Chapter 44 Sports Medicine
Role of the Team Physician
The team physician has multiple, overlapping roles within the framework of the team itself, the individual athletes and their families, the school or overseeing organization, and the community. The primary responsibility is caring for the health and well-being of the individual athlete. The chief duties are to determine initial medical eligibility, provide care for the injured athlete, facilitate and determine return to play, create and maintain emergency preparedness, oversee the healthfulness of the team’s overall training program, supervise the personnel providing health care for the team, and protect against institutional and personal liability.124 The role of intermediary for each of these groups is both a challenging and rewarding one. The team physician is responsible for conveying information to other medical specialists, allied health professionals, athletic trainers, and coaches, as well as for creating and coordinating health care plans for the athletes.3
Beyond the medical obligation to the athlete, there is an assumed medicolegal responsibility of a team physician. Physicians receiving financial or other obvious remuneration for services are bound by normal medicolegal responsibilities. The physician might be held legally liable if injury occurs as a result of negligence or treatment below the professional standard of care. Those physicians who volunteer their services are typically covered by Good Samaritan laws, although these are not present in every state and vary from state to state. These laws often state that the services provided must meet the accepted standard of care to be protected.41 Professional organizations, universities, high schools, and individual physicians also have all been held liable for inadequate emergency preparedness.124 Preparticipation evaluations (PPEs) are not covered under Good Samaritan laws. In some cases, athletes might be legally allowed to participate despite a disqualifying PPE.
The team physician is also responsible for the final decision regarding clearance to play or return to play (RTP) after injury. These issues can be complex, and several fundamentals must be met before the athlete should be cleared. When recovering from an injury, the athlete must have completed appropriate rehabilitation; a health care professional must document the athlete’s recovery; and the risks of return must be discussed and documented. The team physician must obtain the appropriate consultant opinions when dealing with conditions or injuries outside the scope of his or her practice. Although some states have determined that athletic participation implies assumed risk, this is not universal. Team physicians have been held liable for injury occurring after full RTP has been granted. In cases of differing opinion of the team physician and a consultant, the final responsibility typically lies with the team physician.
Event Administration
Both require significant preseason or pre-event planning.72 From an administrative perspective, it is essential that the team physician or chief medical officer develops a chain of command that defines the responsibilities of the medical providers, emergency medical services, event officials, and other parties involved in the athletic event and athlete care. Establishing and regularly rehearsing an emergency action plan (EAP) is critical. Reviewing the medical equipment that will be available at the event as well as the protocols for attending to an injured athlete are standard components of preparation. The team physician must also assess environmental concerns and playing conditions and have a policy to modify or suspend practice or competition if adverse conditions exist.
Although any marathon has a known number of people requiring medical assistance (2% to 20% of participants depending on event size, duration, and weather conditions), if these numbers should increase unexpectedly, there is a potential for the community resources to become overwhelmed if not allocated appropriately.30,133 For this reason, communication among the medical team, race officials, emergency medical services, local hospitals, and participants is critical to help prevent and respond to incidents during the race. It is the role of the chief medical officer of the event to coordinate these effective lines of communication.
Principles of Conditioning and Training
Effective training programs require a fundamental understanding of strength, flexibility, and endurance development, as well as the basic principles of specificity, individuality, periodization, overload, and tapering. Please refer to Chapter 18 on Therapeutic Exercise for an overview of strength, flexibility, and endurance training.
The principle of overload requires that the training stimulus be greater than what the athlete normally performs in competition. Exercise frequency, duration, and intensity are manipulated to produce overload. These specific training variables (frequency, duration, and intensity) must be periodically increased for an athlete to progress (Box 44-1).
BOX 44-1 Training Program Design Variables: FITT
From Frontera WR, et al: Clinical sports medicine: medical management and rehabilitation, Philadelphia, 2007, Saunders.
Periodization
Periodization is one structured training approach that highlights this concept. It was developed by a Russian physiologist in the 1960s but has had a significant resurgence in today’s training programs (Figure 44-1). In periodization, training is divided into defined “periods” to allow buildup of training stresses, time for rest and adaptation to training, and continual progression of fitness. These periods include macrocycles (commonly lasting 1 year), which are divided into shorter mesocycles (commonly lasting 1 month), which are then subdivided into microcycles (commonly lasting 1 week). Microcycles are generally the weekly training programs. Each mesocycle can be made up of four weekly microcycles with a gradual buildup of training frequency and intensity over the first 3 weeks and then a slight decrease in the fourth week to allow for rest and the subsequent metabolic training adaptations to occur. The athlete is then ready to enter the next 4-week mesocycle and progress through another four weekly microcycles. The amount and intensity of training occurring in each mesocycle depend on where the athlete is in relationship to the competitive season, which is how macrocycles are structured.
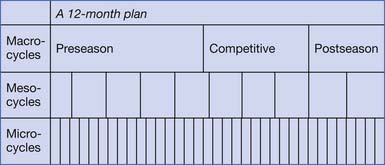
FIGURE 44-1 A template for a periodized training program.
(From Bachl N, Baron R, Smekal G: Principles of exercise physiology and conditioning. In Frontera WR, et al, Clinical sports medicine: medical management and rehabilitation, Philadelphia, 2007, Saunders.)
A typical year-long training program will have three macrocycles: preseason or “build-up phase,” competitive season or “maintenance and fine-tuning phase,” and finally a postseason or “recovery phase.” The preseason macrocycle is typically the longest, occurring before the competitive season. It is designed to develop fitness in anticipation of the more intense training to follow.162 During the preseason macrocycle the athlete generally focuses on higher volume and lower intensity exercise.
The competitive season macrocycle follows the preseason period. The focus of this cycle is to develop and maintain peak fitness with a focus on high-intensity training and sport-specific technique drills.162 Because of the higher intensity training, the volume must be significantly reduced.
Finally, the postseason macrocycle is a time for the athlete to recover from the previous year’s training and physically and mentally prepare for the next year of training and competition.162 The early portion of this period necessitates “active rest.” The athlete generally participates in unstructured, non–sport-specific recreational activities to allow for recovery from the stresses of the competitive season. This is an important period for the athlete to adequately recover from injuries, prevent overtraining, and take a “mental break” from the competitive season.
During the competitive season, a typical microcycle includes a tapering period just before competition. The taper is a short period of reduced training before an important competition with the goal of optimizing performance. Although it is well recognized that a reduction in training before competition improves performance, it not definitively known what is the most ideal taper strategy regarding altering training volume, intensity, and frequency.8,77,113 A recent metaanalysis demonstrated that the most efficient strategy to maximize performance gains appears to be a 2-week taper during which training volume is exponentially reduced by 41% to 60%.19 This study found that the ideal taper for endurance athletes kept training intensity and frequency stable, with only volume being progressively reduced.
Overtraining Syndrome
An important set of symptoms to be aware of for any athlete, coach, or sports medicine physician is that related to overtraining syndrome. When prolonged, excessive training occurs concurrently with insufficient recovery, the athlete might have unexplainable performance decrements resulting in chronic maladaptations leading to overtraining syndrome.109 Common symptoms of overtraining syndrome in addition to an unexplained performance decrement include generalized fatigue, mood disturbance, poor sleep, and increased rates of illness and injury. By definition, these symptoms persist despite more than 2 weeks of rest.158 The treatment is rest, from weeks to months, with gradual resumption of training. Prevention, however, is the best treatment, and following a periodized training program is one method to ensure adequate rest from more intense bouts of training.
Altitude Training
Altitude/hypoxic training is a controversial area of research and sports performance. Many elite endurance athletes incorporate altitude training into their typical training program in hope of enhancing performance in competition. For those athletes who do not live in mountainous areas, there are a variety of “altitude tents” on the market that can be used to simulate living at high altitude. Some controversy arose in 2006 when the World Anti-Doping Agency (WADA) considered placing “artificially-induced hypoxic conditions” on the Prohibited List of Substances/Methods.166 Another area of controversy is the uncertain primary physiologic mechanism(s) responsible for the effect of altitude training on sea-level performance. The proposed mechanisms include accelerated erythropoiesis (increased erythrocyte volume) as the primary hematologic effect, as well as nonhematologic factors such as improved muscle efficiency at the mitochondrial level, glucose transport alterations, and enhanced muscle-buffering capacity via pH regulation.60
Most scientists agree that the most effective form of altitude/hypoxic training is the “live high–train low” method, whereby athletes “live high” to stimulate erythropoietin and subsequently increase erythrocyte volume and “train low” to train at a higher intensity with improved oxygen flux to induce beneficial metabolic and neuromuscular training adaptations.167 The controversy here comes from not knowing what the optimal “dose” of altitude training truly is. So far the research suggests that for athletes to derive the most beneficial hematologic effects while using natural altitude, they need to live at an elevation of 2000 to 2500 meters for 22 hr/day for 4 weeks. For those using simulated altitude environments, 12 to 16 hours of hypoxic exposure might be necessary but at a higher elevation (2500 to 3000 meters167).
Injury Prevention and Rehabilitation
Kinetic Chain Assessment
The kinetic chain model is based on the idea that each complex, athletic movement is the summation of its constituent parts. For example, a quarterback’s throwing motion is created through the action of feet on the playing surface, loading of the lower limbs, rotation of the hips and abdominal muscles, activation of the latissimus dorsi, stabilization of the glenohumeral joint by the periscapular muscles, loading of the throwing arm through the deltoid and biceps, and finally from the upper limb motion of elbow extension and wrist flexion. The upper limb acts as a “funnel” for the energy generated by the core and lower limbs.85 Each of the “links” in the chain must function well to optimize performance and minimize potential tissue trauma.
“Catch-up” occurs when an athlete tries to compensate with one segment for a deficiency in a separate segment. This phenomenon puts higher stress on the tissues of the distal segment and predisposes it to injury.85 For example, a runner mighty present with patellofemoral pain for a variety of these reasons. This could simply be an overtraining phenomenon, or it might be due to weak or inhibited hip musculature, abnormal foot and ankle biomechanics, or poor running technique.105 Relief can be achieved through resting the athlete temporarily, but the pain might consistently return unless the causative issue is corrected. The experienced sports medicine physician will identify the tissue diagnosis and treat it appropriately, but will also identify and correct all predisposing factors. It is important that the physician is able to explain these concepts to the athlete and the treatment team to achieve optimal results.
Rather than view all similar overuse injuries as the same issue, the sports medicine physiatrist is able to break down the complex motions of the athlete into the constituent parts, identify the maladaptive pattern, and create a rehabilitation program that will prevent the injury from recurring. When examining the injured athlete, it is vital to make a biomechanical diagnosis rather than to focus exclusively on the injured tissue. Although it is easy to focus exclusively on the painful tissue, it is essential to assess the entire athlete and his or her mechanics to make appropriate tissue and biomechanical diagnoses (Table 44-1).
| Tissue Diagnosis | Potential Biomechanical Diagnosis |
|---|---|
| Lateral/medial epicondylitis | Posterior deltoid weakness |
| Hamstring strain | Overly tight hamstring, weak gluteal musculature |
| Metatarsal stress fracture | Supinated foot |
| Athletic pubalgia | Weak core musculature, tight hip girdle |
| Shoulder impingement with rotator cuff strain | Periscapular weakness or inhibition |
| Patellofemoral syndrome—patellar cartilage irritation or chondromalacia | Quadriceps and gluteal weakness or inhibition, overpronation |
| Repetitive ankle sprains—anteriotalofibular ligament FL laxity | Weak peroneals, proprioceptive dysfunction |
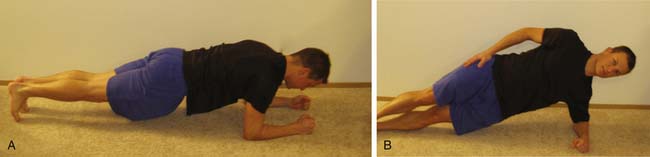
The physical examination of the athlete must be focused on finding the biomechanical culprit, rather than focusing solely on the painful tissue. The athlete must be examined in detail to fully assess the possible causes of the injury. Select upper limb tests that help determine the role of the scapula in upper limb injuries include the scapular assistance test and the scapular retraction test.87 Core muscle function must be assessed dynamically and addressed as part of the rehabilitation plan.88 Examples of physical examination maneuvers to evaluate core muscular function in the athlete are demonstrated in Figures 44-2 to 44-5. The kinetic chain assessment is designed to assess flexibility, strength, and functionality of the affected limb. The examination essentially assesses multiplane sports-specific dynamics rather than single-plane, solitary joint movements.
It is vital for the examining physician to have a thorough understanding of the motions involved in the athlete’s sport to make the appropriate changes; for example, a difference of 10 degrees in knee extension during the cocking phase of a tennis serve increases the valgus load at the elbow by 21%.50 When necessary, this can be accomplished through discussion with the athlete, parent, coach, and/or trainer.
