Chapter 10 Spontaneous Intraparenchymal Hemorrhage
The ability to image the brain using computed tomography (CT) and magnetic resonance imaging (MRI) has greatly enhanced our understanding of intraparenchymal hemorrhages (IPH) in the central nervous system. Before the advent of these modalities, the diagnosis of IPH was based on clinical presentation and indirect angiographic findings. Certainty could only be reached at the time of necropsy. CT and MRI now provide us with means to characterize the hematoma size, its location, and its effect on adjacent structures. Furthermore, they allow us to follow the evolution of the hematomas and taught us that these lesions are highly dynamic and have a dangerous tendency to expand early after their development.1,2 Brott et al. reported substantial hematoma growth (greater than one third of the baseline volume) in 26% of patients within 1 hour of the initial CT scan and in an additional 12% within the first 20 hours among patients presenting for emergency evaluation within 3 hours of symptom onset.1
The cause responsible for the production of the hematoma can often be gleaned from brain imaging, such as in cases of associated tumors and vascular anomalies. Timing and adequacy of interventions may be guided by serial imaging, as is often the case with progressive hydrocephalus requiring ventriculostomy or expanding cerebellar hemorrhages demanding surgical evacuation. Various radiological features predict functional outcome after IPH. Hematoma volume greater than 60 cc, pronounced midline shift, infratentorial location, presence of hydrocephalus, and intraventricular hemorrhage have been shown to be prognosticators of death or poor recovery.3,4
Because hematoma volume carries such remarkable prognostic weight, it is essential to be familiar with the pragmatic technique used to estimate volume on the basis of CT scan appearance. This technique, known as the ABC/2 method and reliably validated in the literature, is simply based on the measurement of the three maximal diameters of the hematoma.5 It is illustrated on Figure 10-1. This measurement method can be applied using axial cuts of MRI sequences, but MRI-based measurements may lead to overestimation of lesion size.6
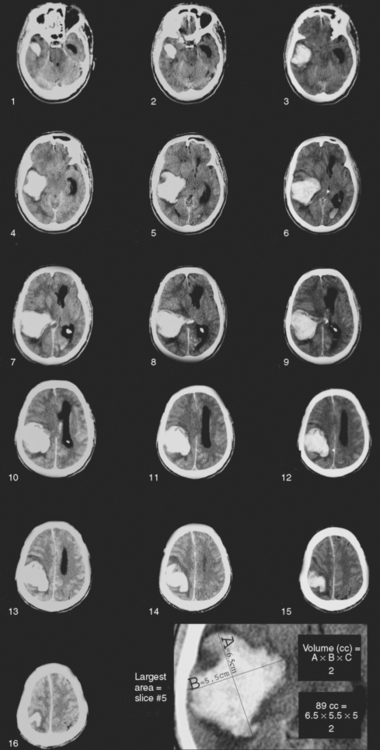
Figure 10-1 An estimation of hematoma volume (cc) can be calculated with the formula (A × B × C)/2.5 First, identify the slice with the largest area of intracerebral hematoma. A represents the longest diameter (cm) to be measured in the slice showing the largest hematoma size. B represents the longest diameter (cm) perpendicular to A. C represents the height of the hematoma, calculated by multiplying the number of slices involved by the slice thickness (cm). To measure the number of slices involved, comparisons should be made to the largest diameter slice using percentages: if the diameter of the hematoma in the slice is greater than 75% of the largest diameter in the scan, that slice is counted as one full slice; if the diameter of the hematoma in the slice is between 25% and 75% of the largest diameter in the scan, that slice is counted as half, if the diameter is less than 25% of the largest diameter, the slice is not counted. The figure exemplifies how hematoma volume can be estimated. Slice 5 was identified as the largest area of intracerebral hematoma. The longest diameter (A) is measured as 6.5 cm. The longest perpendicular diameter (B) is 5.5 cm. C can be measured by calculating the slice thickness (0.5 cm) by the number of slices involved (10) (0.5 × 10=5). Slices 1, 2, and 16 were not counted because the diameter of the hematoma in those slices was less than 25% of the largest diameter in the scan. Slices 3, 9, 12, 13, 14, 15 were counted as half (total of 3); slices 4, 5, 6, 7, 8, 10, 11 were counted as one (total of 7). Values A(6.5) × B(5.5) × C(5) were then multiplied and divided by 2 to reach an estimated volume of 89 cc.
