Chapter 65 Stereotactic Radiosurgery of Skull Base Tumors
Gamma knife surgery, similar to microsurgery, has advantages and disadvantages which must be thoroughly discussed with the patient.1,2 For the patient it is alluring to undergo an outpatient procedure rather than microsurgical management that requires a much longer period of care. Further, gamma knife outcomes show excellent tumor control and, with current methods, low cranial nerve morbidity. Gamma knife surgery is a viable treatment modality for the appropriate patient as defined by age, medical history, tumor characteristics and physical findings. As such, many neurotologists now offer gamma knife surgery as part of their armamentarium for managing vestibular schwannomas and glomus tumors.3
PREOPERATIVE COUNSELING
Informed consent for gamma knife surgery requires the surgeon to discuss alternative options such as observation and microsurgical resection.2 The risks and benefits of these alternatives should be frankly described and compared to gamma knife treatment. Many patients have received information from the Internet or from physicians with limited experience with gamma knife and may have erroneous information. Common misconceptions include the expectation that gamma knife surgery completely removes the tumor and that hearing will improve, or conversely that cranial nerve morbidities are significant. These need to be addressed with evidence-based reports and information.
One statistic, which is particularly alarming to patients considering gamma knife surgery is that there have been eight cases of malignancy within vestibular schwannomas (as of 2002).4 Four of these patients had been previously treated with radiosurgery. While it remains possible that these four malignancies developed after the radiation treatment, it is more likely that these malignant tumors were misdiagnosed as benign at the outset of evaluation and treatment.
Delayed development of radiation-induced neoplasms was addressed by Pollock and colleagues in 1998.5 They reviewed more than 20,000 patients treated with radiosurgery worldwide and found no increased incidence of new neoplasm development (i.e., benign or malignant). A retrospective cohort study comparing the Sheffield, England radiosurgery patient database with the national mortality and cancer registries identified a single new astrocytoma among those treated.6 Based on their national incidence figures, 2.47 cases would have been predicted. The risk of radiosurgery induced malignancy in patients with neurofibromatosis type 2 (NF2) and von Hippel-Lindau disease was similarly studied.7 Of 118 NF2 and 19 von Hippel-Lindau disease patients, totaling 906 and 62 patient-years of follow-up data, respectively, only two cases of intracranial malignancy were found. Both of these were in NF2 patients. One was thought to have arisen before the radiosurgery; the other was a glioblastoma diagnosed three years after radiosurgery. Gliomas may occur in as many as 4% of NF2 patients and the single case may not represent an increased risk. It was suggested that the late risk of malignancy arising after irradiation must be put in the context of the condition being treated, the treatment options available to these individuals, and their life expectancy.
Despite the findings of the studies just reviewed, it is important to counsel patients about the possibility of malignant transformation or induction. A handful of tumors suggestive of radiation induced malignancy have been reported among the tens of thousands who have undergone gamma knife treatment. Lustig and colleagues reported the development of a squamous cell carcinoma following radiation treatment of vestibular schwannoma.8 Hanabusa and colleagues reported the malignant transformation of a vestibular schwannoma following gamma knife surgery.9 There was histologic evidence of vestibular schwannoma following a retrosigmoid resection. Four years after this resection, recidivistic tumor was identified, and the patient was subsequently treated with gamma knife surgery. Six months post-treatment, the tumor had grown, and the patient underwent surgical resection via a combined retrosigmoid-translabyrinthine approach. Abnormal mitotic figures were observed on histologic sections, and the diagnosis of malignancy was assigned.
