that can achieve irregularly shaped beams that can more precisely conform to the target morphology.15 Both approaches are often used with a non-coplanar arc technique, which directs the fields at the target while spreading out the overall delivered energy.16
The ability to create a focal, high-dose distribution does little good in itself if there is no way to precisely and accurately aim at the target. This problem is elegantly solved using the principles of stereotaxy. Traditionally in intracranial radiosurgery a rigid frame is fixed to the skull. This frame defines a coordinate system by which any point within the brain can be localized. Fiducial markers, which are visible in stereotactic imaging studies as part of the procedure, are directly related to this coordinate system; thus, the target can be visualized and localized in “stereotactic space.” Accuracy and precision of treatment are thus guaranteed by the precision and accuracy with which the target can be localized and the assurance that this target will not move during a treatment owing to the rigid head frame. More recent innovations in radiosurgery have included frameless stereotaxy.17 In these systems image-guidance plays a greater role both before and during the procedure. Less invasive restraint systems such as thermoplastic masks are used in place of the rigid head frame, and periodic imaging is used to track and correct for patient motion.
Histopathology
Several histological studies have described the changes of irradiated vessels with progressive narrowing and obliteration of the lumen.18 The earliest changes are endothelial damage and endothelial-intimal separation. These are followed by subendothelial and intimal-medial proliferation of smooth muscle cells with elaboration of extracellular matrix components. Cellular degeneration and hyaline transformation of vessel walls follow, and finally the vessels obliterate completely. The above-mentioned histopathological changes are correlated with time after radiosurgery and tend to occur in smaller vessels.
Gamma Knife Radiosurgery
Treament Planning
Treatment planning is the process of creating a dose distribution that conformally covers the intended target by defining one or more isocenters, or “shots,” which each contribute to the total dose distribution. It is an iterative technique requiring detailed knowledge of neuroanatomy, neuroradiology, the biological effect of single-fraction radiosurgery, and the compromises required to create a treatment that will be effective to obliterate the nidus and at the same time tolerable for the surrounding brain structures. Optimal prescription dose has been described by Steiner and associates within the range of 23 to 25 Gy.19 However, in cases with large nidi or nidi close to critical structures, the dose needed to be adjusted.
Outcomes
Following radiosurgery, angiography reveals that hemodynamic changes occur before changes in the size and shape of an AVM.20 First, the flow rate decreases progressively. This may be related to the changes in the sizes of the feeding arteries and outflow veins. The outcome of an AVM following radiosurgery may be a total, subtotal, or partial obliteration of the nidus.
Total obliteration of the AVM after radiosurgery was defined as “complete absence of former nidus, normalization of afferent and efferent vessels, and a normal circulation time on high-quality rapid serial subtracted angiography.”20 Any remaining nidus, regardless of its size, is considered partial obliteration. Subtotal obliteration of an AVM means the angiographic persistence of an early filling draining veins without demonstrable nidus.21 The early filling venous drainage suggests that some shunting persists. Our studies have shown that these subtotally obliterated AVMs have very low risk of hemorrhage in spite of the fact that per definition the AVM nidus is still patent as indicated by the shunting. It should be noted that more than 70% of them went on to obliterate completely without further treatment.21
The reported obliteration rate following radiosurgery ranged between 30% and 92%.10,22–24 One should be cautious in terms of the interpretation of the results owing to the biases injected from different cutoff time and imaging modality used to conclude total obliteration. Studies excluding patients with short follow-up, reporting only patients undergoing angiography, or including MRI as an imaging study to conclude obliteration tend to overestimate the success rate of radiosurgery.23,25,26
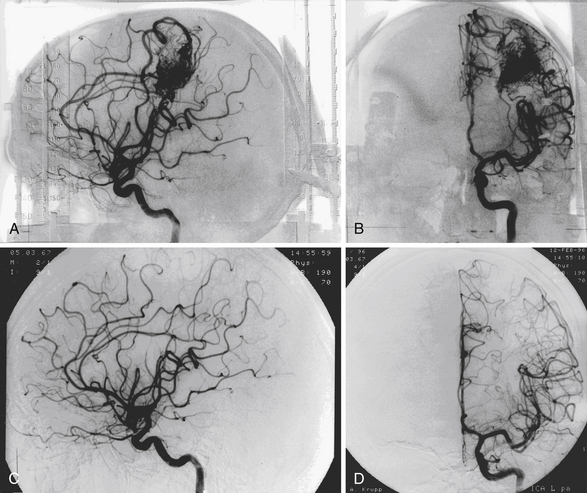
The mean follow-up after GKS was 80 months. GKS yielded a total angiographic obliteration in 552 (54%) and subtotal obliteration in 42 (4.1%) patients (Fig. 47.1). In 290 (28.3%) patients, the AVMs remained patent and in 139 patients (13.6%) no flow voids were observed on the MRI. The angiographic total obliteration was achieved in 65.2% of patients with nidus less than 3 cm3; 43.8% between 3 and 8 cm3, and 27.6% with nidus volume larger than 8 cm3. Small nidus volume, high prescription dose, and low number of isocenters are predictive of obliteration. Preradiosurgical embolization has a negative effect on obliteration.