Prehabilitation
Prehabilitation is based on the concept that many sports injuries can be prevented if the athlete engages in an appropriate preseason “prehabilitation” program. Most of the scientific literature regarding prehabilitation programs focuses on noncontact anterior cruciate ligament tears.73,74,97 Poor form is also linked to numerous athletic injuries. Balance perturbation, plyometric training, and stretching programs all have a role in the prehabilitation of the athlete. Ideally, some of these issues can be assessed during the preseason physical examination and corrected before the start of the season. A large proportion of sports injuries are related to overuse, and it is wise to have a discussion of these issues with athletes and coaches in the preseason. Off-season cross-training, core muscle work, and cardiovascular preparation are generally considered key to a healthy season.
Injury Phases
Rehabilitation is guided by the status of the injured tissue, so an understanding of the timing of injury and recovery is essential. Injuries can be acute, subacute, chronic, or an exacerbation of a chronic condition. A detailed history is required because athletes will present at varying points of their tissue healing, and the treatment plan will have to be created appropriately. Four general phases are typically seen in tissue injury and repair. The physician caters the treatment to the individual tissue and the timing of the injury. The first phase involves the initial injury and the subsequent inflammation, edema, and pain. This phase is typically short, lasting days, depending on the severity of the injury. The reparative phase of the injured tissue might last from 6 to 8 weeks. It involves cell proliferation, granulation tissue formation, and neovascularization. The last phase is remodeling, which occurs as the tissue matures and realigns.
Stages of Rehabilitation
Rehabilitation can be viewed as a three-stage spectrum from the acute stage to the recovery stage, and then to the final functional stage through return to full play (Table 44-2).86 Each stage has individual goals that lead to the overall goal of RTP. The acute stage of rehabilitation is focused on managing the symptoms and signs of the injury. The classic PRICE (protection, rest, ice, compression, and elevation) approach is often followed. Medications, manual therapy, and physical modalities are also used during this phase. If necessary, bracing, injection, or surgery is performed to facilitate protection and future healing. ROM, strength, and cardiovascular fitness must be maintained as much as is possible and tolerated. This can be accomplished by exercising the upper body of an athlete with a lower body injury. Passive ROM must be viewed with caution in the acute to subacute injury phase because it might injure the tissue, leading to increased pain and inflammation. Isometric strength exercises can be prescribed during this stage, if tolerated, to decrease pain, edema, and potential atrophy. The athlete can advance to the next stage of rehabilitation if adequate pain control and near-normal ROM is achieved.
| Phase | Goal | Timing |
|---|---|---|
| Phase 1 (acute) | Allow injured tissue time to heal; decrease symptoms, maintain range of motion | <72 hr |
| Phase 2 (recovery) | Increasing demands on the athlete; flexibility, strength, endurance training; kinetic chain corrections; maintain cardiovascular fitness through cross-training | Variable |
| Phase 3 (functional) | Advance toward full return to play, advance cardiovascular fitness | 2-4 wk |
In the case of an athlete with a shoulder injury, the rehabilitation program moves from predominantly closed-chain exercises, to axial-loaded exercises, to open kinetic chain exercises, to full sport-specific rehabilitation.108 Kinetic chain rehabilitation is modified to the individual athlete’s symptoms and dysfunction. Rather than view this type of rehabilitation as a series of discrete steps, the physiatrist should consider it as a transition from dysfunction to function and adjust the treatment plan and timing accordingly.108
The parameters of the strength training program (repetitions, resistance, speed, multijoint movements) can be adapted during this time, depending on the clinical scenario. Preexisting kinetic chain issues or tissue overload issues from the current injury are addressed at this time as well. As the rehabilitation program continues, increasingly athletic maneuvers (running, jumping, cutting) are used. Neuromuscular control (NMC) involves proprioception, muscle control, and the interplay between the two. Without sufficient NMC, the athlete might continue to be predisposed to future injury. This recovery phase is the most variable and has the highest potential for reinjury, as the tissue is actively remodeling. The athlete must be reevaluated frequently during this phase because setbacks are common and can require modification of the rehabilitation plan. The athlete can advance to the final stage once pain-free ROM is achieved and strength is 75% to 80% or greater (compared with the noninjured side).
During the final functional stage of rehabilitation, the focus remains on kinetic chain issues and technique errors. Strength balance, power, endurance, functional ROM, and NMC are aggressively addressed. Sport-specific drills are used during this stage and advanced to include practice. Full RTP is achieved when the injury is no longer painful; when there is normal flexibility, strength, and proprioception; and when appropriate sport-specific mechanics and sport-specific skills are achieved and reproducible. In many cases, this rehabilitation phase becomes the maintenance program.56
Biomechanics of Sports
Throwing
Approximately 50% of the velocity of a pitch results from the step and body rotation (from the potential energy stored in the large leg and trunk musculature).154 The other 50% comes from the smaller muscles of the shoulder, elbow, wrist, and hand. When forward stride is not allowed, the peak velocity of a pitched ball decreases to 84%. When the lower body is restricted, it decreases to 64%. Peak velocities in water polo are approximately 50% that of baseball because of the lack of a ground reaction force.
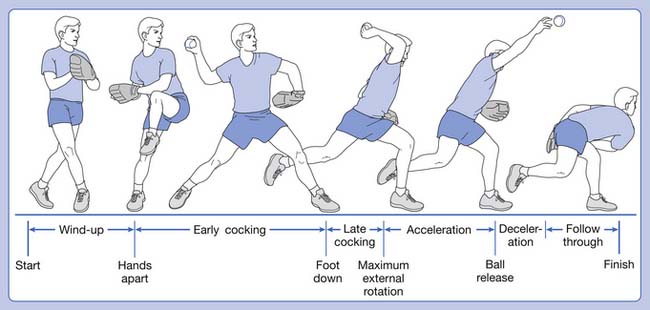
The baseball pitch is composed of six phases (Figure 44-6). Understanding these phases is critical to understanding, diagnosing, preventing, and treating throwing sports’ injuries.
Early cocking is next and is often called the stride phase.110 The stride leg extends toward the batter, as does the knee and hip of the pivot leg, propelling the body forward into the stride. As the hips rotate forward, the trunk follows and subsequently, the throwing shoulder abducts, extends, and externally rotates, leaving the shoulder in a “semicocked” position. Again, in this early phase, there is less risk of injury because most forces are still being generated in the trunk and lower limbs; however, shoulder activity does become more evident. The trapezius and serratus anterior demonstrate moderate to high activity to protract and upwardly rotate the scapula. The middle deltoid generates the abduction force, and the supraspinatus fine-tunes humeral head positioning within the glenoid.
The next phase is late cocking. The hallmark of this phase is when maximal shoulder external rotation is obtained. The shoulder begins this phase in approximately 50 degrees of external rotation and ends in about 175 degrees at maximal external rotation. This extreme amount of rotation allows the greatest accelerating force to the ball over the greatest possible distance. The amount of external rotation obtained correlates with the speed of the pitched ball. A majority of injuries occur in the late cocking and deceleration phases of throwing (Box 44-2).121 For late cocking, this is due to the forces needed to stabilize the shoulder in this extreme ROM. The dynamic stabilizers of the anterior shoulder (long head of the biceps, subscapularis, and pectoralis major) are very active in this phase. The static stabilizers (glenohumeral ligaments, capsule, and labrum) are active as well. The glenohumeral ligaments and capsule increase in laxity because of the extreme ROM in the overhead athlete. This laxity is necessary for performance; however, overstretching these ligaments enhances the work of the dynamic stabilizers with a resultant potential for injury to them.
The deceleration phase is manifested by large eccentric muscular forces of the posterior shoulder girdle to decelerate that rapid internal rotation of the acceleration phase.20 Deceleration begins after ball release and ends when the arm reaches 0 degrees of internal rotation. The posterior shoulder girdle is active in this phase, including the scapular muscles, rotator cuff external rotators (particularly the teres minor), and the posterior deltoid. Because of these large eccentric contractions, injury is common in this phase (Box 44-3).
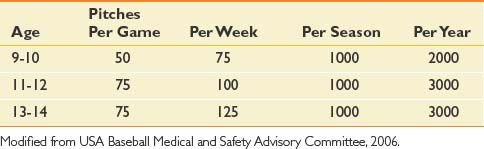
Because of the high risk of overuse injury in the young pitcher’s throwing arm, U.S. baseball has developed age-based pitch count guidelines to decrease this risk (Table 44-3).83 For similar reasons, curve balls and sliders should not be pitched until the athlete reaches puberty. It is also recommended that young pitchers not compete in baseball more than 9 months per year. During those 3 “off” months, they should not compete in other overhead arm sports, such as competitive swimming or javelin throwing.
Running
Notable differences are observed between a walking gait cycle (see Chapter 5) and a running gait cycle.120 One in particular is a third phase in running called the float phase. Float is a period when neither foot is in contact with the ground. It occurs at the beginning of initial swing and the end of terminal swing (Figure 44-7).
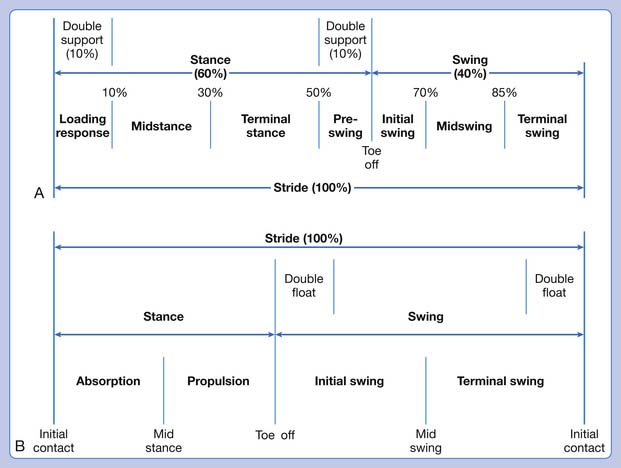
FIGURE 44-7 A comparison of the walking (A) and running (B) gait cycles.
(Modified from Ounpuu s: The biomechanics of walking and running, Clin Sports Med 13(4): 843-863, 1994.)
Faster running also changes foot contact.168 In slower running and walking, contact is typically heel to toe. As running speed increases, foot strike occurs with the forefoot and heel simultaneously, or the forefoot strikes initially followed by the heel lowering to the ground. In sprinting, the athlete maintains weight-bearing on the forefoot from loading response to toe-off.
Swimming
During the propulsive phases of swimming, the arm is moved through adduction and internal rotation starting from a stretched position of abduction and external rotation. The pectoralis major and latissimus dorsi are the major contributors to this motion; however, the serratus anterior and the internal rotator function of the subscapularis and teres major assist.156
General rehabilitation and prehabilitation principles have been established for swimmers with shoulder pain with which all sports medicine clinicians should be familiar. The overarching principle is that most shoulder pain in swimmers (impingement and rotator cuff tendinopathy) is due to dynamic muscle imbalances, weakness, and biomechanical faults, and not hard anatomic factors. A major tenet of shoulder rehabilitation for the swimmer is scapular stabilization, with a prime focus on endurance training of the serratus anterior and lower trapezius. The serratus anterior is a muscle of particular focus because it has been demonstrated to function at 75% of its maximum test ability in swimming and is active throughout the swim stroke cycle.143 Other tenets of shoulder rehabilitation in swimmers include stretching the internal rotators and posterior capsule and cervical and thoracic mobilization.
Jumping and Landing
The biomechanics of jumping and landing in sports is particularly well researched in the setting of noncontact anterior cruciate ligament (ACL) injuries of the knee. Noncontact ACL injuries occur more frequently with the knee in less flexion.172 In this position there are greater knee extensor loads with greater forces creating anterior tibial translation and subsequent ACL injury. A well-defined gender difference is observed in jumping and landing mechanics that is likely one reason for the higher rate of noncontact ACL injuries in female athletes.29 Female athletes land more erect with less knee and hip flexion. They land with less hip external rotation and abduction. They also generally have an imbalance of increased quadriceps to hamstrings activation ratio, creating greater knee extension and lesser knee flexion forces. One goal of ACL injury prevention programs is to improve jumping and landing technique by increasing knee and hip flexion during landing and balancing the quadriceps to hamstring activation ratio.
Pharmacology in Sports
“Doping” refers to any substance or method used to increase performance, possibly at the detriment of the health of the athlete or the ethics of the competition.43 WADA was created to unify the control of the fight against doping. WADA rulings are accepted by all National Olympic committees, the International Olympic Committee (IOC), Paralympics committee, national governments, and international sports federations. The agency, based in Montreal, Canada, consists of government representatives from all continents and members of the Olympic movement. The World Anti-Doping Code was developed in 2003 and enforced in 2004, with a complete listing of banned substances and methods.43 A brief history of doping in sports is presented in Box 44-4.
BOX 44-4 History of Doping and Drug Monitoring
Modified from Calfee R, Fadale P: Popular ergogenic drugs and supplements in young athletes, Pediatrics 117(3):e577-589, 2006; de Rose EH: Doping and sports. In Clinical sports medicine, Philadelphia, 2007, Saunders; Silver MD: Use of ergogenic aids by athletes, J Am Acad Orthop Surg 9(1):61-70, 2001.
