3
Staging and assessment of hepatobiliary malignancies
Colorectal liver metastases
Transabdominal ultrasound
Ultrasound has a diagnostic sensitivity of 36–61% for detecting lesions measuring 1–2 cm, even when performed by experienced radiologists.1 It is very useful in guiding fine-needle aspiration (FNA) to confirm unresectability by cytopathology. However, FNA has a risk of seeding metastases in up to10% of patients and a risk of a false-negative result, which does not justify its use in candidates for potentially curative therapy.2
A meta-analysis of the performance of ultrasound for CRLMs found a pooled sensitivity of 63% (95% confidence interval (CI) 56–70%; five studies) with a specificity of 97.6% (95% CI 95.6–99.5%; four studies).3
Computed tomography and magnetic resonance imaging
Nowadays, CT is the most commonly used imaging modality for staging and follow-up of these patients. Current multidetector scanners allow for multiplanar reformatting with the same resolution as the original axial images. This may improve detection and characterisation of small lesions.3 Multiplanar reformatting also enables the demonstration of hepatic arterial, venous and portal anatomy, which can be useful in preoperative planning.4 CT allows for accurate volumetric assessment of tumour size, liver volume to be resected and future liver remnant volume.5
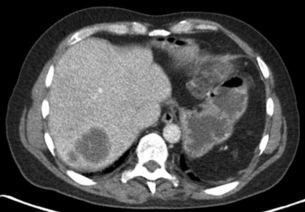
A multiphase contrast-enhanced CT should be performed to stage liver metastases. The study should include an unenhanced, arterial, portovenous and delayed phase. CRLMs may show an enhancing rim on arterial phase imaging and are hypodense on portovenous phase imaging (Fig. 3.1). A slice thickness of 2–4 mm is recommended for axial viewing.
A meta-analysis on the performance of CT found a pooled sensitivity of 74.8% (95% CI 71.2–78.3%; 12 studies) and specificity of 95.6% (95% CI 93.4–97.8%; seven studies).3
The standard MRI protocol includes T2-weighted, unenhanced T1-weighted and dynamic contrast-enhanced T1-weighted sequences. T2-weighted images are used for the detection and characterisation of lesions (haemangioma, cyst).6,7
A meta-analysis on the performance of MRI found a pooled sensitivity of 81.1% (95% CI 76.0–86.1%; five studies) and specificity of 97.2% (95% CI 94.5–99.9%; two studies).3 On per lesion analysis, MRI appeared to be the modality showing higher sensitivities across individual studies compared to CT. Pooled data showed comparable results, with MRI having a combined sensitivity of 88% and accuracy of 87% compared to CT with sensitivity of 74% and accuracy of 78%. Therefore, most centres choose the imaging technique of their preference.
Subgroup analyses of these studies showed that MRI has better sensitivity at picking up the smaller lesions < 1 cm compared to CT and also positron emission tomography (PET)-CT.8 The majority of lesions missed by CT and PET-CT were micrometastases of < 1 cm. This meta-analysis concludes that MRI is preferred as a first-line modality for evaluating the liver. It has high overall sensitivity and specificity estimates and can accurately depict lesions smaller than 10 mm. Secondly, it provides excellent anatomical details.
Positron emission tomography
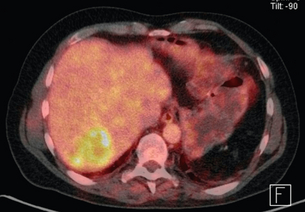
With the PET-CT combination it is possible to produce fusion images with high-resolution anatomical localisation of CT together with the functional data of FDG-PET. This combination of scanning characteristics is becoming more widely available (Fig. 3.2).
Kinkel et al.9 performed a meta-analysis and concluded that, at equivalent specificity, PET-CT is more sensitive than ultrasound (US), CT and MRI for the detection of hepatic metastases from gastrointestinal cancers.
Mainenti et al.11 found that PET-CT showed a trend to perform better than the other modalities. The pooled estimates of a meta-analysis for PET-CT was 93.8% (95% CI 90.0–97.7; six studies) and specificity per patient was 98.7% (95% CI 97.2–100%; six studies).3
Diagnostic laparoscopy and laparoscopic ultrasound
Several centres favour the selective use of laparoscopy. Jarnagin et al.13 have described a clinical risk score (CRS) that predicted survival after hepatic resection and was also suitable in identifying high-risk patients most likely to benefit from laparoscopy. In a study of 103 patients, occult unresectable disease was found in 12% of patients with a low score versus 42% of patients with a high score.13,17 Similarly, in a series of 200 patients, a detection rate of only 6% was reported in patients with the lowest CRS, whereas this was 75% for the highest CRS scores.14 Another study demonstrated a yield of 50% in a selected group of patients, while in the group of 49 patients selected for direct surgical exploration, 46 patients (94%) were eventually resected.15
Staging and assesment of resectability
The significance of resection margins of less than 10 mm is not clear.19 The results of this meta-analysis demonstrate that a negative margin of 1 cm or more confers a survival advantage when compared with subcentimeter negative margins. The management of synchronous liver metastases and extrahepatic abnormalities (particularly the hilar lymph nodes) is also unclear. There is a huge variation in techniques for resection (anatomical vs. non-anatomical, vena cava reconstruction, etc.). Although promising, clear results on the effectiveness of combination treatments with (neo)adjuvant chemotherapy are still lacking.
Modern multidisciplinary consensus defines resectable CRLM simply as tumours that can be resected completely, leaving an adequate liver remnant.21 Before surgical exploration, most surgeons would require that there was no radiographic evidence of involvement of the hepatic artery, major bile ducts, main portal vein, or coeliac/para-aortic lymph nodes, and an adequate predicted functional hepatic remnant.22
In the presence of a limited number of lesions in the lung, liver resection is generally performed first. After radical resection of liver metastases, resection of the lung can follow. There is no reason to refrain from liver resection in a patient with advanced age who has good general (cardiopulmonary) fitness. Risk scoring systems (such as the clinical risk score23 and others24–26) to predict which patients with CRLMs are most likely to benefit from resection are of uncertain clinical utility, particularly since most patients undergo different neoadjuvant chemotherapy regimens. Some studies have shown that the risk scoring system needs refinement27,28 and that the outcome in patients treated by neoadjuvant chemotherapy was not predicted by the traditional clinical scoring system, but rather by response to chemotherapy as evaluated by CT and PET-CT.29
Hepatocellular carcinoma
Transabdominal ultrasound
Transabdominal ultrasound is recommended by the European Associasion for the Study of the Liver (EASL), the American Association for the Study of Liver Disease (AASLC) and the Asian Pacific Association for the Study of Liver Disease (APASL) as a surveillance tool for patients at high risk of developing HCC. Meta-regression analysis has demonstrated a significantly higher sensitivity for early HCC with US every 6 months than with annual surveillance.30
Computed tomography and magnetic resonance imaging
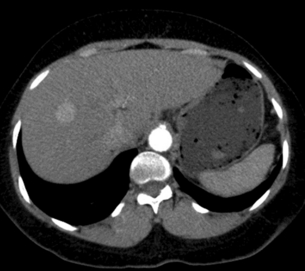
Multiphase contrast-enhanced CT or MRI should be performed when evaluating HCC. The hallmark of HCC on CT and MRI is strong arterial enhancement (Fig. 3.3) and washout of contrast agent in the delayed phase (Fig. 3.4).31 This feature in particular enables differentiation from intrahepatic cholangiocarcinoma, which shows delayed enhancement.32
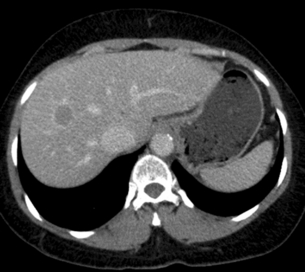
Figure 3.4 CT image of same patient with HCC in the liver showing venous washout of the lesion with enhancing rim.