Complications
Radiation-Induced Changes
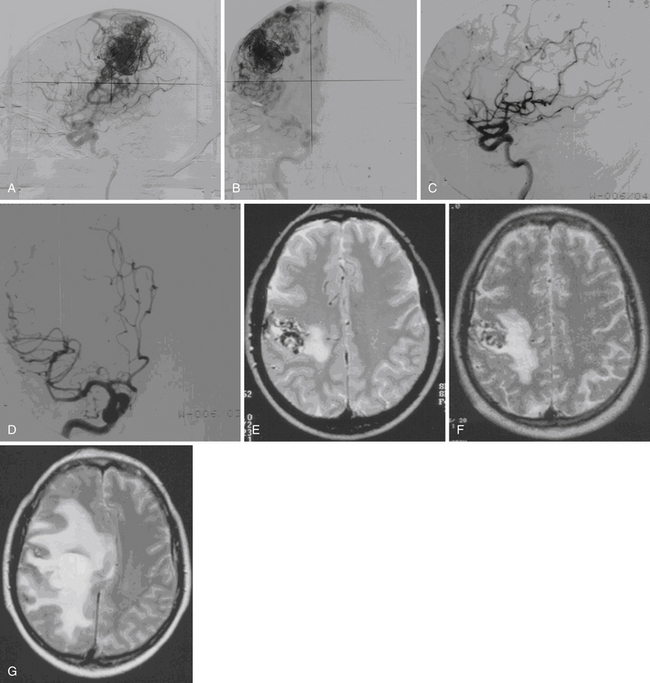
Radiation-induced change is an increased T2 signal around the AVM seen on MRI (Fig. 47.2). Radiation damage of glial cells, endothelial cell damage followed by breakdown of blood-brain barrier, excessive generation of free radicals, or release of vascular endothelial growth factors have been proposed to explain this imaging finding. The severity of radiation-induced changes on images and associated neurological deficits varies, ranging from asymptomatic, being only a few millimeters of increased T2 signal surrounding the treated nidus to massive brain edema with symptoms and signs of increased intracranial pressure. From our 1500 Gamma Knife procedures performed for AVM patients with follow-up MRI available for analysis, 34.4% of patients developed radiation-induced changes. Among them, 60% had mild (a few millimeters of increased T2 signal surrounding the nidus), 33% had moderate (compression of ventricle and effacement of sulci), and 7% had severe (midline shift) radiation-induced changes. The mean time to the development of radiation-induced changes was 13 months after GKS, and the mean duration of the changes was 22 months. Larger nidus volumes, higher prescription doses, history with preradiosurgical embolization, and nidus without previous hemorrhage were associated with higher risk of radiation-induced changes.
Cyst Formation
Cyst formation is a rare complication following GKS. Cysts that develop after resolution of previous hemorrhages or fluid cavities from encephalomalacia after surgeries should not be considered as complications related to GKS. Direct radiation injury to the perilesional brain tissue, increased permeability of the blood-brain barrier with accumulation of the exudative fluid, hemodynamic perturbations during gradual obliteration of the nidus with subsequent ischemic tissue damage, and tissue destruction due to subclinical perilesional hemorrhages have been proposed as the possible mechanisms of cyst formation. From our 1272 patients with follow-up MRI available, we found a total of 20 patients (1.6%) developing a cyst after a mean of 8.1 years after GKS. Four cysts were found in 710 patients with follow-up shorter than 5 years, eight cysts were found in 302 patients with follow-up between 5 and 10 years, and another eight cysts were found in 260 patients with follow-up between 10 and 20 years. Of the 20 patients, 18 had regular MRI follow-up and 14 (78%) of them had radiation-induced changes before the development of cysts. Six patients had large cysts and three of them were symptomatic, requiring surgery. Two patients underwent craniotomy and drainage of the cyst. The cyst wall showed no evidence of neoplasia.
Radiosurgery-Induced Neoplasia
We found two meningiomas from 1333 AVM patients treated with GKS; however, follow-up imaging was performed over a period of at least 10 years in 288 of these patients. If we conservatively estimate that radiosurgery-induced lesions would be evident within a 10-year time interval, then our incidence of radiosurgery-induced neoplasia is 2 in 2880 person-years or 69 in 100,000 person-years. Thus, there is a 0.7% chance that a radiation-induced tumor may develop within 10 years following GKS. This is less than the 1.9% risk detailed by Brada and colleagues,27 but our results encompass a follow-up period of only 10 years. The long latency and relative rarity of these lesions following radiosurgery may defy a conclusive determination of the true incidence.