SURGICAL TECHNIQUE
The Gamma Knife Unit
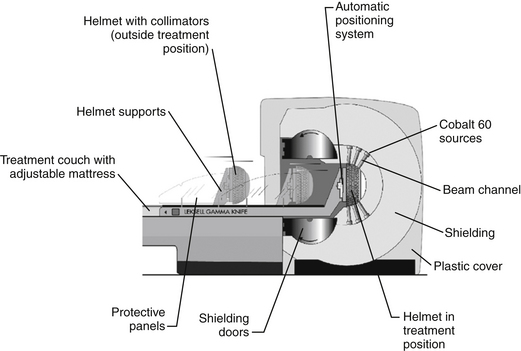
The basic principle of gamma knife surgery is to provide focused radiation to the tumor while minimizing radiation delivery to surrounding tissues. As such, a semicircular shield called the collimator helmet is used to generate approximately 200 individual gamma radiation “beams.” In the center of the helmet, where the beams meet, radiation delivery is maximal, but along each individual radiation tract tissue exposure is relatively low. When the collimator helmet is locked into position, the 201 openings of the collimator helmet coincide with the cobalt sources. There is a shielded chamber within which the 60Co sources are contained, and stainless steel shielding doors protect the treatment room from the 60Co sources. There is a treatment couch with an adjustable mattress that slides into the gamma knife unit together with the collimator helmet and the patient. Figure 65-1 schematically shows the orientation of the components of the gamma knife model, Leksell Gamma Knife® 4C and Figure 65-2 shows the overall appearance of the gamma knife model, Leksell Gamma Knife® 4C.
Frame Attachment
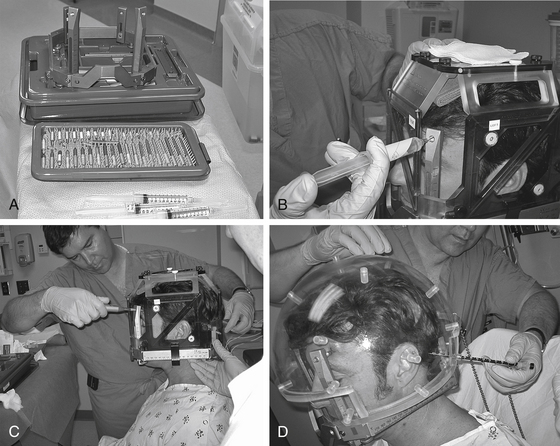
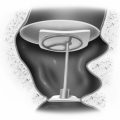
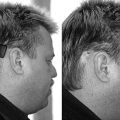
The method of anesthesia used during frame placement is surgeon and patient dependent. In our program, either sedation with versed and fentanyl, or monitored anesthesia with propofol, followed by injection of local anesthetic at the pin sites is used. Figure 65-3A shows the typical array of tools used for the frame attachment. A variety of screw lengths allow the surgeon to choose those ideally suited for the individual location of the posts and tumor. The placement of the frame should begin with an accurate orientation of the location of the target within the patient’s head. Ideally, the target should be located within the fiducial range and placed centrally within the frame thereby avoiding later collisions with the collimator helmet and granting sufficient accuracy for the stereotactic target definition.
The stereotactic frame is assembled and preliminarily supported by using external auditory canal support pins, a Velcro band, or a stereotactic fiducial box. When using a fiducial box to facilitate frame placement, it is important to use the MRI fiducial box, rather than the CT or angiography fiducial box, since this is the smallest of the three plexiglass fiducial boxes (Figs. 65-3B and 65-3C). Asymmetric frame placements are possible and do not impair the accuracy of imaging. The frame can be shifted from side to side or can be moved as far as possible to the front or back to facilitate centering of the tumor. The frame is stabilized against the patient by an assistant and the surgeon should adjust the lengths of the posts to maintain relative tumor position. A low position of the anterior posts can help avoid anterior collisions with the collimator helmet for skull base posterior fossa tumors. In critical positions, collisions can sometimes be avoided by using the curved posts in the anterior position.
Measurements of the frame and placement are then performed to allow the computer to identify any potential collisions after the plan is formulated. These measurements are required for the frame and skull section in Leksell GammaPlan treatment planning software. Measurements include the length of the four posts and the length of the screws that protrude from the posts. Additionally, the volume of the head is measured using the plastic collimator bubble, simulating the relationship of the frame to the treatment collimator helmet (Fig. 65-3D). This concludes frame placement and the patient may proceed to imaging.
Imaging
Many centers acquire only MRI scans for treatment planning. We prefer to also acquire a non-contrast CT scan through the temporal bone to aid in planning. There is evidence of distortion of MR images and correlation with CT scans at the time of planning can aid in reducing radiation delivery to critical structures such as the cochlea and facial nerve.10 A CT fiducial box is affixed to the frame, the patient secured in the holder attached to the table, and an axial scan through the temporal bone and skull base acquired. Both CT and MR images are imported into the Gamma Knife workstation. Axial scans are defined, and coronal and sagittal reconstructions generated for each.