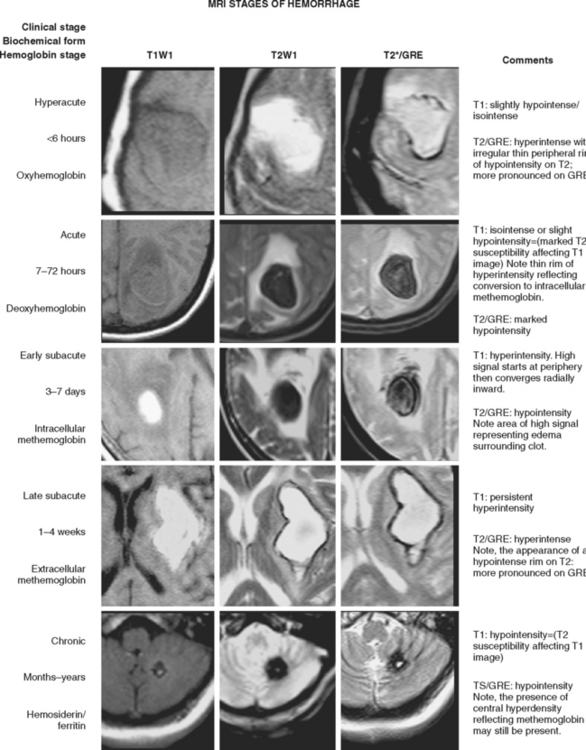
Traditionally, CT scan was considered the modality of choice for the diagnosis of hyperacute IPH.7 However, MRI has now been shown to be as accurate as CT scan for the detection of acute hematomas and offers greater sensitivity for the recognition and temporal staging of chronic hemorrhages.8–10 Susceptibility-weighted sequences, such as gradient-recalled echo (GRE) T2*-weighted imaging, are particularly useful for the detection of acute hemorrhage. GRE also allows optimal visualization of chronic hemorrhages and microhemorrhages.
Figure 10-2 depicts how changes in MRI appearance help determine the age of intraparenchymal hemorrhage.
Trying to establish whether there is an area surrounding the hematoma that is at risk for ischemia (penumbra) has lately been the focus of intensive research. Perfusion- and diffusion-weighted MRI, CT perfusion, single photon emission computed tomography (SPECT), and positron emission tomography have been used as tools to address this question. Most evidence thus far appears to indicate that hematomas are surrounded by a region of hypoperfusion that corresponds to a metabolically quiescent area.11–14 Serial imaging with perfusion-weighted MRI has shown that the hypoperfusion surrounding the hematoma resolves by the second week.15 However, some studies support the notion that hematomas may be surrounded by ischemic penumbra.11,16 Thus, more research is necessary to reach a final answer.
Determining the volume of perihematomal edema and following the evolution of edema over time has important practical implications. Perihematomal edema appears early and, on average, increases by 75% during the first 24 hours in patients with acute spontaneous IPH.17 The volume of baseline relative edema on initial CT scan correlates inversely with the subsequent accumulation of edema in the following hours of the first day.17 More interestingly, presence of greater volume of baseline relative edema (but not of absolute edema) has been found to be associated with better functional outcome at 3 months in patients with IPH without intraventricular extension.18 These observations await confirmation, and their meaning is unclear at present, but they nonetheless highlight the likely importance of edema formation in the outcome of patients with IPH. Late neurological deterioration (in the second and third weeks after acute IPH) from a delayed increase in edema volume is not infrequently encountered in practice and may provoke unexpected brain herniation. For that reason, patients with large hematomas may require serial imaging even many days after the bleeding. The pathophysiology of this late edema demands further investigation.
Classification of IPH is typically based on its anatomical location. The most common sites of origin of spontaneous IPH in the largest imaging series are19,20:
STRIATOCAPSULAR HEMORRHAGES
The striatocapsular region comprises the lenticular and caudate nuclei, the internal capsule, and the external capsule and subinsular area. The presentation and prognosis of striatocapsular hemorrhages depend on the epicenter of the bleeding. Chung and colleagues have proposed a detailed classification that divides these hemorrhages into six types, as listed in Table 10-1.24
TABLE 10-1 Anatomical classification of striatocapsular hemorrhages.
| Type | Site of bleeding | Arterial territory |
|---|---|---|
| Anterior | Caudate nucleus | Heubner’s artery |
| Middle | Globus pallidus or medial putamen | Medial lenticulostriates |
| Posteromedial | Posterior limb of internal capsule | Anterior choroidal |
| Posterolateral | Posterior putamen | Lateral lenticulostriates (posteromedial branches) |
| Lateral | External capsule, subinsular region | Lateral lenticulostriates (lateral branches) |
| Massive | Entire area (may spare caudate and anterior limb of internal capsule) | Variable |
Impaired level of consciousness at presentation and large hematoma size consistently predict poor functional outcome in patients with striatocapsular hemorrhage. Additionally, hydrocephalus and intraventricular extension are markers of large hematoma size in patients with putaminal hemorrhage (unlike in those with bleedings emanating from the caudate) and thus also forecast death or dependency.21–23 Capsular involvement is strongly associated with persistent hemiparesis, but weakness tends to be severe only in patients with larger hematomas.