HCC arising in a non-cirrhotic liver is often large, due to its long asymptomatic course and late presentation.33 This contrasts with the multifocal tumours more commonly found in patients with chronic liver disease, who are often in surveillance programmes. Large HCCs may demonstrate a number of characteristic appearances on CT, which make differentiation from other causes of focal liver masses relatively straightforward. These features include mosaic appearance, with fibrous septa separating areas of variable attenuation which represent internal regions of haemorrhage, necrosis, fatty degeneration and fibrosis. The fibrous capsule has a low attenuation on unenhanced images and enhances on the portal venous phase.
A number of benign lesions, including haemangiomas, focal confluent fibrosis, peliosis, benign regenerative nodules and transient hepatic attenuation difference, can mimic small HCC lesions on CT.34
On MRI, HCCs can have a variable appearance on unenhanced T1-weighted images and typically show an increased signal on T2-weighted images.35 Following gadolinium administration, HCC demonstrates characteristic early enhancement on arterial phase imaging36 and washout on delayed images, resulting in a hypo-intense lesion compared to the surrounding parenchyma.37
Diagnostic laparoscopy and laparoscopic ultrasound
Laparoscopic evaluation can avoid exploratory laparotomy in 45–63% of patients with unresectable disease.38,39 The procedure has been shown to be accurate in assessing the presence of advanced intrahepatic disease and the quality of the liver remnant, but it was less sensitive in determining the extent of local invasion, especially in large (> 10 cm) tumours. Another study clearly reported the value of LUS in detecting small HCCs; 134 new nodules were visualised with this technique in 64 of 186 (34%) patients in whom 28 nodules (in 23 patients) were histologically diagnosed as HCC.40 Of these 23 patients, 18 were diagnosed as having a solitary HCC before laparoscopy. Similarly, new lesions of histologically proven HCC were found in 22% of patients.41 Even when preoperative staging includes a CT, LUS was superior in detecting additional tumours.42 Ultrasound confirmed all 201 tumours seen on CT and detected 21 additional tumours (9.5%) in 11 patients (20%).
Staging and assesment of resectability
Tumour staging to select a treatment regimen is complicated by the fact that many of the staging systems (including the TNM staging system of the American Joint Committee on Cancer (AJCC) – see Table 3.1) are based on surgical findings, while surgery is applicable to only about 5% of these patients. Others recommend the Barcelona Clinic Liver Cancer (BCLC) staging system.43 The Barcelona algorithm does not address the value of resection for some subgroups of patients with HCC who may potentially benefit from this approach, including some patients defined as being ‘early stage’ (with a single tumour size > 2 cm or multiple nodules) and ‘intermediate stage’ (patients who have multinodular tumours but a good performance status). The Milano/Mazzaferro criteria for liver transplantation for HCC (i.e. solitary tumour ≤ 5 cm or up to three tumours all ≤ 3 cm) are widely accepted.44 However, according to the Barcelona algorithm, only selected patients with three nodules ≤ 3 cm (those without ‘associated diseases’) should also undergo liver transplantation.43 The American Hepato-Pancreato-Biliary Association (AHPBA)/American Joint Commission on Cancer (AJCC) Consensus Conference on HCC in 2002 concluded that no single staging system could be used to accurately stage patients across the spectrum of HCCs.44,45
Table 3.1
TNM staging for hepatocellular cancer

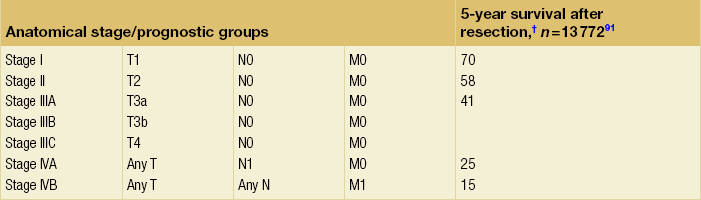
Note: cTNM is the clinical classification, pTNM is the pathological classification.
*The fibrosis score as defined by Ishak is recommended because of its prognostic value in overall survival. This scoring system uses a 0–6 scale.
†Data from AJCC sixth edition.
Modified from the AJCC Cancer Staging Manual, seventh edition (2010), published by Springer, New York.
The ideal patient for resection has a solitary HCC confined to the liver that shows no radiographic evidence of invasion of the hepatic vasculature, no evidence of portal hypertension and well-preserved hepatic function. According to the current TNM/UICC staging system for HCC (Table 3.1), most consider stage IIIB, IIIC, IVA or IVB disease to be incurable by resection. However, hepatic resection for stage IIIB, IIIC and IVA disease may be considered in some centres of excellence as clinical benefits, and long-term survival can be achieved in a selected minority of patients.
Pancreatic and periampullary carcinoma
Most pancreatic tumours are located in the head of the pancreas (60–65%), while 20% are present in the body and 10% in the tail region. Unfortunately only a minority (5–20%) of pancreatic tumours are resectable. Tumours in the pancreatic head often present earlier due to compression of the adjacent common bile duct causing obstructive jaundice. Therefore these tumours are often smaller at time of presentation and more often resectable. These smaller tumours (< 2 cm) without liver metastases have better 5-year survival.46 Hence, by accurately detecting and staging smaller tumours, imaging has the potential to influence survival.
Transabdominal ultrasound
This is usually the first screening examination of the abdomen in patients with obstructive jaundice. It is a useful diagnostic modality with a reasonable sensitivity (> 90%) for detecting bile duct obstruction, determining the level of the obstruction (e.g. intrahepatic, proximal or distal) and identifying the presence of gallstone disease.47 Transabdominal ultrasound is cost-effective, widely available but highly operator dependent and often limited by the inability to adequately visualise the pancreas due to bowel gas interference. The goal of ultrasound is therefore to primarily establish a differential diagnosis among the various causes of obstructive jaundice and in identifying liver metastases. Ultrasound is highly sensitive in detecting gallbladder stones (> 90%),48 but this sensitivity drops to 50–75% for the detection of bile duct stones.49 On ultrasound, a pancreatic tumour appears as a hypoechoic (poorly reflective) mass. A tell-tale sign suggestive of malignant obstruction is the combined presence of a dilated common bile duct and pancreatic duct (double duct sign).