A summarized list of substances banned by WADA is available online. Banned substances are divided into those that are banned in and out of competition and those that are banned in competition only.170 The lists of drugs and supplements banned by the National Football League (NFL), National Collegiate Athletic Association (NCAA), and other organizations are also available online.114
When an athlete has an illness or condition that requires treatment with a banned medication, a therapeutic use exemption (TUE) can give that athlete the authorization to take the needed medicine. Per WADA, all international federations and national antidoping organizations must have a process in place whereby athletes with documented medical conditions can request a TUE.69
Therapeutic Drugs
Analgesics
Acetaminophen can be linked to decreased muscle building after exercise (to an extent similar to that of ibuprofen). Prostaglandins are normally released after eccentric resistance exercise. This response might be blunted after consumption of maximal doses of ibuprofen or acetaminophen, profoundly influencing the anabolic response of muscle to this type of exercise.155
Antiinflammatories
One study found that 15% of high school football players used nonsteroidal antiinflammatory drugs (NSAIDs) daily.160 Although the pain relief provided by NSAIDs might enhance performance, NSAIDs are not considered ergogenic. NSAIDs can mask pain and interrupt a natural defense mechanism for preventing injury. Because of their antiinflammatory effects, NSAIDs likely inhibit the production of prostaglandin E2, which is known to play a role in bone healing.125 Consequently, in those with suspected or known fractures, NSAIDs might be contraindicated.
Corticosteroids are potent antiinflammatories. In general, their use is prohibited by WADA when given orally, rectally, intravenously, or by intramuscular injection. Topical preparations for skin, eye, ear, nose, or buccal cavity, or for iontophoresis are allowed. For applicable athletes, epidural or intraarticular steroid injections or inhaled steroids require a TUE.170
Antihypertensives
Experts recommend diuretics as first-line treatment of hypertension, possibly with early addition of a β-blocker or angiotensin-converting enzyme inhibitor (ACE-I).150 Because of the side effect profile diuretics and β-blockers portend to athletes, however, ACE-I or calcium channel blockers can be considered first-line agents. The detection and management of hypertension and the efficacy of various agents are beyond the scope of this chapter. Rather, this discussion will focus on the aspects of these medications that impact athletes.
Diuretics can decrease plasma volume, cardiac output, and peripheral vascular resistance. Dehydration and electrolyte alterations might result in cramps or even heat stroke. For amateur athletes, the benefits of antihypertensive therapy with diuretics likely outweigh the risks. In the case of elite athletes, diuretics are banned in part for their theoretical ability to increase urine output and mask use of other banned agents (although this method rarely works optimally).125
β-Blockers can reduce exercise tolerance by increasing perceived effort. They can also inhibit glycolysis and glycogenolysis with resulting hypoglycemia after exercise. The negative chronotropic effects of β-blockers can decrease heart rate recovery after exercise. β-Blockers are banned in certain sports because of their anxiolytic effects.125
Diabetes Drugs
Insulin doses might need to be adjusted for persons with insulin-dependent diabetes starting a new exercise program. A 20% to 40% reduction is typical because of increased insulin sensitivity with exercise. High-intensity exercise (i.e., greater than 80% VO2max) can cause a temporary increase in blood glucose secondary to increased sympathoadrenal activation. In that situation, supplemental insulin, if used, should be given at a smaller dose than given for hyperglycemia at rest.13,81
Intramuscular injections of insulin should be avoided because muscle contraction can accelerate insulin absorption. Heat can increase absorption rates of insulin, whereas cold can decrease absorption rates. Therefore athletes with insulin-dependent diabetes mellitus should avoid modalities that use extremes of temperature, for example, hot or cold whirlpools. Extreme ambient temperature can also reduce insulin action in athletes.81
Asthma Drugs
Exercise-induced bronchospasm (EIB) can be treated effectively with a short-acting β-agonist, such as albuterol, within 15 minutes before exercise.150 If not sufficient to prevent symptoms, cromolyn (a mast cell stabilizer) can be added. Inhalation of either cromolyn sodium or a β-agonist, or both, 15 minutes before exercise is almost always successful in blocking EIB and airway inflammation.93
For patients who have chronic persistent asthma (forced expiratory volume in 1 second <80% of predicted and symptoms greater than twice a week), inhaled corticosteroids are standard treatment. Inhaled corticosteroids do not appear to have ergogenic or anabolic effects.125
Performance-Enhancing Drugs
Anabolic Steroids
It is estimated that 1 to 3 million athletes in the United States alone have used anabolic steroids (AS),145 with annual market sales well in excess of $100 million.152 The Centers for Disease Control and Prevention found an overall prevalence of lifetime steroid use in females, grades 9 to 12, to be 3.2%.104 Furthermore, as many as 1 in 10 steroid users is a teenager.94 One study found that 6.3% of high school varsity football players were current or former AS users.63
AS have three general effects that enhance athletic performance. By binding androgen receptors, AS stimulate messenger RNA synthesis, increasing structural and contractile protein synthesis94,152 and producing an anabolic state.22 AS are also anticatabolic via competitive inhibition of the glucocorticoid receptor, inhibiting the catabolic effects of cortisol,152 preserving muscle mass.22 Finally, AS have emotional effects, pushing athletes to train more intensely and more often.22
Because AS are illegal, studying their health risks and side effects is a challenge. Inconsistent formulations, dosing, and training habits make it difficult to derive statistically sound conclusions. Some studies show minimal effects on body composition and strength, whereas others show that supraphysiologic doses of testosterone (or its derivatives) can lead to an increase in lean mass and muscle size in humans (Table 44-4).152 Typical users of steroids can take 10 to 40 times the prescribed dosage for disease states.63,80,104,152 Users often share needles, at a rate of up to 25% in adolescent users, with reports of HIV, hepatitis B and C, and abcesses.152 Premature deaths have resulted from AS use, most commonly as a result of suicide and acute myocardial infarction.80
| System | Effects |
|---|---|
| Cardiovascular |
FSH, Follicle-stimulating hormone; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LH, luteinizing hormone.
From Laos C, Metzl JD: Performance-enhancing drug use in young athletes, Adolesc Med Clin 17(3):719-731, abstract xii, 2006.
The Anabolic Steroid Control Act of 1990 prohibited the use of steroids for any use other than disease treatment, thereby classifying “AS” as Schedule III drugs within the Controlled Substances Act. AS are banned by all major sporting leagues,104 the IOC, and the NCAA.80
Erythropoietin and Blood Doping
Endurance athletes are particularly sensitive to the oxygen-carrying capacity in their blood. For years, athletes sought a competitive advantage by training at altitude or sleeping in altitude tents. This effect can be reproduced by blood doping, blood transfusion, or administration of the drug recombinant human erythropoietin (rhEPO).27,152
Erythropoietin (EPO) is a glycoprotein hormone produced mainly in the kidney in response to tissue hypoxia. EPO is part of a negative feedback cycle that controls tissue oxygen delivery by controlling the number of erythrocytes in the blood.136 Both rhEPO and transfusions have been shown to increase VO2max.16,152
The risks of artificially elevated hemoglobin/hematocrit include stroke, myocardial infarction, and pulmonary embolism. Blood transfusions, even conducted according to hospital procedures, carry risks for infection, including HIV and hepatitis, as well as transfusion reaction.152
Stimulants
Stimulants might be the most common and underrecognized supplement used by high school athletes. Common stimulants include caffeine, ephedrine (ephedra or ma huang), pseudoephedrine, neosynephrine, amphetamines, and methamphetamines.63 Stimulants increase arousal, respiratory rate, heart rate, and blood pressure. Side effects include dizziness, insomnia, agitation, restlessness, anxiety, confusion, paranoia, hallucinations, dyskinesias, gastrointestinal disturbances, heat intolerance, stroke, myocardial infarction, arrhythmia, and death.63
Pseudoephedrine (Sudafed) is a commonly used drug in this class. Although improved cycling power output10 and pace in a 10-km run11 have been demonstrated in studies of this drug, other trials contradict these findings.59 Pseudoephedrine is a chemical precursor in the illicit manufacture of methamphetamine. Federal policies now restrict sales by limiting purchase quantities to consumers of a minimum age.55 Pseudoephedrine is monitored in competition but is not included on the prohibited list.115
The herbals ephedra and ma huang have similar structural properties and physiologic effects to pseudoephedrine.55 Ephedra and ephedra-containing products were taken off the market in 2004.104 Ephedra is banned by the NCAA, IOC, and major league organizations.104
Caffeine is an adenosine receptor antagonist with stimulant properties.55 Studies have shown that caffeine is ergogenic in most, if not all, exercise situations, with few negative effects during exercise.62 Caffeine acts by binding to adenosine receptors in most tissues, including brain, heart, smooth muscle, fat, and skeletal muscle, with a wide spectrum of interacting responses. Caffeine also stimulates the secretion of epinephrine62 and the central nervous system and enhances peripheral neuromuscular transmission and muscle contractility.55 Side effects of caffeine include nausea, heart palpitations, headache, and muscle tension. Caffeine might also act as a diuretic, although no studies have shown a risk for dehydration in athletes.55 Caffeine is monitored in competition but is not prohibited by WADA.170
Supplements
A drug is any substance that exerts an effect on a body system, but a supplement is defined as a substance taken to augment the diet. Regulatory bodies within the U.S. government handle drugs and supplements differently, and supplement manufacturers are not held to the same standards as drug companies. Supplement manufacturing is overseen by the Food and Drug Administration (FDA) and treated as food as long as no drug claims are made.63
Most experts agree that a well-balanced, isocaloric diet of commonly available food is sufficient to guarantee basic nutritional requirements for most athletes, including macronutrients (carbohydrates, proteins, and lipids) and micronutrients (vitamins, minerals, and trace elements).45 Despite this, many athletes take mega-doses of essential nutrients as dietary supplements (amino acids, vitamins, minerals), at times above tolerable levels.45 The supplement industry was estimated to earn $1.2 to $3 billion per year.94 Studies have found that 25% to 38% of high school athletes surveyed used supplements.63 A 2002 metaanalysis found that of the more than 250 dietary products available, only β-hydroxy-β-methylbutyrate and creatine supplements have sufficient scientific evidence to conclude that they significantly augment lean body mass and strength with resistance training.118
Creatine
According to one source, 90% of weightlifters and bodybuilders in the United States are regular users of creatine (Cr). They tend to be male, have an average age of 32 years, attended college, and have a substantial income.127 In a survey of NFL trainers and team physicians, all teams had players actively taking the supplement, with estimated usage 33% to 90%.152
Cr, a naturally occurring compound made from amino acids glycine, arginine, and methionine, is the most popular nutritional supplement on the market.94,152 It is proposed that Cr phosphate supplementation benefits short-duration, high-intensity, repetitive exercise by enhancing adenosine triphosphate regeneration.94,125
Cr appears to be most effective for activities that involve repeated short bouts of high-intensity physical activity,63,152 such as jumping, sprinting, and cycling.12 When maximal force or strength is the outcome measure, Cr has consistently been found to significantly impact force production regardless of sport, sex, or age.152
Common side effects of Cr include muscle cramping and gastrointestinal distress.127 A number of cases of renal failure have been reversed with withdrawal of Cr supplementation.94,152 It is notable that most studies in the literature were short term with healthy subjects. Also, there are insufficient data on the possible effects of Cr on other Cr-containing tissues, such as the brain, cardiac muscle, or the testes.152
Because it is not classified as a drug, Cr is not under direct regulation by the FDA and is widely available over the counter.152 The NCAA does not allow member teams to provide Cr to its players. According to one survey, 40% of NFL teams provided Cr for their players.152
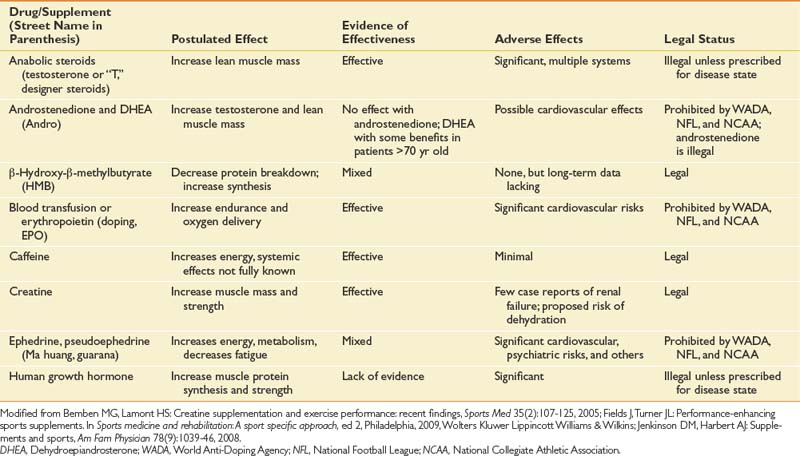
For a summary of drugs and supplements used in sports, see Table 44-5.
