Chapter 104 Stereotactic Radiosurgery Meningiomas
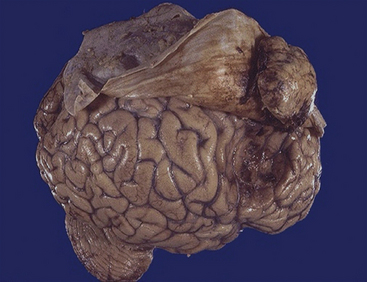
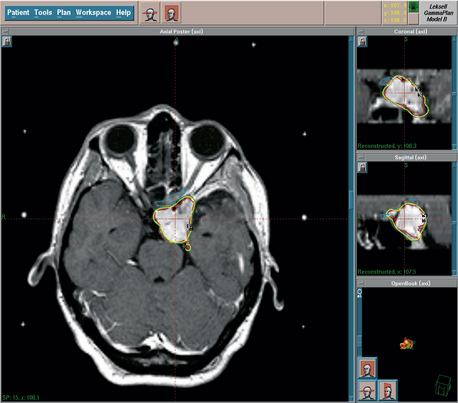
Meningiomas arise from the arachnoid cap cells, or “meningothelial” cells, and are the most frequently reported neuro-oncologic challenge, accounting for 16% to 30% of intracranial tumors,1–6 and for 22% to 25% of spinal cord tumors.7,8 Furthermore, a non-negligible percentage of them is diagnosed on the basis of imaging alone,3,9,10 and an additional 2.3% in autopsy reports (Fig. 104-1).3,11 With the growing contribution of imaging and necroptic observations the relative percentage might become higher.
Epidemiology
The average annual incidence is five to six new cases per 100,000,1–6,12,13 with the age-related risk drastically increasing from the pediatric population to a peak during the sixth and seventh decades.14,15 It is worth stressing that more aggressive clinical and histologic features have been observed in children and adolescents.14 The female/male ratio is approximately 2:1 to 3:1,3,16–18 and this prevalence is presumably related to as yet poorly known progesterone- and estrogen-receptor–mediated cytoactivation.3,15,19
As to their natural history, the few reported series of conservatively managed symptomatic meningiomas have documented a consistent trend to progression in one third of the patients, although with a wide spectrum of variability.20–28 In fact, in a non-negligible percentage, either spontaneously or after subtotal resection, the annual growth rate may suddenly increase up to several millimeters documenting an unexpected aggressiveness.29–32 The main factors associated with growth are younger age and T2-hyperintensity, and presence of calcifications.20 Both growth and recurrence rates are higher in grades 2 and 3 meningiomas29,33,34 and this justifies the advocated multidisciplinary treatments.1–3 Finally, although in anecdotal cases, post-surgical or radiosurgical remnants of a “benign” meningioma have been shown to undergo spontaneous or “induced” malignant cytologic transformation, subsequently confirmed at reoperation.30,35,36
Citopathology
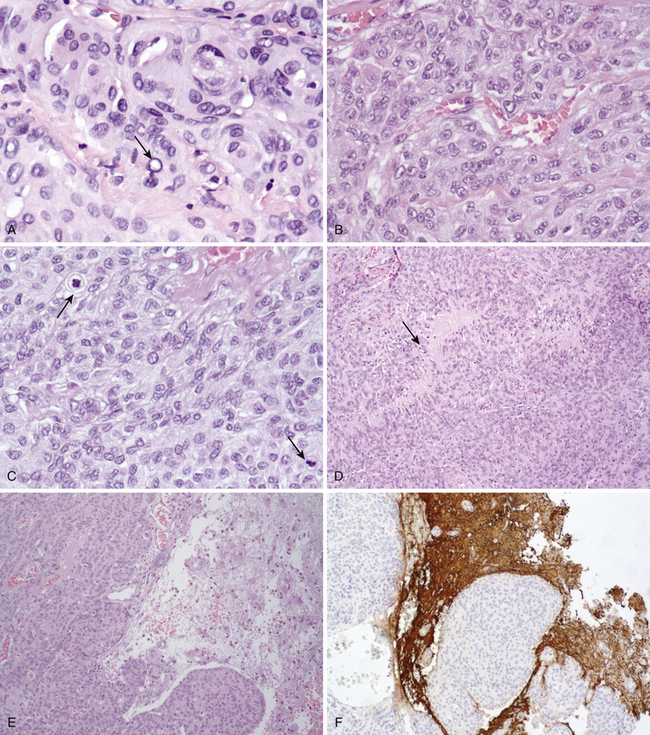
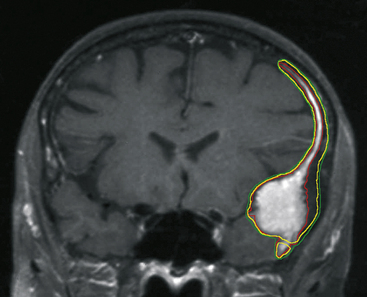
During the last decade, the pathologic criteria for the definition of “atypical” as well as of “anaplastic” meningiomas have been gradually expanded to include not only nuclear pleomorphism, mitoses, atypia, necrosis, and so on, but also other relevant landmarks of parenchymal invasion such as the formation of small cell infiltrates, arachnoidal disruption, sheeting, macronucleoli focal malignant macrodifferentiation (e.g., glial fibrillary acidic protein (GFAP) production, melanocytic foci, etc.), and extra-axial diffusion (Fig. 104-2).2 According to the Mayo Clinic Scheme,2,36,37 substantially endorsed by recent revisions of the World Health Organization (WHO) (Table 104-1),2 these specific parameters may be associated with malignant behavior regardless of their histologic subtype.2 In fact, the very peculiar metastatic potential of these tumors seems to confirm the mentioned cytobiological discrepancy, since histologically benign meningiomas have sometimes shown biological aggressiveness, not only in terms of craniospinal seedings, but also of extraneural colonization (lung, liver, bone, lymph nodes).2,7,38,39 However, the vast majority of these tumors are considered benign or grade 1 in the WHO classification,2,40 currently accounting for a 5-year survival rate exceeding 80%.1,41,42 Atypical (grade 2, 4.7-7.2%, prototypes: clear cell and chordoid varieties) and anaplastic or malignant (grade 3, 1.0%-2.8%, prototypes papillary and rhabdoid) forms are rarer2,40,43–45 and usually bear a worse prognosis, with reported 5-year survivals of 32% to 64%.1,36,42,46–50 Monosomy of chromosome 22 is the most common cytogenetic alteration in the overall oncotype population.51,52 Nearly all neurofibromatosis type 2 (NF2)–associated meningiomas have mutations of the NF2 gene on 22q12.3,51 Moreover, a stepwise change in the genetic characteristics of benign meningiomas undergoing anaplastic transformation has been observed: The loss on 22q, 1p, 6q, 10, 14q, and a gain on 9q, 12q, 15q, 17q, may frequently parallel this evolution as well as amplification on 17q and loss on 9p (CDKN2 gene).2,53–55 Such a theory of correlative clinical-pathologic malignant progression is supported by atypical or anaplastic recurrences of formerly benign lesions, but the oncologic rationale is still debated.2,56
Table 104-1 Meningiomas Grouped by Likelihood of Recurrence and World Health Organization Classification
| Meningiomas with Low Risk of Recurrence and Aggressive Growth | Meningiomas with Greater Likelihood of Recurrence and/or Aggressive Behavior | |
|---|---|---|
| Grade I | Grade II | Grade III |
| Meningothelial Fibrous (fibroblastic) Transitional (mixed) Psammomatous Angiomatous Microcystic Secretory Lymphoplasmacyte-rich Metaplastic |
Atypical: Clear cell/chordoida • ≥ 4 mitoses/10 HPF (≥2.5/mm2) • Or at least three of the following four features: • Sheeting • Macronucleoli • Small cell formation • Hypercellularity (≥53 nuclei/HPF, ≥118/mm2) • Or Brain invasion Clear cell (intracranial) Chordoid |
Anaplastic (Malignant): Rhabdoid, Papillary, etc. Papillary Anaplastic (malignant)a • ≥20 mitotic figures/10 HPF (≥12.5/mm2) • Or: • Focal or diffuse loss of meningothelial differentiation resulting in carcinoma-, sarcoma-, or melanoma-like appearance Meningiomas of any subtype or grade exhibiting high proliferation indices or brain invasion |
Note: World Health Organization meningioma grading according to aggressive behavior (i.e., probability of recurrence).
a Mayo Clinic meningioma grading scheme.
HPF, high-power microscopic fields.
Treatment Options
Surgery
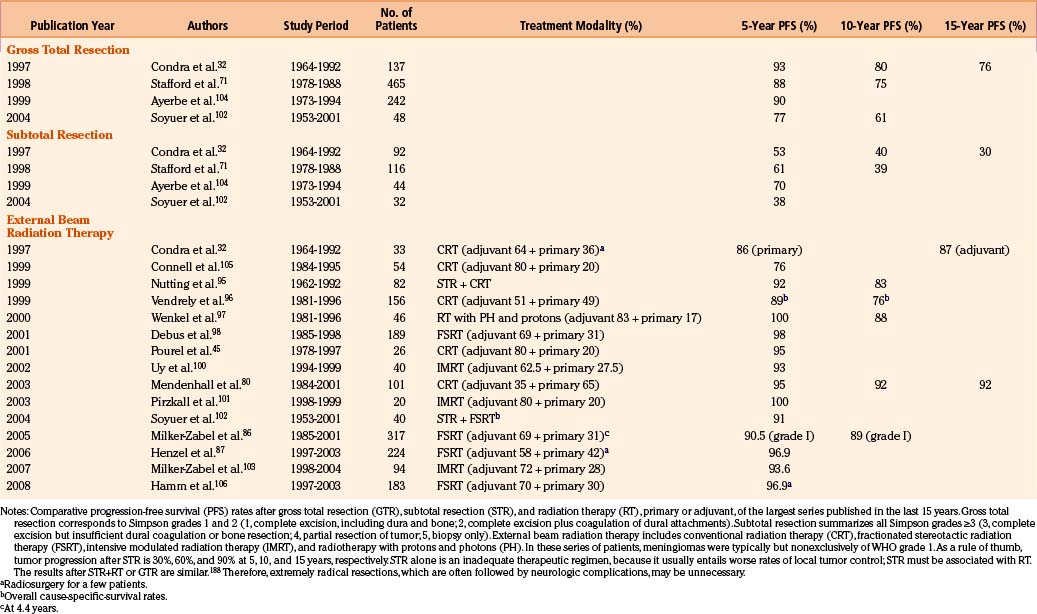
There is general agreement that the optimal treatment for these lesions, whenever feasible, should be a Simpson grade I resection of the tumor—carefully pre-embolyzed if necessary—providing definitive diagnosis, reducing immediately any mass effect, and alleviating clinical signs and symptoms.2,57,59,60 Such a golden therapeutic standard is still the dominant option in the vast majority of convexity meningiomas in fronto-orbital and spinal locations, whereas surgical results are less satisfactory in intraparenchymal or cranial base lesions2,59–62 and particularly grim in grades 2 and 3.3 Unfortunately, despite surgical advances, when these tumors are infiltrating the skull base, cranial nerves, or vascular structures, complete resection may not be feasible without unacceptable morbidity rates. Considering more recently published series, gross removal of basal meningiomas was achieved in 60% to 87.5% of the patients,59–65 with 30% to 56% of severe complications,58,60,65–67 mostly represented by newer or deteriorated pre-existing cranial nerve deficit, occurring temporarily in 20% to 44%, and permanently in 16% to 56% of the cases. Median postoperative mortality rate was 3.6% (range 0%–9%).59–68 Moreover, local recurrence rates are strictly dependent on Simpson’s grade69: at 10-year follow-up, 10% to 33% (average 20% to 25%) after complete resection (Simpson 1–2); and 55% to 75% (average 60%–62%) after partial resection, that is, Simpson >2 (Table 104-2).20,32,70–74 The relevance of these findings may probably explain why, particularly in atypical-anaplastic varieties, adjuvant therapies and sometimes preplanned, combined multidisciplinary approaches may be advocated, to avoid early recurrences and repeat surgery, thereby reducing morbidity.2,12,35
Fractionated Radiotherapy
External beam radiation therapy (EBRT) has been used for decades, either as a primary or as an adjuvant treatment, with documented improvement in local control and rarely in recurrences (see Table 104-2).12,41,42,75–80 In more aggressive cytotypes, most authors seem to favor administering EBRT—mostly Fractionated Stereotactic Radiotherapy (FSRT) early to patients who have undergone subtotal and even total resections.2,3,12,42,43,45,81 FSRT has proven to be successful in primary (imaging defined) meningiomas,12,82–87 particularly in all cases with crucial exposure of extremely radiosensitive structures such as the optic pathways.12,88–91 Series reports comparing progression-free survival (PFS) for patients treated with gross/total removal and subtotal removal (STR), with or without EBRT, have consistently shown the best results with the radical surgery or STR plus radiation, whereas STR alone turned out to be less valuable.3,32,33,41,42,45,70,71,76–78,85,92–107
Stereotactic Radiosurgery
According to the historical definition by Lars Leksell stereotactic radiosurgery (SRS) is “a technique of closed skull destruction of a predetermined intracranial target by a single-fraction high dose of ionizing radiation using a precision stereotactic apparatus”.108 In contrast with spatially less accurate conventional radiotherapy, radiosurgery has the capacity to maximize/optimize the dose exposure on the target volume, while minimizing the irradiation of surrounding critical structures, thereby reducing collateral damages. The concept of delivering a high dose of radiation energy to treat focal pathologic lesions fits all criteria for minimal invasiveness and has gradually become a powerful and attractive therapeutic strategy for many neurosurgical disorders. Recently the impressive advances in neuroimaging, stereotactic techniques, and robotic technology have further improved results, expanding the spectrum of applications. This approach may be increasingly considered not only as potentially adjuvant to microsurgery but also as a valuable alternative option.
