Staging of oesophageal and gastric cancer
Introduction
A review of this chapter from previous editions highlights the huge changes that have, and continue, to occur in the staging process of oesophageal and gastric cancer. The two diseases now have quite distinct staging protocols with an overlap in the often complex area of junctional tumours. The final aim for each patient must be to reach the best treatment option available with the recognition that for the majority of patients this will be a non-curative decision. The extent of change occurring in the last 20 years is clearly demonstrated if we compare the staging investigations employed in the two large MRC trials of neoadjuvant chemotherapy for oesophageal cancer patients undertaken in the UK.1,2 Assessments before treatment in the OEO2 trial (recruitment from 1992 to 1998) included chest radiography, bronchoscopy for upper third and middle third tumours, and ultrasonography or computed tomography (CT) of the liver. In comparison, for entry into the OEO5 trial (recruitment from 2005 to 2011), staging investigations were spiral/multi-slice CT of chest and abdomen (neck and pelvis when indicated) with oral contrast or water, endoscopic ultrasonography (EUS), laparoscopy where clinically indicated with further options of bone scan, positron emission tomography (PET), laparoscopic ultrasonography and intraperitoneal cytology. Although patients rarely require all of these staging modalities, they do all require to be discussed in this chapter with the indications, benefits and limitations outlined for each.
Staging classifications
The TNM (‘tumour–node–metastasis’) staging system was devised by Pierre Denoix between 1943 and 1952, where T represents the extent of the primary tumour, N the absence or presence and extent of regional lymph node involvement, and M the absence or presence of distant metastases.3 A single TNM staging classification was agreed between the American Joint Committee on Cancer (AJCC), the Japanese Joint Committee (JJC) and the International Union Against Cancer (UICC) in 1987.4 This classification system has been revised on several occasions with the latest version, TNM7, coming into effect in January 2010.5 It is now routinely used by the majority of centres in Europe and North America and its use is strongly recommended.
Gastric cancer staging
The development of a staging system for gastric cancer with a worldwide application is difficult as the location of the tumour within the stomach influences survival.6 Distal gastric cancers carry a more favourable prognosis and are more common in Asian populations, with more proximal lesions seen more frequently in western countries. Two thirds of gastric cancers occur in developing countries and the inclusion of molecular or immunohistochemical features of the tumour as part of the staging system would prevent the majority of gastric cancers worldwide being available for comparison.7,8 It is for this reason they do not form part of the current TNM7 classification.
TNM7 included major changes in the classification of gastric carcinomas in comparison to TNM6.4,9 One of the most significant is the use of the oesophageal cancer staging system for junctional tumours or any tumour arising within the proximal 5 cm of the stomach and crossing the oesophagogastric junction. Previously, in TNM6, junctional tumours could be staged according to either the gastric or oesophageal system depending on the judgment of the clinician.9
Within the luminal gastrointestinal (GI) tract the T categories have been amalgamated for tumours occurring anywhere from oesophagus to rectum. The T stage classification for gastric cancer has changed significantly, with subdivision of the T1 category into T1a (tumour invades lamina propria or muscularis mucosae) and T1b (tumour invades submucosa) (Table 3.1). The distinction between these two stages has become increasingly important with the development of endoscopic resectional techniques for early-stage gastric tumours. T3 is now invasion of the subserosa without invasion of the visceral peritoneum (T2b in TNM6). T4a is tumour invasion of the serosa (visceral peritoneum), which was T3 disease in TNM6. T4 disease in TNM6 (invasion into adjacent structures) is T4b in the current classification.
The extent of lymph node involvement is the most important independent prognostic factor in gastric cancer.10 In the history of the development of the TNM classification there has been a move from the location of lymph node metastases (less than or greater than 3 cm from the primary tumour in TNM4) to the total number of involved lymph nodes.9,11–14 The latest edition categorises the number of involved lymph nodes into narrower groups with the aim of improving prognostic accuracy. The seventh edition of TNM will result in upstaging of patients in comparison to TNM6, with fewer nodes required for entry into the N2 and N3 groups.
There is the potential for the N classification to be underestimated if the number of examined nodes is too small. To determine the minimum number required for a correct classification, 926 patients undergoing curative resection for gastric carcinoma were analysed in a study by Ichikura et al.15 The number of metastatic lymph nodes correlated significantly with the number of examined lymph nodes. In patients with pN0 disease those with five to nine examined nodes had a significantly lower survival rate compared to those with 10–14 examined nodes. Interestingly, patients with 10–14 nodes examined had as good a prognosis as those with 15 or more. On the basis of this finding the authors concluded that the minimum number of lymph nodes examined for a correct pN0 classification can be reduced from 15 to 10. However, this is assuming that the cTNM stage of N0 disease is accurate and, although there are reports of improvement in the accuracy rates of preoperative diagnosis in cases of early-stage gastric cancer, it would always be the author’s recommendation that a D2 gastrectomy is performed whenever possible.16 In the pN1 and pN2 categories, patients with 29 or fewer examined nodes tended towards lower survival rates than patients with 30 or more examined nodes. The authors concluded that for pN1–3 classifications, 20 or more nodes should be examined, and examining 30 or more lymph nodes may be desirable.
However, many reports do not reach these numbers and only 31% of gastric resections in a UK-based study included 15 or more lymph nodes for histological analysis.17
The prognostic value of metastatic lymph node ratio (the ratio of the number of metastatic lymph nodes to the number of lymph nodes removed) after curative resection has been reported.18,19 Both studies reported the metastatic lymph node ratio as an independent prognostic factor for survival. Among patients with pN2 by the UICC/TNM6 classification, survival in patients with a metastatic lymph node ratio less than 0.1 was significantly better than in those with a higher metastatic lymph node ratio.19 Maximising the total number of lymph nodes removed at the time of resection could decrease the metastatic lymph node ratio below 0.1, adding further evidence for the need for extended lymphadenectomy. The TNM7 stage groupings for gastric cancer are outlined in Table 3.2.
Oesophageal cancer staging
1. Cervical oesophagus. From the lower border of the cricoid cartilage to the thoracic inlet (suprasternal notch), approximately 18 cm from the upper incisor teeth.
i. Upper thoracic portion. From the thoracic inlet to the level of the tracheal bifurcation, approximately 24 cm from the upper incisor teeth.
ii. Mid-thoracic portion. The proximal half of the oesophagus between the tracheal bifurcation and the oesophagogastric junction. The lower level is approximately 32 cm from the upper incisor teeth.
iii. Lower thoracic portion. The distal half of the oesophagus between the tracheal bifurcation and the oesophagogastric junction. The lower level is approximately 40 cm from the upper incisor teeth. This portion is approximately 8 cm in length and includes the abdominal oesophagus.
Regional lymph nodes (N stage) are those in the oesophageal drainage area including coeliac axis nodes and paraoesophageal nodes in the neck, but not supraclavicular nodes. It is recommended that at least six lymph nodes are examined from the lymphadenectomy specimen. If fewer than six lymph nodes are present and all are negative the classification remains N0. The TNM7 categories for oesophageal cancer are shown in Table 3.3.
The introduction of a stratified classification of nodal involvement has been welcomed by most surgeons. Prior to the publication of the latest TNM7 classification, evidence existed that survival may be predicted by the number of involved lymph nodes.20–22 A review of 336 patients undergoing resection of previously untreated adenocarcinoma and squamous cell carcinoma of the oesophagus and gastro-oesophageal junction reported that patients with more than four involved lymph nodes had survival similar to that of patients with M1 disease.20 Patients with no involved lymph nodes had the best prognosis. The authors identified 18 lymph nodes as the minimal number required for accurate staging. In a multinational, retrospective review of 1053 patients with oesophageal cancer treated with resection alone, recurrent disease had occurred in 40% at 5 years.21 The frequency of systemic disease after oesophagectomy was 16% for those without nodal involvement and progressively increased to 93% in patients with eight or more involved lymph nodes.
A recent UK study of oesophageal and junctional adenocarcinomas used a revised node (N) classification based on number of involved lymph nodes (N0, none; N1, one to five; N2, six or more) and location in relation to the diaphragm.22 This demonstrated that a poorer prognosis was associated with increasing nodal involvement and involvement above and below the diaphragm. The TNM7 stage groupings for oesophageal cancer are outlined in Table 3.4.
Multidisciplinary team
Over time staging investigations have become more numerous and complex, and treatment options more varied. In order to keep abreast of current evidence and to ensure the highest level of expertise is afforded to all patients it is essential that all cancer patients are discussed at a multidisciplinary team (MDT) meeting. This meeting should include surgeons, gastroenterologists, radiologists, radiation and medical oncologists, pathologists, cancer nurse specialists and palliative care physicians. The improved accuracy of CT staging with the involvement of specialist radiologists within the MDT setting has been reported.23 It has also been shown that involvement of the MDT improves overall clinical staging accuracy and is associated with improved outcomes after surgery for gastro-oesophageal cancer.24–26 In a recent review on the introduction of an MDT for patients with oesophageal cancer, there was a significant increase in the percentage of patients receiving complete staging, a multidisciplinary evaluation and adherence to nationally accepted care guidelines.27 The time from diagnosis to treatment significantly decreased, reducing from a mean of 27 to 16 days (P < 0.0001). Dutch guidelines similarly recommend discussion of patients with upper GI malignancies by an MDT. A recent study found that in over one-third of cases the diagnostic work-up or treatment plan proposed by the referring physician was altered after evaluation by the MDT.28
Staging investigations
Contrast radiography
Contrast radiography must be mentioned but cannot be regarded as either a first-line or routine investigation in patients with suspected upper GI malignancy. While there is evidence that double-contrast radiology can diagnose oesophageal and junctional tumours with a sensitivity of 96%, it cannot provide the essential histological confirmation that is obtained at endoscopy and is required for staging purposes.29 It must, however, be stated that in patients with dysphagia with a normal contrast study there is minimal chance that endoscopy will detect any missed oesophageal carcinomas.30,31 If for whatever reason an endoscopy is impossible or not tolerated, a contrast examination may be useful (Fig. 3.1).
Endoscopy
Flexible upper GI endoscopy is the most important investigation in the diagnosis of oesophageal and gastric carcinoma. In experienced hands it is a safe procedure, with a large UK-based audit of 14 149 procedures reporting a perforation rate of 0.05% and an overall mortality rate of 0.008% during diagnostic endoscopy.32 After cardiopulmonary complications, perforation is the second most important complication. Although historically the majority of endoscopies were performed under sedation, there is an ever increasing number being performed without sedation or under topical oropharyngeal anaesthesia with 100 mg lignocaine spray.33–35 Endoscopy provides accurate information on the location and extent of the lesion and its relationship to anatomical landmarks. Crucially, it also provides the opportunity to obtain a tissue diagnosis with the acquisition of biopsies. It has been shown in patients with carcinoma of the oesophagus that two endoscopic biopsies will provide a positive diagnosis in 95.8% of cases, four biopsies in 97.9% and six biopsies in 100% of cases.36 It is recommended that at least six to eight biopsies are taken at the time of endoscopy to improve the chances of reaching a definitive tissue diagnosis. Not all tumours are negotiable at the time of endoscopy and although dilatation can be performed, the risk of perforation is significantly increased with the risk of rendering a potentially operable tumour inoperable or greatly impairing the prognosis.37 It is therefore advisable when a tumour is stenotic to first obtain an urgent tissue diagnosis before any consideration is given to dilatation.