Preparticipation Examination
The primary goals of the PPE are to (1) identify life-threatening conditions, (2) identify conditions that can limit competition, (3) identify factors that predispose the athlete to injury, and (4) meet the legal requirements of the institution and state. Added opportunities of the PPE are to discuss preventative health, high-risk behaviors, establish rapport with the athlete, and evaluate the general health of a potentially underserved population. The Preparticipation Physical Evaluation is a consensus publication by the major sports medicine associations and is an excellent guide for the team physician.4 The PPE encourages safe participation and disqualifies fewer than 1% of high school athletes and 0.2% of college athletes. However, 14% of athletes have been found to require further evaluation.61 The PPE should be performed with enough lead time to allow appropriate subsequent investigation before the season begins.
The most common settings for the PPE include individual examinations performed in the physician’s office, station-based examinations, and group locker room examinations. Although individual examinations are the most commonly performed, station-based examinations might lead to more effective identification of musculoskeletal issues.48 Individual examinations have the advantage of added privacy and offer more opportunity to discuss other general health and safety issues.
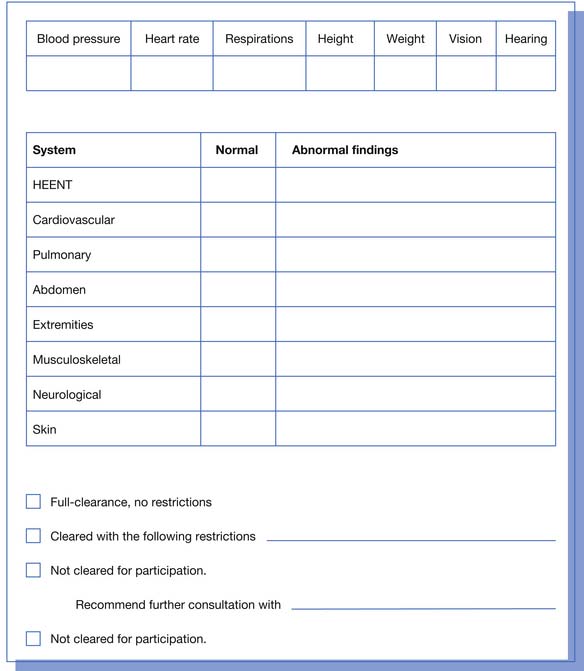
Height, weight, vital signs, hearing examination, and visual examination are performed and recorded. Normal values for blood pressure in the adolescent and pediatric population should be available for the examiner. The physical examination can be performed by different providers but must include the head, ears, eyes, nose, throat, cardiovascular examination, pulmonary auscultation, abdominal examination, genitalia (males only) examination, hernia palpation, skin examination, neurologic examination, and musculoskeletal examination (Figure 44-8). The female genitourinary examination is deferred to the primary care physician.
Many subspecialties might be involved in the PPE. It must be decided who is the lead physician before the decision-making process. It is also vital for the individual providers to be able to identify suspicious findings outside of their own area of specialty and refer to the appropriate specialists when necessary. A provider who is unsure of a finding should always refer to a specialist for further investigation or consultation on final clearance to play, rather than make the decision on his or her own.
Cardiovascular Screening
Sudden cardiac death (SCD) is a rare and devastating condition seen at all levels of sport. The American Heart Association’s (AHA) recommendations for cardiovascular screening include specific questions regarding family history, personal history, and a thorough physical examination (Box 44-5). Controversy exists in sports medicine regarding screening electrocardiograms (ECG) and echocardiography. The AHA currently does not recommend screening 12-lead ECG, echocardiograms, or exercise stress testing for athletes without risk factors.102 The IOC and the European Society of Cardiology recommend screening ECG as part of the PPE, in part because of different etiologies of SCD between the United States and Europe.38 The AHA decision not to recommend this extra screening is based on the low prevalence of SCD, limited resources, impracticality of mass screening, and the lack of a physician base to interpret the ECG, as well as the consequences of false-positive test results.38,102 Hypertrophic cardiomyopathy (HCM) is the most common cause of SCD in American athletes. It is an autosomal dominant disease, and detailed family history might detect premature cardiac death and lead to further diagnostic testing in the athlete. Echocardiography is the most effective way of diagnosing HCM but would require the screening of 200,000 athletes before finding one with the condition.51
Emergency Assessment and Care
The most recent Consensus Conference on CPR (in 2005) made some important changes to CPR guidelines.78 The primary goal of these changes is to maximize blood flow during CPR by provision of effective and uninterrupted chest compressions for longer periods. Recent clinical studies have demonstrated improved survival when a period of 90 to 120 seconds of CPR is used before shock delivery in which the rescuer’s arrival to the patient is greater than 5 mintues.165 Perfusing the myocardium with oxygenated blood before defibrillation improves survival. If the collapse is witnessed (and the victim is down for less than 5 minutes), immediate defibrillation is still indicated. The emphasis of CPR has been taken away from ventilation and placed further on chest compressions as noted by the new compression to ventilation ratio of 30:2 (Table 44-6). In some locations, ventilations have been completed dismissed in favor of solely chest compressions, begging a name change from CPR to CCR (cardiocerebral resuscitation).52
Table 44-6 2005 Consensus Conference on CPR—Important Changes∗
| Measure | 2000 Recommendation | 2005 Recommendation |
|---|---|---|
| Immediate defibrillation for unwitnessed cardiac arrest† | Recommended | Five cycles of CPR before shock is recommended (~2 min) |
| Compression/ventilation ratio | 15:2 | 30:2 |
| Sequence of defibrillation | Three stacked shocks | One shock only followed by immediate CPR |
| Rhythm/pulse check | After each shock | After 5 cycles of CPR following each shock |
CPR, Cardiopulmonary resuscitation.
The goal of the changes is to maximize blood flow during CPR by provision of effective and uninterrupted chest compressions for longer periods.
∗ New 2010 American Heart Association Guidelines are expected to be released in November, 2010.
† Recent clinical studies demonstrate improved survival when a period of 90 to 120 seconds of CPR is used before shock delivery in which the rescuer’s arrival to the patient is more than 5 minutes
Sudden Cardiac Arrest in Athletes
The leading cause of death in young athletes is sudden cardiac arrest (SCA), typically as a result of a structural cardiac abnormality. In a cohort of 387 young athletes, HCM was the most common cause of SCA, accounting for 26% of deaths; commotio cordis was second at 20%; and coronary artery anomalies was the third most common cause at 14%.100 Coronary artery anomalies are the most common cause of SCA in young female athletes. In athletes over the age of 35, coronary artery disease is by far the most common cause of SCA at 75%. Nonstructural causes, such as inherited arrhythmia syndromes and ion channel disorders, are much less common sources of SCA in young athletes, making up only 2% of deaths in that cohort.
Survival after SCA is dismal. In a cohort of 486 young athletes (ages 5 to 22), the survival rate was only 11%.46 Besides the inherently poor prognosis, another factor affecting survival in SCA is delayed early recognition and consequently delayed defibrillation. Three main factors are known to delay treatment in SCA that all medical personnel need to understand. The first is agonal or occasional gasping mistaken for breathing. Second, many lay rescuers falsely identify the presence of a pulse; consequently the updated CPR guidelines eliminate lay rescuer assessment of the pulse and recommend cardiac arrest be assumed if the victim does not demonstrate normal breathing. Finally, the third factor is myoclonic activity falsely identified as seizure and not cardiac arrest. Seizure activity is present in approximately 20% of patients with cardiogenic collapse.47
Cervical Spine
Cervical spinal cord injuries are one of the most catastrophic injuries in all of sports medicine. They have the potential to occur in any sport but are predominantly seen in football, gymnastics, and ice hockey. The data are most robust for high school and collegiate football, and the annual incidence of cervical spinal cord injury is 6:100,000 and 4:100,000, respectively.112 Advances in helmet technology have decreased the incidence of intracranial hemorrhage but paradoxically increased the incidence of cervical spine injury. Rule changes, including the banning of spear-tackling, have been effective at decreasing this rise. The risk of injury can never be fully avoided in contact sports, but the minutes that follow the injury itself are vital to prevent secondary neurologic or cardiopulmonary sequelae.17,153
Removal of the facemask must be done as soon as is prudent to ensure access to the airway, regardless of the patient’s current respiratory status. Current recommendations advise against removal of an athlete’s helmet or shoulder pads in an uncontrolled environment because of the amount of cervical movement that is created.90
Exercise-Associated Collapse in Athletic Endurance Events
Exercise-associated collapse (EAC) in the endurance athlete has a broad differential diagnosis (Box 44-6). Given space constraints, this section focuses on benign EAC, exercise-associated hyponatremia, cardiogenic collapse, and heat-related illnesses. In general, when an athlete collapses before the finish line in a race while still running, the diagnosis is more ominous. The assessment of a collapsed athlete starts with assessing the level of responsiveness and checking airway, breathing, and circulation (ABCs). Depending on the severity of the presentation, specific symptoms, and vital signs, a diagnostic workup can include an assessment of cardiac rhythm, rectal temperature, and blood glucose and sodium levels.
The most common cause of collapse in a marathon runner after crossing the finish line is benign EAC. This is generally considered a form of postural hypotension. While running, the leg muscles act as a venous pump to improve blood flow return to the central circulation. When the athlete stops running, venous pooling of blood in the legs can occur, resulting in a decrease in blood pressure and a subsequent collapse. This is exacerbated in warm environments because blood flow is shunted from the core to the skin to facilitate cooling. It is important to keep the athlete walking after crossing the finish line to keep the muscular venous pump engaged. Treating EAC means treating the primary cause of collapse and providing supportive care. If the underlying cause of the collapse is determined to be benign EAC, oral rehydration and lying the athlete down on a stretcher with legs and pelvis elevated above heart level are typically all that is needed. If the athlete does not improve within 15 to 30 minutes, a search for a more ominous cause of collapse should be undertaken, including orthostatics, if possible, and an electrolyte assessment, followed potentially by administration of intravenous fluids. It is rare to definitively need intravenous fluids after running a marathon.
Exercise-associated hyponatremia (EAH) was first reported in the 1981 Comrades Run (90-km ultramarathon) in South Africa; however, national and international media attention publicized it after the death of a 28-year-old female runner in the 2002 Boston Marathon. It is a hypervolemic hyponatremia causing early symptoms of lightheadedness, a nauseated feeling, later a progressive headache, vomiting, confusion, and finally obtundation, seizures, and death. The pathophysiology is fluid shifts from low osmotic pressure in the blood causing cerebral edema and then neurogenic pulmonary edema. The early symptoms are nonspecific, so medical providers must have an index of suspicion in any marathoner who is not feeling well after the race. Risk factors for EAH include weight gain during the race, marathon race time more than 4 hours, and body mass index extremes.2 Those at risk are runners who ingest too many fluids on the race course and subsequently gain weight. Slower runners have more of an opportunity to drink excessively, and smaller runners generally need less fluid to dilute their serum sodium levels.
Prevention of EAH should be the ultimate goal for any medical director of an endurance athletic event such as a marathon. Prevention starts with educating athletes about the risk of overdrinking on the course and teaching athletes to use their training in the months before the event to determine their individual fluid needs. Emphasizing individual differences and reminding the athletes to replace what they need (sweat losses) and not necessarily more is appropriate (Box 44-7). The mantra “drink when you are thirsty” is generally safe for slower and at-risk runners. Only if the athlete is a very salty sweater or if the competition lasts more than 6 hours should sodium or electrolyte replacement be necessary. Spacing the water/aid stations on the course about every 1.5 miles is another way to limit excessive drinking and potentially prevent EAH.
Given the continued popularity of marathons and other mass participation endurance events, the lay press commonly reports on deaths in these races. With more events and more press coverage, there is an appearance of an increase in marathon-related deaths. The often-quoted risk of SCA in a marathon is 1:50,000. This is an old statistic that came from a study evaluating cardiac-related deaths in the Twin Cities Marathon and Marine Corp Marathon from 1976 to 1994.101 These marathons presented follow-up data from 1995 to 2004, demonstrating a new rate of cardiac-related deaths of 1:220,000.134 The London Marathon also published its rate of 1:80,000 from 1981 to 2006.157 Even though there are more marathons and marathoners, the rate of SCA has improved during the past 10 to 15 years. This could be related to earlier recognition, better preparation, and AED use.
Heat-related illness is another etiology of EAC. Heat exhaustion is the inability to continue to exercise in the heat but is not related to body temperature. It represents the failure of the cardiovascular response to workload, high environmental temperatures, and dehydration. No chronic or harmful effects are known. In contrast, heat stroke is a medical emergency. Heat stroke is defined as multiorgan system failure secondary to hyperthermia. The rectal core temperature in an athlete with heat stroke is generally more than 39°C. Treatment is immediate total body cooling. Disagreement exists on which cooling method is most efficient. Ice bath/cold water immersion, the “taco method” using wet sheets and ice, and finally administering ice to the head, neck, axilla, and groin are all common cooling methods used in the field (at races and in the military). Whatever method is used, it should be simple and safe, provide adequate cooling, and not restrict other forms of treatment, including CPR, defibrillation, and intravenous cannulation.26 The mortality rate and organ damage in athletes with heat stroke are proportional to the length of time between core temperature elevation and the onset of cooling therapy. Finally, it is important to note that heat stroke can occur in cool environments and might be more of a genetic predisposition to excessive endothermy (i.e., endogenous heat production) and not just a factor of extreme environmental conditions.129
Specific Diagnoses in Sports Medicine
Sports Concussion
Concussions are mild traumatic brain injuries sustained as a result of direct blows to the head or forces transmitted through the head and neck. The Centers for Disease Control and Prevention estimates 1.6 to 3.8 million sports- and recreation-related traumatic brain injuries occur each year. They occur in all sports but are most common in contact and collision sports. Concussions can result in symptoms, physical signs, behavioral changes, cognitive impairment, and sleep disturbance (Box 44-8). These symptoms are typically due to a functional disturbance of the brain rather than a true structural injury. It is vital to recognize that concussions rarely result in a loss of consciousness. Concussion symptoms are generally transient, although chronic sequelae might occur.