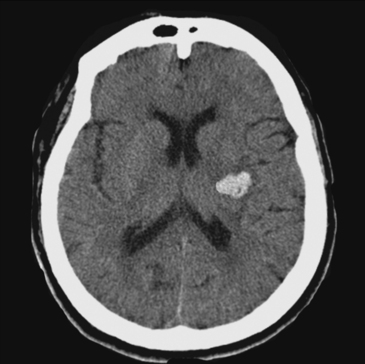
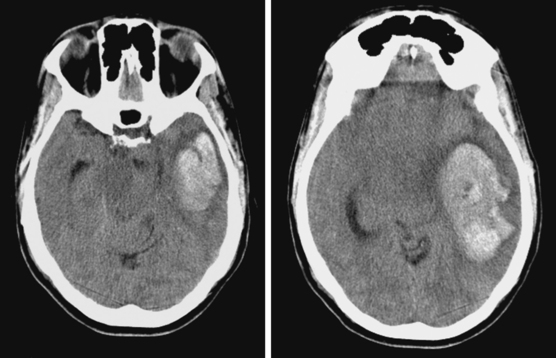
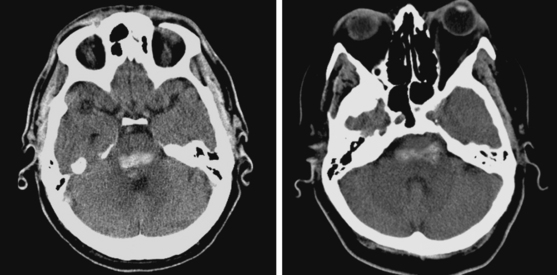
Lateral Putaminal Hemorrhage
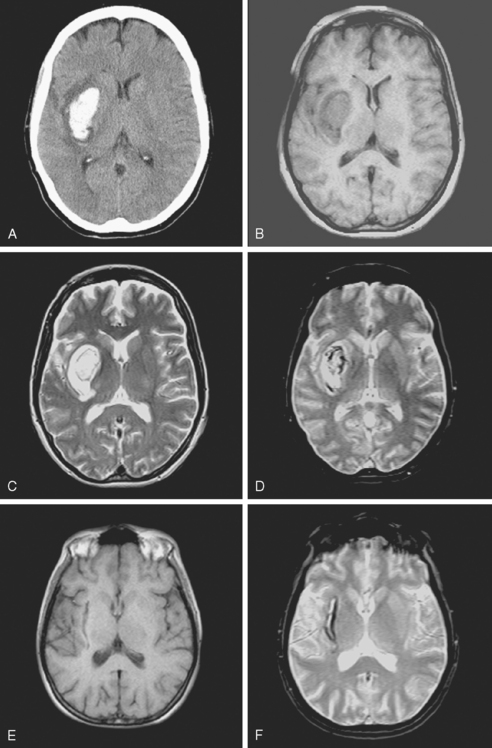
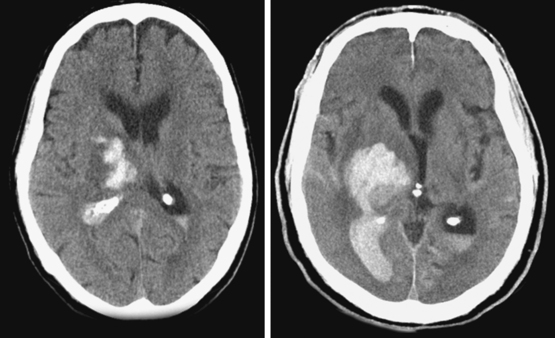
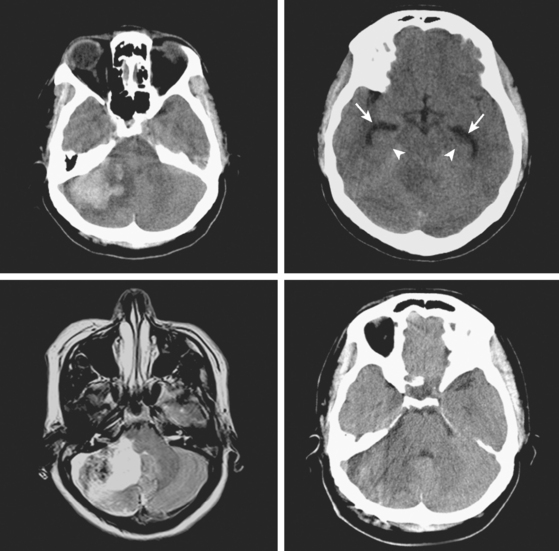
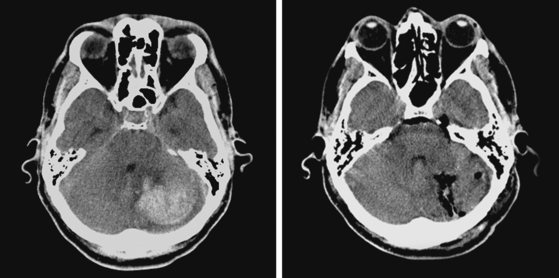
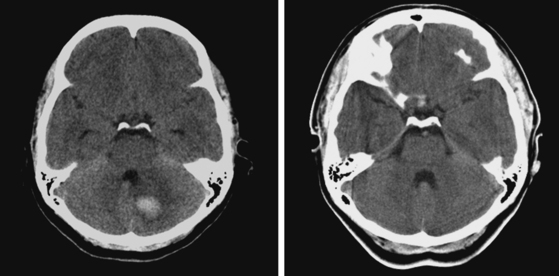
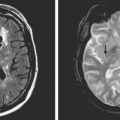
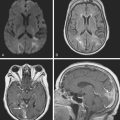
A 48-year-old Hispanic woman with recent diagnosis of hypertension presented with sudden onset of headache and left-sided weakness. Examination revealed an alert patient with a blood pressure of 210/124 mm Hg, mild right horizontal gaze palsy, and severe left hemiparesis. She appeared relatively unconcerned about her weakness and paid less attention to activity occurring over her left visual field. The patient had no complications during her acute hospitalization. Her CT scan of the brain revealed a right lateral putaminal hemorrhage, later confirmed on MRI (Figure 10-3, upper two rows). No underlying vascular anomalies or masses were found. Six months later, she had residual weakness but recovered the ability to walk independently with minimal support and had partial functional use of her left arm. Follow-up imaging is shown in the lower row of Figure 10-3.