Computed tomography (CT)
Once a cancer diagnosis has been made, CT is recommended as the initial imaging investigation for both oesophageal and gastric lesions. This allows detection of nodal involvement and metastatic disease and is the most cost-effective investigation.38 Recent progress in multi-detector row CT (MDCT) allows a thinner section thickness in a single breath hold to be obtained, with subsequent improvement in image quality.
Gastric cancer
Patients with gastric cancer require CT of chest, abdomen and pelvis. Scans are performed with intravenous contrast and oral ingestion of either effervescent granules or 1 litre of water to create gastric distention.39,40 The CT appearances of gastric carcinoma are variable and can present with either focal or diffuse wall thickening (Fig. 3.2). Lesions may project into the lumen of the stomach or ulcerate into the wall. With MDCT the overall accuracy in determining the T stage is now in the region of 77–89%.40–46 The ability of CT to detect organ invasion by the primary tumour remains disappointing even with modern scanners. A study from Japan that assessed high-resolution CT and adjacent organ invasion showed that the finding of an absence of fat plane or an irregularity of the border between the tumour and the adjacent organ was not significantly related to invasion.47 However, when the mean densities at the region of interest were measured they were found to be significantly greater at invasion sites than at non-invasion sites. Although this allowed invasion of the pancreas, liver and colon to be assessed with an accuracy of 75%, 61% and 78%, respectively, these authors still found that CT had limited value in differentiating inflammatory adhesions with fibrosis or oedema from true invasion.
A recent study reported a marked improvement in T-stage accuracy using a new CT vessel probe reconstruction protocol using a 16-row MDCT.48 When compared with standard axial images the overall accuracy rates for T stage improved from 68% to 94% when compared with final histology.
Virtual upper GI endoscopy is a minimally invasive test that utilises three-dimensional (3-D) CT to simulate conventional upper endoscopy images. Images are obtained using both oral and intravenous contrast. The detection rate of gastric lesions using virtual GI endoscopy has been reported to be between 78.7% and 96.7% in early gastric cancer and between 90% and 100% in advanced gastric cancer.49,50 The overall accuracy, sensitivity and specificity for 3-D multi-detector row CT in the preoperative determination of depth of invasion of gastric cancer (T stage) have been reported to be 83.3%, 69.1% and 94.4%, respectively.50 Conventional upper GI endoscopy provides direct visualisation of the mucosa, permits evaluation of colour changes that may be indicative of pathology, and suspicious lesions can be biopsied and the tissue sample evaluated histologically. While virtual upper GI endoscopy using CT is a promising method for the detection and evaluation of upper GI lesions, randomised controlled studies comparing it to conventional upper GI endoscopy are needed to determine its clinical value.
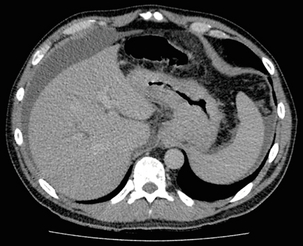
Accuracies ranging between 63% and 80% have been reported for N staging by CT when compared with histological staging of the resected specimen (sensitivity 74%, specificity 65%).42–45,51,52 Limitations to CT nodal staging relate to the detection of involved perigastric nodes close to the primary tumour (Fig. 3.3). These lymph nodes often appear confluent with the primary tumour and therefore CT will continue to lack accuracy in nodal staging of some gastric cancers.
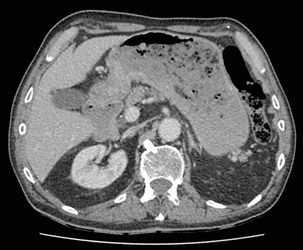
Figure 3.3 CT image of patient with distal gastric cancer, gastric outlet obstruction and food residue within stomach.
One study that retrospectively reviewed the histology of more than 23 000 lymph nodes from gastric cancer resections demonstrated that the mean diameter of a metastatic node was 7.8 mm, and if 5 mm was used as a cut-off, 38% of metastatic nodes would still be missed.53 Improved image quality associated with modern scanners allows the identification of even smaller regional lymph nodes, but the pathological significance of these smaller lymph nodes remains unknown.
A recent study reported an accuracy rate of 93% in the identification of para-aortic lymph node metastases from gastric cancer using MDCT.54 Thirteen of 92 (14%) patients undergoing potentially curative resection had para-aortic lymph node involvement on histological examination. Eleven of these were correctly staged preoperatively using MDCT, a sensitivity of 85%.
The accuracy, sensitivity and specificity of 3-D multi-detector row CT for lymph node staging were reported to be 75%, 57.4% and 89.3%, respectively.51
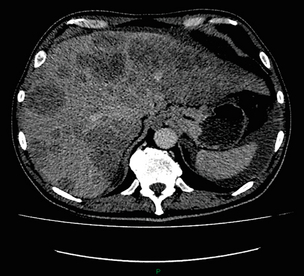
CT is useful in the detection of distant metastases and accuracy figures are similar to those seen for oesophageal malignancy (Fig. 3.4). CT, however, is limited in its ability to detect transcoelomic spread and the presence of peritoneal seedlings. In a study of 78 patients listed for curative gastrectomy for gastric cancer based on CT findings, 23 (29.5%) had undetected peritoneal spread at the time of laparoscopy.55 It is recommended that all patients being considered for curative gastrectomy undergo a staging laparoscopy with peritoneal washings. The presence of CT-defined minimal ascites (< 50 mL) has been reported not to affect survival on a stage-for-stage basis in patients with gastric cancer without peritoneal metastases.56 Of those with CT-defined minimal ascites, 28.1% had peritoneal metastases confirmed at surgery.
Further improvements in the accuracy of CT staging may be achieved through establishment of radiologists with a special interest. One report demonstrated improved levels of sensitivity and specificity among radiologists who regularly stage patients with gastric cancer, with an associated reduction in the open-and-close laparotomy rate.57 Such findings provide additional support for the formation of specialist multidisciplinary teams for the management of gastro-oesophageal cancer.
Oesophageal cancer
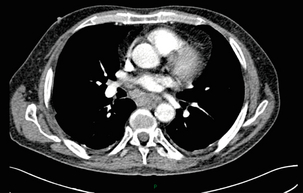
Conventional CT has historically diagnosed T4 lesions with high accuracy rates. This is because the criteria for staging T4 lesions are based on obliteration of the fat layer or the angle between the tumour and the adjacent organs (Fig. 3.5).58–60 Oesophageal wall thickness has been used for earlier T stages because tumour cannot be sufficiently differentiated from normal oesophageal wall.61,62 Accuracy rates for T-stage detection using spiral CT when compared with histopathological stage of resected specimens have been reported as between 43% and 92%.63–65 Wu et al. reported the accuracies of T staging using the following criteria: T1 and T2, oesophageal wall thickness < 5 mm; T3, oesophageal wall thickness > 5 mm; and T4, invasion into adjacent organs.66 In their study, the accuracy values of the respective T staging were 75% for T1/T2, 79% for T3 and 64% for T4. According to these criteria T1 lesions cannot be differentiated from either T2 lesions or from normal oesophageal wall. A recent study from Japan reported improved accuracy rates for T staging of early oesophageal carcinomas (T1a and T1b).67 A dual-phase (arterial and venous phase) contrast-enhanced CT protocol with MDCT was used. All lesions classified as T1 lesions were T1b with no T1a lesions visualised. This differentiation is important as T1a lesions can be considered for endoscopic mucosal resection, with T1b requiring more radical treatment. In addition, the nodal involvement rate increases from 1.3% in T1a lesions to 22% in T1b lesions.68
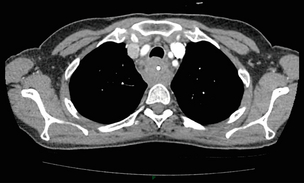
Figure 3.5 CT image of patient with squamous cell carcinoma (SCC) of oesophagus from 20 to 27 cm with T4 invasion into trachea. A fine-bore feeding tube is in situ.
Accuracy for N-stage disease ranges between 27% and 86% (sensitivity 48–68% with a specificity of 90–95%) (Fig. 3.6).63–65,69,70 The difficulty in the accurate identification of involved lymph nodes is the reliance on size to differentiate between malignant and benign pathology.
The size of the lymph node that different authors regard as a criterion for malignant involvement varies from 5 to 15 mm.71 Lymph nodes of more than 1 cm in diameter can, however, be seen within the mediastinum in healthy people, particularly those with coexisting chest problems, and nodes of normal size may contain metastatic deposits.72
CT and EUS are complementary techniques in staging oesophageal cancer patients.73,74 A comparison of EUS and CT identification of involved lymph node stations in 121 patients with squamous cell carcinoma of the oesophagus reported an overall accuracy of 64% for EUS (sensitivity 68%, specificity 58%, positive predictive value (PPV) 68%), 51% for CT (sensitivity 33%, specificity 75%, PPV 64%), and 64% for CT and EUS in combination (sensitivity 74%, specificity 50%, PPV 66%).74 However, some metastatic lymph nodes in the neck and abdomen are only detectable by CT, and it was recommended that both EUS and CT should be undertaken for routine examination prior to treatment of oesophageal cancer.
Positron emission tomography (PET)
The role of fluorodeoxyglucose (FDG)-PET in gastric cancer is not as well established as in oesophageal cancer. Reported detection rates of primary tumours vary between 60% and 90% and depend on the histopathological characteristics of the primary tumour.75,76 Stahl et al. reported on a series of 40 gastric cancer patients and found that tumours with a non-intestinal growth type according to the Lauren classification showed significantly lower FDG uptake than tumours of the intestinal growth type.77 Non-mucinous carcinomas accumulated significantly more FDG than mucinous ones. This finding has also been reported by Yamada et al., who found a higher standardised uptake value (SUV) in the tubular adenocarcinoma group than in the mucinous and signet-ring cell adenocarcinoma group.78 These findings have been confirmed in a more recent study that also showed that the expression of the glucose transporter (GLUT-1) significantly correlated with the maximum calculated SUV: 76% of signet-ring cell carcinomas did not show GLUT-1 expression.79
Oesophageal cancer
Conflict still exists on the role of PET in the management of patients with oesophageal cancer, but it has been widely adopted in most large centres. The Scottish Intercollegiate Guidelines Network (SIGN) previously concluded that PET is not routinely indicated in staging gastro-oesophageal tumours.80 To date, no randomised trial has been undertaken to determine the role of FDG-PET or PET/CT in staging upper GI cancer. Such a trial is unlikely to occur as it is now an established staging tool in upper GI cancer despite the lack of published evidence. In 2009 the Scottish National PET Advisory Group recommended the routine use of PET/CT in staging patients with potentially operable oesophageal cancer. Changes in management with the addition of FDG-PET to the staging protocol in patients with oesophageal cancer are reported to occur in anything from 3% to 41% of patients.81–86 This huge variation may be related to the point in the staging pathway at which PET has been introduced. In a study of 199 patients, FDG-PET was performed only after a full preoperative staging protocol with MDCT, EUS and external ultrasonography of the neck, both combined with selective fine-needle aspiration cytology.86 Only patients considered eligible for curative surgery after these investigations underwent FDG-PET. FDG-PET revealed suspicious hot spots in 15.1% of patients but metastases were confirmed in only 4.0%. All upstaged patients had clinical stage III–IV disease before FDG-PET. In 3.5% the hot spots appeared to be synchronous neoplasms, mainly colonic polyps. The remaining 7.5% were false positive, leading to unnecessary additional investigations. The authors concluded that the diagnostic benefit of the addition of PET is limited after state-of-the-art staging, and so broad implementation in daily clinical practice is questionable. However, it does not appear sensible on a cost basis to introduce PET at the end of the staging pathway. At the time of writing the costs of MDCT in the UK are approximately £500, PET/CT £1000 and EUS £1800. It would therefore seem reasonable to perform the investigations in this order, reserving the most expensive and invasive tests until the end of the pathway.