Additionally, transabdominal ultrasound has a high sensitivity for detecting liver metastases and ascites, and an FNA can be performed. Sensitivity for liver lesions depends on the size of the lesion and is > 90% for lesions larger than 2 cm, 60% for lesions of 1–2 cm and 20% for lesions < 1 cm in diameter.1
Computed tomography and magnetic resonance imaging
The arterial phase optimally demonstrates the arterial vessels (superior mesenteric artery, coeliac artery and hepatic artery) and their relation to the pancreatic tumour (Fig. 3.5). The portal venous phase demonstrates the portal and superior mesenteric veins and the junction (the confluence) at the pancreatic neck (Fig. 3.6). The parenchymal phase is between these two phases and demonstrates pancreatic adenocarinoma as a hypovascular tumour compared to the rest of the parenchyma.
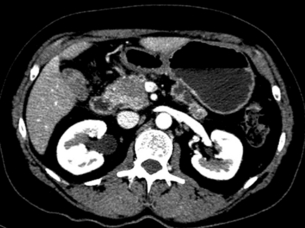
Figure 3.6 CT image of patient with pancreatic head mass with portal vein contact of approximately 90 degrees.
Tumours in the head of the pancreas usually cause dilatation of the common bile duct (CBD). The double duct sign appears when both the CBD and the pancreatic duct are dilated. Smooth dilatation is more suggestive of malignancy, whereas irregular or beaded dilatation is more suggestive of pancreatitis. Tumours that extend beyond the contours of the pancreas with infiltration of the peripancreatic fat are seen as blurring of the normal dark peripancreatic fat. The sensitivity and specificity of CT for detection of a pancreatic head mass were 91% and 85%, respectively, versus 84% and 82% for MRI in a pooled meta-analysis.50 The meta-analysis found that CT was significantly better than MRI and US.
CT has been reported to have a positive predictive value of 100%, negative predictive value of 56% and overall accuracy of 70% for unresectable pancreatic carcinoma.51 This ability to predict unresectability preoperatively is superior to the ability to predict resectability, particularly because the detection of small (< 5 mm) liver and peritoneal metastases is limited.
Endoscopic retrograde cholangiopancreatography (ERCP)
ERCP has been performed as an initial investigation in patients with obstructive jaundice for many years. However, the advent of improved non-invasive diagnostic modalities such as CT, MRI and endoscopic ultrasound (EUS) has led to less frequent use of invasive ERCP. Endoscopic retrograde cholangiopancreatography is associated with a morbidity of 5–10% and a mortality of 0.1–1%. The most common complications include pancreatitis (5–10%), bleeding (1–2%) and perforation (< 0.3%).52
Magnetic resonance cholangiopancreaticography (MRCP) closely mimics the imaging capacity of ERCP but is non-invasive. One advantage of MRCP is that information regarding the tumour and its relation to surrounding structures is also obtained during the same examination. In addition, distant metastases can be detected with MRI. The results of MRCP in visualisation of the biliary system are similar to ERCP in clinical studies, with a sensitivity of 71–93% versus 81–86% and specificity of 92–94% versus 82–100%. When comparing ERCP with EUS, the sensitivity was 75–89% versus 85–89% and specificity was 65–92% versus 80–96%.54 There are no known direct comparisons between ERCP and CT. If an ERCP is performed, it is possible to perform brush cytology, bile aspiration cytology and even intraductal biopsies. These examinations are highly sensitive but lack specificity.
The detection rate of malignancy was not significantly different for brush cytology compared to bile cytology (sensitivity of 33–100% versus specificity of 6–50%, respectively) and also not significantly different for FNA cytology compared to brush cytology (25–91% versus 8–56%, respectively). Forceps biopsy versus brush cytology was also not significantly different (43–81% versus 18–53%, respectively).54 Other new modalities such as intraductal cholangiopancreaticoscopy and intraductal endosonography have yet to be proven in larger series.
Endoscopic ultrasound
EUS allows a sonographic transducer to be placed in close proximity to the pancreas. In doing so, interference from overlying bowel gas is eliminated and higher frequencies can be used, resulting in markedly improved resolution. EUS was considered superior to the other imaging modalities prior to the recent refinements made in CT,55 because most current CT studies date from the period 1994–2000. EUS was considered more sensitive in one systematic review describing nine studies of EUS compared to CT for diagnostic effectiveness.56 The most valuable role of EUS in these studies was in detecting small tumours (< 2 cm). The pooled sensitivity was 85% with a specificity of 94%, but heterogeneity was an issue in this pooled analysis. FNA can be performed when in doubt with a lower risk of causing peritoneal carcinomatosis than with transabdominal aspiration. A study in 62 patients with pancreatic cancer that compared EUS, CT and MRI with the gold standard of surgery found that a sequential approach consisting of helical CT as an initial test and EUS as a confirmatory technique when in doubt seemed to be the most reliable and cost-effective strategy.57 Thus, EUS is not mandatory in the work-up of patients with pancreatic cancer. In those cases with potentially resectable tumours, an EUS-guided (FNA) biopsy has a pooled sensitivity for malignant cytology of 85% (95% CI 84–86%) and a pooled specificity of 98% (95% CI 97–99%).58 The major limitations of this technology are operator dependence and a limited field of visualisation for the detection of distant metastases. This modality should be used on a selective basis (e.g. to obtain preoperative tissue biopsy for patients scheduled for neoadjuvant therapy) and in centres with experience.
Positron emission tomography
Preliminary reports indicate that FDG-PET may provide additional information regarding the M status of a patient and can change the therapeutic option in up to 16% of patients.59 A meta-analysis of PET scanning in patients with a positive, negative and inconclusive CT found sensitivity and specificity of 92% and 68%, respectively, after a positive CT, 73% and 86%, respectively, after a negative CT, and 100% and 68%, respectively, after an inconclusive CT.60 Its usefulness will vary depending upon the pretest probability of the patient and the results of CT. FDG-PET is most often used for response evaluation in neoadjuvant chemoradiotherapy trials.
Diagnostic laparoscopy and laparoscopic ultrasound
Despite best efforts, there are still unexpected occasions when the intraoperative findings are contrary to those reported by the preoperative investigations, especially with regard to resectability. These patients consequently undergo an unnecessary laparotomy, along with its accompanying risks, albeit small, of postoperative morbidity and mortality. The quality of life becomes further diminished in a patient population whose survival is already limited. The detection of small liver and peritoneal metastases (Fig. 3.7) is an important motivation for performing diagnostic laparoscopy, since this might avoid open exploration. CT has excellent accuracy in predicting unresectability; however, sensitivity for assessment of resectability remains much lower. This is due to its inability to detect very small liver lesions (< 1 cm) or peritoneal deposits.