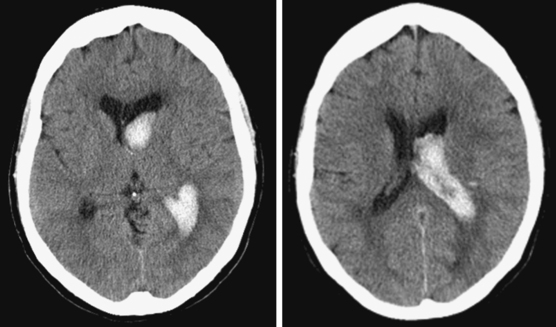
Massive Striatocapsular Hemorrhage
Posterolateral Striatocapsular Hemorrhage
Middle-Posteromedial Striatocapsular Hemorrhage
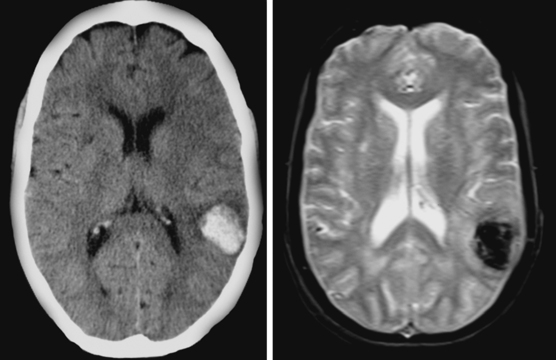
Caudate Hemorrhage
LOBAR HEMORRHAGES
Lobar spontaneous IPH may be less frequent than is traditionally estimated. In recent years, enhanced technology, mainly in the form of MRI and highly improved angiographic equipment, has demonstrated that cases previously believed to be due to hypertension are actually caused by other conditions, such as occult vascular anomalies, venous thrombosis, or, most often, amyloid angiopathy. Secondary causes of IPH are discussed in the next chapter but must be carefully excluded before concluding that a lobar hemorrhage is spontaneous even in a hypertensive patient (Table 10-2). Neuroimaging is an extremely valuable and in fact irreplaceable tool to recognize the correct etiology of bleeding in patients with lobar IPH.
TABLE 10-2 Causes of lobar intracerebral hemorrhage other than primary arterial hypertension
| Cause | Diagnosis |
|---|---|
| Ruptured saccular aneurysm | Angiography |
| AVM | Angiography |
| Cavernous angioma | MRI |
| Cerebral amyloid angiopathy | MRI (GRE) |
| Tumor | MRI with gadolinium |
| Hemorrhagic transformation of ischemic infarction | CT, MRI with DWI |
| Cerebral venous thrombosis | MRV or angiography |
| Coagulopathy (intrinsic or iatrogenic) | History, blood work, CT |
| Recreational drugs (amphetamines, cocaine) | History, toxicology screening |
| Trauma | History, CT |
| Vasculitis | History, blood work, CSF, angiography, cultures if suspicion of infection |
| Ruptured mycotic aneurysm | Angiography, blood cultures, TEE |
| Moyamoya syndrome | Angiography |
AVM, arteriovenous malformation; CSF, cerebrospinal fluid; CT, computed tomography; DWI, diffusion-weighted imagery; GRE, gradient-recall echo; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography.
Lobar hemorrhages tend to originate from the cortical-subcortical or gray-white matter junction and extend into the adjacent white matter. Any lobe can be involved, and the location and laterality of the hematoma determines the clinical manifestations. Seizures, typically of focal onset with secondary generalization, occur frequently occur at onset and during the acute phase.27,28 Multiple hemorrhage locations, simultaneous or sequential, may occur because of hypertension but must be regarded as a potential indicator of an alternative cause (mainly amyloid angiopathy in the elderly with cortical hemorrhages or cavernous malformations in young patients with deep hemorrhages).
The overall outcome of patients with lobar IPH is deemed to be comparatively more favorable than after ganglionic hemorrhages.29,30 Once again, one has to be cautious when analyzing older series reporting favorable outcomes because they may have included patients with more benign secondary hemorrhages in the group of spontaneous lobar IPH. Size is still the most powerful predictor of outcome, and intraventricular extension remains a prognosticator of reduced chances of survival and poor recovery.