Treatment Planning
Dose planning using Leksell GammaPlan involves composing shots to develop a conformal isodose. By definition, this includes the whole target but spares the surrounding healthy tissue. Figure 65-4 shows an example of a vestibular schwannoma. The target is well positioned on the screen and magnified for good visibility. When the shot menu is opened, one can select the size of the collimators. The size of the collimator is selected based on the tumor shape and the gaps in coverage of the 50% isodose line displayed over the tumor. Shots are placed sequentially to cover the target as effectively as possible. Changing the position of the shots, adding additional shots, and adjusting the relative weight of shots quickly leads to a conformal dose plan.
The dose plan can be checked using Leksell GammaPlan with the three-dimensional (3D) image or the measurement tools, such as dose volume histograms. While the subject of conformity index is beyond the scope of this chapter, an excellent review of available methods has been published.11 Leksell GammaPlan indicates the point in the stereotactic space where a global maximal dose can be found. Leksell GammaPlan also calculates the individual shot times. Once the treatment plan has been determined to be appropriate by the gamma knife team (surgeon, radiation oncologist, and radiation physicist), the stereotactic coordinates and irradiation times are printed and used during the gamma knife treatment.
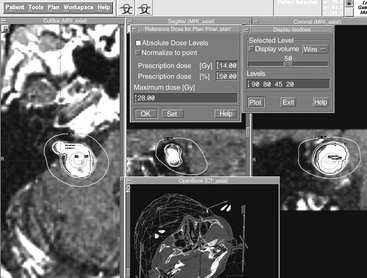
In the final plan, the peripheral dose is set to a value, which is assessed as optimal for a particular patient. Indication, size, and location of the target are taken into account, as well as clinical experience. The peripheral isodose is usually set to the 50% isodose line. This is exactly half the maximum dose in the target, referred to as the hot spot (Fig. 65-5). Along the 50% isodose line the dose gradient is usually the steepest ensuring sufficient dose within the target, while the dose level outside falls steeply, sparing the surrounding healthy tissue. Leksell Gamma Plan can also display the absolute dose values if desired. It will show the point in the stereotactic space where the global maximum dose can be found. With vestibular schwannoma it is valuable to complete this exercise, as the maximal dose at the “hot spot” should be positioned well away from the facial nerve and cochlea. In addition, plotting the absolute dose lines will help in determining the actual level of radiation delivered to surrounding structures.
GAMMA KNIFE SURGERY OUTCOMES
The University of Pittsburgh group has the largest clinical experience in treating vestibular schwannomas with gamma knife surgery. Lunsford and colleagues summarized their experience with 829 vestibular schwannomas treated between 1987 and 2002.12 This extensive clinical experience included an average tumor volume of 2.5 cm3 and a median margin dose to tumor of 13 Gy. They reported tumor control in 97% of patients at 10 years, and facial nerve (motor) dysfunction in < 1% of patients. Trigeminal nerve symptoms occurred in < 3% of patients and typically occurred with large tumors reaching the level of the trigeminal nerve. No reporting of balance function was included in their analyses.
The reporting of hearing preservation has limited representation in the entire 829 patients. Hearing outcomes data were presented in only 267 patients and “5-year actuarial rates of hearing level preservation and speech preservation” were reported in 103 patients. They reported “unchanged hearing preservation” in 50% to 77% of these patients, and this method of reporting auditory performance points to the difficulty in interpreting the outcome of most of the studies reporting hearing outcome in patients with vestibular schwannoma who have been treated with gamma knife surgery. They also stated that “for patients with intracanalicular tumors, hearing preservation rates in those treated with 12.5 to 14 Gy at the margin showed 90% preservation of serviceable hearing.”13 Unfortunately, pretreatment and longitudinal data are not available in these reports.