Specific Applications of Radiosurgery
Embolization of Arteriovenous Malformations
The effectiveness of partial embolization followed by GKS in the management of relatively large AVMs remains controversial. Small series of cases managed with this combined approach reported diverse results with obliteration rates ranging between 50% and 76%.10,28,29 When comparing the outcome in patients treated with Gamma Knife alone to those with combined embolization and Gamma Knife treatment, recent studies reported less favorable outcome in patients with preradiosurgical embolization.10,30
Recanalization of previously embolized parts of the nidus,31 difficulty in nidus delineation following previous embolization,10 and attenuation of radiation dose by embolization materials32 have been proposed to explain the less favorable outcome in patients with pre-GKS embolization. Theoretically, volume reduction following embolization affords a lower chance of GKS-related adverse effect but this expectation was not shown based on our data. Additionally the complications from embolization are not negligible. Therefore, the use of pre-GKS embolization remains problematic and awaits further investigation.
Brainstem Arteriovenous Malformations
Studies have shown that AVMs located in the posterior fossa carry a higher risk of hemorrhage compared to AVMs in other locations.33,34 Furthermore, owing to the critical location in proximity to vital neuronal pathways and nuclei, there is a high risk of morbidity and mortality once the brainstem AVMs rupture. With the advance of microsurgical techniques, extirpation of the AVMs involving the brainstem is feasible but the associated risks are not negligible. Several surgical series have demonstrated a less favorable obliteration rate with a high risk of complications.35–37
Arteriovenous Malformations in Pediatric Patients
Although AVMs only account for 1.4% of intracerebral hemorrhage in the adult population, they represent the underlying cause of 20% to 50% of cerebral hemorrhage in pediatric patients.38,39 Studies have shown that pediatric patients have a high cumulative lifetime risk of hemorrhages, and AVMs in the pediatric population also have a high propensity to rupture.39,40 Radiosurgery has been increasingly used for the management of pediatric AVMs following the success in adult patients.
The locations of the AVMs were hemispheric in 101 (54.3%) patients, thalamus in 24 (12.9%), basal ganglia in 23 (12.4%), corpus callosum in 9 (4.8%), brainstem in 18 (9.7%), insula/sylvian fissure in 5 (2.7%), and cerebellum in 6 (3.2%). Five patients had coexistent intranidal aneurysms, and seven had perinidal aneurysms. Three patients had non-flow-directed aneurysms. The nidus volumes ranged from 0.1 to 24 cm3 (mean 3.2 cm3). Sixty-two nidi had only superficial venous drainage and 124 had deep venous drainage. The Spetzler-Martin grading at the time of initial GKS was grade I in 23 (12.4%) patients, grade II in 55 (29.6%) patients, grade III in 87 (46.8%) patients, grade IV in 20 (10.8%) patients, and grade V in 1 (0.5%) patient. Forty-one patients had a second GKS for still-patent AVM residuals performed at a mean of 2 to 5 years (range years) after the initial GK procedures. The volumes ranged from 0.2 to 15.9 cm3 (mean 2.3 cm3) at the time of repeat GKS.
Only a small series of children went through a systemic psychological test analyzing the cognitive faculties in a long-term follow-up after GKS. However, yearly follow-ups including questioning the parents, the patients, and the referring doctors about the intellectual development and possible cognitive or endocrinological deficits were conducted. According to this information, 95% of the children had normal intellectual development after radiosurgery with satisfactory or good school performance. As adults, they performed from average to excellent and were socially well adjusted. Riva and associates studied patients, ranging in age from 9 to 18 years, treated for AVMs using GKS to record potential effects of radiosurgery on cognitive and neuropsychological performance.41 Tests for general intelligence, nonverbal intelligence, memory and its components, and attention performance were administered to patients and compared with test results of age-matched siblings or first cousins. No statistically significant difference was found between the performance of patients and control subjects in any of the tests administered.
The reported obliteration rate for pediatric AVMs following radiosurgery ranged between 53% and 86%.25,42–45 Some studies had proposed that in pediatric patients the response to radiosurgery seems to be less favorable.25 Hypotheses such as the immature vessels in pediatric patients were more likely to recover from radiation-induced damage and neovascularization in response to radiation have been proposed. Our experience shows comparable result in children compared to adults. Additionally, we observe that radiation-induced damage seems to be more tolerable for kids, suggesting that radiosurgery has a favorable benefit-risk profile in the management of pediatric AVMs. However, the risk of hemorrhage remained in pediatric patients and the development of secondary tumor cannot be overlooked. For pediatric AVMs amenable to surgery, microsurgery should be considered as the first-line treatment.