PET scans have a limited role in evaluating the T stage of a tumour due to their limited spatial resolution of approximately 6 mm (Fig. 3.7). In a comparison of CT, PET and EUS in the initial staging of patients with oesophageal cancer, Lowe et al. reported correct T staging by CT and PET in only 42% of patients compared with 71% with EUS.87 Superficial and in-situ malignancies of the oesophagus can be difficult to detect with PET, with one study reporting 100% of tumours confined to the mucosa (Tis and T1a) being FDG negative.88 Kato et al. reported that just 18% of T1a tumours and 61% of T1b tumours were FDG-PET positive.89 Low FDG uptake has also been reported in tumours with undifferentiated or mucinous features.90,91 Possible explanations for this include differences in the glucose transporter mechanism, reduced intracellular hexokinase activity resulting in a low rate of FDG phosphorylation, a low volume of metabolically active tumour cells or differences in tumour vascularity.
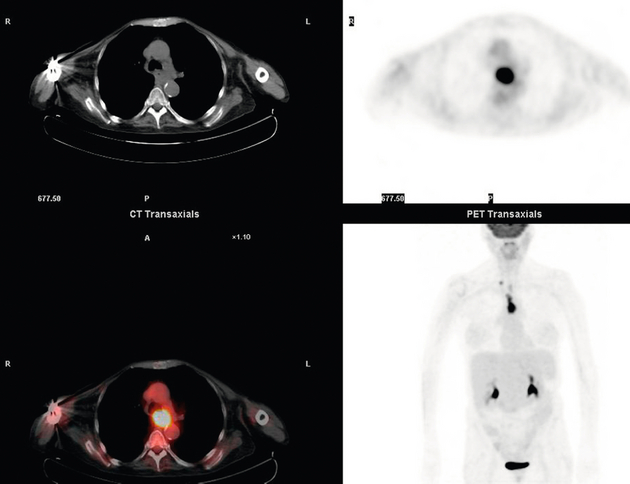
Figure 3.7 FDG-PET/CT image demonstrating increased uptake in a mid-oesophageal SCC. Increased uptake is evident in a right supraclavicular lymph node.
Early studies of FDG-PET in staging oesophageal cancer highlighted the difficulty in differentiating nodal disease adjacent to the primary tumour from the primary tumour itself.92–94
In a review of 12 published studies, pooled sensitivity and specificity for the detection of locoregional metastases were 0.51 (95% confidence interval (CI) 0.34–0.69) and 0.84 (95% CI 0.76–0.91), respectively.95 For distant metastases, pooled sensitivity and specificity were 0.67 (95% CI 0.58–0.76) and 0.97 (95% CI 0.90–1.0), respectively. A more recent meta-analysis reported similar results, where pooled sensitivity and specificity of FDG-PET for regional lymph node metastases were 0.57 (95% CI 0.43–0.70) and 0.85 (95% CI 0.76–0.95), respectively.96
The introduction of integrated CT and PET has improved the accuracy of staging for patients with oesophageal cancer.97,98 In a study of 45 patients with thoracic oesophageal squamous cell cancer, PET/CT was superior to PET alone in the detection of locoregional nodal involvement.98 Sensitivity, specificity and accuracy of PET/CT were 94%, 92% and 92%, respectively, whereas PET alone was 82% sensitive, 87% specific and 86% accurate.
The main role of PET/CT is the identification of distant metastases not evident on CT (Figs 3.8 and 3.9). This prevents unnecessary surgery in patients with incurable disease and allows for appropriate palliative treatments to be offered. In two similar sized meta-analyses, each of over 400 patients, the reported sensitivities and specificities for PET in the detection of distant metastases were similar.95,96 Twelve studies were analysed in the study by van Westreenen et al., with a pooled sensitivity of 67% (95% CI 58–76%) and a specificity of 97% (95% CI 90–100).95 In the report by Van Vliet et al., nine studies were analysed, with a pooled sensitivity of 71% (95% CI 62–79%) and a specificity of 93% (95% CI 89–97).96 Interestingly, only three studies appeared in both meta-analyses.
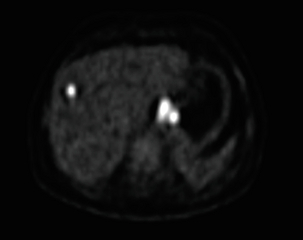
Figure 3.8 FDG-PET/CT image demonstrating increased uptake in abdominal lymph nodes and a solitary metastasis in the right lobe of the liver. The primary lesion was a distal oesophageal adenocarcinoma.
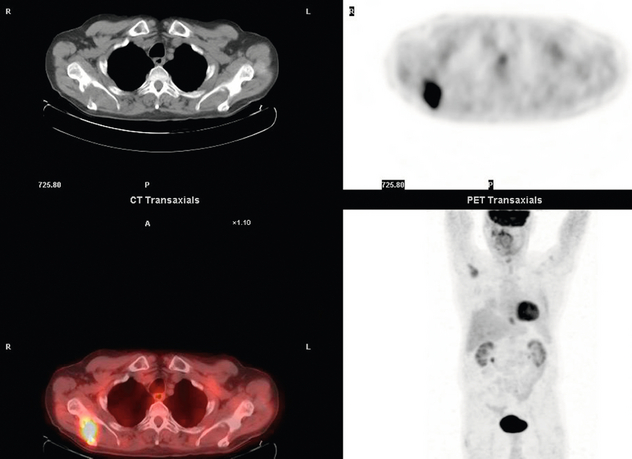
Figure 3.9 FDG-PET/CT image demonstrating a bone metastasis in right scapula from a distal oesophageal adenocarcinoma. The bone lesion was not detected on original review of the CT images.
Accepting that the main role of PET is the detection of metastatic disease, it is debatable whether the routine use of PET is justified in patients with early lesions that are often not visualised on PET and have a low risk of nodal involvement let alone metastatic disease.68,88,89 It is possible that patients with no obvious lymph node involvement on CT gain little from PET but require EUS for confirmation of N0 status and if confirmed should be offered immediate surgery. Those patients with locoregional lymphadenopathy on CT are more likely to have undetected metastases and should be offered PET/CT.
Endoscopic ultrasonography (EUS)
The accuracy of EUS in staging gastric cancer has been varied, with reports that EUS under-stages the depth of invasion and over-stages the nodal invasion because of inflammation around the tumour or in the lymph nodes.99 In a meta-analysis by Puli et al., 22 studies involving 1896 patients were analysed in relation to the accuracy of EUS for staging gastric cancer.100 In relation to T stage, sensitivity and specificity for T1 lesions were 88.1% (95% CI 84.5–91.1) and 100.0% (95% CI 99.7–100.0), respectively. For T2 the sensitivity was 82.3% (95% CI 78.2–86.0) and specificity was 95.6% (95% CI 94.4–96.6). T3 sensitivity was 89.7% (95% CI 87.1–92.0) and specificity was 94.7% (95% CI 93.3–95.9), and T4 had a sensitivity of 99.2% (95% CI 97.1–99.9) and specificity of 96.7% (95% CI 95.7–97.6). The pooled sensitivity and specificity for N1 were 58.2% (95% CI 53.5–62.8) and 87.2% (95% CI 84.4–89.7), respectively. N2 had a pooled sensitivity of 64.9% (95% CI 60.8–68.8) and specificity of 92.4% (95% CI 89.9–94.4). The pooled sensitivity from four studies to diagnose distal metastasis was 73.2% (95% CI 63.2–81.7) and specificity was 88.6% (84.8–91.7).
The presence of low-volume ascites (LVA) on EUS has been shown to be indicative of inoperability in patients with gastric and junctional tumours.101 In patients without evidence of metastatic disease on CT, 6.5% had LVA on EUS. Of these, 76% had either metastatic disease confirmed at laparoscopy or underwent a non-curative resection.
A recent comparison was made of the accuracy of staging using EUS and MDCT in comparison to postoperative pathology patients with gastric cancer undergoing gastrectomy or endoscopic resection.102 In 277 patients the overall accuracy for T staging of EUS was 74.7% and for MDCT was 76.9%. The overall accuracy for N staging was 66% for EUS and 62.8% for MDCT. The performance of EUS and MDCT for large lesions and lesions at the cardia and angle of His had significantly lower accuracy than that of other groups. EUS had significantly lower accuracy rates for early gastric cancer lesions with ulcerative changes compared to those without.
Oesophageal cancer
While EUS has been in use for nearly 30 years, it has not been universally accepted as an essential, routine staging investigation in patients with oesophageal cancer.103 Excellent results have been reported from units that rarely use EUS or have a targeted approach to its application.104 In keeping with ultrasonographic examinations elsewhere its accuracy is operator dependent. Accepting that EUS and EUS-guided fine-needle aspiration (EUS-FNA) are the most accurate techniques for locoregional staging of oesophageal cancer, little evidence exists that they impact on clinical care.105 Pooled sensitivities for the detection of regional lymph node metastases in oesophageal cancer were 0.80 for EUS (95% CI 0.75–0.84), 0.50 for CT (0.41–0.60) and 0.57 for FDG-PET (0.43–0.70) in a meta-analysis by Van Vleit et al.96 Specificities were 0.70 (0.65–0.75), 0.83 (0.77–0.89) and 0.85 (0.76–0.95), respectively.