In an earlier series of 190 patients the average dose to the tumor margin was reduced to 13 Gy, and excellent tumor control was achieved at 97.1%.14 In this study, issues highlighted earlier with reporting of hearing outcome are equally apparent. They reported “hearing-level preservation” in 71 ± 4.7% of patients. They also reported a “preservation of testable speech discrimination ability” in 91 ± 2.6% of subjects. Obviously, testable speech discrimination ability is far different than useful hearing, and it is unfortunate that these authors did not report the actual auditory thresholds or speech discrimination ability. Most importantly, these were not reported as a function of time post-gamma knife surgery. In addition, they reported that “hearing levels improved” in 10 (7%) of 141 patients who exhibited decreased hearing defined as Gardner-Robertson grades II to V before undergoing gamma knife surgery. Based on our clinical observations and those of other centers, this picture is far more complex over time than is represented in these publications.
Prasad and colleagues from the University of Virginia reported their series of 200 vestibular schwannomas treated with gamma knife surgery over a 10-year interval in 2000.15 Of these patients, 153 patients had follow-up data including 96 with primary treatment and 57 with secondary treatment. They reported no hearing pre-gamma knife in 105 patients, including 53 of 96 primary treatment and 52 of 57 secondary treatment patients. The Gardner-Robertson grading system and subjective assessment of hearing was used; however, no pure-tone average or speech discrimination data were reported. Unfortunately, their data set included audiometric data from only 48 patients, and the intervals of audiometric testing were not reported. Despite these limitations, they found that, except for one patient, no change in hearing was observed in the first two years after gamma knife surgery. Their data also showed that the greatest change in Gardner-Robertson grade occurred between years two and four post-gamma knife; however, without understanding the assessment intervals, the precise onset of the hearing loss is unknown. No outcomes regarding balance function were reported.
Kim’s group at the Seoul National University reported the hearing outcomes in 25 patients with vestibular schwannomas with serviceable hearing.16 The median tumor volume was 3.0 cm3 (0.16 to 9.1 cm3), and the dose used was 12 ± 0.7 Gy at the 49.8 ± 1.1% isodose line. They reported the hearing outcomes using the Gardner-Robertson grading system, pure-tone averages, and speech discrimination scores. Pre-gamma knife, interim post-gamma knife, and last post-gamma knife data were reported. Similar to our experience, they found that in 16 patients the hearing deteriorated > 20 dB three to six months post-gamma knife and that this hearing loss continued for 24 months. The only prognostic factor for hearing deterioration that they identified was the maximum dose to the cochlear nucleus.
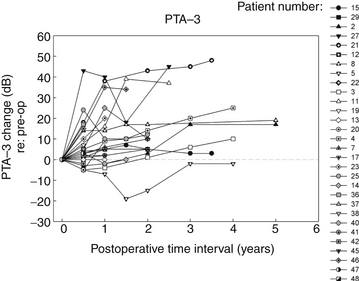
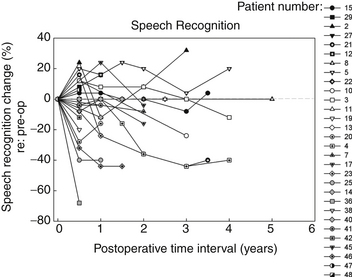
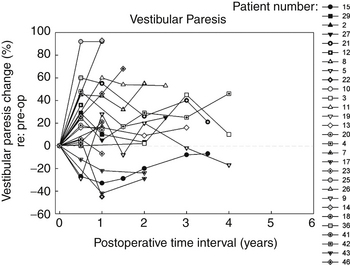
In the Medical College of Wisconsin Acoustic Neuroma and Skull Base Surgery Program, we have established a clinical pathway for all of our patients undergoing gamma knife surgery for primary or secondary treatment of their tumors. Pretreatment they undergo a complete videonystagmography test battery, a complete audiologic assessment, and facial nerve electromyography. At six-months intervals post-treatment, each patient undergoes a gadolinium enhanced MRI as well as an audiologic test battery and caloric testing to assess peripheral vestibular function. In addition to other standard reporting methods, we have also presented the data in a longitudinal manner for their objective auditory thresholds (Fig. 65-6), speech discrimination ability (Fig. 65-7), and degree of vestibular paresis (Fig. 65-8). We have recently published an expanded cohort of 54 patients with a median follow-up interval of 54.7 months.17 This report focused on the longitudinal outcomes in vestibular function and changes in the Dizziness Handicap Inventory before and after gamma knife surgery.