Lars Leksell designed the first arc centered device in 1948,109 and in 1951 he introduced the term and concept of radiosurgery.108,109 His first stereotactic instruments were suitable for replacing a probe (needle electrode) by cross firing intracerebral structures with narrow beams of radiant energy. X-rays were first tried, but both gamma rays and ultrasonic rays were included as alternatives. In close collaboration with Borje Larsson, a physicist at the synchrocyclotron unit in Uppsala, Leksell performed the original experiments with highly focused high-energy proton irradiation of human malignancies.110 As a rule, precise, well-limited lesions were produced, but the synchrocyclotron proved to be too complicated for widespread clinical use. This compelled Leksell to consider other radiation sources and he started designing a 179-source 60Co gamma unit that was fully integrated into the stereotactic system; the first unit was inaugurated in 1967.110 Radiosurgery was initially developed with the aim to offer a bloodless and less risky method, essentially for functional treatments. However, within a few years the machine proved to be extremely effective in a variety of intracranial lesions, provided that the rationale of the approach, that is, the fundamentals of the technique, had been respected. Briefly, the latter are limited target volume, well-defined imaging, compatible site, and adequate cytology. As shown in the following reported experiences, to date it is possible or nearly possible to overcome each of the following constraints: tackling larger volumes with lower dosages,111,112 crucial locations with staged procedures, and, perhaps in the near future, radioresistant oncotypes by means of radiosensitizers.
In recent decades, SRS techniques, particularly gamma knife radiosurgery (GKR), have progressively gained an unquestionable momentum in the therapeutic armamentarium for most brain tumors, particularly for well defined lesions, such as meningiomas. The main reasons for this growing role may be summarized as follows: (1) targeting update, with the advent of computerized and coregistered, morphofunctional neuroimaging; (2) the availability of newer, more powerful and precise irradiation devices; (3) the introduction of computer-guided dose planning; and (4) a deeper radiobiological experience. To date, an estimated half a million people have been treated by GKR worldwide at a continuously increasing annual rate (in 2009, roughly 50,000 patients were treated). In addition, approximately 200,000 patients worldwide have experienced SRS with other dedicated machines. Elective indications currently include metastatic brain tumors, benign endocranial tumors (meningiomas, neuromas, pituitary adenomas, etc.), low-grade neuro-ectodermal tumors, vascular malformations, and some types of functional neurosurgery (e.g., trigeminal neuralgia). Finally, it is generally accepted that the putative mechanism of action of SRS is intimately dependent on the main technical variables (dose–volume integral, timing, target cytology), as well as the goals that we are pursuing (tumor growth control [TGC], necrotic evolution, ephaptic block, etc.). Regarding meningiomas, routine protocols are focused on TGC, probably obtained through a combined mechanism, such as: 1, direct cytotoxicity, presumably promoting apoptosis; 2, damage to the vascular supply, mediated by inhibition of growth factors (vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), factor 8, etc.); and 3, inactivation/destruction of hormonal receptors (e.g., octreotide [OCT] receptor).113,114
Targeting Update
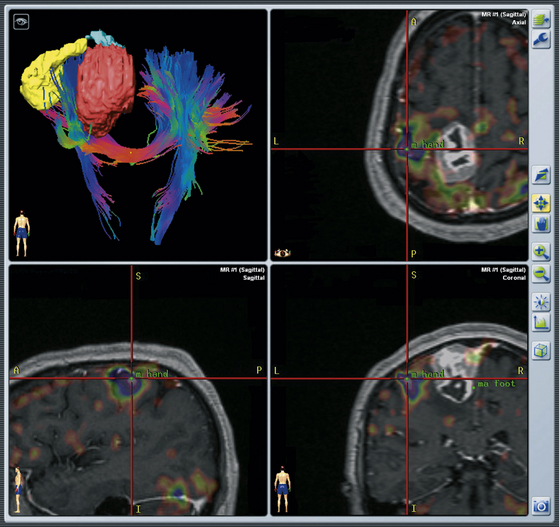
The development of SRS has closely followed the impressive evolution of neuroradiologic techniques. The best example is perhaps the exponential increase of radiosurgical indications after the introduction of computerized imaging, particularly magnetic resonance imaging (MRI). Indeed, for routine SRS procedures, stereo-MRI remains a mainstay in target localization. Coregistered multimodality acquisitions (MRI, computed tomography [CT], angiography, positron emission tomography [PET] scan) have led on one side to the development of fusion algorithms based on contour definition, signal intensity, and voxel matching that allow detailed, interpolated 3D and 4D pictures, speeding up all the preoperative procedures. On the other, advances in computerized integration of stereotactic and nonstereotactic imaging, including functional MRI and diffusion tensor imaging (DTI), are opening unexpected frontiers to SRS treatments.115 Improving safety and efficacy of radiosurgical planning is now possible via fusing the conventional sequences of a stereotactic MRI with the three Tesla pictures of corticospinal, visual, or arcuate DTI, with the aim of preserving the specific tract from undue damage (Fig. 104-3).116 CT and MRI scans sometimes may not adequately identify the regions of functional interest surrounding the planned target. Coregistration of functional MRI becomes mandatory, albeit sometimes still inadequate. Molecular imaging may be the appropriate integration. Using stereo-PET scan metabolic mapping, with FDG or a spectrum of amino acids, particularly fluorodopa and 11C-methionine,117,118 can be merged with conventional pictures, thereby helping to identify the borders of the lesion as well as the metabolically active sites of the tumor or necrotic/radionecrotic foci on the basis of the differential uptake.119,120 Moreover, PET techniques allow us to differentiate the usually octreotide-rich surfaces of meningiomas from other skull base tumors that generally lack these receptors.113,114
Irradiation Techniques and Modalities
1. A single fractionated high dose is used, with very few exceptions, as opposed to multiple small daily fractions.
2. The use of stereotactic technology entails considerable accuracy of the treatment (with a standard mechanical error of 0.3 mm).
3. The dose gradient is extremely steep; thus the resulting lesions are very sharply circumscribed. As a consequence, exposure outside of the target volume is minimized.
4. Radiotherapy relies on the difference in susceptibility between tumor tissue and normal brain tissue, whereas radiosurgery delivers an ablative dose to the target margin with a higher dose delivered centrally.
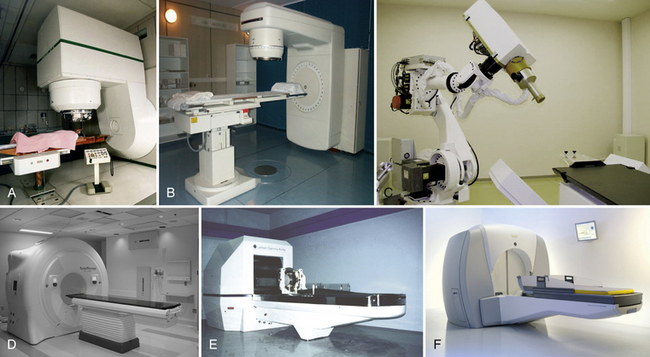
Currently stereotactic radiosurgery techniques may utilize different types of penetrating energy, including cyclotron- or synchrotron-generated particles such as protons or heavy-charged particles, and photon devices such as modified linear accelerators (LINACs) or the gamma knife (GK) (Fig. 104-4).
Proton Beam
High-energy protons represent an extremely important alternative option to photon and electron beams. In fact, proton beams offer the advantage to improve tumor control, especially for treating small volumes, where it is necessary to obtain a localized high-dose distribution in small deep-sited locations, while relatively sparing the surrounding normal tissue.2,12 The tissue depth at which the Bragg ionization peak is maximal can be adjusted by cross-firing systems according to the target position.
Linear Accelerator
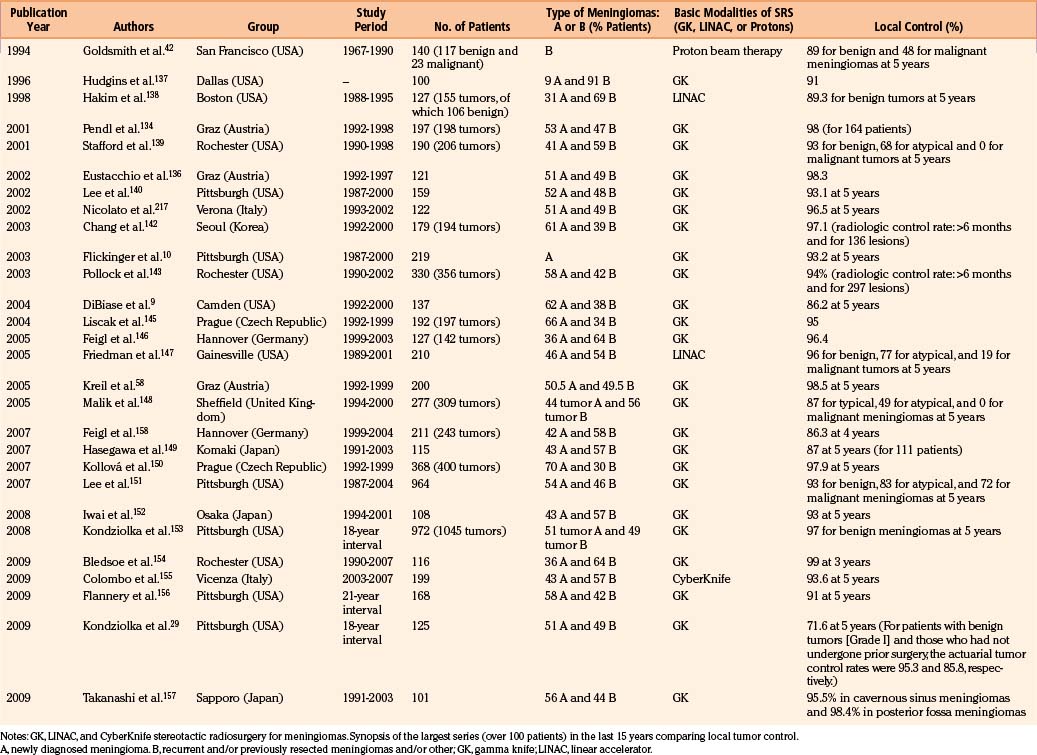
Linear accelerator–based radiosurgery uses x-ray beams that are produced by the collision of accelerated electrons with a metal target (see Fig. 104-4). Multiple noncoplanar arcs converge at a single isocenter, where a nearly sphere-shaped dose distribution is created. These arcs are produced by gantry rotation during irradiation, and each is defined by a different couch angle. By adjusting the number, length, angles, and weights of individual arcs, irradiation of adjacent crucial structures can be reduced. Most LINAC radiosurgery units (e.g., Synergy and Axesse by Elekta, Stockholm; Trilogy by Varian, Palo Alto, CA; Novalis by Brain Lab, Feldkirchen, Germany) use tertiary circular collimators that further decrease beam divergence, thereby protecting normal tissues. For higher conformality while departing from a spherical shape, multiple isocenters are possible, at the cost of greater inhomogeneity and much longer treatment times. This technique has proved to be an effective, alternative radiosurgical option, particularly in cases of larger target volumes, or whenever fractionated radiosurgical procedures are required.2,12,121 Comparative analyses of the literature concerning LINAC versus GK results in meningiomas, however, still show on one side similar tumor control indexes, and on the other, much higher complication rates with LINAC, with comparable or shorter follow-up periods (Table 104-3).9,29,42,58,63,122–159
CyberKnife
This extremely refined instrument was conceived in the mid-1980s and manufactured within a decade. In its 15-year history, the CyberKnife (Accuray, Sunnyvale, CA), has clearly shown basic pros and cons of the technique.121,155,160–163 Regarding the former, the main advantages include (1) easier fractionation, that is, increased protection of sensory cranial nerves,162 and so on; (2) no need of general anesthesia even in young patients; and (3) flexibility to treat lesions throughout the body. Regarding the latter, the absence of a stereotactic frame, as well as the mobility of the radiation source should somehow entail a slightly superior error margin if compared to the gamma knife. It is well known that CyberKnife operative programs are essentially based on CT scan imaging recognition. For certain targets, this might involve less sophisticated imaging.