BOX 44-8 Symptoms Reported After Concussion
Modified from McCrory P, Meeuwisse W, Johnston K, et al: Consensus statement on concussion in sport—The 3rd international conference on concussion in sport held in Zurich, November 2008. PM R 1(5):406-420, 2009.
Pathophysiologically the concussed brain exhibits increased metabolic needs combined with decreased cerebral blood flow.14 This mismatch of “supply and demand” creates a state of tissue vulnerability. Second-impact syndrome is a rare and catastrophic outcome of concussion that might be due to this mismatch. This phenomenon is seen when a youth athlete receives a second impact before the symptoms of the first concussion have subsided. These rare injuries have only been reported in youth athletes and result in severe brain injury and even death from malignant cerebral swelling.
The most vital component of concussion care is the prompt recognition of the injury itself. Athletes, coaches, trainers, and family members should have some degree of education regarding the most common signs and symptoms. An athlete with a suspected concussion should be removed from play immediately and evaluated on the sideline. The Standardized Assessment of Concussion, pocket Sport Concussion Assessment Tool 2, and Maddocks’ questions are commonly used sideline tools. These screening tools do not replace the value of a more thorough assessment that can detect more subtle abnormalities.107 Current consensus recommends that athletes less than 18 years of age should not RTP on the same day as a concussion. In certain professional athletic settings, adult athletes can RTP on the same day.107
Neuropsychologic (NP) testing is a useful component in the management of concussions. Cognitive and somatic symptoms typically improve in parallel but often completely resolve at different times. As such, NP testing can give added information that cannot be gained in a typical clinical setting. Neuropsychologists are best suited to interpret these data, but in situations where no neuropsychologist is available, other medical professionals can be trained to perform the interpretation.49 NP testing is only one component of concussion management, however, and the decision to RTP is ultimately a medical one.
Prognosis after concussion is typically excellent with 80% to 90% of athletes free of symptoms within 7 to 10 days.106 Children and adolescents are a notable exception and might exhibit a longer recovery time.107 RTP decisions can be challenging and must be made by practitioners familiar with concussion management. No athlete should RTP until all the symptoms of the concussion have resolved. Once the athlete is asymptomatic at rest, a stepwise approach RTP protocol must be completed before competition is resumed (Table 44-7). The athlete will be asked to have a 24-hour symptom-free period before advancing to the next step in the protocol. No athlete should be returned to competition while still symptomatic.
| Rehabilitation Stage | Functional Exercise at Each Stage of Rehabilitation | Objective |
|---|---|---|
| 1. No activity | Complete physical and mental rest | Recovery and resolution of symptoms |
| 2. Light aerobic exercise | Walking, swimming, or stationary cycling; low intensity, no resistance training | Increase heart rate |
| 3. Sport-specific exercise | Drills, no head impact activities | Add movement to test coordination and more complex motor skills |
| 4. Noncontact training drills | Progression to more complex training drills, add resistance exercise | Exercise, coordination, and cognitive load |
| 5. Full contact practice | After medical clearance, participate in normal training | Restore confidence and assess functional skills |
| 6. Return to play | Normal game play |
Modified from McCrory P, Meeuwisse W, Johnston K, et al: Consensus statement on concussion in sport—The 3rd international conference on concussion in sport held in Zurich, November 2008. Phys Med Rehabil 1(5):406-420, 2009.
Athletes with repeated concussions must be evaluated on an individual basis. These athletes typically have a prolonged recovery course and might have higher symptom severity.36,37,39,147 An increasing body of literature suggests concussions are an independent risk factor for long-term deficits in executive function and processing speed.37,65 The phenomenon of athletes suffering increasingly severe concussions with decreasing impact severity should raise significant concerns for the physician. These athletes might need to consider giving up contact sports and might not be cleared to participate.23
Stingers
The stinger, or sometimes termed a burner, is probably one of the most common but least understood peripheral nerve injuries that occur in sports. Stingers are nerve injuries that occur within the peripheral neural axis at a specific but variable point from the nerve root to the brachial plexus. The true incidence of stingers is unknown; however, it is estimated that more than 50% of collegiate football players sustain a stinger each year.34
A great deal of controversy exists regarding the pathophysiology of stingers. The symptoms can result from a tensile (stretch) or compressive injury to the cervical nerve root or brachial plexus. Of the two, the cervical nerve root appears to be at greater risk for both tensile and compressive injury than the brachial plexus. However, the literature slightly favors brachial plexus tensile overload∗ over nerve root stretch,32,103,149 nerve root compression,126,138,163 and direct brachial plexus compression44,99 as the primary mechanism of injury in stingers. The pathomechanics of each injury vary by the age and experience of the athlete as well as by specific sport. Tensile injuries typically occur in younger athletes with less experience who have weaker neck and shoulder girdle musculature, leaving them vulnerable to forceful contralateral neck lateral bending with ipsilateral shoulder and arm depression. Cervical root compression is likely to occur in the older, stronger, and more experienced athlete during forceful cervical extension and rotation, narrowing the neuroforamen. This scenario is more commonly seen in professional football defensive backs and offensive lineman. Least commonly, a compressive blow to the brachial plexus can result from equipment issues such as padding.
The patient with a stinger classically presents with sudden onset of a lancinating, burning pain in one upper limb after a traumatic event. The symptoms typically follow a single dermatomal distribution, most commonly in a C5, C6, or C7 pattern. The pain usually lasts seconds to minutes, with the sensory disturbance usually resolving quickly, whereas weakness can be more persistent. Simultaneous, bilateral stingers are very uncommon; therefore any athlete with bilateral upper limb paresthesias or dysesthesias should be evaluated for a spinal cord injury. With the first occurrence of a stinger, symptoms generally resolve quickly and no medical attention is sought. With each subsequent recurrence, more distinct neurologic sequelae might result, including persistent motor weakness. Motor impairment (particularly in the deltoid and biceps, i.e., C5 myotome) is the more common residual neurologic symptom of an athlete with a stinger.68
Although plain radiographs of the cervical spine provide limited information in this setting, they can reveal clues to pathoanatomy contributing to the symptoms. A higher incidence of degenerative changes (creating central canal and neuroforaminal stenosis concomitantly) is noted in more experienced athletes who sustain stingers.95 Levitz et al.95 studied a cohort of football players with chronic stingers and demonstrated that 93% of these players had significant cervical disk disease or neural foraminal narrowing.
Treatment of stingers includes pain control, antiinflammatories, strengthening, and rehabilitation of postural faults and muscle imbalances that might have contributed to the athlete’s risk of sustaining the stinger. Persistent symptoms can be treated with fluoroscopically guided epidural steroid injections, surgical decompression of neuroforaminal stenosis, or even anterior cervical diskectomy and fusion. Equipment modifications should be considered for prevention of recurrent injury, although research is lacking to support their effectiveness.68
Well-established return to competition guidelines do not exist for stingers. The following is an approach based on a combination of clinical experience, neurophysiologic principles, and extrapolation of information from other peripheral nerve injuries. After an initial stinger, if full recovery is demonstrated within 15 minutes, return to same game competition is allowed. If full recovery occurs within 1 week after the initial stinger, then return to competition that next week is allowed. A limited rehabilitation program to address postural dysfunction and relative weaknesses should be prescribed during the in-season strength and conditioning program and definitely in a more comprehensive fashion in the off-season. If the athlete has sustained recurrent stingers, a general rule is to hold the player from competition for the number of weeks that corresponds to the number of stingers sustained in a given season (e.g., 2 weeks for a second stinger). If more than three stingers occur in a season, ending the season should be considered, especially if there is significant weakness, axonopathy on EMG, or focal disk herniation or foraminal stenosis noted on MRI.67
Exercise-Induced Bronchospasm
EIB describes airway narrowing that occurs in association with exercise. EIB can be present with chronic asthma but generally is a separate entity. The prevalence of EIB is significantly higher in athletes than the general population. A diagnosis based solely on clinical symptoms is often inaccurate, and pulmonary function testing and consultation with an asthma specialist is important to definitively diagnose and optimally treat EIB.139
The mechanism of EIB is not fully understood but is certainly different than that of chronic asthma, which is caused by inflammation resulting from overly reactive airways to inhaled stimulants. EIB is thought to result from water loss and cooling in the airway that occurs with hyperventilation, which subsequently triggers bronchoconstriction.24
A variety of diagnostic testing options are available for those in whom EIB is suspected by history but the physical examination is normal (Box 44-9).24 Once a diagnosis of EIB is made, treatment is multifaceted. An adequate warm-up has been demonstrated to reduce the severity of EIB. Some recommend inducing a refractory period by briefly precipitating symptoms with short, vigorous bursts of exercise lasting 15 to 20 minutes before the competitive endurance event. The mainstay of pharmacologic therapy is a short-acting β-agonist 15 minutes before exercise. If this is ineffective, adding cromolyn, a mast cell stabilizer, is indicated first, then inhaled corticosteroids for the final addition to preexercise therapy. If EIB is refractory to the aforementioned treatment, daily chronic asthma therapy should be added to the regimen. This includes inhaled corticosteroids first, and then long-acting β-agonists and leukotriene receptor antagonists can be added.25
Anemia
IDA is most common in menstruating women, and female athletes can be more prone to developing it. The etiology is either blood loss or poor iron intake. Many athletes consume restrictive diets that can have too little iron to meet daily needs. A complete history and physical examination are still important, however, to evaluate for “nonathletic” causes such as significant gastrointestinal or genitourinary blood losses. Usually a serologic workup is diagnostic and includes complete blood count (CBC), serum ferritin, and total iron binding capacity (TIBC). The CBC will show a microcytic anemia.89 Serum ferritin levels that are less than 30 ng/mL in athletes are considered suggestive of IDA.53 The TIBC will be elevated. If IDA is diagnosed, a trial of oral iron supplementation (typically ferrous sulfate or ferrous gluconate, 325 mg three times daily) is undertaken.35 Iron is best absorbed in an acidic environment, so it is concomitantly administered with vitamin C, usually for a 2- to 3-month course.
Foot-strike hemolysis refers to red blood cell destruction in the feet from running impact. However, intravascular hemolysis is seen in swimmers, cyclists, and runners, and whether actual mechanical red blood cell trauma is the source is questionable. Possible reasons are intramuscular destruction, osmotic stress, and membrane lipid peroxidation caused by free radicals released by activated leukocytes. Intravascular hemolysis can even be regarded as a physiologic means to provide heme and proteins for muscle growth in athletes.137 Generally the hemolysis is mild, and treatment is rarely required.
Specific Populations
Women in Sports
Until puberty, boys and girls are similar in body size, shape, and physiology.18 On average, women have a larger surface area-to-mass ratio, lower bone mass, and a wider, shallower pelvis than men. Because of these differences, women might tolerate heat better than men, might be more prone to osteoporosis, and might develop knee problems more frequently.18 The following discussion highlights one issue unique to the female athlete: the female athlete triad.
