Stem cell transplantation
Allogeneic and syngeneic (twin) SCT
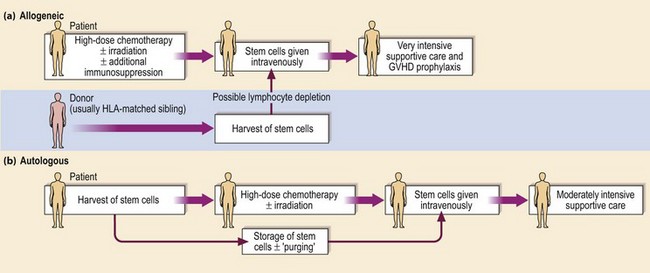
The allogeneic procedure is outlined in Figure 28.1. The patient’s own haematopoietic stem cells, immune system and residual tumour cells are conventionally destroyed by conditioning treatment with high-dose chemotherapy and (usually) radiotherapy prior to intravenous infusion of stem cells harvested from the healthy donor. The ideal patient has a disease curable by allogeneic SCT but not by less toxic treatment and is young (less than 40 years old). The ideal donor, excepting the rare presence of a twin, is a sibling genotypically matched with the recipient for HLA-A, B and DR. The genes for HLA are found on chromosome 6 and so inheritance follows the rules of simple Mendelian inheritance; two siblings have a one in four chance of sharing the same two HLA haplotypes. With relatively small family size in the Western world, only around 30% of patients will have an HLA-identical sibling. Transplants from HLA-haploidentical relatives have been associated with a high rate of morbidity and mortality. An alternative strategy is to search for an unrelated volunteer donor who is a phenotypic HLA match. From the worldwide databases of 18 million HLA-typed volunteers, around 50% of Caucasian patients can be found a suitable donor. Success rates are lower in other ethnic groups. Use of a HLA-mismatched donor leads to an increase in the incidence and severity of the adverse effects of SCT. Weaker transplant reactions result from mismatch for minor histocompatibility antigens, single peptides derived from polymorphic proteins which may differ between donor and recipient.
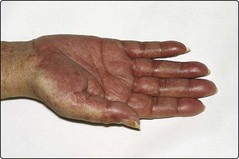
After conditioning treatment there is a period of about 3 weeks before ‘engraftment’ during which the patient is severely pancytopenic and immunosuppressed and requires intensive supportive care with blood products and aggressive treatment of any infection. Major adverse events include graft failure with rejection arising from a failure to immunosuppress the patient adequately, and graft-versus-host disease (GVHD). GVHD is a potentially life-threatening disorder predominantly affecting the skin (Fig 28.2), gastrointestinal tract and liver which may occur early after transplantation (acute GVHD) or after a few months (chronic GVHD). GVHD results from donor immunocompetent cells attacking antigens in the recipient and can be abrogated by removal of T-lymphocytes from the donor stem cells. Such ‘lymphocyte depletion’ may lead to an increased risk of relapse of the underlying malignancy, confirming that much of the curative potential of allogeneic SCT is due to the presence of immunologically active donor cells rather than the preparative regimen (see also non-myeloablative SCT). The profound and prolonged immunosuppression of allogeneic SCT renders the patient vulnerable to life-threatening fungal and viral (e.g. cytomegalovirus) infections. Causes of late death include relapse of disease, chronic GVHD and secondary cancers related to previous chemotherapy and SCT conditioning. Peripheral blood stem cells are now used more often than bone marrow for allogeneic procedures. For related donors, PBSC give quicker engraftment and probably reduced disease relapse and improved survival although there is some increase in GVHD. Common indications for allogeneic SCT include acute leukaemia and chronic myeloid leukaemia.
Autologous SCT
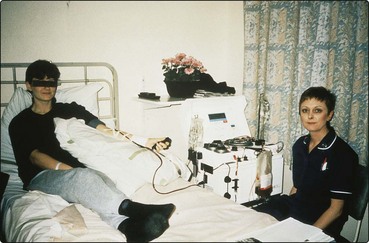
The procedure is outlined in Figure 28.1. High-dose chemotherapy, and sometimes radiotherapy, is followed by reinfusion of previously stored patient stem cells. Autologous SCT has less toxicity than allogeneic SCT and therefore can be performed in older patients. It also has the advantage that the patient is the donor and therefore donor unavailability and GVHD are not issues. The main disadvantage of autologous SCT compared with allogeneic SCT is an increased incidence of relapse of malignant disease. It is not clear whether this arises from resistance to the conditioning treatment or infusion of residual tumour cells in the graft. The ‘purging’ of tumour cells from grafts does not appear to alter survival. Peripheral blood stem cells (PBSC) have now largely replaced the use of bone marrow in autologous procedures. PBSC can be harvested from the patient’s blood by leucapheresis during the recovery phase from moderate doses of chemotherapy (Fig 28.3). The growth factor G-CSF is often used to facilitate the ‘mobilisation’ of stem cells. Compared with the traditional bone marrow autologous transplant, PBSCT gives more rapid recovery of a normal blood count with fewer infections, less intensive supportive care and shortened hospital stay. PBSCT is currently widely used as a convenient and relatively safe method of escalating the dose of chemotherapy in patients with acute leukaemia, lymphoma and myeloma. Its role in the treatment of non-haematopoietic solid tumours and autoimmune disorders is less established.