It is clear that most of the change in hearing and balance function occurs during the first six months after gamma knife surgery; however, continued but less rapid worsening of function can occur up to 12 months. These objective measurements correspond well to the transient facial nerve dysfunction, trigeminal nerve dysfunction, tinnitus, and dysequilibrium occurring in our patients with vestibular schwannomas undergoing gamma knife surgery.3,10,17 A possible mechanism underlying these changes is that there is an initial increased size of the tumor after radiosurgery. Typically this post-treatment edema persists for six months; however, this may remain for up to one year.3,10,17 The labyrinthine artery, a branch of the anterior inferior cerebellar artery, provides essentially all of the blood supply to the cochlea and vestibule and it is likely that the post-radiation edema compromises this blood supply to the inner ear. The resulting inner ear devascularization could certainly explain the rapid change in hearing and balance function seen at the six-month post-treatment assessment in our patients (see Figs. 65-6 and 65-8).
Several of our patients have had tumor control or regression and improvement of hearing and vestibular function. This is clearly divergent from the natural history of vestibular schwannomas. In contrast, worsening of auditory and vestibular function and the development of disequilibrium has occurred in a number of our patients. Continued systematic studies of these patients and expansion of the cohort of patients studied are important to determine the efficacy of gamma knife surgery and to compare to other forms of radiotherapy, as well as microsurgery and expectant management. Recognition of symptoms such as disequilibrium and knowledge regarding the expected time course of vestibular paresis progression are important not only for patient counseling but provide the opportunity to intervene with vestibular rehabilitation or nonspecific vestibular suppression until compensation has been completed, should this be needed clinically.17
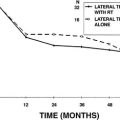
One final issue to consider is tumor growth after radiosurgery (Fig. 65-9). It is important to appreciate that there is an increased size of the tumor after radiosurgery. In fact, we observed a statistically significant increase in tumor size for patients whose tumors extended outside of the internal auditory canal six months after gamma knife surgery and a statistically significant decrease at one year post-treatment.3,10 Typically, post-treatment edema persists for six months; however, this may remain for up to one year. Consequently pretreatment counseling should include this information. There have been anecdotal cases discussed and occasionally reported that describe increased tumor size early after radiosurgery. The challenge is in making a decision about whether to resect these tumors and when.2,5,18–22 Pollock and colleagues emphasized the need to demonstrate sustained tumor growth by serial MRI before making the decision to operate and also to review the case with the surgeon who performed the radiosurgery before a surgical decision is made.5
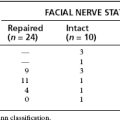
Another related controversy is whether facial nerve dissection and preservation are more difficult during microsurgical resection after radiosurgery. On one end of the spectrum, descriptions of no increased difficulty have been reported;5 and, on the other end of the spectrum,19–22 markedly increased difficulty in separating the tumor from the facial nerve and poorer facial nerve function outcome have also been reported. The report of Watanabe and colleagues included a histopathologic analysis of the resected facial nerve.20 They found microvasculitis of the facial nerve, axonal degeneration, loss of axons, and proliferation of Schwann cells. In light of the mechanism of delayed effects following radiosurgery, these findings are not surprising. Moreover, these findings emphasize the need for the neurotologist to be certain that the treatment plan avoids high radiation doses to the facial nerve. Recall as described earlier that a dose of 12 Gy delivered to the 50% isodose line means that the maximum tumor dose is 24 Gy. If the treatment plan delivers this maximal dose to the area of the facial nerve, it should be expected that greater radiation effects will be observed. For this reason, if the neurotologist and the patient have made a decision to resect a tumor previously treated with radiosurgery, it is important to review the treatment plan to determine the amount of radiation delivered to the facial nerve to counsel the patient appropriately preoperatively.