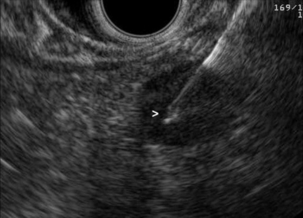
An excellent meta-analysis of the accuracy of EUS in the staging of oesophageal cancer was reported by Puli et al.106 Forty-nine studies comprising 2558 patients were analysed. Pooled sensitivity and specificity of EUS to diagnose T1 were 81.6% (95% CI 77.8–84.9) and 99.4% (95% CI 99.0–99.7), respectively (Fig. 3.10). To diagnose T4, EUS had a pooled sensitivity of 92.4% (95% CI 89.2–95.0) and specificity of 97.4% (95% CI 96.6–98.0) (Fig. 3.11). The addition of fine-needle aspiration (FNA) improved the sensitivity to diagnose N stage from 84.7% with EUS alone (95% CI 82.9–86.4) to 96.7% with EUS-FNA (95% CI 92.4–98.9). They concluded that EUS should be strongly considered for staging oesophageal cancer.
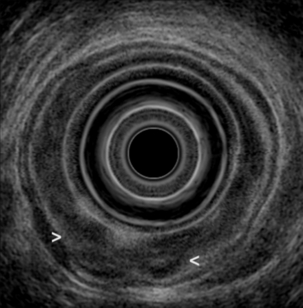
Figure 3.10 EUS image of T1 oesophageal adenocarcinoma. With thanks to Dr Ian Penman, Royal Infirmary of Edinburgh.
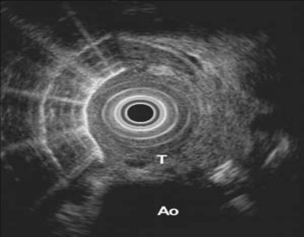
Figure 3.11 EUS image of oesophagus showing a T4 tumour with extension into the aorta. With thanks to Dr Ian Penman, Royal Infirmary of Edinburgh.
An earlier review of 27 studies reported that EUS is highly effective for discrimination of stages T1 and T2 from stages T3 and T4 for primary gastro-oesophageal carcinomas.103 This review included 13 papers for staging oesophageal cancer, 13 for gastric cancer and four for cancers at the gastro-oesophageal junction. The accuracy of EUS was lower for tumours at the gastro-oesophageal junction, thought to be possibly due to the anatomy at this site leading to a tendency to scan obliquely through the bowel wall, giving rise to artefactual misrepresentation of the true depth of penetration.
Failure to intubate and cross oesophageal tumours by EUS is reported to occur in up to 45% of cases and is thought to be associated with an especially poor prognosis.107 It has been reported that over 80% of non-traversable oesophageal tumours are either T3 or T4.108 However, a recent report of 411 consecutive patients undergoing EUS examination by a specialist radiologist reported a failure to cross the tumour in only 12 patients (2.9%).109 Forty (10%) patients required a dilation.
The addition of EUS to history, physical examination, upper endoscopy and CT in patients with oesophageal cancer was reported to change management in 24% of cases (95% CI 12–36%), usually to a more resource-intensive approach.105 EUS-FNA plus cytology results altered management in an additional 8% (95% CI 6–15%) of cases.
The differentiation between inflammatory and neoplastic lymphadenopathy within the mediastinum is essential to ensure patients receive the appropriate treatment. A meta-analysis and systematic review of the accuracy of EUS in evaluating mediastinal lymphadenopathy reported on 76 studies and included 9310 patients.110 Of these, 44 studies used EUS alone and 32 studies used EUS-FNA. FNA improved the sensitivity of EUS from 84.7% (95% CI 82.9–86.4%) to 88.0% (95% CI 85.8–90.0%). With FNA, the specificity of EUS improved from 84.6% (95% CI 83.2–85.9%) to 96.4% (95% CI 95.3–97.4%). As part of the review the EUS studies with FNA were grouped into three time periods and analysed to standardise the criteria and the technology of EUS over two decades. During this time the sensitivity and specificity of EUS with FNA had substantially improved. EUS with FNA should be the diagnostic test of choice for evaluating mediastinal lymphadenopathy (Fig. 3.12).
Ultrasonography (US)
US is not widely adopted as a routine investigation in the staging of oesophagogastric cancer, although it has specific indications. It has an important role in clarifying liver lesions identified on CT or PET and in obtaining guided biopsies for tissue diagnosis where metastatic disease is suspected. External US of the neck has been recommended as part of the routine diagnostic work-up in patients with oesophageal cancer even after normal CT and PET scanning.111 It has been estimated that 10–28% of patients with upper or mid oesophageal tumours have metastatic involvement of neck lymph nodes.112–114 In 176 of 233 patients with oesophageal cancer, CT did not identify any lymphatic metastasis to the neck.110 External US disagreed in 36 patients and FNA confirmed metastasis in nine cases, resulting in an additional value of external US after normal CT scanning of 5% (9/176). In 74 patients with normal CT and PET imaging of the neck, 3 of 74 (4%) had FNA-confirmed metastasis.
In a larger study of 567 patients with oesophageal or gastric cardia cancer US-FNA was the preferred diagnostic modality for the detection of supraclavicular lymph node metastases.115 Sensitivities for US alone were 75%, US-FNA 72%, US plus CT 80%, and US-FNA plus CT 79%, in comparison to a sensitivity of CT alone of 25% (P < 0.001). Specificities were high for US-FNA (100%), CT (99%) and US-FNA plus CT (99%), whereas those of US alone (91%) and US plus CT (91%) were lower (P < 0.001). In 4 of 65 (6%) patients with true-positive malignant lymph nodes, CT was positive with US and/or US-FNA being negative. However, in 36 of 65 (55%) patients, US and/or US-FNA were positive with CT being negative.
Conversely, another group failed to demonstrate any additional staging benefit to performing routine neck US in 180 patients with oesophageal cancer.116 All patients with cervical metastases had stage T3 or T4 disease on EUS. All cervical nodal metastases were detected by the combination of PET and MDCT. The main role of external US is to obtain cytological proof of suspected cervical lesions.
Laparoscopy
Staging laparoscopy should be considered in all patients with gastric cancer being considered for curative resection and in those with oesophageal or junctional cancers with evidence of a significant infradiaphragmatic component. The investigation is performed under general anaesthesia and involves the creation of a CO2 pneumoperitoneum and the insertion of typically three laparoscopic ports. This allows thorough visualisation of the peritoneal cavity, and the opportunity to biopsy any suspicious lesions and obtain peritoneal washings for cytology. The detection of low-volume peritoneal disease remains difficult, with sensitivities for the detection of peritoneal disease by CT alone in the region of 58% for oesophageal cancer and 33% for gastric cancer.117 De Graaf et al. reported on a large series of 416 patients with oesophagogastric cancer staged as having resectable tumours after preoperative staging with CT and/or US.118 Staging laparoscopy changed treatment decision in 84 cases (20.2%), with locally advanced disease present in 17 patients, extensive lymph node disease in four and distant metastases (liver and peritoneum) in 63 cases. Of those patients deemed resectable by staging laparoscopy, 8.1% were found to be unresectable at laparotomy, 16 with locally advanced disease and 11 with metastases. They concluded that staging laparoscopy was most useful in adenocarcinoma, distal oesophageal, gastro-oesophageal junction and gastric cancers, and probably not necessary in lesions of the upper two-thirds of the oesophagus.
Further studies have shown that as a result of preoperative assessment by laparoscopy 10–29.5% of patients avoid unnecessary surgery.119–122 A systematic review has previously recommended the use of laparoscopy for the staging of patients with oesophagogastric cancer.123
Peritoneal cytology
Positive peritoneal cytology is a predictor of poor survival in patients with gastric cancer (Fig. 3.13).124–128 In a series of 118 patients with completely resected gastric carcinoma, 23 patients (20%) had free peritoneal tumour cells (FPTCs).124 The median survival time for patients with positive cytology compared with negative cytology was significantly shorter (11 compared with > 72 months), with estimated 5-year survival rates of 8% vs. 60%. None of the patients with FPTCs had an early gastric cancer. Recurrent disease occurred in 91% of positive and in 38% of negative patients.
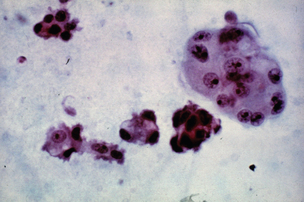
Figure 3.13 Peritoneal washings demonstrating the presence of malignant cells in a patient with gastric adenocarcinoma.
A review of outcomes of 26 consecutive patients with gastric cancer with positive peritoneal washings without peritoneal dissemination reported 1-, 2- and 3-year survival rates of 69%, 35% and 0%, respectively.125 Sixty-nine per cent of patients had peritoneal recurrences and the authors concluded that aggressive surgical resection does not provide any survival benefit for gastric cancer patients with positive peritoneal washings even in the absence of peritoneal dissemination.
In a larger study, 996 consecutive patients with advanced gastric cancer who underwent gastrectomy were studied.126 The 2- and 5-year survival rates of the patients who underwent gastrectomy without any other non-curative factors besides positive peritoneal cytology were 25.3 and 7.8%, respectively. It was concluded that the prognosis of gastric cancer patients with positive peritoneal cytology is so poor that multimodality therapy, including perioperative chemotherapy, is essential.
In a recent report of 1241 patients with gastric cancer undergoing laparoscopy with peritoneal washings, 291 (23%) had positive cytology.127 Of these, 198 patients (68%) had visible metastases but 93 patients (32%) were without gross evidence of advanced disease. The median disease-specific survival for patients with visible metastases was 0.8 years and for those with positive cytology only was 1.3 years. Forty-eight patients had repeat staging laparoscopy after chemotherapy. Compared with patients who had persistently positive cytology (n = 21), those who converted to negative cytology (n = 27) showed a significant improvement in disease-specific survival (2.5 years vs. 1.4 years, P = 0.0003).
It remains unclear whether neoadjuvant chemotherapy can eliminate free peritoneal tumour cells in the peritoneal lavage. In a study of 61 patients with resectable gastric cancer, peritoneal cytology was performed at staging laparoscopy and at the time of tumour resection following neoadjuvant chemotherapy.128 FPTCs were detected immunohistochemically with Ber-EP4 antibody. Forty-two patients (69%) were negative and 19 positive (31%) before chemotherapy. During chemotherapy, 10 (24%) of 42 patients developed FPTCs and 7 (37%) of 19 patients reverted from positive to negative. Patients who became FPTC negative (n = 7) showed an improved median survival (36.1 months) and a longer 2-year survival (71.4%) compared to FPTC-positive patients before and after NAC (n = 12), with a median survival of 9.2 months and a 2-year survival rate of 25%. In contrast, patients who reverted from FPTC negative to positive during NAC (n = 10) had a median survival of 18.5 months and a 2-year survival of only 20%. This study does raise the issue of potential progression during neoadjuvant chemotherapy and demonstrates that the stage of disease prior to starting treatment may be different to that at the time of resection. It is essential that peritoneal washings are obtained at the time of resection.