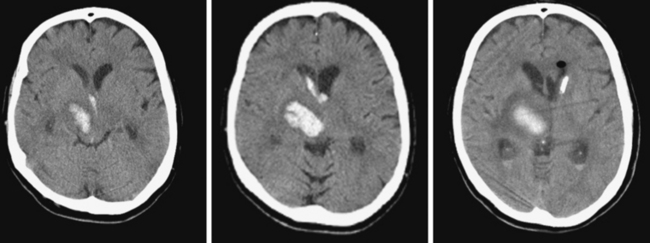
Hematoma Expansion
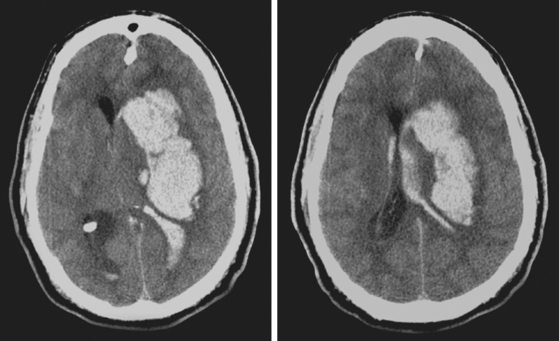
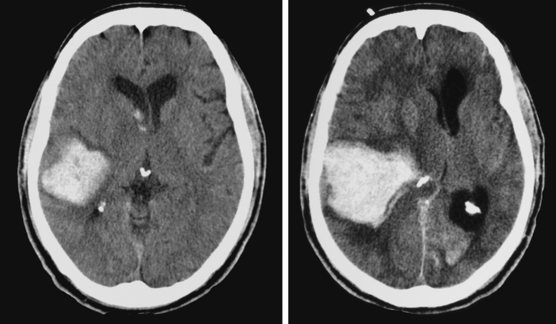
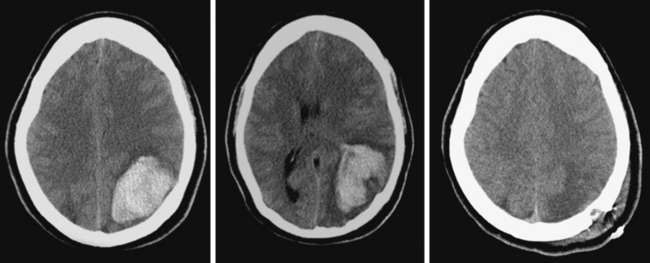
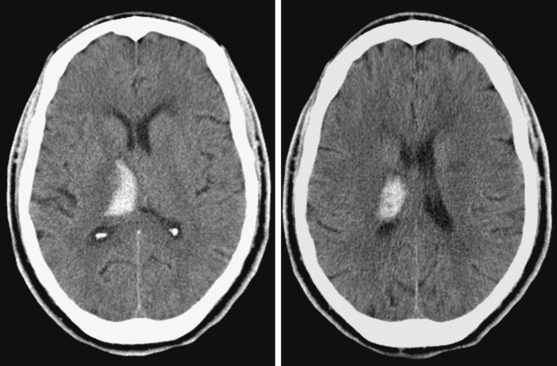
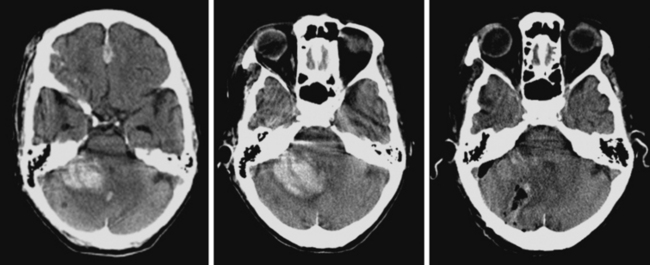
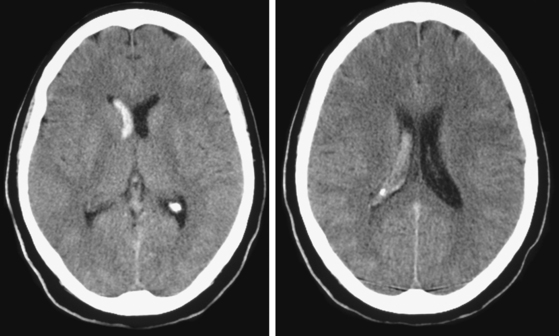
A 74-year-old African American man with long history of hypertension and poor compliance with prescribed antihypertensive medications was brought to the emergency department after being found wandering and limping on the street. Upon arrival, the patient’s blood pressure was 186/126 mm Hg. Neurological examination showed that he was confused and had scant verbal output, right gaze preference with decreased response to visual threat on the left, left hemiplegia, and left hypoesthesia. The degree of weakness had worsened in comparison with the first examination by the paramedics. CT scan showed a right frontoparietal hematoma (Figure 10-8, left) On the second hospital day, the patient was noticed to be slightly more lethargic but otherwise unchanged; repeat CT showed mild increase in the regional mass effect. In the morning of the third day, he became comatose because of massive hematoma enlargement (Figure 10-8, right). His brainstem reflexes were present, but he only responded with extensor posturing to maximal pain. After discussion with the family, further care was limited to comfort measures, and the patient expired hours later.