Large Arteriovenous Malformations
The following strategies are currently available to treat large AVMs with radiosurgery. First, one can embolize a portion of the AVM and then perform radiosurgery if the nidus shrinks to a size manageable with radiosurgery. However, embolization should effectively shrink the nidus for radiosurgery to achieve good results. Also fragmentation of the nidus into a number of segments should be avoided because that will make the radiosurgical planning difficult and increase the probability of radiosurgery failure. Another strategy involves serial staged radiosurgery to selected volumes of the AVMs. Sirin and associates used staged volumetric radiosurgery in 28 large AVMs.45a Of the 21 patients, seven underwent repeat radiosurgery and were eliminated from outcome analysis. Of the remaining 14 patients, 3 had total obliteration on angiograms, and 4 had no flow voids on MRI but had no follow-up angiography. Four patients had hemorrhages after radiosurgery, resulting in two deaths. Worsened neurological deficits occurred in one patient. The third approach is to treat the whole nidus in one session with low-dose radiosurgery. Pan and associates reported an obliteration rate of 25% for AVMs with volume larger than 15 cm3. The obliteration rate increased to 50% at 50 months’ follow-up. The morbidity rate was 3.3%. Post-treatment hemorrhage occurred in 9.2%.
Dural Arteriovenous Fistulas
Intracranial dAVFs comprise a unique subset of vascular malformations from the perspectives of etiology and treatment paradigms. Although dAVFs make up approximately 15% of all intracranial vascular malformations, the precise mechanism of formation remains unknown, with leading theories including adjacent venous sinus stasis as well as alterations in local expression of vasogenic factors, such as vascular endothelial growth factor (VEGF) and fibroblastic growth factor (FGF).46,47 Unfortunately, the presence of venous occlusion proximal or distal to the dAVFs is not an absolute requirement, complicating the interpretation of its role. Similarly, changes in expression levels of VEGF and FGF have been found in animal models of dAVFs as well as samples of patients treated surgically, but no causative relationship has been shown to exist between these vasogenic substances and dAVF formation.48–51
From a treatment standpoint, the experience with dAVFs is distinct from that with AVMs. Whereas size and location of AVMs are correlated with the natural history and optimal treatment protocols, several studies have established that the flow dynamics of dAVFs are the most important indicator of the need to treat and modality of choice, be it endovascular, open microneurosurgical, or radiosurgery.52–54 Specifically, the current classification systems for dAVFs, excluding those involving the cavernous sinus, have been defined based on the direction of fistulous flow as well as the presence or absence of cortical venous reflux (Box 47.1). In an attempt to validate these classification systems based on a large single institution sample, Davies and co-workers55 retrospectively analyzed 102 dAVFs in 98 patients focusing on venous anatomy and direction of flow, confirming that the single best predictor of hemorrhage is retrograde leptomeningeal drainage. As the aggressive natural history of lesions with cortical venous reflux differs significantly from lesions without angiographic evidence of cortical venous reflux, early definitive therapy via endovascular procedures or open surgical resection appears to be preferable to radiosurgery as a first-line treatment. Once again, the ionizing radiation delivered via radiosurgery is thought to be an effective agent for decreasing neovascularization in dAVFs, but the time interval needed for the desired effect is too great to justify radiosurgery as a first-line therapy.48,56
BOX 47.1 Classification of Dural Arteriovenous Fistulas
Cognard Scale
Type I – Drainage into dural venous sinus only with normal antegrade flow
Type IIa – Drainage into dural venous sinus only with retrograde flow
Type IIb – Drainage into dural venous sinus with antegrade flow and CVR
Type IIa + IIb – Drainage into dural venous sinus with retrograde flow and CVR
Type III – CVR only without venous ectasia
Adapted from: Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995;82:166-179; Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671-680.