ALTERNATIVE TECHNIQUES
CyberKnife Stereotactic Radiology
Overview of Treatment Planning
The CyberKnife stereotactic radiosurgery system utilizes a compact 6-MeV linear accelerator, a computer-controlled robotic arm with six degrees of freedom, and an image-guidance technology that does not depend on a rigid stereotactic frame and thereby enables treatment of extracranial sites (Fig. 65-10). Potential benefits of this approach include: 1) increased access to and coverage of any target volume including the ability to treat lesions in and around the cranium that are unreachable with other systems, for example, in the lower posterior fossa and foramen magnum; 2) enhanced ability to avoid critical structures; 3) capability to treat lesions in the neck and spine; 4) ability to treat lesions throughout the body; 5) delivery of highly conformal dose distributions; 6) option of fractionating treatment; and 7) potential to target multiple tumors at different locations during a single treatment, e.g., skull base and neck.
Treatment Delivery
The CyberKnife system’s computer-controlled robotic arm has six degrees of freedom. The robot can position the LINAC to more than 100 specific locations or nodes. Each node has 12 possible approach angles, translating to over 1200 possible beam positions. The treatment planning system determines a set sequence of approach angles, beam weights, and dose distributions. The calculated plan can be incrementally improved by the physicist and physicians. The actual delivery follows a step-and-shoot sequence. The patient is placed in a position approximating that of the CT scan. Image detectors acquire radiographs of the tumor region. The image guidance system software then compares the real time radiographs with the CT information to determine location of the tumor. This information is transmitted to the robot to initialize the pointing of the LINAC beam. The robotic arm then moves the LINAC through the sequence of preset nodes surrounding the patient. At each node, the LINAC stops, and a new pair of images is acquired from which the position is determined again. Corrected position is transmitted to the robot which adapts beam pointing to compensate for any movement. LINAC delivers the preplanned dose of radiation for that position. The entire process is repeated at each node. The total time from imaging to robot compensation is about seven to 10 seconds. The total treatment time depends on the complexity of the plan and delivery paths but is comparable to standard LINAC treatments. Each treatment session ranges from 30 to 90 minutes. Physicians may elect to treat with a single dose, a hypofractionated dose typically of two to five sessions, or a more traditional fractionated regimen. Outcomes following CyberKnife treatment of vestibular schwannomas are emerging at this time.23
ALTERNATIVE APPLICATIONS
Although the majority of stereotactic radiosurgery performed by the neurotologist will be for vestibular schwannoma, other neoplasms and pathologies may be amenable to radiotherapy.24 Several of these were noted earlier in the section on patient selection and this section will focus on a few common pathologies for which the neurotologist may be the primary surgeon.
An evaluation of 42 patients with primary or recurrent/persistent glomus jugulare tumor undergoing gamma knife surgery showed excellent tumor response.25 Approximately 1/3 of tumors shrank and 2/3 showed no size change. A single 3.9 cm tumor was found to have increased 99 months after treatment with 12 Gy at the margin and was re-treated. Progression-free survival was 100% at seven years and 75% at 10 years. Six patients had complications related to treatment. Five of 26 patients with intact hearing at the time of treatment had subjective decline within the first year. Objective measures of hearing were not performed. One patient had facial parasthesias, one had vocal fold paralysis (the re-treated subject), one had vertigo and imbalance, and one had post-treatment migraine requiring admission.
A meta-analysis of glomus jugulare treatment and outcomes compared stereotactic radiosurgery to surgical resection.26 Neurological deficits in those treated with gamma knife, CyberKnife or LINAC showed no change in 58.2%, improved in 39% and permanently worsened in 2.8%. Such deficits included complaints of hearing loss, dizziness, dysphagia, voice change, shoulder dysfunction and headache. Overall, there was an 8.5% incidence of cranial nerve complication with 75% of these being transient. Permanent deficits occurred in three of 141 patients all of which involved facial motor dysfunction; none of which reached House-Brackmann grade VI. Tumor control was achieved in approximately 98% of individuals at 39 months.
Meningiomas are the second most common benign neoplasm of the cerebellopontine angle and can often present with deficits similar to vestibular schwannomas. Total resection results in excellent tumor control rates and, for all cranial locations, shows a 15-year progression-free survival rate of approximately 68% to 75%.27,28 Experience with partially resected or inoperable meningiomas, however, has shown that radiation therapy can produce excellent tumor control in the majority of cases. The use of stereotactic radiosurgery as a primary treatment to avoid or reduce the incidence of surgical and neurological deficits is increasingly common.