The Female Athlete Triad
The female athlete triad is a constellation of interrelated findings of inadequate eating, menstrual abnormalities, and skeletal demineralization.119 The definition of the triad does not require the simultaneous occurrence of all three components. Each facet, in isolation or combination, needs to be considered and treated.117
Inadequate eating describes women who eat insufficient calories for the amount of calories burned, as well as women with frank anorexia or bulimia (Box 44-10). Diminished athletic performance often results, and a vicious cycle develops in which psychologic distress and depression can develop.116 A higher incidence of eating disorders has been reported in female athletes compared with the general population.18,64 Female athletes are also more likely to have disordered eating than their male counterparts.64 A higher rate of eating disorders has been reported among athletes in sports emphasizing leanness, such as gymnastics, figure skating, dancers, distance runners, divers, and swimmers.9,18,116,117
BOX 44-10 Diagnostic and Statistical Manual of Mental Disorders–IV Definitions of Anorexia, Bulimia Nervosa, and Eating Disorders Not Otherwise Specified6
Eating disorders not otherwise specified
Athletic women are 3 times as likely to develop primary or secondary amenorrhea as their nonathletic counterparts.128 Menstrual irregularities range from reduction in luteal phase length and suppression of luteal function to amenorrhea.18 Women with athletic amenorrhea present with a reduction in luteinizing hormone pulse frequencies, which depend on energy availability.116 Dietary supplementation (i.e., providing adequate energy sources) can prevent or eliminate menstrual disturbances.18,64
Skeletal demineralization is the third component of the triad and can lead to premature osteoporosis. The prevalence of osteopenia in female athletes has been reported to be 22% to 50%, whereas the prevalence of osteoporosis remains relatively low.84 Bone undergoes continual resorption and formation, a process called remodeling. Hormonal status, weight-bearing physical activity, and dietary intake (particularly calcium) influence remodeling. Stress fractures pose a health risk to amenorrheic athletes as a result of reduced bone mass. The relative risk for stress fractures is 2 to 4 times greater for amenorrheic athletes compared with those with regular menses.117
Peak bone mass is achieved during the first 3 decades of life, with Tanner stages II and III (early to mid puberty) as the maturational stage in which physical activity has the strongest impact on bone.117 Young athletes with primary amenorrhea might be unable to build bone during formative years, with the end result being that peak bone mass might never be achieved.18
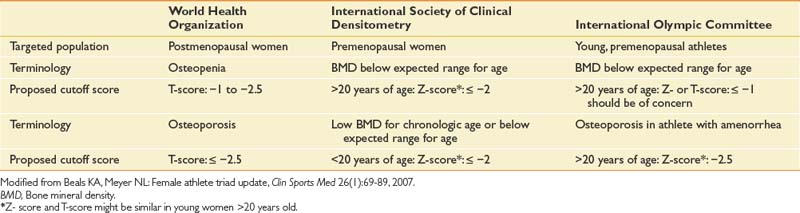
Dual-energy x-ray absorptiometry is the most widely used and validated technique to assess bone mineral density (BMD).82,117 The World Health Organization defines osteoporosis as a BMD greater than 2.5 standard deviations (SD) below the youthful mean (T-score less than or equal to −2.5 SD).82 Osteopenia is defined as a BMD between 1.0 and 2.5 SDs below the youthful mean (T-score 1.0 to 2.5) (Table 44-8).82 Notably, normative data for adolescent girls are not available to definitively define pathologic bone loss in this group; hence Z-scores are best used in the skeletally immature athlete because the Z-score is age matched and the T-score is compared with a 35 year old.9,18
Table 44-8 Current Recommendations for the Diagnosis of Low Bone Mineral Density and Osteoporosis in Premenopausal and Postmenopausal Women and Young Athletes
Randomized controlled trials have shown that oral contraceptive pills might increase lumbar and total body BMD in patients with hypothalamic amenorrhea.71,161 In addition, 1200 to 1500 mg of calcium and 400 to 800 international units of vitamin D supplementation per day are recommended (see Chapter 41).64More recently, attention has turned to the cardiovascular risks associated with the hypoestrogenic state in women with hypothalamic amenorrhea. Estrogen has cardioprotective effects, such as increasing high-density lipoprotein and decreasing low-density lipoprotein cholesterol.159 Estrogens also stimulate the production of nitric oxide (NO), a potent vasodilator and inhibitor of platelet aggregation, leukocyte adhesion, and vascular smooth muscle proliferation and migration, all of which are part of the atherosclerotic process. Flow-mediated vasodilatation (FMD) results from NO release in response to shear stress and increased blood flow.76 Athletic amenorrhea is associated with decreased endothelium-dependent dilation of the brachial artery (a measure of FMD)131,173 and unfavorable lipid profile.131 Treatment with oral contraceptive pills has been associated with improved FMD.132
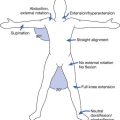
Female athletes presenting with features of the female athlete triad require appropriate workup and referrals (Figure 44-9). Although referrals to a nutritionist, psychologist/therapist, and other medical specialists, such as endocrinologists, cardiologists, and gastroenterologists, might be necessary, a primary treatment focus should be to restore energy balance by improving caloric intake and potentially decreasing energy expenditure (limiting aerobic exercise). Finally, pregnancy should be excluded in any amenorrheic female of childbearing age.
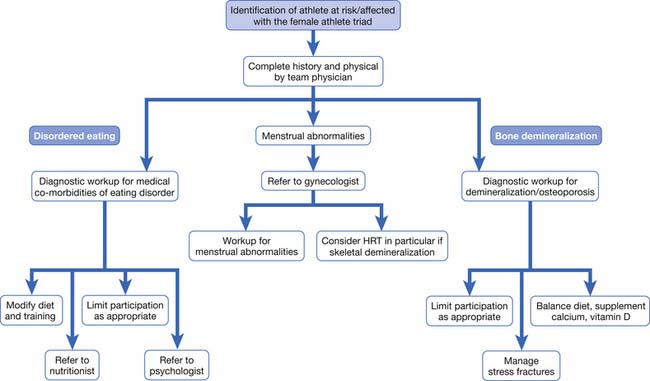
FIGURE 44-9 Overview of the management of the female athlete triad. HRT, Hormone-replacement therapy.
(Modified from Borg-Stein J, Dugan SA, Solomon JL: Special considerations in the female athlete. In Frontera WR, Micheli LJ, Herring SA, et al, editors: Clinical sports medicine: medical management and rehabilitation, Philadelphia, 2007, Elsevier.)
The Pediatric and Adolescent Athlete
According to one estimate, 30 million children and adolescents participate in organized sports activities in the United States.1,70 As athletic involvement increases, the incidence of sports-related injuries also increases.91,148
Physically active adults had better standardized fitness scores as children,42 suggesting that an active lifestyle as a child promotes continued lifelong physical fitness. Physical activity in children and adolescents prevents osteoporosis, improves self-esteem, and reduces anxiety and depression.15,28 Furthermore, there is a positive relationship between physical fitness and academic achievement in English and mathematics.31 High school athletes are more likely to engage in positive health behaviors and less likely to engage in negative health behaviors compared with nonathletes.123
When advising pediatric athletes in their training program, the physiologic and anatomic differences between children and adults must be considered. Strength training has been historically discouraged in the pediatric population because of concerns of growth disturbance and potential for other injuries.91 According to a position statement by the American Academy of Pediatrics Committee on Sports Medicine, “short-term programs in which prepubescent athletes are trained and supervised by knowledgeable adults can increase strength without significant injury risk.”5 Although growth plate disturbances have been reported, these have been attributed to poor lifting techniques, lifting maximal weights, and lack of adult supervision.66
Training programs for pediatric athletes should include stretching routines. Children tend to lose flexibility with age, with the greatest losses occurring at puberty because of muscle–tendon imbalances that occur with rapid growth.92 Loss of flexibility can make the athlete vulnerable to acute and overuse injuries involving the low back, pelvis, and knee.111 Excessive flexibility, however, is associated with increased risk of sports-related injury, especially in sports that necessitate rapid change of direction or acceleration.111
The pediatric skeletal system is distinguished from that of adults by the presence of the active growth plate, or physis. The physis is situated between the epiphysis and metaphysis. Two types of epiphyses exist: traction and pressure.21 The traction epiphysis, also known as the apophysis, is the point of attachment of a tendon to bone.1 Because apophyses contribute to bone shape, but not bone length, acute or chronic injuries involving apophyses are not usually associated with growth disturbance. Osgood-Schlatter disease, Sever’s disease, and medial epicondylopathy are examples.1,21
Pressure epiphyses are found at the end of long bones such as the distal femur and proximal tibia (Figure 44-10). In contrast to apophyseal injuries, injuries to active pressure epiphysis can result in limb-length discrepancies or angular deformities.21,146 The most common cause of injury to the pressure epiphysis acutely is a fall. Acute physeal injuries are widely described using the system described by Salter and Harris (Figure 44-11). Chronic injuries result from ongoing stress to the physis, as is seen in distal radial injuries in young gymnasts (Figure 44-12).21
FIGURE 44-10 Physes of the distal femur and proximal tibia.
(Modified from Caine D, DiFiori J, Maffulli N: Physeal injuries in children’s and youth sports: reasons for concern? Br J Sports Med 40[9]:749-760, 2006.)
Older Athlete
According to the Healthy People 2000 report, 60% of adults 65 and older engage in leisure-time physical activity.75 Popular activities among seniors include gardening, dancing, golf, hunting, fishing, woodworking, tennis, bowling, biking, and swimming. Masters athletes have achieved impressive improvements in peak exercise performance. In fact, athletes more than 70 years of age have surpassed the winning time at the first modern Olympic Games held in Athens in running events from 100 meters to the marathon.151
Benefits of Exercise in the Elderly
The benefits of exercise in elderly persons are many, although not entirely understood. Higher levels of physical activity are associated with lower rates of many afflictions associated with aging, including coronary heart disease and incidence of cancer, although the underlying mechanisms for these findings are largely unknown.98 One theory is that exercise might reduce inflammation and oxidative stress, both of which are known to increase with aging.33 Even short bouts of exercise might boost immune function, decreasing the likelihood of acute infection.141 Weight-bearing exercise increases BMD.117 A 2009 Cochrane Review found exercise programs focusing on strength, balance, flexibility, and endurance were effective in reducing falls in elderly individuals living in the community.58
Together, these benefits confer to the elderly individual a lower risk of mortality. A 2006 study of healthy older adults living in the community found that for every 287 kcal/day in activity energy expenditure, there is approximately a 30% lower risk of mortality.98 Exercise also provides a sense of psychologic well-being and enhanced quality of life. In one survey, 68% of athletic seniors regarded their quality of life as much better than that of their sedentary friends.144
The observed benefits are not limited to the very healthy in this age-group. A 2002 systematic review of randomized controlled trials of exercise training in elderly, institutionalized adults found strong evidence to support that exercise increases strength and mobility in this population.140 Moderate evidence was found for an effect on ROM, and contradictory evidence was seen regarding gait, activities of daily living, balance, and endurance. The authors concluded that more research is necessary to know the extent of possible advantages of exercise in this population. Notably, there were no ill effects reported as a result of exercise.140
Athletes With Disabilities
Physiatrists are uniquely equipped to treat athletes with disabilities, given their training in disabling diseases and musculoskeletal problems. Organized athletic competition among disabled persons dates back to 1944, when Sir Ludwig Guttmann formally introduced sporting activities as part of a rehabilitation program at Stoke Mandeville Hospital in England. He believed that “by restoring activity of mind and body—by instilling self respect, self discipline, a competitive spirit and comradeship—sport develops mental attitudes that are essential for social reintegration.”164
The Paralympic Games provide opportunities for thousands of elite athletes with various disabilities to compete. The first games took place in Rome in 1960.40 Currently people with spinal cord injuries, amputations, cerebral palsy, and visual impairments compete in the Paralympic Games. People with other disabilities participate as well, in a group collectively known as Les Autres (“the others”). This group includes those athletes with multiple sclerosis, muscular dystrophy, Friedreich’s ataxia, arthrogryposis, osteogenesis imperfecta, and Ehlers-Danlos syndrome.40
Organized competitions use a classification system to ensure that training and skill provide the advantage, not level of disability. Various classification schemes are based on medical, disability-specific, combined, or functional/sport-specific models. Table 44-9 is one example of such a system.
Table 44-9 Classification System for Training the Disabled Athlete
| Cycling | |
|---|---|
CP, Cerebral palsy.
From Crandell DM: Special considerations in the disabled athlete. In Frontera WR, et al, editors: Clinical sports medicine: medical management and rehabilitation, Philadelphia 2007, Saunders.
Several studies have shown that disabled persons can participate in competitive sports with low rates of injury. A study of disabled high school students reported a rate of 2.0 injuries per 1000 athlete exposures.130 A survey of athletes who competed in the Junior National Wheelchair Games reported injuries ranging from minor (bruises, blisters, and abrasions) to more serious problems (bladder infections, hyperthermia, and soft tissue injuries).169 To date, no catastrophic injuries have been reported.40 A review of injury data from the Paralympic Games dating back to 1976 found that the number and type of injuries and illnesses were not significantly different from elite athletes without disabilities.54
These athletes require disability-specific evaluation and counseling before participation, often as part of the preparticipation physical. For example, 15% of people with Down syndrome have atlantoaxial instability caused by abnormal collagen resulting in ligamentous laxity and hypotonia. As a result, the Special Olympics require that all athletes with Down syndrome be screened with lateral neck radiographs before participating in sports programs.122
1. Adirim T.A., Cheng T.L. Overview of injuries in the young athlete. Sports Med. 2003;33(1):75-81.
2. Almond C.S., Shin A.Y., Fortescue E.B., et al. Hyponatremia among runners in the Boston Marathon. N Engl J Med. 2005;352(15):1550-1556.
3. American Academy of Family Physicians, American Academy of Orthopaedic Surgeons, American Academy of College Sports Medicine, et al. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-442.
4. American Academy of Family Physicians. Preparation Physical Evaluation Task Force: Preparticipation physical evaluation. Minneapolis, MN: Physician and Sports Medicine; 1996.
5. American Academy of Pediatrics Committee on Sports Medicine. Strength training, weight and power lifting, and body building by children and adolescents. Pediatrics. 1990;86(5):801-803.