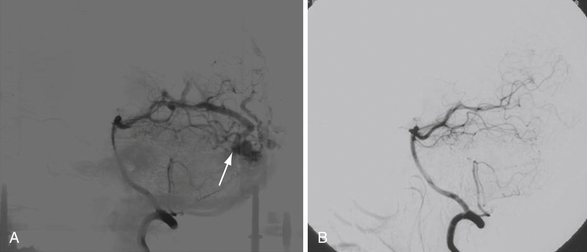
Given the relatively low incidence of clinically identified lesions with respect to other types of vascular malformations and the percentage of those diagnosed dAVFs treated with radiosurgery, large clinical series are few in number and published studies report the findings of single institutions.57–60 The long-term analysis of radiosurgery for dAVFs over 25 years at the Karolinska University Hospital in Stockholm, Sweden, included 52 patients treated between 1978 and 2003. The obliteration rate reported in this study was 68% with 16 dAVFs presenting as less aggressive Borden I or Cognard I/IIa lesions. Moreover, 43 of 52 patients received GKS only, with the remaining 9 patients having undergone prior surgical or endovascular treatment.60 In a similar institutional experience at the University of Virginia between 1989 and 2005, 55 patients with dAVFs were treated with GKS, primarily as an adjunct to surgery or embolization. Obliteration rates measured by angiography at 3 years ranged from 54% to 65%, with the 16 patients classified as Borden I lesions (Fig. 47.3). Unlike the Karolinska study, the majority of patients treated at the University of Virginia received GKS as a secondary therapy, with 41 of the 54 patients receiving surgical or endovascular intervention prior to radiosurgery. Regardless of the difference in utilization of radiosurgery as a primary or secondary treatment modality, the results of these long-term studies indicate that GKS is an effective and safe treatment for intracranial dAVFs. From a purely logistic standpoint, the role of GKS may remain a second-tier therapy as the majority of lesions are still initially examined via angiography, facilitating treatment at the time of radiographic diagnosis. Of course, radiosurgery may serve as a first-line therapy for deep lesions that are either inaccessible to endovascular treatment given current microcatheter technology or in situations in which there is low risk of intracranial hemorrhage.
Cavernous Malformations
Cavernous malformations (CMs) are another type of intracranial vascular malformation. They can also be referred to as cavernous hemangiomas, angiographically occult vascular malformations, or cavernous angiomas. From a histological standpoint, they are discrete and lobulated lesions composed of dilated sinusoidal vascular channels formed by a single layer of endothelial lining and variable layers of fibrous adventitia. CMs do have a hereditary component and may occur as multiple lesions within the same patient. The proportion of CMs that are developmental anomalies as opposed to acquired malformations and the extent to which the etiology of a cavernous malformation alters its natural history remain the subject of much debate.61,62 Annual rates of hemorrhage have been reported to be less than 1% to as high as 7% for those with multiple recent hemorrhages.63,64 Acquired malformations are frequently associated with deep venous anomalies and may point to an underlying pathophysiology associated with venous hypertension.65,66 Radiosurgery and radiation therapy have even been linked to the formation of CMs.67,68
CMs have been associated with headaches, seizures, focal neurological deficits, and intracerebral hemorrhages. In some patients who have symptomatic yet microsurgically inaccessible lesions, radiosurgery has been employed. However, unlike the consensus regarding radiosurgical indications and outcomes for AVMs and dAVFs, opinions vary greatly as to the risk-to-benefit profile for radiosurgical treatment of CMs. Moreover, few proponents of radiosurgery for CM patients advocate treatment unless there has been at least one if not several prior hemorrhages. In addition, as compared to AVMs or dAVFs, histopathological studies of CMs resected after prior radiosurgery show little in the way of protective changes associated with the treatment.69
Proponents of radiosurgery for CMs maintain a reduction in the risk of hemorrhage within 2 years after radiosurgery.70,71 Less favorable assessments of the benefits of radiosurgery for altering the natural history of hemorrhage associated with CMs have been put forth by others.24,72,73 It does seem clear that radiosurgery of CMs is associated with a higher rate of complications than for AVMs or dAVFs. This may be related to radiosensitization of surrounding brain parenchyma by iron deposition from repeated clinical and subclinical hemorrhages associated with CMs.74 If radiosurgery is attempted for a young patient with a surgically inaccessible CM that has repeatedly hemorrhaged (e.g., a deep-seated thalamic lesion or one intrinsic to the brainstem), a lower radiosurgical dose (15 Gy or less) compared to AVMs seems warranted.24,75,76
The results in terms of reduction of CM-associated epilepsy following radiosurgery seem a bit more promising.77 In a randomized, multicenter study of 49 cases, Bartolomei and associates showed that 53% of patients achieved a seizure-free status (Engel’s class I) using a mean marginal dose of 19.2 Gy. Two patients in this cohort experienced severe complications.77 Hsu and colleagues showed Engel’s class I seizure control in 64.3% of patients treated with linear accelerator (linac)–based radiosurgery.78 The improvement in seizures need not be accompanied by a protection from hemorrhage associated with these vascular lesions. Further investigation of the role of radiosurgery for symptomatic CMs in inaccessible or eloquent brain tissue is required. Until then, radiosurgery for CMs must be employed only after careful scrutiny of the risks and benefits likely afforded the patient.
Adler J.R.Jr., Chang S.D., Murphy M.J., et al. The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124-128.