Hydrocephalus
Temporal Hematomas
Focal Lobar Hemorrhage
Surgical Evacuation (Figure 10-12)
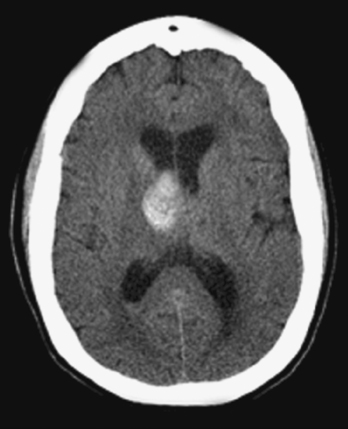
THALAMIC HEMORRHAGES
The thalamus is a complex structure comprising multiple relay nuclei with distinct functions. Nuclear groups also differ in their arterial supply and may harbor anatomically confined hemorrhages that produce fairly consistent clinical syndromes based on their precise primary location. Functional outcome mainly depends on the size of the hematoma and whether extension into the lower diencephalon and midbrain occurs.35 Hematoma diameter greater than 3 cm is associated with high mortality rate,36,37 whereas volumes less than 10 cc often foretell favorable recovery. Unlike what is seen with most other hemorrhages, ventricular extension is compatible with good outcome in patients with thalamic bleeding.36,38 In fact, patients with medial thalamic hemorrhages may benefit from decompression into the ventricular space, thus avoiding the dangerous caudal spread into the midbrain. However, when ventricular extension is accompanied by hydrocephalus the prognosis worsens substantially.36,38,39
Careful analysis of neuroimaging findings has allowed the development of a clinic-anatomical classification that divides thalamic hemorrhages into five types, as listed in Table 10-3.39
TABLE 10-3 Anatomical classification of thalamic hemorrhages.
| Type | Arterial territory |
|---|---|
| Anterior | Polar or tuberothalamic artery |
| Posteromedial | Thalamo-subthalamic paramedian artery |
| Posterolateral | Thalamogeniculate arteries |
| Dorsal | Posterior choroidal arteries |
| Global | Entire thalamic region |
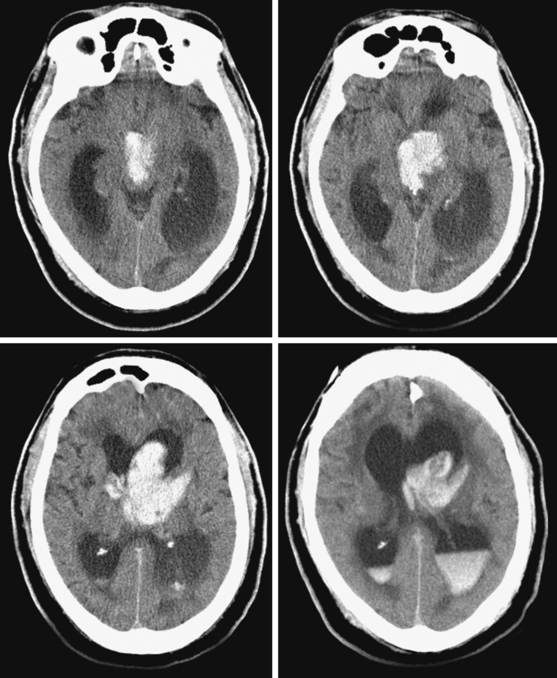
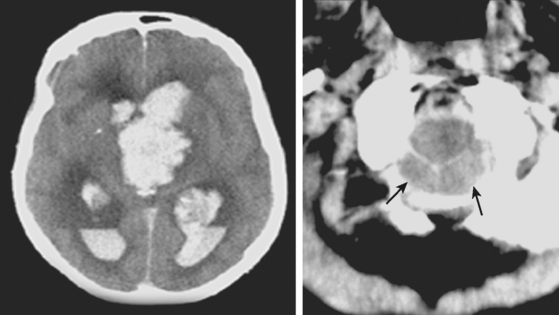
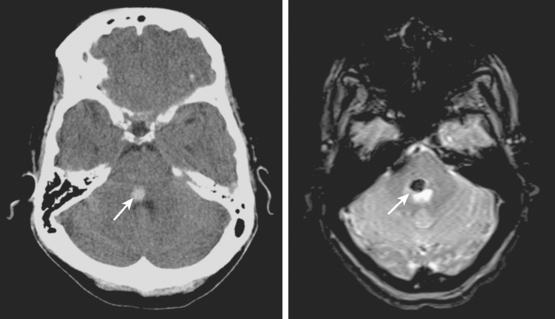
Global Thalamic Hematomas
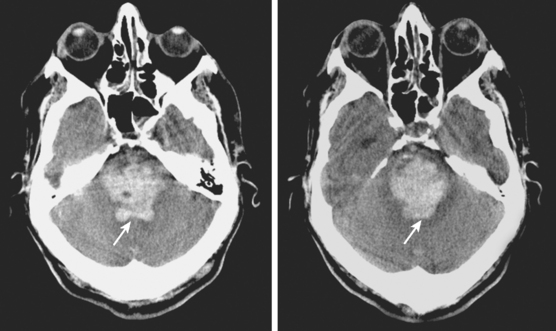
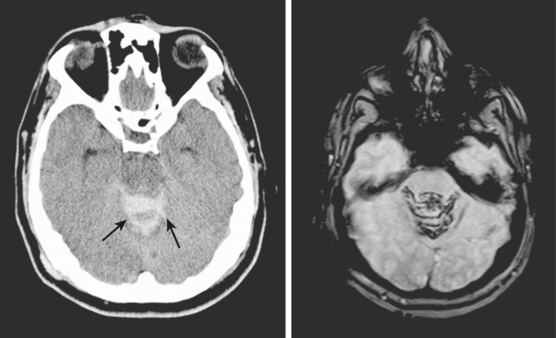
A 66-year-old African American man with background of hypertension and diabetes type 2 developed sudden headache and vomiting shortly after waking up. Initially conscious, he rapidly became less responsive. Upon arrival to the emergency department, the patient had a Glasgow coma scale sum score of 6; he exhibited small sluggishly reactive pupils and forced gaze deviation to the left and downward. His blood pressure was 198/122 mm Hg, and he had hyperpnea. CT scan showed a massive thalamic hemorrhage with intraventricular extension causing severe hydrocephalus (Figure 10-13). Emergency ventriculostomy failed to improve his clinical status. After discussing the ominous prognosis with the patient’s family, intubation was withheld. A short hospitalization was complicated by aspiration pneumonia and sepsis, and the patient was subsequently discharged in persistent coma to a hospice.