The CyberKnife (see Fig. 104-4) combines a lightweight 6-MeV LINAC designed for radiosurgery and mounted onto a highly maneuverable robotic arm that can position and point the LINAC. Internally controlled real-time image guidance eliminates the need of skeletal fixation for either positioning or rigid immobilization of the target. This system acquires radiographs of skeletal features associated with the treatment site, uses image registration techniques to determine the target’s coordinates with respect to the LINAC and to the manipulator, which finally directs the beam to the planned point. Whenever the patient moves, internal controls detect the change, stop radiation, correct the trajectory of the beam, and start irradiating again nearly in the real time. Complex radiosurgical treatments may be performed, in which beams originate at arbitrary points in the workspace to target arbitrary points within the lesion. Even nonisocentric beams can be focused anywhere within a volume around the center. Total treatment time depends on the complexity of the plan and delivery paths but it is comparable to standard LINAC treatments.
Tomotherapy
Helical tomotherapy unit is a new modality for radiation treatments, the first dedicated to intensity-modulated irradiation using a fully integrated image-guided radiotherapy machine with on-board megavoltage CT capability. The tomotherapy system (see Fig. 104-4) uses a 6-megavoltage accelerator, and a 64-leaf binary multileaf collimator and xenon image detector array mounted on a rotating gantry. Radiation is delivered in a helical way, obtained by concurrent gantry rotation and couch/patient movements. Altogether, these components allow continuous, intensity-modulated rotational irradiation with fan beam entry from 360 degrees. A megavoltage CT scan before the treatment (nominal energy of 3.5 megavoltages) can be fused with a planning CT scan to determine the correct patient setup every time that the radiotherapist needs to check it. Finally, ultimate dose calculation is refined by means of convolution/superimposition. Tomotherapy has been used for the treatment of benign brain tumors (meningiomas and neurinomas), resulting in good target coverage with high-dose homogeneity and respecting organs-at-risk constraints.164 In addition, this technique allows treatment of larger brain lesions than GKS. Once again the most important difference seems to be that the latter still maintains a better conformity index, and non-negligible advantages in terms of the integral dose to the brain.
Gamma Knife
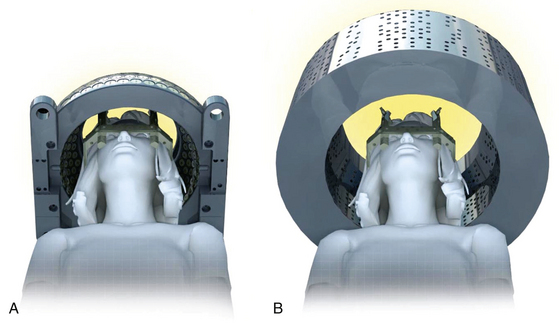
Depending on the model, the gamma knife (GK) (Elekta, Sweden) contains an array of 201 (Model B or C) or 192 (Perfexion) (see Fig. 104-4) individual 60Co sources aligned with a collimation system that directs each of the radiation beams to a very precise focal point. As a consequence, even very small targets can be treated by a high radiation dosage, whereas peripheral dose levels remain low. GK treatments are therefore quite heterogeneous in terms of dose distribution inside and outside of the target; tumor control and tissue sparing is achieved via the steep dose gradient at the periphery rather than exploiting the radiobiological differential between normal and pathologic tissue as in fractionated radiotherapy. At installation, GK units have an initial dose rate of approximately 4 Gy/min. Given that the 60Co half-life of 5.28 years, the primary sources must be replaced every 6 to 7 years. Due to the low energy of the isotope sources, most treatments are referred to a normalization of 50%. With the multiple isocenters routinely used, GK plans tend to be more conformal but less homogeneous than LINAC-based plans. Due to the typically steep dose gradients of radiosurgery, an accurate alignment of the plan’s isocenter with the physical isocenter of the machine is a quite challenging issue.12 Details of the structure and operation of the central body and collimation system are among the biggest differences between the Perfexion Gamma Knife (GKPx) and earlier B and C models. While the external hemispheric helmet in the B and C models is fundamental in providing the final beam collimation, in the Perfexion model the central body and collimation system are somewhat more complex because the 60Co sources are mobile and the conical collimator body is entirely internal to the unit, so that there are no external helmets (Fig. 104-5). Moreover the surgeon can modify the shooting collimator size using the sector drive motors that move the sources along their bushing to the correct position; the localization of each sector is monitored by linear and rotational encoders characterized by a positioning repeatability of less than 0.01 mm.165,166 Critical structures such as optic pathways and cranial nerves may be shielded through the application of beam blocking patterns that minimize the contribution of possibly dangerous shots.
Dose Planning
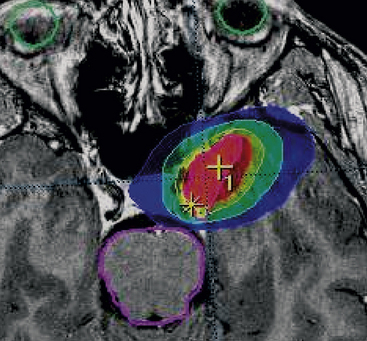
The goal of dose planning is to create an extremely conformal and selective isodose configuration, with complete covering of the tumor, and minimal exposure of the surrounding tissues (Fig. 104-6). Using a combination of multiple isocenters of different sizes, differential weighting of the crucial shots, selective blocking of the collimated beams and—typical of the Perfexion Gamma Knife—using hybrid shots,165 it is possible to produce dose plans that closely conform not only to the main shape of the meningioma, but also to all the MRI-documented dural attachments (Fig. 104-7), while sparing the neighboring neural structures. Probabilistic models, quadrature-sum analysis, and phantom studies have repeatedly confirmed the reliability of such operative models.107,108,166 These newer approaches in treatment planning have consistently improved Paddick’s conformity index,167 meanwhile reducing treatment times for both GKR and GKPx88,165,169 and for MML LINAC.88,168 To date, the recommended surface (SD), edge (ED), or peripheral (PD) doses for meningiomas range from 11 to 15 Gy at the 50% isodose; the higher levels are currently reserved for more aggressive histotypes.2,3 The “ideal”—that is, the most biologically justified—planning target volume, is still a matter of debate, with a spectrum of options, ranging from including the gross, T1 contrast–enhancing image plus a supposedly infiltrated margin of a few millimeters,3,32,41,98,103,170 to the controversial inclusion of the “dural tail” that—according to studies of extremely refined doses—should be essentially composed of hypervascular dura with none of the expected tumor colonies.3,92,171,172 Another deceptive variable in the definition of the target volume is represented by hyperostosis,3,173,174 particularly after the studies of Pieper et al.174 showing that (in a series of 26 consecutively operated patients) hyperostotic bone was almost constantly (25/26) present, even without any imaging evidence. In these cases, ablative radiosurgery on the hyperostotic bone might have the same meaning of Simpson’s grade 1 in surgical approaches. As mentioned previously, the novel and impressive advances in radiosurgical treatment strategies, particularly coregistration and fusion algorithms–dose-planning software, and automatic positioning systems are helping to overcome some of the major SRS, and specifically GKR, constraints. We can reasonably target unusually larger volumes, even in crucial sites, by using lower dosages or adopting “staged” SRS procedures, via means of dose and/or volume fractionation.111,112
Published results are interesting, and there is no evidence of increased adverse radiation effects (AREs).111,112,152 In fact, the well-known relationship between the dose–volume integral and the risk of adverse radiation effects does not seem to apply at lower-dose regimens.135,152,175,176 Nonetheless, in meningioma treatments, relevant limits, pitfalls, and risks remain to be tackled. An instructive example is represented by dosimetry planning for cavernous sinus meningiomas (Fig. 104-8). The dose heterogeneity of these treatments usually requires an extremely careful evaluation of dose-distribution algorithms,177–179 because of the occasional reported cases of radio-induced vascular injury to the carotid arteries,177–179 the still observed morbidity of this technique on sensory nerves,151 and the sometimes disappointing results in atypical and anaplastic lesions.2,151 Finally, a controversial issue in treatment planning pertains to the so-called “radiation-induced meningiomas.180–182 These tumors usually appear at a variable interval from radiation exposure, depending on the dose and timing. They are non-infrequently deep seated, multifocal in 4.6% to 18.7% of the cases.180,181 Surgical resection is considered to be the best therapeutic option. However, when dealing with frail patients or with particularly crucial locations, radical surgery may be too risky, opening the question on the SRS alternative. The published literature is rather scanty.180,182 Eligibility criteria (limited volume, well-defined imaging, etc.) adopted in these cases do not differ from standard protocols. Clinical-radiologic results—particularly the LTC (75% to 78%) and the 5-year PFS (similar)—are satisfactory, and only slightly inferior to the published data regarding regular meningiomas. Morbidity remains low (5%) with no reported cases of newer carcinogenesis.180–182
Radiobiology
Radiosurgery, like most radiation treatments, results in the formation of free radicals as electrons are freed from their atoms. Their main biological effect occurs at the DNA level: The transfer of energy results in breaks in the DNA strands (direct effect). Additional radiation damage to the DNA is mediated through reactive species of water (indirect effect). Whenever single-strand breaks occurs, the aberrations are of little eventual consequence, because the breaks are easily repaired. Conversely, lethal aberrations may occur leading to mutagenesis, or to cell death, due either to double-strand breaks or to chromosome breaks with the “sticky ends” rearranging and rejoining in grossly distorted, nonviable formations. The number of lethal aberrations and subsequent killed cells is closely related to several conditioning factors: the specific oncotype and a complex series of cellular parameters (“alpha–beta ratio,” superoxide-enzyme characterization, etc.) defining the specific radiosensitivity, the radiation dose, the tumor volume, and the microscopic model of energy deposition. On the basis of these features, meningiomas mostly belong to relatively radiosensitive, late-responding tissues.
Effective dosages are in the lower range, not far from normal-cell thresholds, while the time interval for the effect is close to the maximum in-vivo doubling time.21,22,183–185 Moreover, all kinds of ionizing radiations are currently identified, not only according to dose level, but also characteristic pattern of energy transfer and consequent relative biological effectiveness (RBE).186 Linear energy transfer (LET) is defined as the average energy that is locally delivered by a particle in an absorbing medium divided by unit length of the crossed distance. Larger particles are correlated with higher LET values, with increasing ionization density and greater RBE. The differential cytotoxicity of low- versus high-LET radiation beams is particularly emphasized in hypoxic tumors. Indeed, some of the main reactive molecules produced by radiation beams (i.e., ion pairs and free radicals) are represented by superoxide compounds with unpaired valence electrons, breaking DNA-protein chemical bonds. Therefore, in most cases the permanent effect of this free radical–mediated injury is dependent on the presence of oxygen.
Radiosensitizers and radioenhancers are supposed to concurrently increase the toxicity of ionizing radiation on tumor cells reducing the required dose and therefore protecting peripheral brain tissues. The literature of the last decade apparently abounds in experimental—and a few clinical—reports analyzing the potentially beneficial role of a variety of radiosensitizers and radioenhancers in specific trials.183,187
1. Hypoxic cell sensitizers are able to increase the radiosensitivity of tumor cells deficient of oxygen by inducing the formation and stabilization of DNA-toxic radicals, mimicking the effect of oxygen. These drugs include nitroimidazole, misonidazole, etanidazole, nimorazole, and efaproxaril.
2. Hypoxic cytotoxins presumably play their role by selectively killing hypoxic cells, which are generally more resistant to radiation. This group includes three classes of drugs: quinone antibiotics, nitroaromatic compounds, and benzotriazine di-N-oxides.
3. Promising non-hypoxic sensitizers include the halogenated pyridines working as DNA base analogues, becoming incorporated in newly synthesized DNA, and rendering tumors more sensitive to ionizing radiations.