Laparoscopic ultrasonography (lapUS)
This technique is performed at the time of staging laparoscopy. Commonly used linear array probes have a frequency of 5–10 MHz with a depth of penetration of 4–10 cm. Early reports suggested that lapUS was more accurate at staging gastric and oesophageal cancer than CT or laparoscopy alone, with accuracies quoted between 80% and 90%.129,130 It can provide additional information on tumour depth, regional lymphadenopathy, small metastases deep within the liver parenchyma and assessment of invasion of adjacent organs. In patients with gastric cancer the addition of lapUS to laparoscopy alone provided additional information in 1 of 28 patients.131 The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recently recommended staging laparoscopy with lapUS if routine preoperative staging investigations in patients with gastric cancer demonstrate no evidence of metastatic disease.131 The evidence for its use in oesophageal cancer is limited.131 Recent improvements in the quality of alternative imaging techniques have resulted in the increased detection of smaller liver lesions and enlarged lymph nodes. As such the additional benefit of performing lapUS over and above conventional laparoscopy is now less clear.
Magnetic resonance imaging (MRI)
MRI is mainly reserved as a complementary staging modality and is often used when additional information is required on particular abnormalities identified by CT, in particular liver and bone lesions or adrenal gland abnormalities (Fig. 3.14). A systematic review comparing local staging accuracy of MRI, EUS and spiral CT for stomach cancer found overall T-stage accuracies for EUS of 65–92%, for CT of 77–89% and for MRI of 71–83%.132 A recent study reported similar accuracies for 64-slice multi-detector computed tomography (MDCT) and MRI in the T staging of gastric carcinoma in comparison with histopathology.133 Forty patients were imaged. The accuracy of MRI was slightly higher than that of MDCT in identifying T1 lesions (50% vs. 37.5%), whereas the accuracy of MDCT was higher in differentiating T2 lesions (81.2% vs. 68.7%). The accuracy of MRI and MDCT did not differ significantly in the evaluation of T3–T4 lesions (P > 0.05). Understaging was observed in 20% of cases with MR imaging and in 17.5% with MDCT.
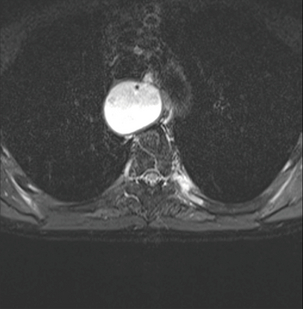
Figure 3.14 MRI image of a bone metastasis in the left proximal sixth rib. This had arisen from an oesophageal adenocarcinoma and was not evident on CT but had appeared on PET/CT.
MRI was significantly worse at assessing accuracy of T stage of oesophageal cancer (60%) when compared with EUS (84%).134 A study comparing CT, MRI and endobronchial ultrasonography (EBUS) for the assessment of invasion of thyroid or oesophageal cancer in cases with suspected tracheobronchial invasion reported sensitivity and specificity of CT for invasion of 59% and 56%, for MRI 75% and 73%, and for EBUS 92% and 83%, respectively.135
Endobronchial ultrasonography (EBUS)
EBUS is a technique that enables ultrasound examination of the endobronchial tree using a modified bronchoscope, similar in principle to EUS examination of the upper GI tract. Several studies have reported improved accuracy rates compared with CT assessment in cases where endobronchial invasion by the tumour is suspected. Accuracy rates in the region of 90–95% have been reported for EBUS (sensitivity values around 90%, specificity 80–100%), whereas the reported accuracy of CT in distinguishing endobronchial invasion from compression by the tumour is much lower, at around 50–60%.135–137 EBUS may also be used to examine carinal and mediastinal lymph nodes and EBUS-directed FNA can also be performed on any suspicious nodes noted at the time of examination.
Restaging following neoadjuvant or radical therapy
With the increasing number of patients being offered neoadjuvant or radical non-surgical treatment, the requirement for repeat staging investigations is also increasing. A clinical improvement in swallowing ability in those patients with dysphagia may or may not be a useful indicator of tumour response.138,139 A scoring system has been devised as follows: 0, no dysphagia; 1, mild, i.e. with solids, requiring modification of diet to soft foods; 2, moderate, i.e. difficulty with soft foods, predominantly liquid diet; 3, severe, i.e. obstructed, needing medical intervention for dilatation or bolus obstruction.140 Clearly this will have limited application in patients without dysphagia.
A study assessing the impact of baseline nutritional status on treatment response and survival in 105 patients with locally advanced oesophageal cancer treated with definitive chemoradiotherapy reported that serum albumin level > 35 g/L was the only independent predictive factor of complete response.141
Endoscopic assessment of response to neoadjuvant therapy has been reported in 100 consecutive patients with oesophageal cancer.142 Thirty patients were considered to have had a complete response but this was confirmed pathologically in only 15 patients. Survival was improved in those with a pathologically confirmed complete response (3-year survival rate 62.4%, SE 12.9%) compared with non-responders (16.3%, SE 6.6%). Those with microscopic residual disease also had an improved 3-year survival rate (46.3%, SE 12.2%).
EUS assessment of response is limited by disintegration of the involved anatomical structures.143 In a study of 40 patients who completed chemoradiotherapy and underwent oesophagectomy, EUS measurements of maximal tumour thickness were made pre- and post-chemoradiotherapy. A tumour thickness after chemoradiotherapy of less than or equal to 6 mm or a reduction in thickness greater than or equal to 50% correlated significantly with histopathological tumour regression grade and overall survival. However, the study was limited as 10 of 56 patients could not have repeat EUS due to the development of severe oesophageal stenosis.
The most commonly used assessment of response is follow-up CT, but historically the value of CT in predicting response to chemotherapy has been disappointing.144 One study of patients with oesophageal cancer who underwent CT before and after preoperative chemotherapy found that 93% of patients had a reduction in tumour volume following chemotherapy, but this showed no correlation to histological evidence of tumour response or to survival.145 One limitation with conventional imaging is the reliance on a large change in tumour volume, often requiring a greater than 50% reduction in tumour volume to reliably predict response.
In recent years there has been an increasing interest in the use of FDG-PET to identify evidence of response. Malignant tumours generally exhibit an increased rate of glycolysis that is most evident in rapidly growing, poorly differentiated neoplasms.146 Malignant cells accumulate more glucose than normal cells due to a predominantly glycolytic catabolism instead of a citric acid cycle catabolism.147 The original study of FDG-PET assessment of response to chemotherapy in patients with upper GI cancer reported that a wide range of changes in uptake were evident between pre-treatment scans and those performed on completion.148 There are now several subsequent studies. In the first of these, Brucher et al. took a reduction of 52% in tumour FDG uptake as evidence of response on PET.149 Histologically there were 11 non-responders and 13 responders. Using this cut-off, 5 of 11 patients determined as non-responders histologically would, on PET imaging, be classified as responders. In the histological partial response group the change in SUV ranged from +1% to − 68%. This remains a major issue, with considerable overlap between PET identified and histologically identified responders and non-responders.
The level of reduction in FDG uptake chosen to identify response has varied from 30% to 80% in published studies.148–153 There has been much recent debate on the MUNICON trial, which tailored patient management according to the change in FDG uptake following one cycle of platinum and fluorouracil-based induction chemotherapy.154 Those patients with decreases of 35% or more were defined as metabolic responders and continued to receive neoadjuvant chemotherapy for 12 weeks and then surgery. Non-responders, according to PET, discontinued chemotherapy after the first cycle and proceeded to surgery. After a median follow-up of 2.3 years (interquartile range 1.7–3.0), median overall survival was not reached in metabolic responders, whereas median overall survival was 25.8 months (19.4–32.2) in non-responders (hazard ratio 2.13 (1.14–3.99), P = 0.015). No histological response was reported in metabolic non-responders, but this is perhaps not surprising as they did not receive a full course of chemotherapy. In addition, the PET non-responders essentially had surgery alone and, when compared to those receiving neoadjuvant chemotherapy and surgery, had a poorer overall survival, as would be expected from the results of the phase III trials of neoadjuvant chemotherapy.1,155
The overlap between metabolic and pathological responders has been widely reported and remains a major issue. In a study by Kim et al., a complete metabolic response to preoperative chemoradiotherapy on FDG-PET showed the highest correlation with pathological complete response when compared with endoscopic biopsies or CT.156 However, the concordance was 71% and basing management decisions on FDG-PET response could result in incorrect and suboptimal management in some cases. Accuracy in predicting complete histological response has been reported as 89% for FDG-PET/CT, 67% for EUS-FNA and 71% for CT.157 Other studies report no correlation between FDG-PET response and histopathological response.158,159 More research is needed before FDG-PET can accurately determine which patients with oesophageal carcinoma should continue with neoadjuvant treatment or be offered early surgery.
A similar situation has been reported for assessment of response to neoadjuvant chemotherapy in patients with gastric cancer.160 In a study by Vallbohmer et al., 40 patients underwent gastrectomy following neoadjuvant chemotherapy.160 FDG-PET was performed before and 2 weeks after the end of neoadjuvant chemotherapy. There was no significant correlation between the pre-treatment SUV, post-treatment SUV or change in SUV and response or prognosis.
Sentinel lymph nodes
A sentinel lymph node (SLN) is the node in direct communication with the primary tumour and the first node to be involved in lymphatic metastasis. The role of SLN biopsy is well established in cancers of the breast and melanoma, but its role in oesophageal and gastric cancer is still evolving and is controversial.161–163 The main aim of the SLN concept is to reduce the extent of dissection necessary to detect lymph node metastases. Ideally, the SLN is confined to a single lymph node or lymph node station but published studies show that this is not the case in oesophageal cancer, with a mean number of SLNs of 4.7 reported by Takeuchi et al. and a median of 4 by Grotenhuis et al.164,165 In addition, the lymphatic drainage of the oesophagus is comprised of abundant lymph–capillary networks, especially in the submucosa. The resultant longitudinal lymphatic drainage can result in skipping metastases leading to positive distant nodes in the presence of negative local nodes.165,166 The finding of multiple, dispersed SLNs in patients with oesophageal cancer would indicate that a radical lymphadenectomy is indicated in most patients.
This finding also applies to gastric cancer. In a review of 88 patients with gastric cancer with a solitary lymph node metastasis, 65 occurred in the perigastric nodes while 23 showed skipping metastases.167 In relation to the location of the tumour, the authors identified several different lymph node stations that could be involved with identically placed tumours. The same finding was reported in a similar sized study by Kunisaki et al.168 Of 102 patients with single lymph node metastases, over 60% occurred in specific lymph nodes for each tumour but the remainder were scattered in an unpredictable manner, including the para-aortic lymph nodes. With such an unpredictable pattern of spread a radical lymphadenectomy is essential to ensure that no residual disease is left in patients with potentially curable disease.
Future developments
The next version of this chapter will undoubtedly again require major revision as technology improves and available evidence on the role of each staging method is strengthened. One important study result awaited is the COGNATE trial, in which patients with oesophageal and gastric cancer without evidence of metastatic disease are randomised to receive EUS or not.169 The two groups will be compared with regards to the treatment received and the rate of complete resection. Length and quality of survival will be compared between the two groups.