Hsu P.W., Chang C.N., Tseng C.K., et al. Treatment of epileptogenic cavernomas: surgery versus radiosurgery. Cerebrovasc Dis. 2007;24:116-120.
Karlsson B., Lindquist C., Steiner L. Prediction of obliteration after Gamma Knife surgery for cerebral arteriovenous malformations. Neurosurgery. 1997;40:425-430.
Kondziolka D., Lunsford L.D., Kestle J.R. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
Pan D.H., Kuo Y.H., Guo W.Y., et al. Gamma Knife surgery for cerebral arteriovenous malformations in children: a 13-year experience. J Neurosurg Pediatr. 2008;1:296-304.
Soderman M., Edner G., Ericson K., et al. Gamma Knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg. 2006;104:867-875.
Please go to expertconsult.com to view the complete list of references.
1. Katsaridis V., Papagiannaki C., Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589-597.
2. Panagiotopoulos V., Gizewski E., Asgari S., et al. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol. 2009;30:99-106.
3. Magnus V. Bidrat til hjernechirurgiens klinik og resultater. Norsk Mag Laegevidensk (Suppl Merkur Bok Akcidenstrykkeri). 1921;82:1-138.
4. Cushing H., Bailey P. Tumors Arising From the Blood Vessels of the Brain. Springfield, IL: Charles C Thomas; 1928.
5. Kjellberg R.N., Davis K.R., Lyons S., et al. Bragg peak proton beam therapy for arteriovenous malformation of the brain. Clin Neurosurg. 1983;31:248-290.
6. Svien H.J., Peserico L. Regression in size of arteriovenous anomaly. J Neurosurg. 1960;17:493-496.
7. Johnson R. Radiotherapy of Cerebral Angiomas with a Note on Some Problems in Diagnosis. Berlin: Springer-Verlag; 1975.
8. Nataf F., Schlienger M., Lefkopoulos D., et al. Radiosurgery of cerebral arteriovenous malformations in children: a series of 57 cases. Int J Radiat Oncol Biol Phys. 2003;57:184-195.
9. Saunders W.M., Winston K.R., Siddon R.L., et al. Radiosurgery for arteriovenous malformations of the brain using a standard linear accelerator: rationale and technique. Int J Radiat Oncol Biol Phys. 1988;15:441-447.
10. Schlienger M., Atlan D., Lefkopoulos D., et al. Linac radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys. 2000;46:1135-1142.
11. Voges J., Treuer H., Lehrke R., et al. Risk analysis of LINAC radiosurgery in patients with arteriovenous malformation (AVM). Acta Neurochir Suppl. 1997;68:118-123.
12. Colombo F., Cavedon C., Casentini L., et al. Early results of CyberKnife radiosurgery for arteriovenous malformations. J Neurosurg. 2009;111:807-819.
13. Lindquist C., Paddick I. The Leksell Gamma Knife Perfexion and comparisons with its predecessors. Neurosurgery. 2007;61:130-140.
14. Friedman W.A., Bova F.J. The University of Florida radiosurgery system. Surg Neurol. 1989;32:334-342.
15. Benedict S.H., Cardinale R.M., Wu Q., et al. Intensity-modulated stereotactic radiosurgery using dynamic micro-multileaf collimation. Int J Radiat Oncol Biol Phys. 2001;50:751-758.
16. Solberg T.D., Boedeker K.L., Fogg R., et al. Dynamic arc radiosurgery field shaping: a comparison with static field conformal and noncoplanar circular arcs. Int J Radiat Oncol Biol Phys. 2001;49:1481-1491.
17. Adler J.R.Jr., Chang S.D., Murphy M.J., et al. The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124-128.
18. Schneider B.F., Eberhard D.A., Steiner L.E. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87:352-357.
19. Steiner L., Greitz T., Leksell L. Radiosurgery in Intracranial Arteriovenous Malformation. Sixth International Congress of Neurological Surgeons. Amsterdam: Excerpta Medica; 1977.
20. Lindquist C., Steiner L. Stereotactic radiosurgical treatment of arteriovenous malformations. In: Lunsford L.D., editor. Modern Stereotactic Neurosurgery. Boston: Martinus Nijhoff; 1988:491-505.
21. Yen C.P., Varady P., Sheehan J., et al. Subtotal obliteration of cerebral arteriovenous malformations after gamma knife surgery. J Neurosurg. 2007;106:361-369.
22. Karlsson B., Lindquist C., Steiner L. Prediction of obliteration after gamma knife surgery for cerebral arteriovenous malformations. Neurosurgery. 1997;40:425-430.
23. Liscak R., Vladyka V., Simonova G., et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005-1014.
24. Pollock B.E., Garces Y.I., Stafford S.L., et al. Stereotactic radiosurgery for cavernous malformations. J Neurosurg. 2000;93:987-991.