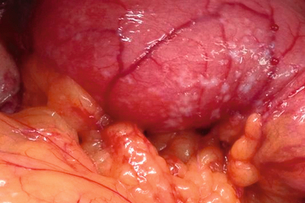
Figure 3.7 Peritoneal metastases on the first part of the duodenum found during laparotomy in a patient with a periampullary carcinoma.
Despite the logical rationale behind its use, laparoscopy continues to provoke considerable debate. Advocates have reported that laparoscopy can identify occult metastases, which were not detected by a preceding CT, in 30% of patients.61 Consequently, the resection rates after laparoscopy have been reported to be 75–92%.62 Because of these results, some centres strongly recommend the use of diagnostic laparoscopy as a routine procedure.
Critics argue that routine laparoscopy is not cost-effective. With the newer generations of CT scanners, the incidence of missed hepatic or peritoneal metastases is less than 20%. The implication is that performing routine laparoscopy adds unnecessary surgical time and expense to the remaining 80% of patients with resectable disease or, if locally unresectable, precludes them from surgical palliation, which is considered superior.63,64
The yield of additional laparoscopic staging is influenced greatly by the quality of the prelaparoscopic staging process. Incorrect staging with poor quality CT, for example, results in an overestimation in the yield of diagnostic laparoscopy and LUS. While the most important objective in laparoscopy is to prevent an unnecessary laparotomy, a number of patients do need a subsequent laparotomy for further palliation (e.g. bypass procedure for gastrointestinal obstruction). The limited detection rate for unresectable metastatic disease and the likely absence of a large gain after switching from surgical to endoscopic palliation prompted many centres not to routinely perform laparoscopy in patients with peripancreatic carcinoma.64 In a study assessing 233 patients with upper gastrointestinal malignancy, of whom 114 patients had a periampullary tumour, laparotomy was avoided initially in 17 patients (15%), but five of these patients subsequently required laparotomy for duodenal obstruction.61 This reduced the overall efficacy of laparoscopy in preventing unnecessary laparotomies from 15% to 11%. In a more recent study of 297 patients, the laparoscopic yield decreased to 13% (39 patients), probably due to improved radiological staging techniques.64 This, combined with an increasingly critical view of resectability and palliation, has resulted in a decreased benefit of laparoscopic staging.
Staging and assesment of resectability
Staging is according to the TNM atlas, seventh edition, 2009 (Tables 3.2–3.4). Most clinicians agree that a tumour is considered incurable if there are distant metastases (liver, lung, lymph nodes outside the (radical) lymph node dissection area as defined by Pedrazzoli et al.65) or if there is local invasion into arterial structures such as superior mesenteric artery, coeliac trunk or common hepatic artery (Table 3.5).
Table 3.2
TNM staging system for exocrine and endocrine tumours of the pancreas

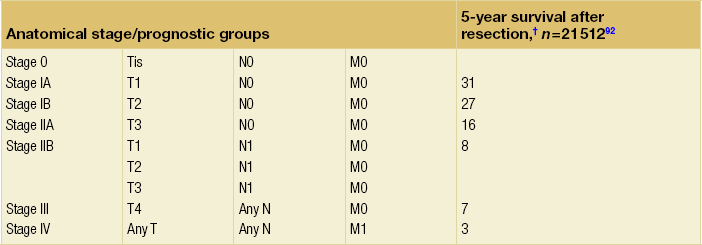
Note: cTNM is the clinical classification, pTNM is the pathological classification.
*This includes lesions classified as PanIn III classification.
†Data from AJCC sixth edition.
Modified from the AJCC Cancer Staging Manual, seventh edition (2010), published by Springer, New York.
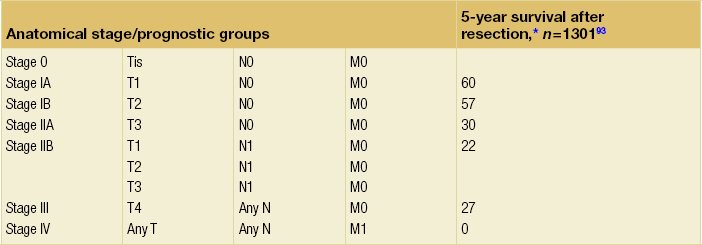
Table 3.3
TNM staging for ampullary carcinoma

Note: cTNM is the clinical classification, pTNM is the pathological classification.
*Data from AJCC sixth edition.
Modified from the AJCC Cancer Staging Manual, seventh edition (2010), published by Springer, New York.
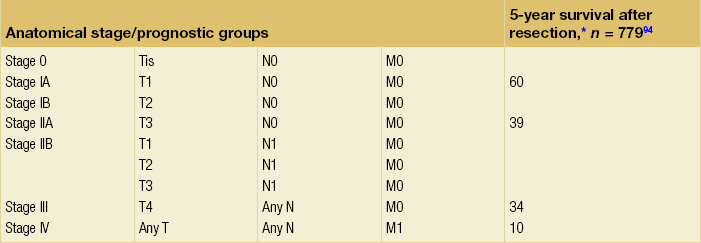
Table 3.4
TNM staging system for distal cholangiocarcinoma

Note: cTNM is the clinical classification, pTNM is the pathological classification.
*Data from AJCC sixth edition.
Modified from the AJCC Cancer Staging Manual, seventh edition (2010), published by Springer, New York.
Table 3.5
Resectability of pancreatic tumours
| Resectable | Borderline | Unresectable |
| No tumour abutment with vessel | > 90° tumour abutment with PV of SMV | > 180° tumour abutment with PV or SMV |
| > 5 mm length of PV or SMV contact | PV/SMV constriction or thrombus or teardrop deformation of SMV | |
| Any ingrowth in SMA | ||
| No distant metastasis | Distant metastasis |
PV, portal vein; SMA, superior mesenteric artery; SMV, superior mesenteric vein.
Criteria to assess vascular ingrowth on CT include tumour involvement of any of the major pancreatic vessels (coeliac artery, hepatic artery, superior mesenteric artery, superior mesenteric vein or portal vein) that exceeds one-half of the circumference of the vessels (Fig. 3.8). This is especially specific for the involved arteries. Contour deformity, obliteration and thrombosis of the veins is also highly suspicious of vascular involvement.67 Additional radiological features that suggest vascular invasion include perivascular cuffing, described as increased attenuation of the normal perivascular fat, and the presence of dilated collateral veins. The ‘teardrop’ sign, which describes the deformity of the otherwise round shape of the superior mesenteric vein, suggests venous invasion. Nevertheless, there is much debate over the exact definition of vascular involvement.
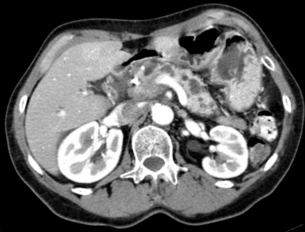
Figure 3.8 CT image of patient with pancreatic head mass with portal contact of more than 180 degrees.