Radioenhancers are agents aimed to reduce the amount of radiation required to kill a given population of tumor cells. More recently, interesting protocols using gene therapy–guided radiosurgical procedures (i.e., viral vectors) have further expanded the spectrum of radioenhancers available.187
Radiosurgery Treatment Results
Since the early 1990s, the role of radiosurgery in the spectrum of therapeutic options for intracranial meningiomas has been increasingly emphasized even as a primary treatment alternative, especially in the elderly, and in tumors in critical locations. Indeed, the impact of the significant surgical morbidity, mortality, and postoperative recurrences, on patient’s and caregiver’s quality of life, has triggered such dramatic change in the therapeutic paradigm. Skull base lesions are classic examples of this phenomenon. To date the majority of these tumors are treated with SRS procedures, limiting surgical approaches to the debulking of larger tumors. If we analyze the largest (over 100 patients) published radiosurgical series of the last decade, GKR is by far the most diffused technical option (4,608 reported patients) followed by MicroMultiLeaf LINAC (337) and CyberKnife (301) (see Table 104-3).10,114,142,144,145,148,188–193 In terms of localization, meningiomas of the cranial base—and specifically of the cavernous sinus and of the petroclival region—are the most represented. As a rule the cytologic grading is the main determinant of the radiosurgical effectiveness.2,3,12 It is generally accepted that for benign (grade 1) meningiomas, a similar, effective, and durable LTC may be obtained using lower SRS dosages of 11 to 16 Gy at the isodose prescription line (ED or SD), and slightly higher dosages with aggressive oncotypes. The overall neuroradiologic results are rewarding and stable. In spite of the fact that the available literature is mostly limited to Evidence Based Medicine class 3 Data, with only a few studies presenting class 2 information,75 the overall neuroradiologic results seem rewarding. Given the definition of “local tumor control” as a post-treatment computerized target volume equal to or smaller than the original, the 5-year actuarial tumor control rates range from 86.2% to 97.9% both after GKR, and CyberKnife treatment.155,161–163 In the former and larger group (GKR), primary or “imaging-diagnosed” meningiomas and recurrences show comparable 5-year PFS (93%–98%)144,145,148,154 than recurrences (34%–97%144,154). Instead, patients with benign histotypes (grade 1) are usually characterized by 5-year actuarial tumor control rates (87%–96%) much higher than those with atypical (49%–77%) or anaplastic (0%–19%) lesions.144,145,149–154 The still limited number of reports with a mean follow-up period of 7 to 10 years have consistently confirmed such findings.58,144,154,156,176 Adopting the concept of clinical improvement as resolution or reduction of preoperative neurologic symptoms,152 the vast majority of cases shows stable or improved KPS and neurologic gradings at 5 to 7 years or longer follow-up.140 A recent review published by the Pittsburgh Gamma Knife Center confirms in a cohort of 972 patients, with a long-term follow-up (for some of them up to 20 years) an overall tumor control rate up to 97% and morbidity rate of 7.7%.29,151,153 Morbidity rates are usually low, albeit slightly higher for crucial locations such as the cavernous sinus and petroclival region.156,175
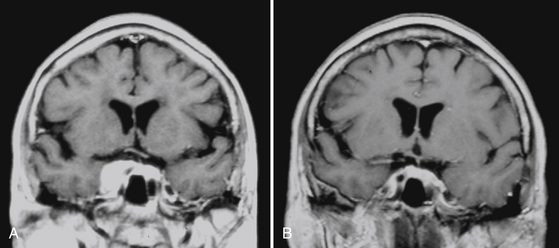
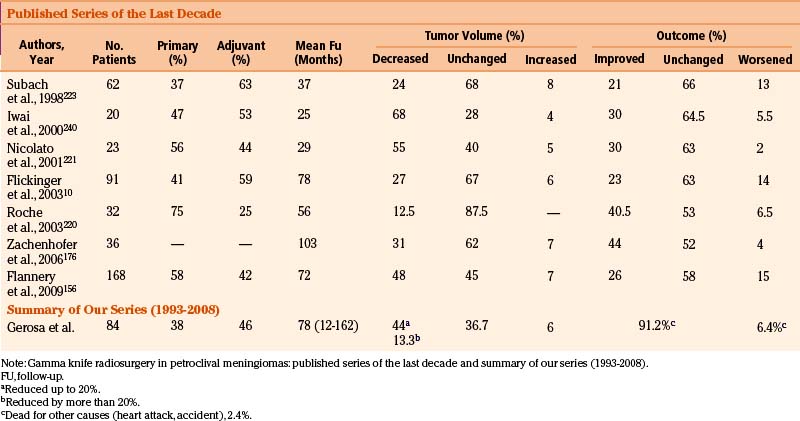
Our experience in GK meningioma treatment (1993–2009) is briefly summarized in Table 104-4. Clinical radiologic results have been extremely rewarding (Figs. 104-9 and 104-10). A comparative analysis of LINAC-based radiosurgical experiences in meningiomas versus GKR experiences clearly shows that follow-up period is longer for GKR (as seen previously), with several reports reaching 6 to 7 years mean follow-up versus 3 to 4 years for the LINAC series, targeted tumor volumes are extremely variable with both approaches, whereas the relative marginal dosages (12–15 Gy) as well as the tumor control rates (usually over 90%) are quite similar. The incidence of sequelae with both techniques is quantitatively (3%–13%) and qualitatively reasonable,58 with severe neurologic worsening extremely rare and no mortality.
Table 104-4 Gamma Knife Radiosurgery in Meningiomas: Synopsis of Our Experience (1993-2009)
| Total number of treated patients/treated lesions | 1062/1207 |
| Imaging-defined lesions (primary treatments) | 58% |
| Remnants/recurrences | 42% |
| Grade | |
| Benign (grade 1) | 92% |
| Atypical (grade 2) | 5.5% |
| Anaplastic (grade 3) | 2.5% |
| Site | |
| Skull base | 68% |
| •Cavernous sinus | 31% |
| •Petroclival | 18% |
| Other | 32% |
| Dose Planning and Local Tumor Control | |
| Average tumor volume | 8.4 cc (0.8–24) |
| Superficial dose | 14.3 Gy (9–22) |
| Absorbed dose | 23.7 Gy (14.6–31) |
| Integral dose | 246 mJoule (42–475) |
| Number of isocenters | 14 (1–34) |
| Local tumor control (overall) | 91% |
| Mean follow-up | 70 months |
Special Issues
Site
Paraoptic Meningiomas
The group includes all meningiomas located in close proximity to, or in direct contact with, the anterior optic pathways (AOPs), that is, meningiomas of the optic nerve sheaths, spheno-orbital, clinoid, para- or supra-sellar lesions, and so on. There is no documented evidence that encasement of the optic nerve and chiasm by one of these tumors is an absolute contraindication to radiation therapy.121 Instead, extremely valuable PFS rates, with stable or improved visual fields and acuity have been described in the vast majority of patients treated with definitive radiation therapy for tumors of these regions.88–91,121,194 Dose ranges were at or above 50 to 53 Gy in conventional fractionation.121 This seems to confirm that LTC is the primary factor in maintaining functional vision.90,91,121,195 It is generally accepted that for benign (grade 1) meningiomas, a similar, effective and durable LTC may be obtained using SRS doses of 11 to 15 Gy at the isodose prescription line. However, AOPs currently represent, together with the cochlear region, the most radiosensitive intracranial structure.90,121,196 As a consequence, meningiomas of this region require an extremely careful dosimetry planning to avoid any possible damage to the visual function that is documented at the time of the treatment. In fact, a radiosurgical dose exposure of more than 9 Gy to an AOP volume exceeding 10 cubic mm—even less in children—may eventually produce irreversible visual damage, particularly if administered on a previously suffering target.121,196 NF2, with the complex imbalance of the visual function (neurotubular deficit, etc.), which is frequently bilateral, is a classic example of such situation.197,198 In an accurate analysis of the risk for radiation optic neuropathy (RON), a series of 215 patients received in 73% of cases doses exceeding the 8- to 9-Gy threshold on a short segment of the AOP: Only 1.1% of the patients eventually developed a RON.199 Several different alternatives have been recommended and used with rather successful results, including fractionated GKR88 or CyberKnife12,121,155,162 procedures, to fractionated STR to proton-photon therapy to Perfexion-guided hybrid shots.61 The growing experience in this area is leading to an improved therapeutic ratio.88,121,162,200,201
Cavernous Sinus
The first published case of sphenocavernous meningioma dates back to 1910 with an autopsy report by Frotscher and Becker,20,28 but systematic nosography of these challenging tumors came only in 1938 with the famous monograph by Cushing and Eisenhardt.20,173 For decades they had been considered mostly inoperable and treatment options for these patients included simple observation, palliation, and fractionated radiation therapy, with aggressive and risky surgical attempts only in young patients with large progressing tumors.20,75,202,203
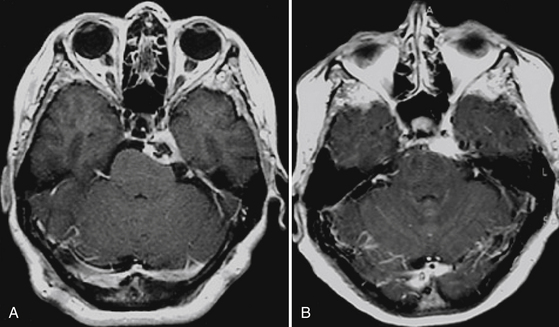
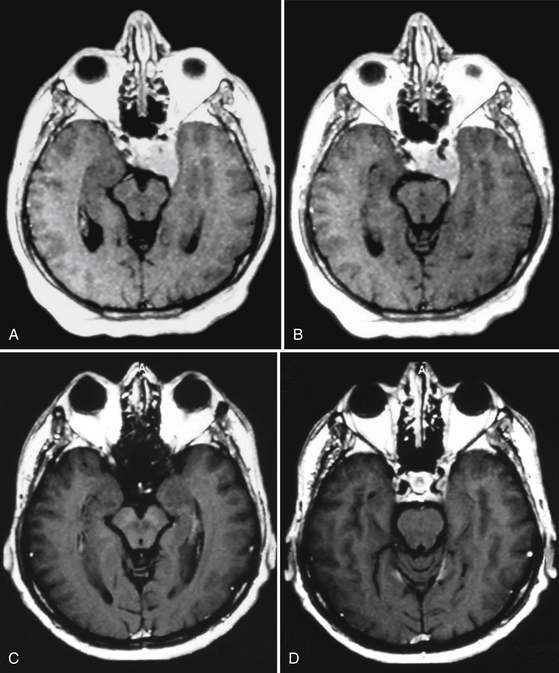
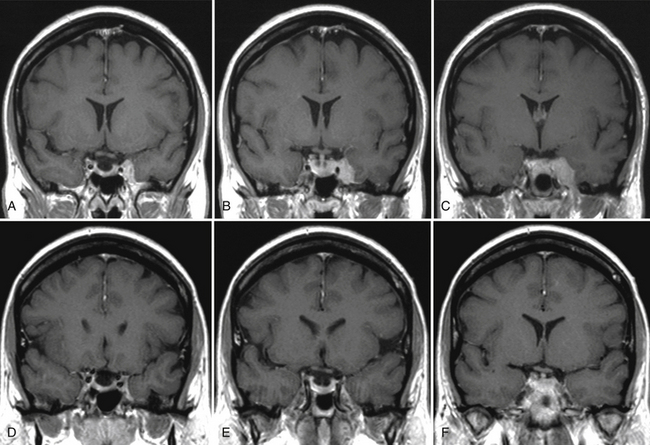
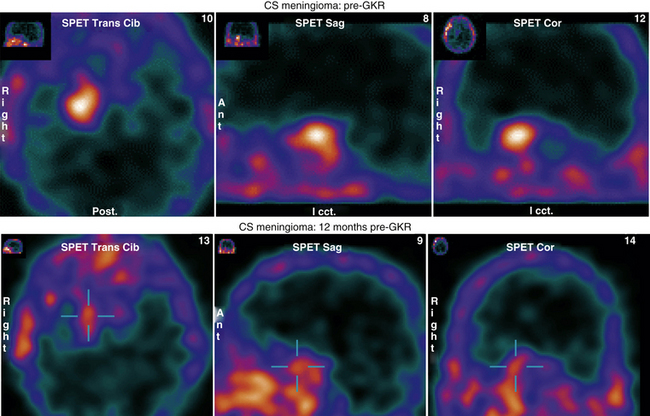
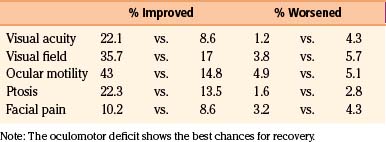
The intensive, multidimensional anatomic investigation of pioneer microsurgeons led in the 1970s and 1980s to newer, relatively safer, and more sophisticated surgical procedures, improving the chances for a better outcome.20,61,62,204–206 Nonetheless, on the one hand, morbidity and mortality rates remained non-negligible, basically due to the almost constant infiltration, on the part of the tumor, of the carotid artery and its branches,20,207–209 and to the encasement of the oculomotor and trigeminal nerves.20,208,209 Furthermore, recurrence rates varied from 5% to 10% at 1- to 8-year follow-up, after radical resection; this rate is double that observed after partial resection.20,61,62,205,206,210 These are the principal reasons that explain the growing role of radiotherapy and subsequently of SRS as a valid therapeutic option for these patients. Indeed, published results indicate for fractionated radiotherapy, at a mean follow-up of 30 to 70 months, progression rates of 0%–10%, and for cranial neuropathy, rates from 0% to 22%. Gamma knife results reported in the radiosurgical literature are yet more rewarding than those reported for linac213 and proton therapy218 (Table 104-5).20,128,140,211–216 Clinical radiologic results in our experience support these findings.141,217 LTC may vary from a slight-to-substantial reduction (Figs. 104-9 and 104-10), to—in a minority of cases—a dramatic shrinkage (Figs. 104-11 and 104-12). Furthermore, neurologic recovery, particularly of the oculomotor nerves, has been noted (Table 104-6), occurring more frequently in primary treatments. Finally, it is worth mentioning that in cavernous sinus meningiomas, SRS has shown to be effective (e.g., in restoring ocular motility), not only by reducing the tumor volume and its mechanical compression, but also via an octreotide receptor-mediated effect, that seems to prevent an anticolinergic activity of those lesions even without modifying the actual tumor size.114,141,217 Once again, like in functional radiosurgery models, the clinical results of this technique cannot be classified as a mere mechanical consequence of a radiation-induced necrosis with volumetric shrinkage and decreased pressure on the neighboring cranial nerves, and an additional factor plays a role (Fig. 104-13).2,50,217
Table 104-6 Gamma Knife Radiosurgery in Cavernous Sinus Meningioma: Our Clinical Results in Primary versus Adjuvant Gamma Knife Radiosurgery
Petroclival Meningiomas
In these patients, during the last decade SRS treatments have gained an increasing role, either as a primary or as an adjuvant procedure. Microsurgery remains the basic option for the non-infrequent large petroclival lesions, sometimes with the goal of a gross debulking giving substantial relief to intracranial pressure. Newer techniques, particularly via orbitozygomatic and retrosigmoid approaches, have gradually improved the surgical outcome, thanks to reduced morbidity.57,219 Nevertheless, in small- to medium-sized tumors of this region, SRS has become an accepted alternative, both in terms of LTC in medium-to-long term follow-up and of reduced morbidity (Table 104-7).10,156,176,220–224 This treatment is characterized by an absolute low rate of sequelae10,156,176,220–224 and favorable symptomatic results often obtained on secondary trigeminal neuralgia.225 We recently reviewed our series of GKR-treated petroclival meningiomas, updating previously published results,221 to a longer mid-term follow-up. Preliminary, positive results were confirmed on both outcome score and neuroradiologic pictures (see Table 104-7).