6. American Psychiatric Association. Diagnostic and statistical manual of medical disorders, IV. Arlington, VA: American Psychiatric Association; 1994.
7. Archambault J.L. Brachial plexus stretch injury. J Am Coll Health. 1983;31(6):256-260.
8. Banister E.W., Carter J.B., Zarkadas P.C. Training theory and taper: validation in triathlon athletes. Eur J Appl Physiol Occup Physiol. 1999;79(2):182-191.
9. Beals K.A., Meyer N.L. Female athlete triad update. Clin Sports Med. 2007;26(1):69-89.
10. Bell D.G., Jacobs I., Ellerington K. Effect of caffeine and ephedrine ingestion on anaerobic exercise performance. Med Sci Sports Exerc. 2001;33(8):1399-1403.
11. Bell D.G., McLellan T.M., Sabiston C.M. Effect of ingesting caffeine and ephedrine on 10-km run performance. Med Sci Sports Exerc. 2002;34(2):344-349.
12. Bemben M.G., Lamont H.S. Creatine supplementation and exercise performance: recent findings. Sports Med. 2005;35(2):107-125.
13. Berg K. The Athlete with type I diabetes. In Primary care sports medicine. Philadelphia: Lippincott Williams & Wilkins; 2001.
14. Bergsneider M., Hovda D.A., Shalmon E., et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86(2):241-251.
15. Bernhardt D.T. The young athlete. Philadelphia: Lippincott Williams & Wilkins; 2001.
16. Birkeland K.I., Stray-Gundersen J., Hemmersbach P., et al. Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc. 2000;32(7):1238-1243.
17. Boden B.P., Tacchetti R.L., Cantu R.C., et al. Catastrophic cervical spine injuries in high school and college football players. Am J Sports Med. 2006;34(8):1223-1232.
18. Borg-Stein J., Dugan S.A., Solomon J.L. Special considerations in the female athlete. In: Frontera W.R., Micheli L.J., Herring S.A., et al, editors. Clinical sports medicine: medical management and rehabilitation. Philadelphia: Elsevier, 2007.
19. Bosquet L., Montpetit J., Arvisais D., et al. Effects of tapering on performance: a meta-analysis. Med Sci Sports Exerc. 2007;39(8):1358-1365.
20. Burkhart S.S., Morgan C.D., Kibler W.B. The disabled throwing shoulder: spectrum of pathology. Part I. Pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
21. Caine D., DiFiori J., Maffulli N. Physeal injuries in children’s and youth sports: reasons for concern? Br J Sports Med. 2006;40(9):749-760.
22. Calfee R., Fadale P. Popular ergogenic drugs and supplements in young athletes. Pediatrics. 2006;117(3):e577-e589.
23. Cantu R.C. Recurrent athletic head injury: risks and when to retire. Clin Sports Med. 2003;22(3):593-603.
24. Carlsen K.H., Anderson S.D., Bjermer L., et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy. 2008;63(4):387-403.
25. Carlsen K.H., Anderson S.D., Bjermer L., et al. Treatment of exercise-induced asthma, respiratory and allergic disorders in sports and the relationship to doping: part II of the report from the Joint Task Force of European Respiratory Society (ERS) and European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA(2)LEN. Allergy. 2008;63(5):492-505.
26. Casa D.J., Armstrong L.E., Ganio M.S., et al. Exertional heat stroke in competitive athletes. Curr Sports Med Rep. 2005;4(6):309-317.
27. Catlin D.H., Fitch K.D., Ljungqvist A. Medicine and science in the fight against doping in sport. J Intern Med. 2008;264(2):99-114.
28. Chang D.S., Mendelbaum B.R., Weiss J.M., editors. Special considerations in the pediatric and adolescent athlete. St Louis: Elsevier, 2007.
29. Chappell J.D., Creighton R.A., Giuliani C., et al. Kinematics and electromyography of landing preparation in vertical stop-jump: risks for noncontact anterior cruciate ligament injury. Am J Sports Med. 2007;35(2):235-241.
30. Chiampas G., Jaworski C.A. Preparing for the surge: perspectives on marathon medical preparedness. Curr Sports Med Rep. 2009;8(3):131-135.
31. Chomitz V.R., Slining M.M., McGowan R.J., et al. Is there a relationship between physical fitness and academic achievement? Positive results from public school children in the northeastern United States. J Sch Health. 2009;79(1):30-37.
32. Chrisman O.D., Snook G.A., Stanitis J.M., et al. Lateral-flexion neck injuries in athletic competition. JAMA. 1965;192(7):613-615.
33. Chung H.Y., Kim H.J., Kim J.W., et al. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001;928:327-335.
34. Clancy W.G.Jr., Brand R.L., Bergfield J.A. Upper trunk brachial plexus injuries in contact sports. Am J Sports Med. 1977;5(5):209-216.
35. Clark S.F. Iron deficiency anemia: diagnosis and management. Curr Opin Gastroenterol. 2009;25(2):122-128.
36. Collie A., McCrory P., Makdissi M. Does history of concussion affect current cognitive status? Br J Sports Med. 2006;40(6):550-551.
37. Collins M.W., Grindel S.H., Lovell M.R., et al. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282(10):964-970.
38. Corrado D., Pelliccia A., Bjornstad H.H., et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516-524.
39. Covassin T., Stearne D., Elbin R. Concussion history and postconcussion neurocognitive performance and symptoms in collegiate athletes. J Athl Train. 2008;43(2):119-124.
40. Crandell DM: Special considerations in the disabled athlete. In Clinical sports medicine, Philadelphia, 2006, Saunders.
41. Delee J., Drez D., et al. DeLee and Drez’s orthopaedic sports medicine: principles and practice. Philadelphia: Saunders; 2003.
42. Dennison B.A., Straus J.H., Mellits E.D., et al. Childhood physical fitness tests: predictor of adult physical activity levels? Pediatrics. 1988;82(3):324-330.
43. DeRose E.H. Doping and sports. In: In Clinical sports medicine. Philadelphia: Saunders; 2007.
44. Di Benedetto M., Markey K. Electrodiagnostic localization of traumatic upper trunk brachial plexopathy. Arch Phys Med Rehabil. 1984;65(1):15-17.
45. Di Luigi L. Supplements and the endocrine system in athletes. Clin Sports Med. 2008;27(1):131-151.
46. Drezner J.A., Chun J.S., Harmon K.G., et al. Survival trends in the United States following exercise-related sudden cardiac arrest in the youth: 2000-2006. Heart Rhythm. 2008;5(6):794-799.
47. Drezner J.A., Courson R.W., Roberts W.O., et al. Inter-association Task Force recommendations on emergency preparedness and management of sudden cardiac arrest in high school and college athletic programs: a consensus statement. J Athl Train. 2007;42(1):143-158.
48. DuRant R.H., Seymore C., Linder C.W., et al. The preparticipation examination of athletes. Comparison of single and multiple examiners. Am J Dis Child. 1985;139(7):657-661.
49. Echemendia R.J., Herring S., Bailes J. Who should conduct and interpret the neuropsychological assessment in sports-related concussion? Br J Sports Med. 43(suppl 1), i32-35, 2009.
50. Elliott B., Fleisig G., Nicholls R., et al. Technique effects on upper limb loading in the tennis serve. Sports Med. 2003;6(1):76-87.
51. Epstein S.E., Maron B.J. Sudden death and the competitive athlete: perspectives on preparticipation screening studies. J Am Coll Cardiol. 1986;7(1):220-230.
52. Ewy G.A. Cardiocerebral resuscitation: a better approach to cardiac arrest. Curr Opin Cardiol. 2008;23(6):579-584.
53. Fallon K.E. Utility of hematological and iron-related screening in elite athletes. Clin J Sport Med. 2004;14(3):145-152.
54. Ferrara M.S., Peterson C.L. Injuries to athletes with disabilities: identifying injury patterns. Sports Med. 2000;30(2):137-143.
55. Fields J., Turner J.L. Performance-enhancing sports supplements. In Sports Medicine and rehabilitation: a sport specific approach, ed 2. Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins, 2009.
56. Frontera W.R., Micheli L.J., Herring S.A., et al. Clinical sports medicine: medical management and rehabilitation. Philadelphia: Saunders/Elsevier; 2007.
57. Funk F.J.Jr., Wells R.E. Injuries of the cervical spine in football. Clin Orthopaed and Relat Res. 1975;109:50-58.
58. Gillespie L.D., Robertson M.C., Gillespie W.J., et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
59. Gillies H., Derman W.E., Noakes T.D., et al. Pseudoephedrine is without ergogenic effects during prolonged exercise. J Appl Physiol. 1996;81(6):2611-2617.
60. Gore C.J., Clark S.A., Saunders P.U. Nonhematological mechanisms of improved sea-level performance after hypoxic exposure. Med Sci Sports Exerc. 2007;39(9):1600-1609.
61. Grafe M.W., Paul G.R., Foster T.E. The preparticipation sports examination for high school and college athletes. Clin Sports Med. 1997;16(4):569-591.
62. Graham T.E. Caffeine and exercise: metabolism, endurance and performance. Sports Med. 2001;31(11):785-807.
63. Gregory A.J., Fitch R.W. Sports medicine: performance-enhancing drugs. Pediatr Clin North Am. 2007;54(4):797-806. xii
64. Griffin L.Y., Kercher J., Reifsteck F. The female athlete. In: In Clinical sports medicine. Philadelphia: Mosby; 2006.
65. Guskiewicz K.M., McCrea M., Marshall S.W., et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
66. Guy J.A., Micheli L.J. Strength training for children and adolescents. J Am Acad Orthop Surg. 2001;9(1):29-36.
67. Harrast M.A. Epidural steroid injections for lumbar spinal stenosis. Curr Rev Musculoskelet Med. 2008;1(1):32-38.
68. Harrast M.A., Weinstein S. The Cervical Spine. In: Kibler B., editor. OKU orthopaedic knowledge update: sports medicine 4. Rosemont, IL: American Orthopaedic Society for Sports Medicine, 2009.
69. Hawkins S., Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 2003;33(12):877-888.
70. Hergenroeder A.C. Prevention of sports injuries. Pediatrics. 1998;101(6):1057-1063.
71. Hergenroeder A.C., Smith E.O., Shypailo R., et al. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months. Am J Obstet Gynecol. 1997;176(5):1017-1025.
72. Herring S.A., Bergfeld J., Boyd J., et al. Sideline Preparedness for the Team Physician: A Consensus Statement. Med Sci Sports Exer. 2001;33(5):846-849.
73. Hewett T.E., Ford K.R., Myer G.D. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490-498.
74. Hewett T.E., Myer G.D., Ford K.R. Anterior cruciate ligament injuries in female athletes. Part 1. Mechanisms and risk factors. Am J Sports Med. 2006;34(2):299-311.
75. Hill C. Caring for the aging athlete. Geriatr Nurs. 2001;22(1):43-45.
76. Hoch A.Z., Lal S., Jurva J.W., et al. The female athlete triad and cardiovascular dysfunction. Phys Med Rehabil Clin N Am. 2007;18(3):385-400. vii-viii
77. Houmard J.A., Scott B.K., Justice C.L., et al. The effects of taper on performance in distance runners. Med Sci Sports Exerc. 1994;26(5):624-631.
78. International Liaison Committee on Resuscitation. European Resuscitation Council Guidelines for Resuscitation 2005 Section 1. Introduction. Resuscitation. 2005;67:181-186.
79. Jackson D.W., Lohr F.T. Cervical spine injuries. Clin Sports Med. 1986;5(2):373-386.
80. Jenkinson D.M., Harbert A.J. Supplements and sports. Am Fam Physician. 2008;78(9):1039-1046.
81. Jimenez C.C., Corcoran M.H., Crawley J.T., et al. National athletic trainers’ association position statement: management of the athlete with type 1 diabetes mellitus. J Athl Train. 2007;42(4):536-545.
82. Kanis J.A., McCloskey E.V., Johansson H., et al. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467-475.
83. Kerut E.K., Kerut D.G., Fleisig G.S., et al. Prevention of arm injury in youth baseball pitchers. J La State Med Soc. 2008;160(2):95-98.
84. Khan K.M., Liu-Ambrose T., Sran M.M., et al. New criteria for female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36(1):10-13.
85. Kibler W.B. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
86. Kibler W.B., Herring S.A., Press J.M., et al. Functional rehabilitation of sports and musculoskeletal injuries. Aspen: Pro-Ed; 2005.
87. Kibler W.B., McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
88. Kibler W.B., Press J., Sciascia A. The role of core stability in athletic function. Sports Med. 2006;36(3):189-198.
89. Killip S., Bennett J.M., Chambers M.D. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671-678.
90. Kleiner D.M. Prehospital care of the spine-injured athlete: monograph summary. Clin J Sport Med. 2003;13(1):59-61.
91. Kocher M., Tucker R., Siparsky P. The Pediatric athlete. In: Johnson D.L., Mair S.D., editors. Clinical sports medicine. Philadelphia: Mosby, 2006.