Elia and colleagues reviewed stereotactic radiosurgery outcomes for meningioma published since 2001.28 In over 1500 patients the 5-year progression-free control rate was 93.4%. The complication rate ranged from 2.5% to 13% and included neurological and vascular toxicities. Many of these were for tumors around the optic chiasm and carotid arteries and included dosages up to 20 Gy. Kreil and colleagues recently published their series on the treatment of 200 skull base meningiomas with gamma knife surgery.29 There were 21 patients with cerebellopontine angle lesions. Of 20 patients with preoperative hearing loss (not quantified), one improved and 19 remained stable; none showed deterioration. Tinnitus remained stable in seven of seven patients. Vertigo was present in 25 skull base meningiomas and improved in eight and worsened in none. Given the low incidence of complication and the high rate of tumor control, stereotactic radiosurgery should be strongly considered in tumors around sensitive neural structures and in patients medically unsuitable for conventional surgery.
In addition to tumors, the neurotologist is often consulted for facial pain syndromes, most notably trigeminal neuralgia. Functional stereotactic radiosurgery using gamma knife has been employed in the treatment of trigeminal neuralgia. In the series of meningiomas reported by Kriel there were 25 patients with preoperative trigeminal neuralgia due to tumor of which 16 improved.29 There were two induced cases of trigeminal neuralgia but these were transient. Such findings indicate that radiation to the trigeminal nerve can induce functional changes.
Gorgulho and De Salles reviewed surgical and stereotactic treatments for trigeminal neuralgia.30 Among current treatments, long-term improvement was noted in 70% to 75% of microvascular decompressions, 58% to 77% of radiofrequency rhizotomies, 32% of balloon compressions, 17% to 50% of glycerol rhizotomies, and 45% to 57% of stereotactic radiosurgeries. Immediate improvement was noted in over 90% of patients with stereotactic radiosurgery. Recurrence rates were highest with glycerol rhizotomy and much lower and very similar among the other modalities. Stereotactic radiosurgery was noted to be particularly attractive because it is the least invasive of these methods.
Many different treatment protocols for trigeminal neuralgia have been attempted.30 In their review, Gorgulho and De Salles identified several patterns with regards to gamma knife treatment for trigeminal neuralgia that affect outcomes. The root entry zone of the trigeminal nerve, not the nerve proper, should be the preferred target as dosage delivery to this area seems to correlate with pain relief. A minimal dosage of 70 Gy and maximal dosage of 90 Gy should be prescribed. The incidence of post-treatment numbness with this prescription dose ranges from 3% to 55% but bothersome numbness persists in only about 4% to 12%. Treating a longer section of the trigeminal nerve proper does not improve pain control and increases the incidence of post-treatment numbness. Likewise, higher dosage to the nerve does not improve pain control and increases numbness. The overall incidence of complications with stereotactic radiosurgery for trigeminal neuralgia is significantly lower than all other techniques. As with other benign diseases, potential long-term effects of radiation treatment need to be considered in younger individuals.
FINANCIAL DISCLOSURE
None of the authors has a financial interest in any of the companies discussed in this chapter.
1. Kondziolka D., Lunsford L.D., McLaughlin M.R., Flickinger J.C. Long-term outcomes after radiosurgery for acoustic neuroma. N Engl J Med. 1998;339:1426-1433.
2. Wackym P.A. Stereotactic radiosurgery, microsurgery, and expectant management of acoustic neuroma: basis of informed consent. Otolaryngol Clin North Am. 2005;38:653-670.
3. Wackym P.A., Runge-Samuelson C.L., Poetker D.M., et al. Gamma knife radiosurgery for acoustic neuromas performed by a neurotologist: early experiences and outcomes. Otol Neurol. 2004;25:752-761.
4. Bari M.E., Forster D.M., Kemeny A.A., et al. Malignancy in a vestibular schwannoma. Report of a case with central neurofibromatosis, treated by both stereotactic radiosurgery and surgical excision, with a review of the literature. Br J Neurosurg. 2002;16:284-289.
5. Pollock B.E., Lunsford L.D., Kondziolka D., et al. Vestibular schwannoma management. Part II. Failed radiosurgery and the role of delayed microsurgery. J Neurosurg. 1998;89:949-955.