25. Pan D.H., Guo W.Y., Chung W.Y., et al. Gamma Knife radio surgery as a single treatment modality for large arterioenous malformations. J Neurosurg. 93(Suppl 3), 2000. 113-110
26. Shin M., Maruyama K., Kurita H., et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: the University of Tokyo experience. J Neurosurg. 2004;101:18-24.
27. Brada M., Rajan B., Traish D., et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf). 1993;38:571-578.
28. Miyawaki L., Dowd C., Wara W., et al. Five year results of LINAC radiosurgery for arteriovenous malformations: outcome for large AVMs. Int J Radiat Oncol Biol Phys. 1999;44:1089-1106.
29. Zabel-du Bois A., Milker-Zabel S., Huber P., et al. Risk of hemorrhage and obliteration rates of LINAC-based radiosurgery for cerebral arteriovenous malformations treated after prior partial embolization. Int J Radiat Oncol Biol Phys. 2007;68:999-1003.
30. Andrade-Souza Y.M., Ramani M., Scora D., et al. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery. 2007;60:443-451.
31. Gobin Y.P., Laurent A., Merienne L., et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
32. Andrade-Souza Y.M., Ramani M., Beachey D.J., et al. Liquid embolisation material reduces the delivered radiation dose: a physical experiment. Acta Neurochir (Wien). 2008;150:161-164.
33. Hernesniemi J.A., Dashti R., Juvela S., et al. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63:823-829.
34. Khaw A.V., Mohr J.P., Sciacca R.R., et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 2004;35:660-663.
35. Batjer H., Samson D. Arteriovenous malformations of the posterior fossa. Clinical presentation, diagnostic evaluation, and surgical treatment. J Neurosurg. 1986;64:849-856.
36. Drake C.G., Friedman A.H., Peerless S.J. Posterior fossa arteriovenous malformations. J Neurosurg. 1986;64:1-10.
37. Nozaki K., Hashimoto N., Kikuta K., et al. Surgical applications to arteriovenous malformations involving the brainstem. Neurosurgery. 2006;58:270-278. ONS-
38. Al-Jarallah A., Al-Rifai M.T., Riela A.R., et al. Nontraumatic brain hemorrhage in children: etiology and presentation. J Child Neurol. 2000;15:284-289.
39. Celli P., Ferrante L., Palma L., et al. Cerebral arteriovenous malformations in children. Clinical features and outcome of treatment in children and in adults. Surg Neurol. 1984;22:43-49.
40. Gerosa M.A., Cappellotto P., Licata C., et al. Cerebral arteriovenous malformations in children (56 cases). Childs Brain. 1981;8:356-371.
41. Riva D., Pantaleoni C., Devoti M., et al. Radiosurgery for cerebral AVMs in children and adolescents: the neurobehavioral outcome. J Neurosurg. 1997;86:207-210.
42. Levy E.I., Niranjan A., Thompson T.P., et al. Radiosurgery for childhood intracranial arteriovenous malformations. Neurosurgery. 2000;47:834-841.
43. Nicolato A., Lupidi F., Sandri M.F., et al. Gamma knife radiosurgery for cerebral arteriovenous malformations in children/adolescents and adults. Part I: differences in epidemiologic, morphologic, and clinical characteristics, permanent complications, and bleeding in the latency period. Int J Radiat Oncol Biol Phys. 2006;64:904-913.
44. Nicolato A., Lupidi F., Sandri M.F., et al. Gamma Knife radiosurgery for cerebral arteriovenous malformations in children/adolescents and adults. Part II: differences in obliteration rates, treatment-obliteration intervals, and prognostic factors. Int J Radiat Oncol Biol Phys. 2006;64:914-921.
45. Reyns N., Blond S., Gauvrit J.Y., et al. Role of radiosurgery in the management of cerebral arteriovenous malformations in the pediatric age group: data from a 100-patient series. Neurosurgery. 2007;60:268-276. discussion 276
45a. Sirin S., Kondziolka D., Niranjan A., et al. Prospective staged volume radiosurgery for large arteriovenous malformations; indications and outcomes in otherwise untreatable patients. Neurosurg. 2006;58(1):17-27.
46. Kojima T., Miyachi S., Sahara Y., et al. The relationship between venous hypertension and expression of vascular endothelial growth factor: hemodynamic and immunohistochemical examinations in a rat venous hypertension model. Surg Neurol. 2007;68:277-284.
47. Terada T., Higashida R.T., Halbach V.V., et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884-889.
48. Kilic K., Konya D., Kurtkaya O., et al. Inhibition of angiogenesis induced by cerebral arteriovenous malformations using gamma knife irradiation. J Neurosurg. 2007;106:463-469.