Successful resection of (a part of) the superior mesenteric vein or portal vein has been described and could be an advantage, provided that an R0/R1 resection can be achieved. In a review by Ramacciato et al.,68 12 series are described with a total of 399 patients; the morbidity rate was 16.7–54% and mortality rate 0–7.7%, with a median survival of 13–22 months and a 5-year survival of 9–18%. Muller et al.69 also described 110 patients following resection of venous invasion with a morbidity rate of 41.8% and mortality rate of 3.6%. The survival was not increased by the addition of a venous resection with bypass, because earlier local recurrence and/or distant metastases arose in this group of patients compared to patients who had no invasion of the vein.
Size is the only characteristic of a pancreatic tumour that can be determined preoperatively. A review by Garcea et al.71 analysed studies and used the size measured at histopathology. In these studies the cut-off value varied, but most studies suggested 2 cm. In all these studies, the median survival of patients with tumours < 2 cm is 35.5 months versus 14 months for larger tumours. Although the studies varied in quality, this meta-analysis concluded that size (< 2 cm or > 2 cm) is an (independent) prognostic factor for median survival (odds ratio (OR) = 2.52, 95% CI 1.95–3, P < 0.001). One relatively small study used size of the tumour on preoperative imaging and found that prognosis correlated with the size determined on CT.72
Proximal bile duct tumours
Patients with proximal bile duct tumours generally present with jaundice. Patients with jaundice and a hilar stricture will either have a benign biliary stricture or a malignancy that has obstructed the hepatic confluence.73,74 The differential diagnosis includes benign biliary strictures (postoperative bile duct injury, primary sclerosing cholangitis, HIV cholangiopathy, Mirizzi syndrome) or another malignancy such as gallbladder cancer or lymphoma. Diagnosis of a hilar cholangiocarcinoma can be challenging, particularly in patients with primary sclerosing cholangitis, with multiple stenosis and mass lesions often identified on imaging without significant intrahepatic biliary dilation.
Transabdominal ultrasound
Abdominal ultrasound is often the first diagnostic study to confirm biliary duct dilatation, identify the level of obstruction and exclude gallstones.75 Duplex ultrasound has also been useful to assess vascular involvement.76
Computed tomography and magnetic resonance imaging
CT is used to establish the presence of a tumour, its location as well as local spread, vascular ingrowth and metastases. Magnetic resonance cholangiography is comparable to ERCP in the detection of biliary malignancy. An advantage offered by magnetic resonance cholangiography is that it can identify the luminal involvement and thus give a better staging of the tumour without cannulation of the bile ducts and risk of infection. It allows visualisation of both the obstructed and non-obstructed ducts, and gives important information such as the extent of tumour within the biliary tree and in periductal tissue, vascular and nodal involvement, lobar atrophy, invasion of adjacent liver parenchyma and distant metastases, without the risk of biliary intubation.77 If imaging studies demonstrate a focal stenotic lesion of the bile duct in the absence of previous biliary tract surgery, an assumptive diagnosis of hilar cholangiocarcinoma is made until proven otherwise.74
Endoscopic retrograde cholangiopancreatography
ERCP and percutaneous transhepatic cholangiography (PTC) both allow for the collection of tissue samples for pathology and bile for cytology. The value of cytology has recently been stressed.78 Non-invasive imaging does not allow tissue diagnosis, but histological confirmation is not mandatory before surgical exploration. It is notable that, in patients that require biliary drainage preoperatively, PTC is preferable to ERCP, as it allows selective evaluation of the intrahepatic biliary tree much more clearly.
Positron emission tomography
PET has been shown to have a high sensitivity for diagnosing biliary malignancy. The limitation of the method is that patients with an inflammatory process of the biliary tree (as in primary sclerosing cholangitis) can have false-positive findings. In a prospective study by Kim et al.,79 PET proved significantly more accurate in identifying distant metastases compared with CT (58% vs. 0%). Other studies have confirmed the usefulness of PET for detecting metastases not found by other imaging,80 ultimately influencing clinical management in up to 25% of patients.81 The sensitivity of FDG-PET for detecting primary intrahepatic cholangiocarcinoma has been estimated at 78%.81 However, Petrowsky et al.82 found PET-CT to be more sensitive (93% vs. 55%) and specific (80% vs. 33%) in detecting intrahepatic compared to extrahepatic cholangiocarcinoma.78 Another limitation reported by Kluge et al.83 was a sensitivity of 92% in detecting hilarcholangiocarcinoma, but the rate of detection of extrahepatic tumours was dependent on the shape of the tumour.
Diagnostic laparoscopy and laparoscopic ultrasound
These results were explained by a change in diagnosis after laparoscopy from a primary bile duct tumour to locally invasive gallbladder cancer. Another study of 110 consecutive patients confirmed these data.85 Laparoscopy revealed histologically proven incurable disease in 44 patients (41%). Of the 65 patients who underwent laparotomy, 35 patients (54%) were unresectable. Although laparotomy was avoided in 41% of cases, laparoscopy was unable to assess resectability correctly in 44% of patients. These findings are in agreement with the study from the Memorial Sloan Kettering Cancer Center involving 100 patients with carcinoma of the extrahepatic biliary tree.86 Thirty-five patients (35%) were identified as having unresectable disease at laparoscopy. Of the 65 patients who underwent laparotomy, a further 34 tumours (52%) were unresectable, resulting in an overall accuracy of 51%. Finally, in a series of 401 patients with hepatobiliary cancer, the highest yield for laparoscopy was found in patients with biliary cancer, but the study emphasised that the surgeon’s preoperative impression of resectability was as important as the laparoscopic staging procedure itself.12 Recently, a study found that the yield has decreased to 12% in recent years due to better preoperative imaging.87
LUS has not been very useful in staging the local tumour spread of proximal bile duct cancer. A previous study confirmed that patients with unresectable disease most often had locally advanced tumours, but LUS did not contribute to the assessment of resectability in these patients.86 Furthermore, extensive biliary and vascular involvement can be determined with high accuracy (91%) using external colour Doppler ultrasound, as well as thin-slice contrast-enhanced multislice CT.88 The additional value of LUS is therefore too low for it to be performed routinely.