6. Rowe J., Grainger A., Walton L., et al. Risk of malignancy after gamma knife stereotactic radiosurgery. Neurosurgery. 2007;60:60-65.
7. Rowe J., Grainger A., Walton L., et al. Safety of radiosurgery applied to conditions with abnormal tumor suppressor genes. Neurosurgery. 2007;60:860-864.
8. Lustig L.R., Jackler R.K., Lanser M.J. Radiation-induced tumors of the temporal bone. Am J Otol. 1997;18:230-235.
9. Hanabusa K., Morikawa A., Murata T., Taki W. Acoustic neuroma with malignant transformation. Case report. J Neurosurg. 2001;95:518-521.
10. Poetker D.M., Jursinic P.A., Runge-Samuelson C.L., Wackym P.A. Distortion of magnetic resonance images used in gamma knife radiosurgery treatment planning: implications for acoustic neuroma outcomes. Otol Neurotol. 2005;26:1220-1228.
11. Paddick I. A simple scoring ration to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Suppl 3):219-222.
12. Lunsford L.D., Niranjan A., Flickinger J.C., et al. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195-199.
13. Niranjan A., Lunsford L.D., Flickinger J.C., et al. Dose reduction improves hearing preservation rates after intracanalicular acoustic tumor radiosurgery. Neurosurgery. 1999;45:753-762.
14. Flickinger J.C., Kondziolka D., Niranjan A., Lunsford L.D. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001;94:1-6.
15. Prasad D., Steiner M., Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:745-759.
16. Paek S.H., Chung H.-T., Jeong S.S., et al. Hearing preservation after gamma knife radiosurgery of vestibular schwannoma. Cancer. 2005;104:580-590.
17. Wackym PA, Hannley MT, Runge-Samuelson CL, et al: Gamma knife surgery of vestibular schwannomas: Longitudinal changes in vestibular function and measurement of the Dizziness Handicap Inventory. J Neurosurg 109(Suppl):137-143.
18. Pitts L.A., Jackler R.K. Treatment of acoustic neuromas. N Engl J Med. 1998;339:1471-1473.
19. Ho S.Y., Kveton J.F. Rapid growth of acoustic neuromas after stereotactic radiotherapy in type 2 neurofibromatosis. Ear Nose Throat J. 2002;81:831-833.
20. Watanabe T., Saito N., Hirato J., et al. Facial neuropathy due to axonal degeneration and microvasculitis following gamma knife surgery for vestibular schwannoma: a histological analysis. J Neurosurg. 2003;99:916-920.
21. Lee D.J., Westra W.H., Staecker H., et al. Clinical and histopathologic features of recurrent vestibular schwannoma (acoustic neuroma) after stereotactic radiosurgery. Otol Neurol. 2003;24:650-660.
22. Friedman R.A., Brackmann D.E., Hitselberger W.E., et al. Surgical salvage after failed irradiation for vestibular schwannoma. Laryngoscope. 2005;115:1827-1832.
23. Chang S.D., Gibbs I.C., Sakamoto G.T., et al. Staged stereotactic irradiation for acoustic neuroma. Neurosurgery. 2005;56:1254-1261.
24. Knisely J.P.S., Linskey M.E. Less common indications for stereotactic radiosurgery or fractionated radiotherapy for patients with benign brain tumors. Neurosurg Clin N Am. 2006;17:149-167.
25. Pollock B.E. Stereotactic radiosurgery in patients with glomus jugulare tumors. Neurosurg Focus. 2004;17:63-67.
26. Gottfried O.N., Liu J.K., Couldwell W.T. Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors. Neurosurg Focus. 2004;17:22-30.
27. Goldsmith B., McDermott M.W. Meningioma. Neurosurg Clin N Am. 2006;17:111-120.
28. Elia A.E., Shih H.A., Loeffler J.S. Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus. 2007;23:1-9.
29. Kreil W., Luggin J., Fuchs I., et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76:1425-1430.
30. Gorgulho A.A., De Salles A.A.F. Impact of radiosurgery on the surgical treatment of trigeminal neuralgia. 2006;66:350-356.