Intraventricular Meningiomas
Meningiomas growing within the ventricular system are rare, and probably originate from arachnoidal “buds” partially embedded in the choroid plexus.226–228 Incidence has been reported as the 0.5% to 3.7% of all intracranial locations.226–229 The rationale for primary SRS, as in several other instances, is closely related to the limitations and morbidity that frequently characterize radical removal of these lesions. However, as recently reported,230 with the combined use of the most sophisticated operative techniques (advanced neuronavigation, third-generation ultrasonic aspirator, and molecular coagulation), total excision of these deep-seated and usually highly vascularized tumors may be safely accomplished. When planning primary SRS, it should be emphasized that dealing with “imaging-defined” lesions, a reliable diagnosis is extremely relevant. Current strategies include a clearly defined picture via MRI—typical site and morphology, homogeneous contrast enhancement, and hyperintense signal on T2-weighted images226—and a spectrum MRI analysis. However, SRS has been used in adjuvant protocols on surgical residual or—as an alternative to surgery—in recurrences. Considering the very peculiar localization of the target—surrounded by variably thick, cerebral spinal fluid (CSF) films—prescription doses higher (>20 Gy) than usual had been used before 1995.150,226,230 The most recent literature has shown that reduced (12–15 Gy) dosages may obtain the same satisfactory results, while decreasing undue neurologic risks.150,154
Convexity and Parasagittal Meningiomas
Microsurgery still represents the major therapeutic option for meningiomas arising in these regions, because both the tumor mass and the involved dural attachment can be radically removed in most cases and with acceptable risks.29,161,231,232 The role of SRS in convexity meningiomas is usually marginal, whereas in the parasagittal variety radiosurgery may be a relevant alternative—in deeply located in small- to medium-sized lesions—either as a primary treatment for elderly patients (general anesthesia too dangerous or refused, etc.) or as an adjuvant to surgery in the case of residual tumors invading the sagittal sinus or aggressive recurrences.29,161,232 In these special localizations, the SRS procedure appears to carry an increased risk of ARE, particularly brain edema and peritumoral imaging changes (PRICs).3,75,161 These increased risks have also been confirmed in CyberKnife series155,161 and extensively analyzed in the literature.3,190,233–237 Indeed, parasagittal tumor location has been shown to be a potential predictor of peritumoral edema,161 which is significantly more common in this location than in skull base radiosurgery, regardless of dose–volume influence.161 Such peculiar, radio-induced “regional” sequelae presumably develop because of the crucial and “vulnerable” local venous drainage. Clinical consequences usually appear 3 to 6 months after treatment, and often require steroids, diuretics, and pentoxifylline.3,29,75,161
Sequelae: Adverse Radiation Effects
The variable (2.6%–9.6%)29,111,142 occurrence of these AREs is essentially a function of the dose–volume integral, only marginally modulated by individual radiosensitivity thresholds.9,10,111,142,238,239 As a consequence, in most treatment protocols, large tumors have been receiving lower doses than the smaller ones.9,10,12,111,137,146,168,214,224,238,240,241 According to a broadly accepted notion, SRS should never treat targets exceeding 3 to 3.5 cm in diameter and 14 to 22.5 cm3 in volume. Violations of these principles might in time trigger the multistep process of AREs, starting with the formation or the worsening of perifocal edema, followed by the focal vasculopathy and by other, more severe sequelae.
Peritumoral Imaging Changes
“Satellite” edema, particularly common in the parasagittal or in the convexity region, is the dominant feature—together with minor vascular sequelae—in the early stages of the peritumoral imaging changes. Accurately reported series have shown the described influence of the dose–volume factor in determining the severity and the timing of these processes, at least in standard dosimetry protocols.3,190,233,234,236,237 However, recent reports have extensively emphasized the chances for maintaining an adequate LTC without increasing side effects9,111,128,136,146,214,224,238 by treating larger meningiomas with reduced dosages.111,126,131,240 According to Ganz et al.,111,137,178 at least in WHO grade 1 meningiomas, the straightforward relation between the dose–volume integral and the incidence of AREs, may be too simplistic for lower-dose protocols.111,112 Furthermore, the predominance of PRICs and AREs following SRS in non–skull-base meningiomas compared with basal tumors observed with GKS,29 MML-LINAC,168 and CyberKnife,161 might be strictly dependent on the prescription dose adopted, becoming irrelevant at reduced dose regimens.111,112
Vasculopathy—Radiation Necrosis
These neuropathologic entities represent the next steps of the worsening AREs sequence, taking place in 1.5% to 4.6% of the cases during mid- to long-term follow-up.242 The typical process is comparable to the parenchymal multistep damage described in irradiated arteriovenous malformation by Nataf et al.,242 including the specific radiation-tolerance limits of the neural tissue.243 The final pathophysiology of the radionecrotic stage is still poorly known. In a minority of cases, an obvious, solid fibromatous nodule further increases the edema—presumably related to vascular permeability factors. Surgical removal of the nodule is usually the most appropriate solution.244
Cranial Neuropathy
Various cranial nerves are characterized by a wide spectrum of radiosensitivity. As a rule, special sensory nerves—and especially their primary receptor fibers—such as those belonging to the cochlear (radiation threshold on 10–15 mm3 in single fraction, 5–6 Gy)245 and to the optic nerve (same landmark, 8–9 Gy)121,196,199 may be easily damaged. Somatic motor nerves like the oculomotor usually tolerate dose exposures superior to 20 Gy.151,196,203
Oncogenicity
In a very limited number of cases, putative cancerogenic mechanisms have been advocated to explain the rapid growth of previously small, quiescent meningiomas following SRS.30,35 The surgically verified atypical-anaplastic oncotypes were the most reasonable explanation. The definition of radiation-induced tumors is still based on the indirect criteria devised by Cahan246: 5-year-plus latency time, localization overlapping the irradiated one, and different oncotype. The relative risk of carcinogenesis after radiosurgery in the central nervous system has been calculated by means of probabilistic methods,247 and varies from 1.57 to 8.75 for a dose of 1 Gy, increasing in time up to 18.4 between 20 and 25 years. The long-term (30-year) risk of newer radiation-induced tumors in meningioma patients has been estimated in 1 per 1000 treated patients.247 The natural incidence of new gliomas in the population (1/10,000 every year), and the number of meningiomas treated over three decades with SRS worldwide (75,000) must be the basic reference for any statistical evaluation. As a consequence, the thus far extremely rare (two cases) reported instances of malignant tumors diagnosed in SRS-treated meningioma patients are probably an underestimation of the real incidence; that, however, should not affect further development of this technique.
Other Effects
Rare to extremely rare putative radiosurgical sequelae include persisting headache (2.5%),29 seizures (7%),29 and normal pressure248 or hypertensive29 hydrocephalus (1%). In the few reported cases of ventricular dilatation, the trigger mechanism is reminiscent of vestibular schwannoma–related hydrocephalus, increased albumin concentration in the CSF.29,248
The Future
During the last decade the evolution of radiosurgical techniques has been essentially supported by serial advancements either in terms of stereotactic imaging—such as particularly high Tesla MRI, image-fusion algorithms, virtual “nonrigid” reproductions—and of computerized dosimetry, using multi-isocentric targeting allowing highly conformal and selective planning. The latter actually represents the most precise and recent form of intensity-modulated irradiation. Indeed, by 1994 several LINAC units were adopting multiple isocenter techniques, switching to multiple static conformal fields to improve conformality, or switching to fractionated techniques and lower radiation doses,249 in order to limit radiotoxicity on normal brain. In fact, prescription doses for single-stage radiosurgery began to decrease in the early 1990s, and we still do not know how much further they will be lowered. In the near future, further improvements in clinical results may be reasonably expected from some of the main investigative trials presently in progress.
Additional therapeutic support should come from newer radioprotective agents presently under investigation—for instance, free radical scavengers, membrane stabilizers, 21-aminosteroids—as well as from compounds limiting the incidence of adverse radiation effects, such as cyclooxygenase 2 inhibitors.250
Al-Mefty O., Kadri P.A., Pravdenkova S., et al. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004;101:210-218.
Elia A.E., Shih H.A., Loeffler J.S. Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus. 2007;23:E5.
Eustacchio S., Trummer M., Fuchs I., et al. Preservation of cranial nerve function following gamma knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir. 2002;84(Suppl):71-76.
Flickinger J.C., Kondziolka D., Maitz A.H., et al. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801-806.
Friedman W.A., Murad G.J., Bradshaw P., et al. Linear accelerator surgery for meningiomas. J Neurosurg. 2005;103:206-209.
Ganz J.C., Reda W.A., Abdelkarim K. Adverse radiation effects after gamma knife surgery in relation to dose and volume. Acta Neurochir. 2009;151:9-19.
Kondziolka D., Mathieu D., Lunsford L.D., et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53-58.
Kreil W., Luggin J., Fuchs I., et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76:1425-1430.
Malik I., Rowe J.G., Walton L., et al. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13-20.
Marosi C., Hassler M., Roessler K., et al. Meningioma. Crit Rev Oncol Hematol. 2008;67:153-171.
Minniti G., Amichetti M., Enrici R.M. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat Oncol. 2009;4:42-53.
Modha A., Gutin P.H. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57:538-550.
Nakamura M., Roser F., Michel J., et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62-70.
Nicolato A., Foroni R., Alessandrini F., et al. Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients. Neurosurgery. 2002;51:1153-1159.
Patil C.G., Hoang S., Borchers D.J.3rd, et al. Predictors of peritumoral edema after stereotactic radiosurgery of supratentorial meningiomas. Neurosurgery. 2008;63:435-440.
Perry A., Dehner L.P. Meningeal tumors of childhood and infancy. An update and literature review. Brain Pathol. 2003;13:386-408.
Perry A., Gutmann D.H., Reifenberger G. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70:183-202.
Perry A., Louis D.N., Scheithauer B.W., et al. Meningeal tumors. In: Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K. World Health Organization Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2007:164-172.
Pollock B.E. Stereotactic radiosurgery of benign intracranial tumors. J Neurooncol. 2009;92:337-343.
Pollock B.E. Stereotactic radiosurgery for intracranial meningiomas: indications and results. Neurosurg Focus. 2003;14:e4.
Rogers L., Mehta M. Role of radiation therapy in treating intracranial meningiomas. Neurosurg Focus. 2007;23:E4.
Stafford S.L., Pollock B.E., Foote R.L., et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1037.
Stieber V.W. Radiation therapy for visual pathway tumors. J Neuroophthalmol. 2008;28:222-230.