References
1. Medical Research Council Oesophageal Cancer Working Party, Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002; 359:1727–1733. 12049861
2. Medical Research Centre Clinical Trials Unit. Available from www.ctu.mrc.ac.uk, 2012. [accessed 12.09.12].
3. Denoix, P.F., Enquete permanente dans les centres anticancereaux. Bull Inst Nat Hyg 1946; 1:12–17. 20986738
4. Hermanek, P., Sobin, L.H. UICC TNM classification of malignant tumors. Berlin: Springer; 1987.
5. Sobin L.H., Gospodarowicz M.K., Wittekind C.H., eds. TNM classification of malignant tumors, 7th ed., Oxford: Wiley-Blackwell, 2009. The current TNM classification that all units should adopt.
6. Hundahl, S.A., Phillips, J.L., Menck, H.R., The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88(4):921–932. 10679663
7. Parkin, D.M., Bray, F., Ferlay, J., et al, Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. 15761078
8. Washington, K., 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–3079. 20882416
9. Greene, F., Page, D., Morrow, M. AJCC cancer staging manual, 6th ed. New York: Springer; 2002.
10. Siewert, J.R., Bottcher, K., Stein, H.J., et al, Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228(4):449–461. 9790335
11. Oliver, H.B., Donald, E.H., Robert, V.P., et al. Manual for staging of cancer, American Joint Committee on Cancer, 4th ed. Philadelphia: JB Lippincott; 1992.
12. Fleming, I., Cooper, J.S., Henson, D.E., et al. Manual for staging of cancer, American Joint Committee on Cancer, 5th ed. Philadelphia: JB Lippincott; 1997.
13. Sobin, L., Wittekind, C. UICC TNM classification of malignant tumors, 5th ed. New York: Wiley-Liss; 1997.
14. Sobin, L., Wittekind, C. International Union Against Cancer (UICC): TNM classification of malignant tumors, 6th ed. New York: Wiley; 2002.
15. Ichikura, T., Ogawa, T., Chochi, K., et al, Minimum number of lymph nodes that should be examined for the International Union Against Cancer/American Joint Committee on Cancer TNM classification of gastric carcinoma. World J Surg. 2003;27(3):330–333. 12607061
16. Tsujimoto, H., Sugasawa, H., Ono, S., et al, Has the accuracy of preoperative diagnosis improved in cases of early-stage gastric cancer? World J Surg. 2010;34(8):1840–1846. 20407771
17. Mullaney, P.J., Wadley, M.S., Hyde, C., et al, Appraisal of compliance with the UICC/AJCC staging system in the staging of gastric cancer. Union Internacional Contra la Cancrum/American Joint Committee on Cancer. Br J Surg 2002; 89:1405–1408. 12390382
18. Saito, H., Fukumoto, Y., Osaki, T., et al, Prognostic significance of level and number of lymph node metastases in patients with gastric cancer. Ann Surg Oncol. 2007;14(5):1688–1693. 17245613
19. Kunisaki, C., Shimada, H., Nomura, M., et al, Clinical impact of metastatic lymph node ratio in advanced gastric cancer. Anticancer Res. 2005;25(2B):1369–1375. 15865093
20. Rizk, N., Venkatramen, E., Park, B., et al, The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2006;132(6):1374–1381. 17140960
21. Peyre, C.G., Hagen, J.A., DeMeester, S.R., et al, Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg. 2008;248(6):979–985. 19092342 A multinational trial of 1053 patients with oesophageal cancer treated by resection alone. Systemic disease occurred in 16% of node-negative patients and with increasing rates with increasing nodal involvement, occurring in 93% of patients with > 8 nodes positive.
22. Peters, C.J., Hardwick, R.H., Vowler, S.L., et al, Generation and validation of a revised classification for oesophageal and junctional adenocarcinoma. Oesophageal Cancer Clinical and Molecular Stratification Study Group. Br J Surg. 2009;96(7):724–733. 19526624
23. Barry, J.D., Edwards, P., Lewis, W.G., et al, Special interest radiology improves the perceived preoperative stage of gastric cancer. Clin Radiol 2002; 57:984–988. 12409108
24. Davies, A.R., Deans, D.A.C., Penman, I., et al, The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro-esophageal cancer. Dis Esophagus. 2006;19(6):496–503. 17069595
25. Stephens, M.R., Lewis, W.G., Brewster, A.E., et al, Multidisciplinary team management is associated with improved outcomes after surgery for oesophageal cancer. Dis Esophagus 2006; 19:164–171. 16722993
26. Adams, R., Morgan, M., Mukherjee, S., et al, A prospective comparison of multidisciplinary treatment of oesophageal cancer with curative intent in a UK cancer network. Eur J Surg Oncol. 2007;33(3):307–313. 17123775
27. Freeman, R.K., Van Woerkom, J.M., Vyverberg, A., et al, The effect of a multidisciplinary thoracic malignancy conference on the treatment of patients with esophageal cancer. Ann Thorac Surg. 2011;92(4):1239–1242. 21867990
28. van Hagen, P., Spaander, M.C., van der Gaast, A., et al, Impact of a multidisciplinary tumour board meeting for upper-GI malignancies on clinical decision making: a prospective cohort study. Int J Clin Oncol 2011; Dec 23. Epub ahead of print. 22193638
29. Levine, M.S., Chu, P., Furth, E.E., et al, Carcinoma of the esophagus and esophagogastric junction: sensitivity of radiographic diagnosis. AJR Am J Roentgenol. 1997;168(6):1423–1426. 9168701
30. DiPalma, J.A., Pretchter, G.C., Brady, C.E., 3rd., X-ray-negative dysphagia: is endoscopy necessary. J Clin Gastroenterol. 1984;6(5):409–411. 6501826
31. Halpert, R.D., Feczko, P.J., Spickler, E.M., et al, Radiological assessment of dysphagia with endoscopic correlation. Radiology. 1985;157(3):599–602. 4059545
32. Quine, M.A., Bell, G.D., McCloy, R.F., et al, Prospective audit of perforation rates following upper gastrointestinal endoscopy in two regions of England. Br J Surg. 1995;82(4):530–533. 7613903
33. Quine, M.A., Bell, G.D., McCloy, R.F., et al, Prospective audit of upper gastrointestinal endoscopy in two regions of England: safety, staffing, and sedation methods. Gut. 1995;36(3):462–467. 7698711
34. Fisher, N.C., Bailey, S., Gibson, J.A., A prospective, randomized controlled trial of sedation vs. no sedation in outpatient diagnostic upper gastrointestinal endoscopy. Endoscopy 1998; 30:21–24. 9548039
35. Al-Atrakchi, H.A., Upper gastrointestinal endoscopy without sedation: a prospective study of 2000 examinations. Gastrointest Endosc 1989; 35:79–81. 2714608
36. Lal, N., Bhasin, D.K., Malik, A.K., et al, Optimal number of biopsy specimens in the diagnosis of carcinoma of the oesophagus. Gut 1992; 33:724–726. 1624148
37. Di-Franco, F., Lamb, P.J., Karat, D., et al, Iatrogenic perforation of localised oesophageal cancer. Br J Surg 2008; 95:837–840. 18457352
38. Hadzijahic, N., Wallace, M.B., Hawes, R.H., et al, CT or EUS for the initial staging of esophageal cancer? A cost minimization analysis. Gastrointest Endosc 2000; 52:715–720. 11115901 CT scan is the most cost-effective initial staging investigation.
39. Mani, N.B., Suri, S., Gupta, S., et al, Two-phase dynamic contrast-enhanced computed tomography with water-filling method for staging of gastric carcinoma. Clin Imaging 2001; 25:38–43. 11435038
40. Park, H.S., Lee, J.M., Kim, S.H., et al, Three-dimensional MDCT for preoperative local staging of gastric cancer using gas and water distention methods: a retrospective cohort study. AJR Am J Roentgenol. 2010;195(6):1316–1323. 21098189
41. Kim, H.J., Kim, A.Y., Oh, S.T., et al, Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005;236(3):879–885. 16020558
42. Kim, A.Y., Kim, H.J., Ha, H.K., Gastric cancer by multi-detector row CT: preoperative staging. Abdom Imaging. 2005;30(4):465–472. 15785907
43. Yang, D.M., Kim, H.C., Jin, W., et al, 64 multidetector row computed tomography for preoperative evaluation of gastric cancer: histological correlation. J Comput Assist Tomogr. 2007;31(1):98–103. 17259840
44. Chen, C.Y., Hsu, J.S., Wu, D.C., et al, Gastric cancer: preoperative local staging with 3D multidetector row CT – correlation with surgical and histopathologic results. Radiology. 2007;242(2):472–482. 17255419
45. Hur, J., Park, M.S., Lee, J.H., et al, Diagnostic accuracy of multidetector row computed tomography in T and N staging of gastric cancer with histopathologic correlation. J Comput Assist Tomogr. 2006;30(3):372–377. 16778609
46. Hwang, S.W., Lee, D.H., Lee, S.H., et al, Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25(3):512–518. 20370729
47. Tsubnraya, A., Naguchi, Y., Matsumoto, A., et al, A preoperative assessment of adjacent organ invasion by stomach carcinoma with high resolution computed tomography. Surg Today 1994; 24:299–304. 8038502
48. Moschetta, M., Stabile Ianora AA, Anglani A, et al. Preoperative T staging of gastric carcinoma obtained by MDCT vessel probe reconstructions and correlations with histological findings. Eur Radiol. 2010;20(1):138–145. 19504100
49. Kim, J.H., Eun, H.W., Choi, J.H., et al, Diagnostic performance of virtual gastroscopy using MDCT in early gastric cancer compared with 2D axial CT: focusing on interobserver variation. AJR Am J Roentgenol. 2007;189(2):299–305. 17646454
50. Bhandari, S., Shim, C.S., Kim, J.H., et al, Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc. 2004;59(6):619–626. 15114303
51. Cho, J.S., Kim, J.K., Rho, S.M., et al, Pre-operative assessment of gastric carcinoma: value of two-phase dynamic CT with mechanical i.v. injection of contrast material. AJR Am J Roentgenol 1994; 163:69–75. 8010251
52. Inamoto, K., Kouzai, K., Ueeda, T., et al, CT virtual endoscopy of the stomach: comparison study with gastric fiberscopy. Abdom Imaging. 2005;30(4):473–479. 15688107
53. Noda, N., Sasako, M., Yamaguchi, N., et al, Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. Br J Surg 1998; 85:831–834. 9667718
54. Marrelli, D., Mazzei, M.A., Pedrazzani, C., et al, High accuracy of multislices computed tomography (MSCT) for para-aortic lymph node metastases from gastric cancer: a prospective single-center study. Ann Surg Oncol. 2011;18(8):2265–2272. 21267792
55. Kapiev, A., Rabin, I., Lavy, R., et al, The role of diagnostic laparoscopy in the management of patients with gastric cancer. Isr Med Assoc J. 2010;12(12):726–728. 21348398
56. Lee, H., Hwang, H.S., Chang, D.K., et al, Clinical significance of minimal ascites of indeterminate nature in gastric adenocarcinoma without peritoneal carcinomatosis: long-term follow-up stud. Hepatogastroenterology. 2011;58(105):137–142. 21510301
57. Barry, J.D., Edwards, P., Lewis, W.G., et al, Special interest radiology improves the perceived preoperative stage of gastric cancer. Clin Radiol 2002; 57:984–988. 12409108
58. Vilgrain, V., Mompoint, D., Palazzo, L., et al, Staging of esophageal carcinoma: comparison of results with endoscopic sonography and CT. AJR Am J Roentgenol 1990; 155:277–281. 2115251
59. Picus, D., Balfe, D.M., Koehler, R.E., et al, Computed tomography in the staging of esophageal carcinoma. Radiology 1983; 146:433–438. 6849089
60. Kumbasar, B., Carcinoma of esophagus: radiologic diagnosis and staging. Eur J Radiol 2002; 42:170–180. 12044696
61. Botet, J.F., Lightdale, C.J., Zauber, A.G., et al, Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology 1991; 181:419–425. 1924783
62. Iyer, R.B., Silverman, P.M., Tamm, E.P., et al, Diagnosis, staging, and follow-up of esophageal cancer. AJR Am J Roentgenol 2003; 181:785–793. 12933482
63. Kim, S.H., Lee, J.M., Han, J.K., et al, Three-dimensional MDCT imaging and CT esophagography for evaluation of esophageal tumors: preliminary study. Eur Radiol. 2006;16(11):2418–2426. 16775691
64. Panebianco, V., Grazhdani, H., Iafrate, F., et al, 3D CT protocol in the assessment of the esophageal neoplastic lesions: can it improve TNM staging? Eur Radiol. 2006;16(2):414–421. 16041528
65. Onbas, O., Eroglu, A., Kantarci, M., et al, Preoperative staging of esophageal carcinoma with multidetector CT and virtual endoscopy. Eur J Radiol. 2006;57(1):90–95. 16122893
66. Wu, L.F., Wang, B.Z., Feng, J.L., et al, Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol 2003; 9:219–224. 12532435
67. Umeoka, S., Koyama, T., Watanabe, G., et al, Preoperative local staging of esophageal carcinoma using dual-phase contrast-enhanced imaging with multidetector row computed tomography: value of the arterial phase images. J Comput Assist Tomogr. 2010;34(3):406–412. 20498545
68. Leers, J.M., DeMeester, S.R., Oezcelik, A., et al, The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma: a retrospective review of esophagectomy specimens. Ann Surg. 2011;253(2):271–278. 21119508
69. Moorjani, N., Junemann-Ramirez, M., Judd, O., et al, Endoscopic ultrasound in oesophageal carcinoma: comparison with multislice computed tomography and importance in the clinical decision making process. Minerva Chir. 2007;62(4):217–223. 17641581
70. Pfau, P.R., Perlman, S.B., Stanko, P., et al, The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007;65(3):377–384. 17321235
71. Fekete, F., Gayet, B., Frija, J. CT scanning in the diagnosis of oesophageal disease. In: Jamieson G.G., ed. Surgery of the oesophagus. Edinburgh: Churchill Livingstone; 1988:85–89.