Anterior Thalamic Hemorrhage
Posteromedial Thalamic Hemorrhage
Posterolateral Thalamic Hemorrhage
PONTINE HEMORRHAGES
Before the introduction of CT, pontine hemorrhages were thought to be nearly always fatal. However, modern brain imaging has taught us that not only survival but also meaningful and sometimes fairly complete recovery is possible after bleeding in the pons. The question is how often spontaneous (hypertensive) pontine hemorrhages are followed by a favorable course, because evidence of cavernous angiomas is often found on MRI of patients with benign evolution. Thus CT allowed us to recognize the greater spectrum of prognosis in patients with pontine hemorrhages, and MRI is showing us that cause is the most important predictor of outcome in these patients.44 In practice, patients with nonmassive pontine hemorrhage and a favorable clinical evolution should undergo MRI to exclude cavernous angiomas, particularly when they are young and have no previously documented hypertension.
Prognosis is highly dependent on clinical severity at presentation, but radiological signs including hydrocephalus, larger hemorrhage size, and extension into the midbrain and thalamus are also reliable prognosticators of poor outcome.45–47 Conversely, patients with small hematomas (< 4 cc) restricted to the tegmentum tend to have minor neurological sequelae.46,48 Hemorrhage location serves as the foundation for a CT classification with useful prognostic implications (Table 10-4).48
| Type | Region involved |
|---|---|
| Unilateral tegmental | One side of dorsal pons |
| Bilateral tegmental or basal-tegmental | Both sides of dorsal pons or the junction between basis pontis and tegmentum |
| Massive | Entire pons |
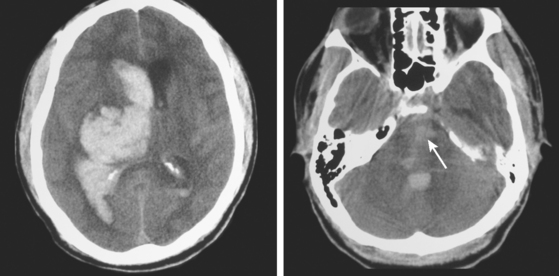
Massive Pontine Hemorrhage
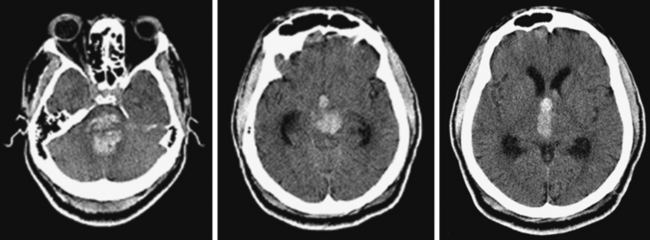
An adult man of unknown identity was found face down on the street, unconscious and surrounded by vomit. He was emergently intubated by paramedics and transported to the emergency department. On arrival, his vital signs revealed hypertension (190/100 mm Hg), sinus tachycardia (115 bpm), and hyperthermia (101°F). He was comatose and had 3-mm nonreactive pupils, absent corneal reflexes, and minimal oculocaloric response to irrigation of the right ear. There was no motor response to pain. CT scan disclosed a massive pontine hemorrhage (Figure 10-20). Over the following hours, the patient lost all signs of brainstem function and, after formal demonstration of apnea on the apnea test, he was declared brain dead.