Takanashi M., Fukuoka S., Hojyo A., et al. Gamma knife radiosurgery for skull-base meningiomas. Prog Neurol Surg. 2009;22:96-111.
Walsh M.T., Couldwell W.T. Management options for cavernous sinus meningiomas. J Neurooncol. 2009;92:307-316.
1. Rosenberg L.A., Prayson R.A., Lee J., et al. Long-term experience with World Health Organization grade III (malignant) meningiomas at a single institution. Int J Radiat Oncol Biol Phys. 2009;74:427-432.
2. Modha A., Gutin P.H. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57:538-550.
3. Rogers L., Mehta M. Role of radiation therapy in treating intracranial meningiomas. Neurosurg Focus. 2007;23:E4.
4. Central Brain Tumor Registry in the United States. Statistical Report: Primary Brain Tumors in the United States, 1992-1997. Hinsdale, IL: CBTRUS; 2001.
5. Central Brain Tumor Registry of the United States. Statistical Report: Primary Brain Tumors in the United States, 1998-2002. Hinsdale, IL: CBTRUS; 2005.
6. Claus E.B., Bondy M.L., Schildkraut J.M., et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095.
7. Eom K.S., Kim D.W., Kim T.Y. Diffuse craniospinal metastases of intraventricular rhabdoid papillary meningioma with glial fibrillary acidic protein expression: a case report. Clin Neurol Neurosurg. 2009;111:619-623.
8. Celli P., Palma L., Domenicucci M., et al. Histologically benign recurrent meningioma metastasizing to the parotid gland: case report and review of the literature. Neurosurgery. 1992;31:1113-1116.
9. DiBiase S.J., Kwok Y., Yovino S., et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60:1515-1519.
10. Flickinger J.C., Kondziolka D., Maitz A.H., et al. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801-806.
11. Nakasu S., Hirano A., Shimura T., et al. Incidental meningiomas in autopsy study. Surg Neurol. 1987;27:319-322.
12. Elia A.E., Shih H.A., Loeffler J.S. Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus. 2007;23:E5.
13. Whittle I.R., Smith C., Navoo P., et al. Meningiomas. Lancet. 2004;363:1535-1543.
14. Perry A., Dehner L.P. Meningeal tumors of childhood and infancy. An update and literature review. Brain Pathol. 2003;13:386-408.
15. Perry A., Louis D.N., Scheithauer B.W., et al. Meningeal tumors. In: Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K. World Health Organization Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer, 2007.
16. Goldsmith B. Meningioma. In: Leibel S., Phillips T. Textbook of Radiation Oncology. Philadelphia: WB Saunders, 1998.
17. Longstreth W.T.Jr., Dennis L.K., McGuire V.M., et al. Epidemiology of intracranial meningioma. Cancer. 1993;72:639-648.
18. McDermott M.W., Quiñones-Hinojosa A., Fuller G.N., et al. Meningiomas. In: Levin V.A., editor. Cancer in the Nervous System. 2nd ed. New York: Oxford University; 2002:269-299.
19. Wahab M., Al-Azzawi F. Meningioma and hormonal influences. Climacteric. 2003;6:285-292.
20. Walsh M.T., Couldwell W.T. Management options for cavernous sinus meningiomas. J Neurooncol. 2009;92:307-316.
21. Olivero W.C., Lister J.R., Elwood P.W. The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg. 1995;83:222-224.
22. Go R.S., Taylor B.V., Kimmel D.W. The natural history of asymptomatic meningiomas in Olmsted County, Minnesota. Neurology. 1998;51:1718-1720.
23. Kuratsu J., Kochi M., Ushio Y. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. 2000;92:766-770.
24. Niiro M., Yatsushiro K., Nakamura K., et al. Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry. 2000;68:25-28.
25. Yoneoka Y., Fujii Y., Tanaka R. Growth of incidental meningiomas. Acta Neurochir. 2000;142:507-511.
26. Nakamura M., Roser F., Michel J., et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62-70.
27. Herscovici Z., Rappaport Z., Sulkes J., et al. Natural history of conservatively treated meningiomas. Neurology. 2004;63:1133-1134.
28. Yano S., Kuratsu J. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006;105:538-543.
29. Kondziolka D., Madhok R., Lunsford L.D., et al. Stereotactic radiosurgery for convexity meningiomas. J Neurosurg. 2009;111:458-463.
30. Kunert P., Matyja E., Janowski M., et al. Rapid growth of small, asymptomatic meningioma following radiosurgery. Br J Neurosurg. 2009;23:206-208.
31. Jung H.W., Yoo H., Paek S.H., et al. Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000;46:567-574.
32. Condra K.S., Buatti J.M., Mendenhall W.M., et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427-436.
33. Mahmood A., Qureshi N.H., Malik G.M. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta Neurochir. 1994;126:53-58.
34. Naumann M., Meixensberger J. Factors influencing meningioma recurrence rate. Acta Neurochir. 1990;107:108-111.
35. Couldwell W.T., Cole C.D., Al-Mefty O. Patterns of skull base meningioma progression after failed radiosurgery. J Neurosurg. 2007;106:30-35.
36. Perry A., Scheithauer B.W., Stafford S.L., et al. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046-2056.
37. Perry A., Stafford S.L., Scheithauer B.W., et al. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455-1465.
38. Abboud M., Haddad G., Kattar M., et al. Extraneural metastases from cranial meningioma: a case report. Radiat Oncol. 2009;4:20.
39. Kuroda H., Kashimura H., Ogasawara K., et al. Malignant intracranial meningioma with spinal metastasis—case report. Neurol Med Chir. 2009;49:258-261.
40. Kleihues P., Cavenee W. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Nervous System. Lyon: International Agency for Research on Cancer Press, 2000.
41. Glaholm J., Bloom H.J., Crow J.H. The role of radiotherapy in the management of intracranial meningiomas: the Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys. 1990;18:755-761.
42. Goldsmith B.J., Wara W.M., Wilson C.B., et al. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
43. Dziuk T.W., Woo S., Butler E.B., et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998;37:177-188.
44. Goyal L.K., Suh J.H., Mohan D.S., et al. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46:57-61.
45. Pourel N., Auque J., Bracard S., et al. Efficacy of external fractionated radiation therapy in the treatment of meningiomas: a 20-year experience. Radiother Oncol. 2001;61:65-70.
46. Ware M.L., Larson D.A., Sneed P.K., et al. Surgical resection and permanent brachytherapy for recurrent atypical and malignant meningioma. Neurosurgery. 2004;54:55-63.
47. Harris A.E., Lee J.Y., Omalu B., et al. The effect of radiosurgery during management of aggressive meningiomas. Surg Neurol. 2003;60:298-305.
48. Hug E.B., Devries A., Thornton A.F., et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151-160.
49. Palma L., Celli P., Franco C., et al. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86:793-800.
50. Ojemann S.G., Sneed P.K., Larson D.A., et al. Radiosurgery for malignant meningioma: results in 22 patients. J Neurosurg. 2000;93(Suppl 3):62-67.
51. Louis D.N., Ramesh V., Gusella J.F. Neuropathology and molecular genetics of neurofibromatosis 2 and related tumors. Brain Pathol. 1995;5:163-172.
52. Riemenschneider M.J., Perry A., Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045-1054.
53. Bostrom J., Meyer-Puttlitz B., Wolter M., et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159:661-669.
54. Perry A., Banerjee R., Lohse C.M., et al. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol. 2002;12:183-190.
55. Weber R.G., Bostrom J., Wolter M., et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci USA. 1997;94:14719-14724.
56. Al-Mefty O., Kadri P.A., Pravdenkova S., et al. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004;101:210-218.
57. Bambakidis N.C., Kakarla U.K., Kim L.J., et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery. 2008;62(Suppl 3):1182-1191.
58. Kreil W., Luggin J., Fuchs I., et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76:1425-1430.
59. Sekhar L.N., Swamy N.K., Jaiswal V., et al. Surgical excision of meningiomas involving the clivus: preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg. 1994;81:860-868.
60. Couldwell W.T., Fukushima T., Giannotta S.L., et al. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84:20-28.
61. De Jesus O., Sekhar L.N., Parikh H.K., et al. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996;39:915-919.
62. DeMonte F., Smith H.K., al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
63. Villavicencio A.T., Black P.M., Shrieve D.C., et al. Linac radiosurgery for skull base meningiomas. Acta Neurochir. 2001;143:1141-1152.
64. Mathiesen T., Lindquist C., Kihlstrom L., et al. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39:2-7.
65. Bricolo A.P., Turazzi S., Talacchi A., et al. Microsurgical removal of petroclival meningiomas: a report of 33 patients. Neurosurgery. 1992;31:813-828.
66. Samii M., Klekamp J., Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery. 1996;39:1086-1094.
67. Cusimano M.D., Sekhar L.N., Sen C.N., et al. The results of surgery for benign tumors of the cavernous sinus. Neurosurgery. 1995;37:1-9.
68. Thomas N.W., King T.T. Meningiomas of the cerebellopontine angle. A report of 41 cases. Br J Neurosurg. 1996;10:59-68.
69. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:2208-2213.
70. Mirimanoff R.O., Dosoretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
71. Stafford S.L., Perry A., Suman V.J., et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73:936-942.
72. Wara W.M., Sheline G.E., Newman H., et al. Radiation therapy of meningiomas. Am J Roentgenol Radium Ther Nucl Med. 1975;123:453-458.
73. Saberi H., Meybodi A.T., Rezai A.S. Levine-Sekhar grading system for prediction of the extent of resection of cranial base meningiomas revisited: study of 124 cases. Neurosurg Rev. 2006;29:138-144.
74. Levine Z.T., Buchanan R.I., Sekhar L.N., et al. Proposed grading system to predict the extent of resection and outcomes for cranial base meningiomas. Neurosurgery. 1999;45:221-230.
75. Pollock B.E. Stereotactic radiosurgery of benign intracranial tumors. J Neurooncol. 2009;92:337-343.
76. Barbaro N.M., Gutin P.H., Wilson C.B., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525-528.
77. Taylor B.W.Jr., Marcus R.B.Jr., Friedman W.A., et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
78. Miralbell R., Linggood R.M., de la Monte S., et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol. 1992;13:157-164.
79. Forbes A.R., Goldberg I.D. Radiation therapy in the treatment of meningioma: the Joint Center for Radiation Therapy experience 1970 to 1982. J Clin Oncol. 1984;2:1139-1143.
80. Mendenhall W.M., Morris C.G., Amdur R.J., et al. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer. 2003;98:1473-1482.
81. Milosevic M.F., Frost P.J., Laperriere N.J., et al. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys. 1996;34:817-822.
82. Korah M.P., Nowlan A.W., Johnstone P.A., et al. Radiation Therapy Alone for Imaging-Defined Meningiomas. Int J Radiat Oncol Biol Phys. 2010;76(1):181-186.
83. Philip M.A., Nowlan A.P., Johnstone P., et al. Radiation therapy alone for imaging defined meningiomas. Int J Radiat Oncol Biol Phys. 2007;69:S264.
84. Alheit H., Saran F.H., Warrington A.P., et al. Stereotactically guided conformal radiotherapy for meningiomas. Radiother Oncol. 1999;50:145-150.
85. Selch M.T., Ahn E., Laskari A., et al. Stereotactic radiotherapy for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2004;59:101-111.
86. Milker-Zabel S., Zabel A., Schulz-Ertner D., et al. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61:809-816.
87. Henzel M., Gross M.W., Hamm K., et al. Stereotactic radiotherapy of meningiomas: symptomatology, acute and late toxicity. Strahlenther Onkol. 2006;182:382-388.
88. Smee R.I., Schneider M., Williams J.R. Optic nerve sheath meningiomas—non-surgical treatment. Clin Oncol (R Coll Radiol). 2009;21:8-13.
89. Andrews D.W., Faroozan R., Yang B.P., et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002;51:890-902.
90. Becker G., Jeremic B., Pitz S., et al. Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2002;54:1422-1429.
91. Baumert B.G., Villa S., Studer G., et al. Early improvements in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma. Radiother Oncol. 2004;72:169-174.
92. Adegbite A.B., Khan M.I., Paine K.W., et al. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51-56.
93. Peele K.A., Kennerdell J.S., Maroon J.C., et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology. 1996;103:1761-1766.
94. Maguire P.D., Clough R., Friedman A.H., et al. Fractionated external-beam radiation therapy for meningiomas of the cavernous sinus. Int J Radiat Oncol Biol Phys. 1999;44:75-79.
95. Nutting C., Brada M., Brazil L., et al. Radiotherapy in the treatment of benign meningioma of the skull base. J Neurosurg. 1999;90:823-827.
96. Vendrely V., Maire J.P., Darrouzet V., et al. [Fractionated radiotherapy of intracranial meningiomas: 15 years’ experience at the Bordeaux University Hospital Center]. Cancer Radiother. 1999;3:311-317.