72. Schnyder, P.A., Gamsu, G., CT of the pretracheal retrocaval space. AJR Am J Roentgenol 1981; 136:303–308. 6781251
73. Blackshaw, G., Lewis, W.G., Hopper, A.N., et al, Prospective comparison of endosonography, computed tomography, and histopathological stage of junctional oesophagogastric cancer. Clin Radiol. 2008;63(10):1092–1098. 18774355
74. Takizawa, K., Matsuda, T., Kozu, T., et al, Lymph node staging in esophageal squamous cell carcinoma: a comparative study of endoscopic ultrasonography versus computed tomography. J Gastroenterol Hepatol. 2009;24(10):1687–1691. 19788609
75. Chen, J., Cheong, J.H., Yun, M.J., et al, Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 2005; 103:2383–2390. 15856477
76. Mochiki, E., Kuwano, H., Katoh, H., et al, Evaluation of 18F-2-deoxy-2-fluoro-d-glucose positron emission tomography for gastric cancer. World J Surg 2004; 28:247–253. 14961197
77. Stahl, A., Ott, K., Weber, W.A., et al, FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 2003; 30:288–295. 12552348
78. Yamada, A., Oguchi, K., Fukushima, M., et al, Evaluation of 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med 2006; 20:597–604. 17294670
79. Alakus, H., Batur, M., Schmidt, M., et al, Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010;31(6):532–538. 20220543
80. http:www.sign.ac.uk, website of SIGN, [accessed 10.10.12].
81. Blackstock, A.W., Farmer, M.R., Lovato, J., et al, A prospective evaluation of the impact of 18-F-fluoro-deoxy-d-glucose positron emission tomography staging on survival for patients with locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys 2006; 64:455–460. 16213103
82. Heeren, P.A., Jager, P.L., Bongaerts, F., et al, Detection of distant metastases in esophageal cancer with 18F-FDG PET. J Nucl Med 2004; 45:980–987. 15181133
83. Katsoulis, I.E., Wong, W.L., Mattheou, A.K., et al, Fluorine-18 fluorodeoxyglucose positron emission tomography in the preoperative staging of thoracic oesophageal and gastro-oesophageal junction cancer: a prospective study. Int J Surg. 2007;5(6):399–403. 17631431
84. Lerut, T., Flamen, P., Role of FDG-PET scan in staging of cancer of the esophagus and gastroesophageal junction. Minerva Chir. 2002;57(6):837–845. 12592225
85. Meltzer, C.C., Luketich, J.D., Friedman, D., et al, Whole-body FDG positron emission tomographic imaging for staging esophageal cancer: comparison with computed tomography. Clin Nucl Med 2000; 25:882–887. 11079584
86. van Westreenen, H.L., Westerterp, M., Sloof, G.W., et al, Limited additional value of positron emission tomography in staging oesophageal cancer. Br J Surg. 2007;94(12):1515–1520. 17902092
87. Lowe, V.J., Booya, F., Fletcher, J.G., et al, Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005; 7:422–430. 16270235
88. Himeno, S., Yasuda, S., Shimada, H., et al, Evaluation of esophageal cancer by positron emission tomography. Jpn J Clin Oncol 2002; 32:340–346. 12417599
89. Kato, H., Miyazaki, T., Nakajima, M., et al, The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 2005; 103:148–156. 15558794
90. Sihvo, E.I., Rasanen, J.V., Knuuti, M.J., et al, Adenocarcinoma of the esophagus and the esophagogastric junction: positron emission tomography improves staging and prediction of survival in distant but not in locoregional disease. J Gastrointest Surg 2004; 8:988–996. 15585386
91. Flamen, P., Lerut, T., Haustermans, K., et al, Position of positron emission tomography and other imaging diagnostic modalities in esophageal cancer. Q J Nucl Med Mol Imaging 2004; 48:96–108. 15243407
92. Strauss, L.G., Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med Mol Imaging 1996; 23:1409–1415. 8781149
93. Flanagan, F.L., Dehdashti, F., Siegel, B.A., Staging of esophageal cancer with 18-F-fluorodexoglucose positron emission tomography. AJR Am J Roentgenol. 1997;168(2):417–424. 9016218
94. McAteer, D., Wallis, F., Couper, G.W., et al, Evaluation of 18F-FDG positron emission tomography in gastric and oesophageal cancer. Br J Radiol 1999; 72:525–529. 10560332
95. van Westreenen, H.L., Westerterp, M., Bossuyt, P.M., et al, Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22(18):3805–3812. 15365078
96. Van Vliet, E.P., Heijenbrok-Kal, M.H., Hunink, M.G., et al, Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 2008; 3:547–557. 18212745
97. Bar-Shalom, R., Guralnik, L., Tsalic, M., et al, The additional value of PET/CT over PET in FDG imaging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2005;32(8):918–924. 15838691
98. Yuan, S., Yu, Y., Chao, K.S., et al, Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med 2006; 47:1255–1259. 16883002
99. Pollack, B.J., Chak, A., Sivak, M.V., Jr., Endoscopic ultrasonography. Semin Oncol 1996; 23:336–346. 8658217
100. Puli, S.R., Reddy, J.B., Bechtold, M.L., et al, How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol. 2008;14(25):4011–4019. 18609685 Twenty-two studies involving 1896 patients with gastric cancer.
101. Sultan, J., Robinson, S., Hayes, N., et al, Endoscopic ultrasonography-detected low-volume ascites as a predictor of inoperability for oesophagogastric cancer. Br J Surg. 2008;95(9):1127–1130. 18655220
102. Hwang, S.W., Lee, D.H., Lee, S.H., et al, Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25(3):512–518. 20370729
103. Kelly, S., Harris, K., Berry, E., et al, A systematic review of the staging performance of endoscopic ultrasound in gastro-esophageal carcinoma. Gut 2001; 49:534–539. 11559651
104. Smithers, B.M., Gotley, D.C., Martin, I., et al, Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg. 2007;245(2):232–240. 17245176
105. Gines, A., Cassivi, S.D., Martenson, J.A., Jr., et al, Impact of endoscopic ultrasonography and physician specialty on the management of patients with esophagus cancer. Dis Esophagus. 2008;21(3):241–250. 18430106
106. Puli, S.R., Reddy, J.B., Bechtold, M.L., et al, Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14(10):1479–1490. 18330935 This study analysed 49 studies comprising 2558 patients. It reported high accuracy rates for EUS for both T and N staging in patients with oesophageal cancer.
107. Heintz, A., Höhne, U., Schweden, F., et al, Preoperative detection of intrathoracic tumour spread of esophageal cancer: endosonography versus computed tomography. Surg Endosc 1991; 5:75–78. 1948618
108. Hordijk, M.L., Zander, H., van Blankenstein, M., et al, Influence of tumour stenosis on the accuracy of endosonography in preoperative T staging of esophageal cancer. Endoscopy 1993; 25:171–175. 8491135
109. Morgan, M.A., Twine, C.P., Lewis, W.G., et al, Prognostic significance of failure to cross esophageal tumors by endoluminal ultrasound. Dis Esophagus. 2008;21(6):508–513. 18430190
110. Puli, S.R., Reddy, J.B., Bechtold, M.L., et al, Endoscopic ultrasound: its accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14(19):3028–3037. 18494054 A meta-analysis of 9310 patients with mediastinal lymphadenopathy demonstrating improved accuracy rates with the addition of EUS-FNA.