Staging and assesment of resectability
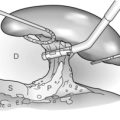
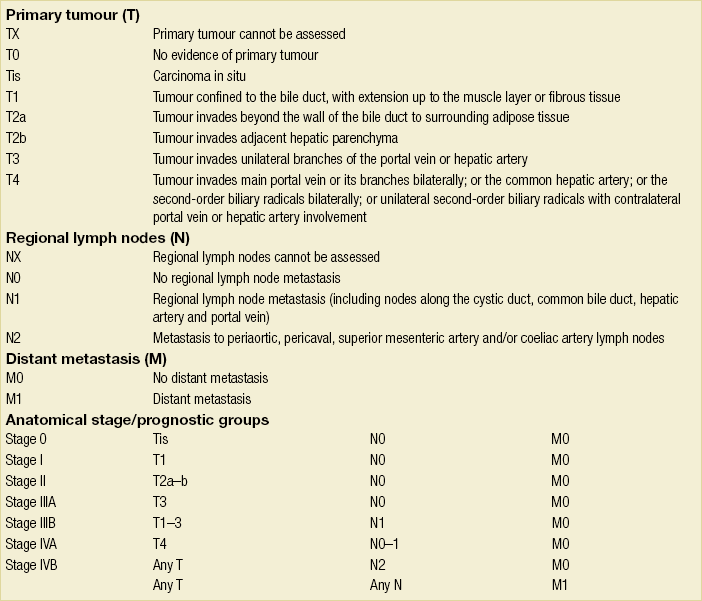
The American Joint Committee on Cancer (AJCC) TNM staging system is most commonly used to stage hilar cholangiocarcinoma (Table 3.6). However, this system is based on pathology criteria and does not provide information on the potential for resectability. Therefore, other staging systems have been used to predict resectability and to assess the extent of resection. The Bismuth–Corlette classification (Fig. 3.9) stratifies patients based on extent of biliary involvement by tumour. In brief: type I tumours are below the confluence of the left and right hepatic duct; type II tumours reach the confluence; type IIIa and IIIb tumours occlude the common hepatic duct and either the right or left hepatic duct, respectively; and type IV tumours involve the confluence and both the right and left hepatic ducts.
Table 3.6
TNM staging system for perihilar cholangiocarcinoma
Note: cTNM is the clinical classification, pTNM is the pathological classification.
Modified from the AJCC Cancer Staging Manual, seventh edition (2010), published by Springer, New York.
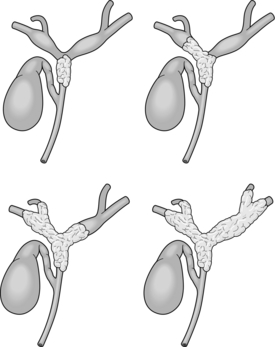
Figure 3.9 Classification of Klatskin tumours according to Bismuth–Corlette, types I, II, IIIa and IV. Type I and II tumours are limited to the confluence of the right and left hepatic duct. In type III tumours, the segmental branches of the right or left hepatic duct are involved (type IIIa or IIIb respectively). In type IV tumours, the tumour extends into the segmental branches of both right and left hepatic duct.
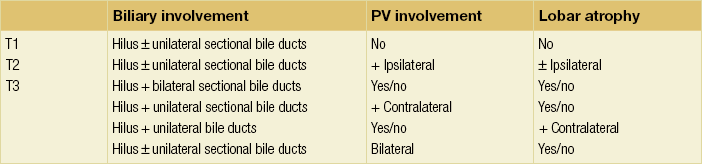
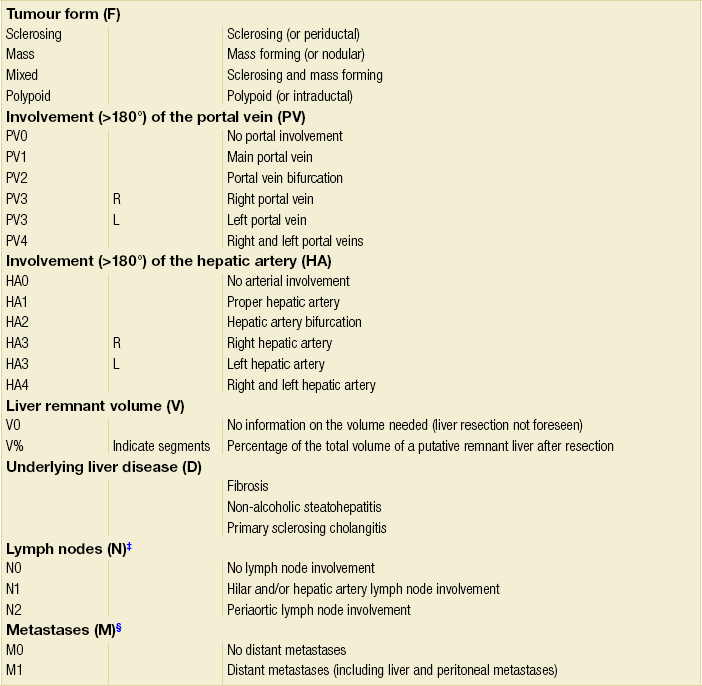
The preoperative clinical T staging system (Table 3.7) as proposed by Jarnagin and colleagues89,90 defines both the radial and longitudinal extension of hilar cholangiocarcinoma, which are critical factors in the determination of resectability. This Memorial Sloan-Kettering Cancer Center (MSKCC) staging system incorporates three factors based on preoperative imaging studies: (1) location and extent of ductal involvement; (2) presence or absence of portal vein invasion; and (3) presence or absence of hepatic lobar atrophy. Criteria for unresectable disease include locally advanced tumour extending to secondary biliary radicles (i.e. sectional bile ducts (right anterior, right posterior, left lateral and left medial) bilaterally), to unilateral sectional bile ducts with contralateral portal vein branch involvement, encasement or occlusion of the main portal vein proximal to its bifurcation, atrophy of one hepatic lobe with contralateral portal vein involvement, or atrophy of one hepatic lobe with contralateral tumour extension to sectional bile ducts. A recently designed new system of staging incorporates the size of the tumour, the extent of the disease in the biliary system, the involvement of the hepatic artery and portal vein, the involvement of lymph nodes, distant metastases, and the volume of the putative remnant liver after resection (Table 3.8). These staging systems are relatively new and have to be proven in future studies.