92. Kulling F.A. Special considerations in the pediatric and adolescent athlete. In DeLee J.C., Drez D., editors: DeLee and Drez’s orthopaedic sports medicine, ed 2, Philadelphia: Saunders, 2003.
93. Landry G.L. Exercise-induced anaphylaxis and urticaria. In: In principles and practice of primary care sports medicine. Philadelphia: Lippincott Williams & Wilkins; 2001.
94. Laos C., Metzl J.D. Performance-enhancing drug use in young athletes. Adolesc Med Clin. 2006;17(3):719-731. abstract xii
95. Levitz C.L., Reilly P.J., Torg J.S. The pathomechanics of chronic, recurrent cervical nerve root neurapraxia: the chronic burner syndrome. Am J Sports Med. 1997;25:73-76.
96. Manaster B.J., May D.A., Disler D.G. Musculoskeletal Imaging: the requisites, ed 3. Philadelphia: Mosby; 2007.
97. Mandelbaum B.R., Silvers H.J., Watanabe D.S., et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
98. Manini T.M., Everhart J.E., Patel K.V., et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296(2):171-179.
99. Markey K.L., Di Benedetto M., Curl W.W. Upper trunk brachial plexopathy. The stinger syndrome. Am J Sports Med. 1993;21(5):650-655.
100. Maron B.J. Sudden death in young athletes. N Engl J Med. 2003;349(11):1064-1075.
101. Maron B.J., Poliac L.C., Roberts W.O. Risk for sudden cardiac death associated with marathon running. J Am Coll Cardiol. 1996;28(2):428-431.
102. Maron B.J., Thompson P.D., Ackerman M.J., et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 115(12), 1643-1455, 2007.
103. Marshall T.M. Nerve pinch injuries in football. J Ky Med Assoc. 1970;68(10):648-649.
104. Matich A.J. Performance-enhancing drugs and supplements in women and girls. Curr Sports Med Rep. 2007;6(6):387-391.
105. McConnell J. The physical therapist’s approach to patellofemoral disorders. Clin Sports Med. 2002;21(3):363-387.
106. McCrea M., Guskiewicz K., Randolph C., et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65(5):876-882. discussion 82–3
107. McCrory P., Meeuwisse W., Johnston K., et al. Consensus statement on concussion in sport: the 3rd international conference on concussion in sport held in Zurich, November 2008. Phys Med Rehabil. 2009;1(5):406-420.
108. McMullen J., Uhl T.L. A Kinetic chain approach for shoulder rehabilitation. J Athl Train. 2000;35(3):329-337.
109. Meeusen R., Nederhof E., Buyse L., et al. Diagnosing overtraining in athletes using the two bout exercise protocol. Br J Sports Med. 2008.
110. Meister K. Injuries to the shoulder in the throwing athlete. Part one: Biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
111. Micheli L.J., Greene H.S., Cassella M., et al. Assessment of flexibility in young female skaters with the modified Marshall Test. J Pediatr Orthop. 1999;19(5):665-668.
112. Mueller F.O., Cantu R. Twenty-fifth annual report. Chapel Hill, NC: University of North Carolina; 2007.
113. Mujika I., Padilla S. Scientific bases for precompetition tapering strategies. Med Sci Sports Exerc. 2003;35(7):1182-1187.
114. National Collegiate Athletic Association Drug Testing Program. NCAA 2009-10 Banned Drug List. [Retrieved: April 19, 2009]. Available from http://www.ncaa.org/wps/ncaa?key=/ncaa/ncaa/legislation+and+governance/eligibility+and+recruiting/drug+testing/drug_testing.html. Accessed April 19, 2009.
115. National Football League. NFL Players Association player policies: list of prohibited substances. Available from www.nfllayers.com. Accessed April 19, 2009.
116. Nattiv A., Arendt E.A., Hecht S.S. The female athlete. In: William E., Garrett J., Kirkendall D.T., Squire D.L., editors. Principles & practice: primary care sports Medicine. Philadelphia: Lippincott Williams & Wilkins; 2001:93-113.
117. Nichols D.L., Sanborn C.F., Essery E.V. Bone density and young athletic women: an update. Sports Med. 2007;37(11):1001-1014.
118. Nissen S.L., Sharp R.L. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol. 2003;94(2):651-659.
119. Otis C.L., Drinkwater B., Johnson M., et al. American College of Sports Medicine position stand: the Female Athlete Triad. Med Sci Sports Exerc. 1997;(29(5)):i-ix.
120. Ounpuu S. The biomechanics of walking and running. Clin Sports Med. 1994;13(4):843-863.
121. Park S.S., Loebenberg M.L., Rokito A.S., et al. The shoulder in baseball pitching: biomechanics and related injuries. Part 1. Bull Hosp Jt Dis. 2002;61(1-2):68-79.
122. Patel D.R., Greydanus D.E. The pediatric athlete with disabilities. Pediatr Clin North Am. 2002;49(4):803-827.
123. Patel R.R., Trost S.G., Levin S., et al. Sports participation and health-related behaviors among US youth. Arch Pediatr Adolesc Med. 2000;154(9):904-911.
124. Pearsall A.W.t., Kovaleski J.E., Madanagopal S.G. Medicolegal issues affecting sports medicine practitioners. Clin Orthop Relat Res. 2005;(433):50-57.
125. Phillips G.C. Medications, supplements, and ergogenic drugs. In: In Clinical sports medicine. Philadelphia: Mosby; 2006.
126. Poindexter D.P., Johnson E.W. Football shoulder and neck injury: a study of the “stinger,”. Arch Phys Med Rehabil. 1984;65(10):601-602.
127. Poortmans J.R., Francaux M. Adverse effects of creatine supplementation: fact or fiction? Sports Med. 2000;30(3):155-170.
128. Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2008;90(suppl 5):S219-S225.
129. Rae D.E., Knobel G.J., Mann T., et al. Heatstroke during endurance exercise: is there evidence for excessive endothermy? Med Sci Sports Exerc. 2008;40(7):1193-1204.
130. Ramirez M., Yang J., Bourque L., et al. Sports injuries to high school athletes with disabilities. Pediatrics. 2009;123(2):690-696.
131. Rickenlund A., Eriksson M.J., Schenck-Gustafsson K., et al. Amenorrhea in female athletes is associated with endothelial dysfunction and unfavorable lipid profile. J Clin Endocrinol Metab. 2005;90(3):1354-1359.
132. Rickenlund A., Eriksson M.J., Schenck-Gustafsson K., et al. Oral contraceptives improve endothelial function in amenorrheic athletes. J Clin Endocrinol Metab. 2005;90(6):3162-3167.
133. Roberts W.O. Administration and medical management of mass participation endurance events. In: Mellion E., Walsh W.M., Madden C., et al, editors. Team physician’s handbook. Philadelphia: Hanley & Belfus, 2002.
134. Roberts W.O., Maron B.J. Evidence for decreasing occurrence of sudden cardiac death associated with the marathon. J Am Coll Cardiol. 2005;46(7):1373-1374.
135. Robertson W.C.Jr., Eichman P.L., Clancy W.G. Upper trunk brachial plexopathy in football players. JAMA. 1979;241(14):1480-1482.
136. Robinson N., Giraud S., Saudan C., et al. Erythropoietin and blood doping. Br J Sports Med. 40(suppl 1), i30-34, 2006.
137. Robinson Y., Cristancho E., Boning D. Intravascular hemolysis and mean red blood cell age in athletes. Med Sci Sports Exerc. 2006;38(3):480-483.
138. Rockett F.X. Observations on the “burner”: traumatic cervical radiculopathy. Clin Orthop Relat Res. 1982;(164):18-19.
139. Rundell K.W., Jenkinson D.M. Exercise-induced bronchospasm in the elite athlete. Sports Med. 2002;32(9):583-600.
140. Rydwik E., Frandin K., Akner G. Effects of physical training on physical performance in institutionalised elderly patients (70+) with multiple diagnoses. Age Ageing. 2004;33(1):13-23.
141. Sakamoto Y., Ueki S., Kasai T., et al. Effect of exercise, aging and functional capacity on acute secretory immunoglobulin. a response in elderly people over 75 years of age. Geriatr Gerontol Int. 2009;9(1):81-88.
142. Salter R., Harris W. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
143. Scovazzo M.L., Browne A., Pink M., et al. The painful shoulder during freestyle swimming: an electromyographic cinematographic analysis of twelve muscles. Am J Sports Med. 1991;19(6):577-582.
144. Shephard R.J., Kavanagh T., Mertens D.J., et al. Personal health benefits of masters athletics competition. Br J Sports Med. 1995;29(1):35-40.
145. Silver M.D. Use of ergogenic aids by athletes. J Am Acad Orthop Surg. 2001;9(1):61-70.
146. Siow H.M., Cameron D.B., Ganley T.J. Acute knee injuries in skeletally immature athletes. Phys Med Rehabil Clin N Am. 2008;19(2):319-345. ix
147. Slobounov S., Slobounov E., Sebastianelli W., et al. Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery. 2007;61(2):338-344. discussion 44
148. Soprano J.V. Musculoskeletal injuries in the pediatric and adolescent athlete. Curr Sports Med Rep. 2005;4(6):329-334.
149. Speer K.P., Bassett F.H.3rd. The prolonged burner syndrome. Am J Sports Med. 1990;18(6):591-594.
150. Storms W.W. Review of exercise-induced asthma. Med Sci Sports Exerc. 2003;35(9):1464-1470.
151. Tanaka H., Seals D.R. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol. 2008;586(1):55-63.
152. Tokish J.M., Kocher M.S., Hawkins R.J. Ergogenic aids: a review of basic science, performance, side effects, and status in sports. Am J Sports Med. 2004;32(6):1543-1553.
153. Torg J.S., Vegso J.J., Sennett B., et al. The National Football Head and Neck Injury Registry. 14-year report on cervical quadriplegia, 1971 through 1984. JAMA. 1985;254(24):3439-3443.
154. Toyoshima S., Herashina T., Miyashita M., et al. Contribution of the body parts to throwing performance. In: Nelson R.C., Morehouse C.A., editors. Biomechanics IV. Baltimore: University Park Press, 1974.
155. Trappe T.A., Fluckey J.D., White F., et al. Skeletal muscle PGF(2)(alpha) and PGE(2) in response to eccentric resistance exercise: influence of ibuprofen acetaminophen. J Clin Endocrinol Metab. 2001;86(10):5067-5070.
156. Troup J.P. The physiology and biomechanics of competitive swimming. Clin Sports Med. 1999;18(2):267-285.
157. Tunstall Pedoe D.S. Marathon cardiac deaths: the London experience. Sports Med. 2007;37(4-5):448-450.
158. Urhausen A., Kindermann W. Diagnosis of overtraining: what tools do we have? Sports Med. 2002;32(2):95-102.
159. Walsh B.W., Schiff I., Rosner B., et al. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17):1196-1204.
160. Warner D.C., Schnepf G., Barrett M.S., et al. Prevalence, attitudes, and behaviors related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. J Adolesc Health. 2002;30(3):150-153.
161. Warren M.P., Miller K.K., Olson W.H., et al. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in women with hypothalamic amenorrhea and osteopenia: an open-label extension of a double-blind, placebo-controlled study. Contraception. 2005;72(3):206-211.
162. Wathen D., Baechle T.R., Earl R.W. Training variation: periodization. In: Baechle T., Earle R.W., editors. Essentials of strength training and conditioning. Champaign, IL: Human Kinetics, 2000.
163. Watkins R.G. Neck injuries in football players. Clin Sports Med. 1986;5(2):215-246.
164. Webborn A.D. Fifty years of competitive sport for athletes with disabilities: 1948-1998. Br J Sports Med. 1999;33(2):138.
165. White R.D. 2005 American Heart Association Guidelines for cardiopulmonary resuscitation: physiologic and educational rationale for changes. Mayo Clin Proc. 2006;81(6):736-740.
166. Wilber R.L. Application of altitude/hypoxic training by elite athletes. Med Sci Sports Exerc. 2007;39(9):1610-1624.
167. Wilbur R.L. Live high + train low: thinking in terms of an optimal hypoxic dose. Int J Sports Physiol Perform. 2007;2(3):223-238.
168. Williams K.R. Biomechanics of running. Exerc Sport Sci Rev. 1985;13:389-441.
169. Wilson P.E., Washington R.L. Pediatric wheelchair athletics: sports injuries and prevention. Paraplegia. 1993;31(5):330-337.
170. World Anti-Doping Agency: list of banned Substances. April 19, 2009]. Available http://www.usantidoping.org/files/active/what/wallet_card.pdf. Accessed.
171. Wroble R.R., Albright J.P. Neck and low back injuries in wrestling. Clin Sports Med. 1986;5(2):295-325.
172. Yu B., Lin C.F., Garrett W.E. Lower extremity biomechanics during the landing of a stop-jump task. Clin Biomech (Bristol, Avon). 2006;21(3):297-305.
173. Zeni Hoch A., Dempsey R.L., Carrera G.F., et al. Is there an association between athletic amenorrhea and endothelial cell dysfunction? Med Sci Sports Exerc. 2003;35(3):377-383.