97. Wenkel E., Thornton A.F., Finkelstein D., et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363-1370.
98. Debus J., Wuendrich M., Pirzkall A., et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. J Clin Oncol. 2001;19:3547-3553.
99. Dufour H., Muracciole X., Metellus P., et al. Long-term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: is there an alternative to aggressive tumor removal? Neurosurgery. 2001;48:285-294.
100. Uy N.W., Woo S.Y., Teh B.S., et al. Intensity-modulated radiation therapy (IMRT) for meningioma. Int J Radiat Oncol Biol Phys. 2002;53:1265-1270.
101. Pirzkall A., Debus J., Haering P., et al. Intensity modulated radiotherapy (IMRT) for recurrent, residual, or untreated skull-base meningiomas: preliminary clinical experience. Int J Radiat Oncol Biol Phys. 2003;55:362-372.
102. Soyuer S., Chang E.L., Selek U., et al. Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol. 2004;71:85-90.
103. Milker-Zabel S., Zabel-du Bois A., Huber P., et al. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68:858-863.
104. Ayerbe J., Lobato R.D., de la Cruz J., et al. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir. 1999;141:921-932.
105. Connell P.P., Macdonald R.L., Mansur D.B., et al. Tumor size predicts control of benign meningiomas treated with radiotherapy. Neurosurgery. 1999;44:1194-1199.
106. Hamm K., Henzel M., Gross M.W., et al. Radiosurgery/stereotactic radiotherapy in the therapeutical concept for skull base meningiomas. Zentralbl Neurochir. 2008;69:14-21.
107. Minniti G., Amichetti M., Enrici R.M. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat Oncol. 2009;4:42-53.
108. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
109. Leksell L. A stereotaxic apparatus for intracerebral surgery. Acta Chir Scand. 1949;99:229-233.
110. Meyerson B.A., Linderoth B. History of stereotactic neurosurgery in the nordic countries. In: Lozano A.M., Gildenberg P.L., Tasker R.R. Textobook of Stereotactic and Functional Neurosurgery. New York: Springer; 2009:65-72.
111. Ganz J.C., Reda W.A., Abdelkarim K. Adverse radiation effects after gamma knife surgery in relation to dose and volume. Acta Neurochir. 2009;151:9-19.
112. Ganz J.C., Reda W.A., Abdelkarim K. Gamma knife surgery of large meningiomas: early response to treatment. Acta Neurochir. 2009;151:1-8.
113. Nicolato A., Foroni R., Grigolato D., et al. 111 Indium-octreotide brain scintigraphy: a prognostic factor in skull base meningiomas treated with gamma knife radiosurgery. Q J Nucl Med. 2004;48:26-32.
114. Nicolato A., Giorgetti P., Foroni R., et al. Gamma knife radiosurgery in skull base meningiomas: a possibile relationship between somatostatin receptor decrease and early neurological improvement without tumor shrinkage at short-term imaging follow-up. Acta Neurochir. 2005;147:367-375.
115. Graves E.E., Nelson S.J., Vigneron D.B., et al. Serial proton MRI spectroscopic imaging of recurrent malignant gliomas after gamma knife radiosurgery. AJNR. 2001;22:598-601.
116. Maruyama K., Kamada K., Shin M., et al. Integration of three dimensional corticospinal tractography into treatment planning for gamma knife surgery. J Neurosurg. 2005;102:673-677.
117. Astner S.T., Dobrei-Ciuchendea M., Essler M., et al. Effect of 11C-methionine-positron emission tomography on gross tumor volume delineation in stereotactic radiotherapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2008;72:1161-1167.
118. Grosu A.L., Weber W.A., Astner S.T., et al. 11C-methionine PET improves the target volume delineation of meningiomas treated with stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:339-344.
119. Levivier M., Wikler D., Goldman S., et al. Integration of metabolic data of positron emission tomography in the dosimetry planning of radiosurgery with the gamma knife: early experience with brain tumors—Technical note. J Neurosurg. 2000;93(Suppl 3):233-238.
120. Koga T., Maruyama K., Igaki H., et al. The value of image co-registration during stereotactic radiosurgery. Acta Neurochir. 2009;151:465-471.
121. Stieber V.W. Radiation therapy for visual pathway tumors. J Neuroophthalmol. 2008;28:222-230.
122. Engenhart R., Kimmig B.N., Hover K.H., et al. Stereotactic single high dose radiation therapy of benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 1990;19:1021-1026.
123. Chang S.D., Adler J.R.Jr. Treatment of cranial base meningiomas with linear accelerator radiosurgery. Neurosurgery. 1997;41:1019-1025.
124. Kondziolka D., Lunsford L.D., Coffey R.J., et al. Stereotactic radiosurgery of meningiomas. J Neurosurg. 1991;74:552-559.
125. Duma C.M., Lunsford L.D., Kondziolka D., et al. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32:699-704.
126. Ganz J.C., Backlund E.O., Thorsen F.A. The results of Gamma knife surgery of meningiomas, related to size of tumor and dose. Stereotact Funct Neurosurg. 1993;61(Suppl 1):23-29.
127. Nicolato A., Ferraresi P., Foroni R., et al. Gamma knife radiosurgery in skull base meningiomas. Preliminary experience with 50 cases. Stereotact Funct Neurosurg. 1996;66(Suppl 1):112-120.
128. Liscak R., Simonova G., Vymazal J., et al. Gamma knife radiosurgery of meningiomas in the cavernous sinus region. Acta Neurochir. 1999;141:473-480.
129. Iwai Y., Yamanaka K., Yasui T., et al. Gamma knife surgery for skull base meningiomas. The effectiveness of low-dose treatment. Surg Neurol. 1999;52:40-44.
130. Morita A., Coffey R.J., Foote R.L., et al. Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg. 1999;90:42-49.
131. Nakaya K., Hayashi M., Nakamura S., et al. Low-dose radiosurgery for meningiomas. Stereotact Funct Neurosurg. 1999;72(Suppl 1):67-72.
132. Kondziolka D., Levy E.I., Niranjan A., et al. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91:44-50.
133. Aichholzer M., Bertalanffy A., Dietrich W., et al. Gamma knife radiosurgery of skull base meningiomas. Acta Neurochir. 2000;142:647-652.
134. Pendl G., Eustacchio S., Unger F. Radiosurgery as alternative treatment for skull base meningiomas. J Clin Neurosci. 2001;8(Suppl 1):12-14.
135. Kobayashi T., Kida Y., Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001;55:325-331.
136. Eustacchio S., Trummer M., Fuchs I., et al. Preservation of cranial nerve function following gamma knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir. 2002;84(Suppl):71-76.
137. Hudgins W.R., Barker J.L., Schwartz D.E., et al. Gamma knife treatment of 100 consecutive meningiomas. Stereotact Funct Neurosurg. 1996;66(Suppl 1):121-128.
138. Hakim R., Alexander E.3rd, Loeffler J.S., et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42:446-453.
139. Stafford S.L., Pollock B.E., Foote R.L., et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1037.
140. Lee J.Y., Niranjan A., McInerney J., et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97:65-72.
141. Nicolato A., Foroni R., Alessandrini F., et al. Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients. Neurosurgery. 2002;51:1153-1159.
142. Chang J.H., Chang J.W., Choi J.Y., et al. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003;74:226-230.
143. Pollock B.E. Stereotactic radiosurgery for intracranial meningiomas: indications and results. Neurosurg Focus. 2003;14:e4.
144. Pollock B.E., Stafford S.L., Utter A., et al. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55:1000-1005.
145. Liscak R., Kollova A., Vladyka V., et al. Gamma knife radiosurgery of skull base meningiomas. Acta Neurochir. 2004;91(Suppl):65-74.
146. Feigl G.C., Bundschuh O., Gharabaghi A., et al. Volume reduction in meningiomas after gamma knife surgery. J Neurosurg. 2005;102(Suppl):189-194.
147. Friedman W.A., Murad G.J., Bradshaw P., et al. Linear accelerator surgery for meningiomas. J Neurosurg. 2005;103:206-209.
148. Malik I., Rowe J.G., Walton L., et al. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13-20.
149. Hasegawa T., Kida Y., Yoshimoto M., et al. Long-term outcomes of Gamma knife surgery for cavernous sinus meningioma. J Neurosurg. 2007;107:745-751.
150. Kollova A., Liscak R., Novotny J.Jr., et al. Gamma knife surgery for benign meningioma. J Neurosurg. 2007;107:325-336.
151. Lee J.Y., Kondziolka D., Flickinger J.C., et al. Radiosurgery for intracranial meningiomas. Prog Neurol Surg. 2007;20:142-149.
152. Iwai Y., Yamanaka K., Ikeda H. Gamma knife radiosurgery for skull base meningioma: long-term results of low-dose treatment. J Neurosurg. 2008;109:804-810.
153. Kondziolka D., Mathieu D., Lunsford L.D., et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53-58.
154. Bledsoe J.M., Link M.J., Stafford S.L., et al. Radiosurgery for large-volume (>10 cm3) benign meningiomas. J Neurosurg Sept. 18, 2009. [Epub ahead of print]
155. Colombo F., Casentini L., Cavedon C., et al. Cyberknife radiosurgery for benign meningiomas: short-term results in 199 patients. Neurosurgery. 2009;64:A7-13.
156. Flannery T.J., Kano H., Lunsford L.D., et al. Long-term control of petroclival meningiomas through radiosurgery. J Neurosurg 2009 Sept. 4, 2009. [Epub ahead of print]
157. Takanashi M., Fukuoka S., Hojyo A., et al. Gamma knife radiosurgery for skull-base meningiomas. Prog Neurol Surg. 2009;22:96-111.
158. Feigl G.C., Samii M., Horstmann G.A. Volumetric follow-up of meningiomas: a quantitative method to evaluate treatment outcome of gamma knife radiosurgery. Neurosurgery. 2007;61:281-286.
159. Sheehan J., Pouratian N., Sansur C.A., Steiner L. Gamma knife surgery for meningiomas. In: Lee J.H., editor. Meningiomas: Diagnosis, Treatment and Outcome. London: Springer-Verlag; 2009:267-276.
160. Lartigau E., Mirabel X., Prevost B., et al. Extracranial stereotactic radiotherapy: preliminary results with the CyberKnife. Onkologie. 2009;32:209-215.
161. Patil C.G., Hoang S., Borchers D.J.3rd, et al. Predictors of peritumoral edema after stereotactic radiosurgery of supratentorial meningiomas. Neurosurgery. 2008;63:435-440.
162. Adler J.R.Jr., Gibbs I.C., Puataweepong P., et al. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2008;62(Suppl 2):733-743.
163. Adler J.R.Jr., Murphy M.J., Chang S.D., et al. Image-guided robotic radiosurgery. Neurosurgery. 1999;44:1299-1306.
164. Sterzing F., Schubert K., Sroka-Perez G., et al. Helical tomotherapy. Experiences of the first 150 patients in Heidelberg. Strahlenther Onkol. 2008;184:8-14.
165. Petti P.L., Larson D.A., Kunwar S. Use of hybrid shots in planning Perfexion Gamma Knife treatments for lesions close to critical structures. J Neurosurg. 2008;109(Suppl):34-40.
166. Ma L., Chuang C., Descovich M., et al. Whole-procedure clinical accuracy of gamma knife treatments of large lesions. Med Phys. 2008;35:5110-5114.
167. Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Suppl 3):219-222.
168. Clark B.G., Candish C., Vollans E., et al. Optimization of stereotactic radiotherapy treatment delivery technique for base-of-skull meningiomas. Med Dosim. 2008;33:239-247.
169. Kim M.S., Park K., Kim J.H., et al. Gamma knife radiosurgery for orbital tumors. Clin Neurol Neurosurg. 2008;110:1003-1007.
170. Sibtain A., Plowman P.N. Stereotactic radiosurgery. VII. Radiosurgery versus conventionally-fractionated radiotherapy in the treatment of cavernous sinus meningiomas. Br J Neurosurg. 1999;13:158-166.
171. Kawahara Y., Niiro M., Yokoyama S., et al. Dural congestion accompanying meningioma invasion into vessels: the dural tail sign. Neuroradiology. 2001;43:462-465.
172. Perry A., Gutmann D.H., Reifenberger G. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70:183-202.
173. Cushing H., Eisenhardt L. Meningiomas. Their Classification, Regional Behaviour, Life History, and Surgical End Results. Springfield: Charles C. Thomas; 1938.
174. Pieper D.R., Al-Mefty O., Hanada Y., et al. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-746.
175. Kondziolka D., Nathoo N., Flickinger J.C., et al. Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery. 2003;53:815-821.
176. Zachenhofer I., Wolfsberger S., Aichholzer M., et al. Gamma-knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery. 2006;58:28-36.
177. Abeloos L., Levivier M., Devriendt D., et al. Internal carotid occlusion following gamma knife radiosurgery for cavernous sinus meningioma. Stereotact Funct Neurosurg. 2007;85:303-306.