111. Omloo, J.M., van Heijl, M., Smits, N.J., et al, Additional value of external ultrasonography of the neck after CT and PET scanning in the preoperative assessment of patients with esophageal cancer. Dig Surg. 2009;26(1):43–49. 19155627
112. Bressani Doldi, S., Lattuada, E., Zappa, M.A., et al, Ultrasonographic evaluation of the cervical lymph nodes in preoperative staging of eosphageal neoplasms. Abdom Imaging 1998; 23:275–277. 9569295
113. Van Overhagen, H., Lameris, J.S., Zonderland, H.M., et al, Ultrasound and ultrasound-guided fine needle aspiration biopsy of supraclavicular lymph nodes in patients with esophageal carcinoma. Cancer 1991; 67:585–587. 1985752
114. Bonvalot, S., Bouvard, N., Lothaire, P., et al, Contribution of cervical ultrasound and ultrasound fine-needle aspiration biopsy to the staging of thoracic oesophageal carcinoma. Eur J Cancer 1996; 32A:893–895. 9081373
115. Van Vliet, E.P., van der Lugt, A., Kuipers, E.J., et al, Ultrasound, computed tomography, or the combination for the detection of supraclavicular lymph nodes in patients with esophageal or gastric cardia cancer: a comparative study. J Surg Oncol. 2007;96(3):200–206. 17455243
116. Schreurs, L.M., Verhoef, C.C., van der Jagt, E.J., et al, Current relevance of cervical ultrasonography in staging cancer of the esophagus and gastroesophageal junction. Eur J Radiol. 2008;67(1):105–111. 17681735
117. Lightdale, C.J., Endoscopic ultrasonography in the diagnosis, staging and follow-up of esophageal and gastric cancer. Endoscopy 1992; 24:297–303. 1633769
118. De Graaf, G.W., Ayantunde, A.A., Parsons, S.L., et al, The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol. 2007;33(8):988–992. 17344017
119. Yau, K.K., Siu, W.T., Cheung, H.Y., et al, Immediate preoperative laparoscopic staging for squamous cell carcinoma of the esophagus. Surg Endosc. 2006;20(2):307–310. 16362473
120. Smith, A., Finch, M.D., John, T.G., et al, Role of laparoscopic ultrasonography in the management of patients with oesophagogastric cancer. Br J Surg 1999; 86:1083–1087. 10460650
121. Molloy, R.G., McCourtney, J.S., Anderson, J.R., Laparoscopy in the management of patients with cancer of the gastric cardia and oesophagus. Br J Surg 1995; 82:352–354. 7796006
122. Kapiev, A., Rabin, I., Lavy, R., et al, The role of diagnostic laparoscopy in the management of patients with gastric cancer. Isr Med Assoc J. 2010;12(12):726–728. 21348398
123. Rao, B., Hunerbein, M., Diagnostic laparoscopy: indications and benefits. Langenbecks Arch Surg. 2005;390(3):187–196. 15156319
124. Nekarda, H., Gess, C., Stark, M., et al, Peritoneal cytology. Immunocytochemically detected free peritoneal tumour cells (FPTC) are a strong prognostic factor in gastric carcinoma. Br J Cancer. 1999;79(3–4):611–619. 10027338
125. Nakagohri, T., Yoneyama, Y., Kinoshita, T., et al, Prognostic significance of peritoneal washing cytology in patients with potentially resectable gastric cancer. Hepatogastroenterology. 2008;55(86–87):1913–1915. 19102421
126. Fukagawa, T., Katai, H., Saka, M., et al, Significance of lavage cytology in advanced gastric cancer patients. World J Surg. 2010;34(3):563–568. 20054543
127. Mezhir, J.J., Shah, M.A., Jacks, L.M., et al, Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17(12):3173–3180. 20585870
128. Lorenzen, S., Panzram, B., Rosenberg, R., et al, Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol. 2010;17(10):2733–2739. 20490698 This study demonstrated changes in the positivity of peritoneal washings in patients with gastric cancer receiving neoadjuvant chemotherapy.
129. Anderson, D.N., Campbell, S., Park, K.G., Accuracy of laparoscopic ultrasonography in the staging of upper gastrointestinal malignancy. Br J Surg 1997; 84:580. 9112924
130. Finch, M.D., John, T.G., Garden, O.J., et al, Laparoscopic ultrasonography for staging gastroesophageal cancer. Surgery 1997; 121:10–17. 9001545
131. Richardson, W., Stefanidis, D., Mittal, S., et al, SAGES guidelines for the use of laparoscopic ultrasound. Surg Endosc. 2010;24(4):745–756. 19768506
132. Kwee, R.M., Kwee, T.C., Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25(15):2107–2116. 17513817
133. Anzidei, M., Napoli, A., Zaccagna, F., et al, Diagnostic performance of 64-MDCT and 1.5-T MRI with high-resolution sequences in the T staging of gastric cancer: a comparative analysis with histopathology. Radiol Med. 2009;114(7):1065–1079. 19774440
134. Wu, L.F., Wang, B.Z., Feng, J.L., et al, Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol 2003; 9:219–224. 12532435
135. Herth, F., Ernst, A., Schulz, M., et al, Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest. 2003;123(2):458–462. 12576366
136. Osugi, H., Nishimura, Y., Takemura, M., et al, Bronchoscopic ultrasonography for staging supracarinal esophageal squamous cell carcinoma: impact on outcome. World J Surg. 2003;27(5):590–594. 12715229
137. Wakamatsu, T., Tsushima, K., Yasuo, M., et al, Usefulness of preoperative endobronchial ultrasound for airway invasion around the trachea: esophageal cancer and thyroid cancer. Respiration. 2006;73(5):651–657. 16675895
138. Geh, J.I., Crellin, A.M., Glynne-Jones, R., Preoperative chemoradiotherapy in oesophageal cancer. Br J Surg 2001; 88:338–356. 11260097
139. Zhang, X., Shen, L., Li, J., et al, A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. Am J Clin Oncol. 2008;31(1):29–33. 18376224
140. Ilson, D.H., Oesophageal cancer: new developments in systemic therapy. Cancer Treat Rev 2003; 29:525–532. 14585262
141. Di Fiore, F., Lecleire, S., Pop, D., et al, Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol. 2007;102(11):2557–2563. 17680847
142. Brown, W.A., Thomas, J., Gotley, D., et al, Use of oesophagogastroscopy to assess the response of oesophageal carcinoma to neoadjuvant therapy. Br J Surg. 2004;91(2):199–204. 14760668
143. Jost, C., Binek, J., Schuller, J.C., et al, Endosonographic radial tumor thickness after neoadjuvant chemoradiation therapy to predict response and survival in patients with locally advanced esophageal cancer: a prospective multicenter phase ll study by the Swiss Group for Clinical Cancer Research (SAKK 75/02). Gastrointest Endosc. 2010;71(7):1114–1121. 20304399
144. Jones, D.R., Parker, L.A., Detterbeck, F.C., et al, Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer 1999; 85:1026–1032. 10091784
145. Griffith, J.F., Chan, A.C., Chow, L.T., et al, Assessing chemotherapy response of squamous cell oesophageal carcinoma with spiral CT. Br J Radiol 1999; 72:678–684. 10624325
146. Younes, M., Lechago, L.V., Somoano, J.R., Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res 1996; 56:1164–1167. 8640778
147. Warburg, O., On the origin of cancer cells. Science 1956; 123:309–314. 13298683
148. Couper, G.W., McAteer, D., Wallis, F., et al, The detection of response to chemotherapy using positron emission tomography in patients with oesophageal and gastric cancer. Br J Surg 1998; 85:1403–1406. 9782025
149. Brucher, B.L., Weber, W., Bauer, M., et al, Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001;233(3):300–309. 11224616
150. Weber, W.A., Ott, K., Becker, K., et al, Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19(12):3058–3065. 11408502
151. Kato, H., Kuwano, H., Nakajima, M., et al, Usefulness of positron emission tomography for assessing the response of neoadjuvant chemoradiotherapy in patients with esophageal cancer. Am J Surg. 2002;184(3):279–283. 12354600
152. Flamen, P., Van Cutsem, E., Lerut, A., et al, Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13(3):361–368. 11996465
153. Downey, R.J., Akhurst, T., Ilson, D., et al, Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21(3):428–432. 12560430
154. Lordick, F., Ott, K., Krause, B.J., et al, PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8(9):797–805. 17693134
155. Boonstra, J.J., Kok, T.C., Wijnhoven, B.P., et al, Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomised controlled trial. BMC Cancer 2011; 11:181. 21595951
156. Kim, T.J., Kim, H.Y., Lee, K.W., et al, Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2009;29(2):403–421. 19325056
157. Cerfolio, R.J., Bryant, A.S., Ohja, B., et al, The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129(6):1232–1241. 15942562
158. Smithers, B.M., Couper, G.W., Watts, N., et al, Positron emission tomography and pathological evidence of response to neoadjuvant therapy in adenocarcinoma of the oesophagus. Dis Esophagus. 2008;21(2):151–158. 18269651
159. Schmidt, M., Bollschweiler, E., Dietlein, M., et al, Mean and maximum standardized uptake values in [18F]FDG-PET for assessment of histopathological response in oesophageal squamous cell carcinoma or adenocarcinoma after radiochemotherapy. Eur J Nucl Med Mol Imaging. 2009;36(5):735–744. 19096843
160. Vallbohmer, D., Holscher, A.H., Schneider, P.M., et al, [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemotherapy in gastric cancer. J Surg Oncol. 2010;102(2):135–140. 20648583
161. Kato, H., Miyazaki, T., Nakajima, M., et al, Sentinel lymph nodes with technetium-99m colloidal rhenium sulfide in patients with esophageal carcinoma. Cancer. 2003;98(5):932–939. 12942559
162. Lamb, P.J., Griffin, S.M., Burt, A.D., et al, Sentinel node biopsy to evaluate the metastatic dissemination of oesophageal adenocarcinoma. Br J Surg 2005; 92:60–67. 15584066
163. Kosugi, S., Nakagawa, S., Kanda, T., et al, Radio-guided sentinel node mapping in patients with superficial esophageal carcinoma: feasibility study. Minim Invasive Ther Allied Technol 2007; 16:181–186. 17573623
164. Takeuchi, H., Fujii, H., Ando, N., et al, Validation study of radio-guided sentinel lymph node navigation in esophageal cancer. Ann Surg 2009; 249:757–763. 19387329
165. Grotenhuis, B.A., Wijnhoven, B.P., van Marion, R., et al, The sentinel node concept in adenocarcinomas of the distal esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg 2009; 138:608–612. 19698844
166. Dresner, S.M., Lamb, P.J., Bennett, M.K., et al, The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery 2001; 129:103–109. 11150040
167. Liu, C.G., Lu, P., Lu, Y., et al, Distribution of solitary lymph nodes in primary gastric cancer: a retrospective study and clinical implications. World J Gastroenterol. 2007;13(35):4776–4780. 17729400
168. Kunisaki, C., Shimada, H., Nomura, M., et al, Distribution of lymph node metastasis in gastric carcinoma. Hepatogastroenterology. 2006;53(69):468–472. 16795994
169. Bangor University. Cancer of the oesophagus or gastricus: new assessment of the technology of endosonography (Cognate). Available at http://www.bangor.ac.uk/imscar/cognate/trialsummary; [accessed 12.09.12]. The results of this multicentre randomised trial are awaited and should provide guidance on the current role of EUS.