References
1. Hagspiel, K.D., Neidl, K.F., Eichenberger, A.C., et al, Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995;196(2):471–478. 7617863
2. Ohlsson, B., Nilsson, J., Stenram, U., et al, Percutaneous fine-needle aspiration cytology in the diagnosis and management of liver tumours. Br J Surg. 2002;89(6):757–762. 12027987
3. Floriani, I., Torri, V., Rulli, E., et al, Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31(1):19–31. 20027569
4. Sahani, D., Mehta, A., Blake, M., et al, Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics. 2004;24(5):1367–1380. 15371614
5. Yim, P.J., Vora, A.V., Raghavan, D., et al, Volumetric analysis of liver metastases in computed tomography with the fuzzy C-means algorithm. J Comput Assist Tomogr. 2006;30(2):212–220. 16628034
6. Bennett, G.L., Petersein, A., Mayo-Smith, W.W., et al, Addition of gadolinium chelates to heavily T2-weighted MR imaging: limited role in differentiating hepatic hemangiomas from metastases. Am J Roentgenol. 2000;174(2):477–485. 10658728
7. Schima, W., Saini, S., Echeverri, J.A., et al, Focal liver lesions: characterization with conventional spin-echo versus fast spin-echo T2-weighted MR imaging. Radiology. 1997;202(2):389–393. 9015063
8. Wiering, B., Krabbe, P.F., Jager, G.J., et al, The impact of fluoro-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer. 2005;104(12):2658–2670. 16315241
9. Kinkel, K., Lu, Y., Both, M., et al, Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224(3):748–756. 12202709
10. Bipat, S., van Leeuwen, M.S., Comans, E.F., et al, Colorectal liver metastases: CT, MR imaging, and PET for diagnosis – meta-analysis. Radiology. 2005;237(1):123–131. 16100087
11. Mainenti, P.P., Mancini, M., Mainolfi, C., et al, Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging. 2010;35(5):511–521. 19562412
12. D’Angelica, M., Fong, Y., Weber, S., et al, The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol. 2003;10(2):183–189. 12620915
13. Jarnagin, W.R., Conlon, K., Bodniewicz, J., et al, A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001;91(6):1121–1128. 11267957
14. Mann, C.D., Neal, C.P., Metcalfe, M.S., et al, Clinical Risk Score predicts yield of staging laparoscopy in patients with colorectal liver metastases. Br J Surg. 2007;94(7):855–859. 17380479
15. Metcalfe, M.S., Close, J.S., Iswariah, H., et al, The value of laparoscopic staging for patients with colorectal metastases. Arch Surg. 2003;138(7):770–772. 12860759
16. Jarnagin, W.R., Bodniewicz, J., Dougherty, E., et al, A prospective analysis of staging laparoscopy in patients with primary and secondary hepatobiliary malignancies. J Gastrointest Surg. 2000;4(1):34–43. 10631360
17. Grobmyer, S.R., Fong, Y., D’Angelica, M., et al, Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg. 2004;139(12):1326–1330. 15611458
18. Abbas, S., Lam, V., Hollands, M., Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis. ISRN Oncol 2011; 2011:763245. 22091431
19. Dhir, M., Lyden, E.R., Wang, A., et al, Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta-analysis. Ann Surg. 2011;254(2):234–242. 21694583
20. Poston, G.J., Adam, R., Alberts, S., et al, OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23(28):7125–7134. 16192596
21. Berri, R.N., Abdalla, E.K., Curable metastatic colorectal cancer: recommended paradigms. Curr Oncol Rep. 2009;11(3):200–208. 19336012
22. Adam, R., de Haas, R.J., Wicherts, D.A., et al, Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26(22):3672–3680. 18669451
23. Fong, Y., Fortner, J., Sun, R.L., et al, Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318. 10493478
24. Konopke, R., Kersting, S., Distler, M., et al, Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29(1):89–102. 18673436
25. Nagashima, I., Takada, T., Matsuda, K., et al, A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg. 2004;11(2):79–83. 15127268
26. Nordlinger, B., Guiguet, M., Vaillant, J.C., et al, Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77(7):1254–1262. 8608500
27. Reissfelder, C., Rahbari, N.N., Koch, M., et al, Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16(12):3279–3288. 19688403
28. Zakaria, S., Donohue, J.H., Que, F.G., et al, Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246(2):183–191. 17667495
29. Small, R.M., Lubezky, N., Shmueli, E., et al, Response to chemotherapy predicts survival following resection of hepatic colo-rectal metastases in patients treated with neoadjuvant therapy. J Surg Oncol. 2009;99(2):93–98. 19065637
30. Santi, V., Trevisani, F., Gramenzi, A., et al, Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53(2):291–297. 20483497
31. Baron, R.L., Oliver, J.H., III., Dodd, G.D., III., et al, Hepatocellular carcinoma: evaluation with biphasic, contrast-enhanced, helical CT. Radiology. 1996;199(2):505–511. 8668803
32. Rimola, J., Forner, A., Reig, M., et al, Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50(3):791–798. 19610049
33. Iannaccone, R., Piacentini, F., Murakami, T., et al, Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: helical CT and MR imaging findings with clinical–pathologic comparison. Radiology. 2007;243(2):422–430. 17356175
34. Ariff, B., Lloyd, C.R., Khan, S., et al, Imaging of liver cancer. World J Gastroenterol. 2009;15(11):1289–1300. 19294758
35. Beavers, K.L., Semelka, R.C., MRI evaluation of the liver. Semin Liver Dis. 2001;21(2):161–177. 11436570
36. Hussain, S.M., Zondervan, P.E., Ijzermans, J.N., et al, Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22(5):1023–1036. 12235331
37. Semelka, R.C., Helmberger, T.K., Contrast agents for MR imaging of the liver. Radiology. 2001;218(1):27–38. 11152776
38. Lo, C.M., Lai, E.C., Liu, C.L., et al, Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg. 1998;227(4):527–532. 9563541
39. De Castro, S.M., Tilleman, E.H., Busch, O.R., et al, Diagnostic laparoscopy for primary and secondary liver malignancies: impact of improved imaging and changed criteria for resection. Ann Surg Oncol. 2004;11(5):522–529. 15123462
40. Ido, K., Nakazawa, Y., Isoda, N., et al, The role of laparoscopic US and laparoscopic US-guided aspiration biopsy in the diagnosis of multicentric hepatocellular carcinoma. Gastrointest Endosc. 1999;50(4):523–526. 10502174
41. Montorsi, M., Santambrogio, R., Bianchi, P., et al, Laparoscopy with laparoscopic ultrasound for pretreatment staging of hepatocellular carcinoma: a prospective study. J Gastrointest Surg. 2001;5(3):312–315. 11360055
42. Foroutani, A., Garland, A.M., Berber, E., et al, Laparoscopic ultrasound vs triphasic computed tomography for detecting liver tumors. Arch Surg. 2000;135(8):933–938. 10922255
43. Llovet, J.M., Di Bisceglie, A.M., Bruix, J., et al, Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. 18477802
44. Liu, C.L., Fan, S.T., Lo, C.M., et al, Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19(17):3725–3732. 11533094
45. Henderson, J.M., Sherman, M., Tavill, A., et al, AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB (Oxford). 2003;5(4):243–250. 18332995
46. Nix, G.A., Dubbelman, C., Wilson, J.H., et al, Prognostic implications of tumor diameter in carcinoma of the head of the pancreas. Cancer. 1991;67(2):529–535. 1985745
47. Laing, F.C., Jeffrey, R.B., Jr., Wing, V.W., et al, Biliary dilatation: defining the level and cause by real-time US. Radiology. 1986;160(1):39–42. 3012631
48. Shea, J.A., Berlin, J.A., Escarce, J.J., et al, Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med. 1994;154(22):2573–2581. 7979854
49. Cronan, J.J., US diagnosis of choledocholithiasis: a reappraisal. Radiology. 1986;161(1):133–134. 3532178
50. Bipat, S., Phoa, S.S., van Delden, O.M., et al, Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29(4):438–445. 16012297
51. Kalra, M.K., Maher, M.M., Mueller, P.R., et al, State-of-the-art imaging of pancreatic neoplasms. Br J Radiol. 2003;76(912):857–865. 14711772
52. Loperfido, S., Angelini, G., Benedetti, G., et al, Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48(1):1–10. 9684657
53. van der Gaag, N.A., Rauws, E.A., van Eijck, C.H., et al, Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362(2):129–137. 20071702
54. Agency for Healthcare Research and Quality Evidence report/technology assessment: Number 50Endoscopic retrograde cholangiopancreatography. Summary. Agency for Healthcare Research and Quality, 2002.