178. Pollock B.E., Stafford S.L. Results of stereotactic radiosurgery for patients with imaging defined cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2005;62:1427-1431.
179. Ma L., Larson D., Petti P., et al. Boosting central target dose by optimizing embedded dose hot spots for gamma knife radiosurgery. Stereotact Funct Neurosurg. 2007;85:259-263.
180. Kondziolka D., Kano H., Kanaan H., et al. Stereotactic radiosurgery for radiation-induced meningiomas. Neurosurgery. 2009;64:463-469.
181. Zattara-Cannoni H., Roll P., Figarella-Branger D., et al. Cytogenetic study of six cases of radiation-induced meningiomas. Cancer Genet Cytogenet. 2001;126:81-84.
182. Jensen A.W., Brown P.D., Pollock B.E., et al. Gamma knife radiosurgery of radiation-induced intracranial tumors: local control, outcomes, and complications. Int J Radiat Oncol Biol Phys. 2005;62:32-37.
183. Iwata H., Shibamoto Y., Murata R., et al. Estimation of errors associated with use of linear-quadratic formalism for evaluation of biologic equivalence between single and hypofractionated radiation doses: an in vitro study. Int J Radiat Oncol Biol Phys. 2009;75:482-488.
184. Fowler J.F. Sensitivity analysis of parameters in linear-quadratic radiobiologic modeling. Int J Radiat Oncol Biol Phys. 2009;73:1532-1537.
185. Kocher M., Wilms M., Makoski H.B., et al. Alpha/beta ratio for arteriovenous malformations estimated from obliteration rates after fractionated and single-dose irradiation. Radiother Oncol. 2004;71:109-114.
186. Suntharalingam N., Podgorsak E.B., Hendry J.H., et al. Basic radiobiology (chapter 14). In: Podgorsak E.B., editor. Radiation Oncology Physics: A Handbook for Teachers and Students. Vienna: International Atomic Energy Agency; 2005:485-504.
187. Oh B.C., Liu C.Y., Wang M.Y., et al. Stereotactic radiosurgery: adjacent tissue injury and response after high-dose single fraction radiation. Part II: Strategies for therapeutic enhancement, brain injury mitigation, and brain injury repair. Neurosurgery. 2007;60:799-814.
188. Marosi C., Hassler M., Roessler K., et al. Meningioma. Crit Rev Oncol Hematol. 2008;67:153-171.
189. Linskey M.E., Davis S.A., Ratanatharathorn V. Relative roles of microsurgery and stereotactic radiosurgery for the treatment of patients with cranial meningiomas: a single-surgeon 4-year integrated experience with both modalities. J Neurosurg. 2005;102(Suppl):59-70.
190. Kim D.G., Kim Ch H., Chung H.T., et al. Gamma knife surgery of superficially located meningioma. J Neurosurg. 2005;102(Suppl):255-258.
191. Mindermann T., de Rougemont O. The significance of tumor location for gamma knife treatment of meningiomas. Stereotact Funct Neurosurg. 2004;82:194-195.
192. Kuo J.S., Yu C., Giannotta S.L., et al. The Leksell gamma knife Model U versus Model C: a quantitative comparison of radiosurgical treatment parameters. Neurosurgery. 2004;55:168-172.
193. Torres R.C., Frighetto L., De Salles A.A., et al. Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus. 2003;14:e5.
194. Jensen R.L., Wendland M.M., Chern S.S., et al. Novalis intensity-modulated radiosurgery: methods for pretreatment planning. Neurosurgery. 2008;62:A2-A9.
195. Narayan S., Cornblath W.T., Sandler H.M., et al. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56:537-543.
196. Leber K.A., Bergloff J., Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43-50.
197. Kiratli H., Yildiz S., Soylemezoglu F. Neurofibromatosis type 2: optic nerve sheath meningioma in one orbit, intramuscular schwannoma in the other. Orbit. 2008;27:451-454.
198. Wentworth S., Pinn M., Bourland J.D., et al. Clinical experience with radiation therapy in the management of neurofibromatosis-associated central nervous system tumors. Int J Radiat Oncol Biol Phys. 2009;73:208-213.
199. Stafford S.L., Pollock B.E., Leavitt J.A., et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
200. Shin M., Kurita H., Sasaki T., et al. Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurg. 2001;95:435-439.
201. Pitz S., Becker G., Schiefer U., et al. Stereotactic fractionated irradiation of optic nerve sheath meningioma: a new treatment alternative. Br J Ophthalmol. 2002;86:1265-1268.
202. Pichierri A., Santoro A., Raco A., et al. Cavernous sinus meningiomas: retrospective analysis and proposal of a treatment algorithm. Neurosurgery. 2009;64:1090-1099.
203. Lee J., Stippler M., Niranjan A., et al. Gamma knife radiosurgery for cavernous sinus meningiomas. Radiosurgery. 2006;6:118-130.
204. Dolenc V. Anatomy and Surgery of the Cavernous Sinus. New York: Springer-Verlag; 1989.
205. Abdel-Aziz K.M., Froelich S.C., Dagnew E., et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery. 2004;54:1375-1383.
206. Sekhar L.N., Patel S., Cusimano M., et al. Surgical treatment of meningiomas involving the cavernous sinus: evolving ideas based on a ten year experience. Acta Neurochir. 1996;65(Suppl):58-62.
207. Kotapka M.J., Kalia K.K., Martinez A.J., et al. Infiltration of the carotid artery by cavernous sinus meningioma. J Neurosurg. 1994;81:252-255.
208. Larson J.J., van Loveren H.R., Balko M.G., et al. Evidence of meningioma infiltration into cranial nerves: clinical implications for cavernous sinus meningiomas. J Neurosurg. 1995;83:596-599.
209. Sen C., Hague K. Meningiomas involving the cavernous sinus: histological factors affecting the degree of resection. J Neurosurg. 1997;87:535-543.
210. Sindou M., Wydh E., Jouanneau E., et al. Long-term follow-up of meningiomas of the cavernous sinus after surgical treatment alone. J Neurosurg. 2007;107:937-944.
211. Chang S.D., Adler J.R.Jr., Martin D.P. LINAC radiosurgery for cavernous sinus meningiomas. Stereotact Funct Neurosurg. 1998;71:43-50.
212. Roche P.H., Regis J., Dufour H., et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000;93(Suppl 3):68-73.
213. Spiegelmann R., Nissim O., Menhel J., et al. Linear accelerator radiosurgery for meningiomas in and around the cavernous sinus. Neurosurgery. 2002;51:1373-1379.
214. Iwai Y., Yamanaka K., Ishiguro T. Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas. Neurosurgery. 2003;52:517-524.
215. Maruyama K., Shin M., Kurita H., et al. Proposed treatment strategy for cavernous sinus meningiomas: a prospective study. Neurosurgery. 2004;55:1068-1075.
216. Metellus P., Regis J., Muracciole X., et al. Evaluation of fractionated radiotherapy and gamma knife radiosurgery in cavernous sinus meningiomas: treatment strategy. Neurosurgery. 2005;57:873-886.
217. Nicolato A., Foroni R., Alessandrini F., et al. The role of Gamma knife radiosurgery in the management of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2002;53:992-1000.
218. Noel G., Habrand J.L., Mammar H., et al. Highly conformal therapy using proton component in the management of meningiomas. Preliminary experience of the Centre de Protontherapie d’Orsay. Strahlenther Onkol. 2002;178:480-485.
219. Yamakami I. Surgical removal of petroclival meningiomaas:what is the most appropriate treatment option in the multimodal treatment era? No Shinkei Geka. 2008;36:1069-1079.
220. Roche P.H., Pellet W., Fuentes S., et al. Gamma knife radiosurgical management of petroclival meningiomas results and indications. Acta Neurochir. 2003;145:883-888.
221. Nicolato A., Foroni R., Pellegrino M., et al. Gamma knife radiosurgery in meningiomas of the posterior fossa. Experience with 62 treated lesions. Minim Invasive Neurosurg. 2001;44:211-217.
222. Van Havenbergh T., Carvalho G., Tatagiba M., et al. Natural history of petroclival meningiomas. Neurosurgery. 2003;52:55-62.
223. Subach B.R., Lunsford L.D., Kondziolka D., et al. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437-443.
224. Iwai Y., Yamanaka K., Nakajima H., et al. [Gamma knife radiosurgery for skull base meningiomas: the treatment results and patient satisfaction expressed in answers to a questionnaire]. No Shinkei Geka. 2000;28:411-415.
225. Huang C.F., Tu H.T., Liu W.S., et al. Gamma knife surgery for trigeminal pain caused by benign brain tumors. J Neurosurg. 2008;109(Suppl):154-159.
226. Kim I.Y., Kondziolka D., Niranjan A., et al. Gamma knife radiosurgery for intraventricular meningiomas. Acta Neurochir. 2009;151:447-452.
227. Guidetti B., Delfini R., Gagliardi F.M., et al. Meningiomas of the lateral ventricles. Clinical, neuroradiologic, and surgical considerations in 19 cases. Surg Neurol. 1985;24:364-370.
228. Liu M., Wei Y., Liu Y., et al. Intraventricular meninigiomas: a report of 25 cases. Neurosurg Rev. 2006;29:36-40.
229. Lang I., Jackson A., Strang F.A. Intraventricular hemorrhage caused by intraventricular meningioma: CT appearance. AJNR Am J Neuroradiol. 1995;16:1378-1381.
230. Gelabert-Gonzalez M., Garcia-Allut A., Bandin-Dieguez J., et al. Meningiomas of the lateral ventricles. A review of 10 cases. Neurocirugia (Astur). 2008;19:427-433.
231. Morokoff A.P., Zauberman J., Black P.M. Surgery for convexity meningiomas. Neurosurgery. 2008;63:427-433.
232. Black P.M., Morokoff A.P., Zauberman J. Surgery for extra-axial tumors of the cerebral convexity and midline. Neurosurgery. 2008;62:1115-1121.
233. Ganz J.C., Schrottner O., Pendl G. Radiation-induced edema after gamma knife treatment for meningiomas. Stereotact Funct Neurosurg. 1996;66(Suppl 1):129-133.
234. Nakamura S., Hiyama H., Arai K., et al. Gamma knife radiosurgery for meningiomas: four cases of radiation-induced edema. Stereotact Funct Neurosurg. 1996;66(Suppl 1):142-145.
235. Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-413.
236. Vermeulen S., Young R., Li F., et al. A comparison of single fraction radiosurgery tumor control and toxicity in the treatment of basal and nonbasal meningiomas. Stereotact Funct Neurosurg. 1999;72(Suppl 1):60-66.
237. Ramsey A.F., Blurton M., Ekstrand K. Edema following gamma knife radiosurgery for intracranial meningiomas. Int J RadiatOncol Biol Phys. 2002;54(Suppl 1):146-147. (abstract)
238. Chin L.S., Ma L., DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94:899-904.
239. Flickinger J.C., Kondziolka D., Lunsford L.D., et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys. 2000;46:1143-1148.
240. Iwai Y., Yamanaka K., Nakajima H. The treatment of skull base meningiomas—combining surgery and radiosurgery. J Clin Neurosci. 2001;8:528-533.
241. Kondziolka D., Lunsford L.D. Radiosurgery of meningiomas. Neurosurg Clin N Am. 1992;3:219-230.
242. Nataf F., Ghossoub M., Missir O., et al. [Parenchymal changes after radiosurgery of cerebral arteriovenous malformations. Clinical and MRI data]. Neurochirurgie. 2001;47:355-368.
243. Sharma M.S., Kondziolka D., Khan A., et al. Radiation tolerance limits of the brainstem. Neurosurgery. 2008;63:728-732.
244. Dropcho E.J. Neurotoxicity of radiation therapy. Neurol Clin. 2010;28:217-234.
245. Regis J., Roche P.H. Modern management of acoustic neuroma. Prog Neurol Surg. 2008;21:1-5.
246. Kirova Y., Vilcoq J.R., Asselain B., et al. [Radiation-induced sarcomas after breast cancer: experience of Institute Curie and review of literature]. Cancer Radiother. 2006;10:83-90.
247. Muracciole X., Regis J. Radiosurgery and carcinogenesis risk. Prog Neurol Surg. 2008;21:207-213.
248. Kajiwara M., Yamashita K., Ueba T., et al. Normal pressure hydrocephalus after radiosurgery for sphenoid ridge meningioma. J Clin Neurosci. 2009;16:162-164.
249. Varlotto J.M., Shrieve D.C., Alexander E.3rd, et al. Fractionated stereotactic radiotherapy for the treatment of acoustic neuromas: preliminary results. Int J Radiat Oncol Biol Phys. 1996;36:141-145.
250. Kondziolka D., Lunsford L.D. Future perspectives in acoustic neuroma management. Prog Neurol Surg. 2008;21:247-254.