49. Sandalcioglu I.E., Wende D., Eggert A., et al. Vascular endothelial growth factor plasma levels are significantly elevated in patients with cerebral arteriovenous malformations. Cerebrovasc Dis. 2006;21:154-158.
50. Shin Y., Nakase H., Nakamura M., et al. Expression of angiogenic growth factor in the rat DAVF model. Neurol Res. 2007;29:727-733.
51. Tirakotai W., Bertalanffy H., Liu-Guan B., et al. Immunohistochemical study in dural arteriovenous fistulas and possible role of local hypoxia for the de novo formation of dural arteriovenous fistulas. Clin Neurol Neurosurg. 2005;107:455-460.
52. Borden J.A., Wu J.K., Shucart W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
53. Brown R.D.Jr., Wiebers D.O., Nichols D.A. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994;81:531-538.
54. Cognard C., Gobin Y.P., Pierot L., et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
55. Davies M.A., Ter Brugge K., Willinsky R., et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830-837.
56. Hosobuchi Y., Fabricant J., Lyman J. Stereotactic heavy-particle irradiation of intracranial arteriovenous malformations. Appl Neurophysiol. 1987;50:248-252.
57. Guo W.Y., Pan D.H., Wu H.M., et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol. 1998;19:1081-1087.
58. Koebbe C.J., Singhal D., Sheehan J., et al. Radiosurgery for dural arteriovenous fistulas. Surg Neurol. 2005;64:392-398. discussion 398-399
59. Pollock B.E., Nichols D.A., Garrity J.A., et al. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999;45:459-466.
60. Soderman M., Edner G., Ericson K., et al. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg. 2006;104:867-875.
61. Gonzalez L.F., Lekovic G.P., Eschbacher J., et al. Are cavernous sinus hemangiomas and cavernous malformations different entities? Neurosurg Focus. 2006;21:e6.
62. Mindea S.A., Yang B.P., Shenkar R., et al. Cerebral cavernous malformations: clinical insights from genetic studies. Neurosurg Focus. 2006;21:e1.
63. Kondziolka D., Lunsford L.D., Kestle J.R. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
64. Mathiesen T., Edner G., Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg. 2003;99:31-37.
65. Perrini P., Lanzino G. The association of venous developmental anomalies and cavernous malformations: pathophysiological, diagnostic, and surgical considerations. Neurosurg Focus. 2006;21:e5.
66. Sheehan J., Lunsford L.D., Kondziolka D., et al. Development of a posterior fossa cavernous malformation associated with bilateral venous anomalies: case report. J Neuroimaging. 2002;12:371-373.
67. Motegi H., Kuroda S., Ishii N., et al. De novo formation of cavernoma after radiosurgery for adult cerebral arteriovenous malformation—case report. Neurol Med Chir (Tokyo). 2008;48:397-400.
68. Nimjee S.M., Powers C.J., Bulsara K.R. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006;21:e4.
69. Gewirtz R.J., Steinberg G.K., Crowley R., et al. Pathological changes in surgically resected angiographically occult vascular malformations after radiation. Neurosurgery. 1998;42:738-742.
70. Hasegawa T., McInerney J., Kondziolka D., et al. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197.
71. Kondziolka D., Lunsford L.D., Flickinger J.C., et al. Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg. 1995;83:825-831.
72. Karlsson B., Kihlstrom L., Lindquist C., et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293-297.
73. Shih Y.H., Pan D.H. Management of supratentorial cavernous malformations: craniotomy versus gamma knife radiosurgery. Clin Neurol Neurosurg. 2005;107:108-112.
74. St. George E.J., Perks J., Plowman P.N. Stereotactic radiosurgery XIV: the role of the haemosiderin “ring” in the development of adverse reactions following radiosurgery for intracranial cavernous malformations: a sustainable hypothesis. Br J Neurosurg. 2002;16:385-391.
75. Kim M.S., Pyo S.Y., Jeong Y.G., et al. Gamma knife surgery for intracranial cavernous hemangioma. J Neurosurg. 2005;102(Suppl):102-106.
76. Liscak R., Vladyka V., Simonova G., et al. Gamma knife surgery of brain cavernous hemangiomas. J Neurosurg. 2005;102(Suppl):207-213.
77. Bartolomei F., Regis J., Kida Y., et al. Gamma Knife radiosurgery for epilepsy associated with cavernous hemangiomas: a retrospective study of 49 cases. Stereotact Funct Neurosurg. 1999;72(Suppl 1):22-28.
78. Hsu P.W., Chang C.N., Tseng C.K., et al. Treatment of epileptogenic cavernomas: surgery versus radiosurgery. Cerebrovasc Dis. 2007;24:116-120.