55. Long, E.E., Van, D.J., Weinstein, S., et al, Computed tomography, endoscopic, laparoscopic, and intra-operative sonography for assessing resectability of pancreatic cancer. Surg Oncol. 2005;14(2):105–113. 16125619
56. Dewitt, J., Devereaux, B.M., Lehman, G.A., et al, Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4(6):717–725. 16675307
57. Soriano, A., Castells, A., Ayuso, C., et al, Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99(3):492–501. 15056091
58. Hewitt, M.J., McPhail, M.J., Possamai, L., et al, EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75(2):319–331. 22248600
59. Heinrich, S., Goerres, G.W., Schafer, M., et al, Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242(2):235–243. 16041214
60. Orlando, L.A., Kulasingam, S.L., Matchar, D.B., Meta-analysis: the detection of pancreatic malignancy with positron emission tomography. Aliment Pharmacol Ther. 2004;20(10):1063–1070. 15569108
61. Jimenez, R.E., Warshaw, A.L., Rattner, D.W., et al, Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135(4):409–414. 10768705
62. Jimenez, R.E., Warshaw, A.L., Fernandez-Del Castillo C. Laparoscopy and peritoneal cytology in the staging of pancreatic cancer. J Hepatobiliary Pancreat Surg. 2000;7(1):15–20. 10982586
63. Van Heek, N.T., De Castro, S.M., van Eijck, C.H., et al, The need for a prophylactic gastrojejunostomy for unresectable periampullary cancer: a prospective randomized multicenter trial with special focus on assessment of quality of life. Ann Surg. 2003;238(6):894–902. 14631226
64. Nieveen van Dijkum, E.J., Romijn, M.G., Terwee, C.B., et al, Laparoscopic staging and subsequent palliation in patients with peripancreatic carcinoma. Ann Surg. 2003;237(1):66–73. 12496532
65. Pedrazzoli, S., Pasquali, C., Sperti, C., General aspects of surgical treatment of pancreatic cancer. Dig Surg. 1999;16(4):265–275. 10449970
66. Siriwardana, H.P., Siriwardena, A.K., Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006;93(6):662–673. 16703621
67. Lu, D.S., Vedantham, S., Krasny, R.M., et al, Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199(3):697–701. 8637990
68. Ramacciato, G., Mercantini, P., Petrucciani, N., et al, Does portal–superior mesenteric vein invasion still indicate irresectability for pancreatic carcinoma? Ann Surg Oncol. 2009;16(4):817–825. 19156463
69. Muller, M.W., Friess, H., Koninger, J., et al, Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008;195(2):221–228. 18154768
70. Glanemann, M., Shi, B., Liang, F., et al, Surgical strategies for treatment of malignant pancreatic tumors: extended, standard or local surgery? World J Surg Oncol 2008; 6:123. 19014474
71. Garcea, G., Dennison, A.R., Pattenden, C.J., et al, Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9(2):99–132. 18326920
72. Phoa, S.S., Tilleman, E.H., van Delden, O.M., et al, Value of CT criteria in predicting survival in patients with potentially resectable pancreatic head carcinoma. J Surg Oncol. 2005;91(1):33–40. 15999356
73. Allema, J.H., Reinders, M.E., van Gulik, T.M., et al, Results of pancreaticoduodenectomy for ampullary carcinoma and analysis of prognostic factors for survival. Surgery. 1995;117(3):247–253. 7878528
74. Wetter, L.A., Ring, E.J., Pellegrini, C.A., et al, Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg. 1991;161(1):57–62. 1846276
75. Saini, S., Imaging of the hepatobiliary tract. N Engl J Med. 1997;336(26):1889–1894. 9197218
76. Hann, L.E., Greatrex, K.V., Bach, A.M., et al, Cholangiocarcinoma at the hepatic hilus: sonographic findings. Am J Roentgenol. 1997;168(4):985–989. 9124155
77. Manfredi, R., Barbaro, B., Masselli, G., et al, Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):155–164. 15192788
78. Boberg, K.M., Jebsen, P., Clausen, O.P., et al, Diagnostic benefit of biliary brush cytology in cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2006;45(4):568–574. 16879890
79. Kim, J.Y., Kim, M.H., Lee, T.Y., et al, Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103(5):1145–1151. 18177454
80. Moon, C.M., Bang, S., Chung, J.B., et al, Usefulness of 18F-fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. J Gastroenterol Hepatol. 2008;23(5):759–765. 17931372
81. Corvera, C.U., Blumgart, L.H., Akhurst, T., et al, 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206(1):57–65. 18155569
82. Petrowsky, H., Wildbrett, P., Husarik, D.B., et al, Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45(1):43–50. 16690156
83. Kluge, R., Schmidt, F., Caca, K., et al, Positron emission tomography with [18F]fluoro-2-deoxy-d-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33(5):1029–1035. 11343227
84. Nieveen van Dijkum, E.J., de Wit, L.T., van Delden, O.M., et al, Staging laparoscopy and laparoscopic ultrasonography in more than 400 patients with upper gastrointestinal carcinoma. J Am Coll Surg. 1999;189(5):459–465. 10549734
85. Tilleman, E.H., De Castro, S.M., Busch, O.R., et al, Diagnostic laparoscopy and laparoscopic ultrasound for staging of patients with malignant proximal bile duct obstruction. J Gastrointest Surg. 2002;6(3):426–430. 12022996
86. Weber, S.M., DeMatteo, R.P., Fong, Y., et al, Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235(3):392–399. 11882761
87. Ruys, A.T., Busch, O.R., Gouma, D.J., et al, Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann Surg Oncol. 2011;18(9):2647–2653. 21347792
88. Smits, N.J., Reeders, J.W., Imaging and staging of biliopancreatic malignancy: role of ultrasound. Ann Oncol. 1999;10(Suppl. 4):20–24. 10436778
89. Jarnagin, W.R., Fong, Y., DeMatteo, R.P., et al, Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517. 11573044
90. Burke, E.C., Jarnagin, W.R., Hochwald, S.N., et al, Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228(3):385–394. 9742921
91. Minagawa, M., Ikai, I., Matsuyama, Y., et al, Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909–922. 17522517
92. Bilimoria, K.Y., Bentrem, D.J., Ko, C.Y., et al, Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110(4):738–744. 17580363
93. O’Connell, J.B., Maggard, M.A., Manunga, J., Jr., et al, Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008;15(7):1820–1827. 18369675
94. Miyakawa, S., Ishihara, S., Horiguchi, A., et al, Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16(1):1–7. 19110652
95. DeOliveira, M.L., Schulick, R.D., Nimura, Y., et al, New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53(4):1363–1371. 21480336