Bilateral Tegmental Hemorrhage
Unilateral Tegmental Hemorrhage
CEREBELLAR HEMORRHAGES
Cerebellar hemorrhages differ fundamentally from other forms of IPH in that timely surgery can lead to dramatic recovery of function and result in meaningful recovery. Noncomatose patients with hemispheric cerebellar hematomas typically present with inability to walk, as well as headache, vomiting, and dizziness. Appendicular ataxia without hemiplegia (although variable degrees of hemiparesis may be present) and dysarthria are common expressions of cerebellar dysfunction. Compression of the pons is typically manifested by peripheral facial palsy and ipsilateral horizontal gaze palsy. Appearance of these pontine signs may herald subsequent depression in the level of consciousness, but sudden coma may develop unannounced after a seemingly benign initial course.49,50
Large Cerebellar Hematomas with Surgical Decompression
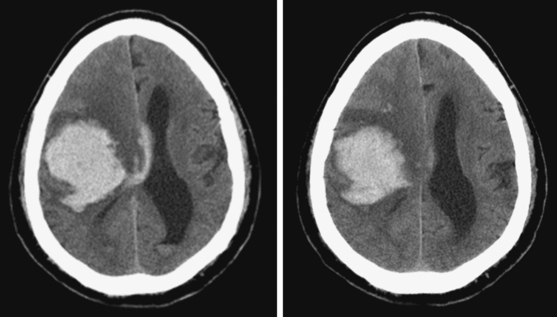
A 56-year-old man with history of hypertension and coronary artery disease presented with sudden onset of headache, dizziness, and inability to walk. On examination, he was fully lucid, and his blood pressure was 185/110 mm Hg; his neurological deficits were a very mild right peripheral facial palsy, slight right hemiparesis, right dysmetria, and pronounced gait ataxia. Brain imaging showing a large hematoma in the right cerebellar hemisphere is presented in Figure 10-24. Within 8 hours of admission to the ward, the patient became confused and then lethargic, and developed right sixth nerve palsy and right facial paralysis. Emergency surgical evacuation through suboccipital craniotomy was followed by good functional recovery. Postsurgical CT scan is shown in Figure 10-24, lower right. Six months later, the patient had returned to his regular activities as a hospital administrator and complained only of mild balance problems and some slowing of his executive abilities.
Benign Hemispheric Cerebellar Hemorrhages
Vermian Hemorrhages
INTRAVENTRICULAR HEMORRHAGES
Hemorrhages primarily located in the ventricular system are most frequently due to underlying causes, such as vascular anomalies (AVM, cavernous angiomas, or, rarely, aneurysms that may be mycotic), coagulation disorders (from blood dyscrasias; iatrogenic, such as following thrombolysis), trauma (including manipulation of a ventriculostomy catheter), and tumors (papilloma, subependymoma, astrocytoma, metastasis, etc.); much less often, they may be spontaneous (with the exception of germinal matrix hemorrhages in premature newborns). We have also seen cases of pure intraventricular hemorrhage in patients with moyamoya-like disease. This classification excludes parenchymal hypertensive hemorrhages with secondary extension into the ventricular space, which largely represent the most common cause of intraventricular clots. The distinction becomes challenging when the parenchymal hematoma is difficult to visualize, as is usually the case with caudate hemorrhages. When the cause of intraventricular hemorrhage remains obscure, the patient should be studied with contrast MRI and conventional angiography. An example of spontaneous intraventricular hemorrhage is presented in Figure 10-29.
Prognosis depends on the clinical severity at presentation and underlying cause of the bleeding.60 Coma from onset in patients with massive intraventricular hemorrhage (“packed ventricles”) and obstructive hydrocephalus that fails to improve rapidly with ventriculostomy portends extremely poor outcome. In patients with subarachnoid or intraparenchymal hemorrhage, moderate to large amounts of intraventricular blood are strong predictors of lethality.61 However, patients with spontaneous intraventricular hemorrhage appear to tolerate even large amounts of ventricular blood much better; they exhibit considerably less tendency to develop impaired consciousness or hydrocephalus and often retain full neurological capacities.60,61 To our knowledge, the reason for this prognostic divergence is unclear at present.
Intraventricular hemorrhage is a poor prognostic factor in patients with IPH;4,62 larger volume and early growth of intraventricular clot are predictive of mortality and unfavorable functional outcome.22,63 External ventricular drainage catheters are inserted to treat the resulting hydrocephalus, but all too often drainage is insufficient because of obstruction of the catheter by the clotted blood. Furthermore, drainage through ventriculostomy has never been shown to accelerate the clearance of intraventricular blood. Intraventricular infusion of thrombolytics aimed at hastening this clearance is currently being tested with promising initial results.64
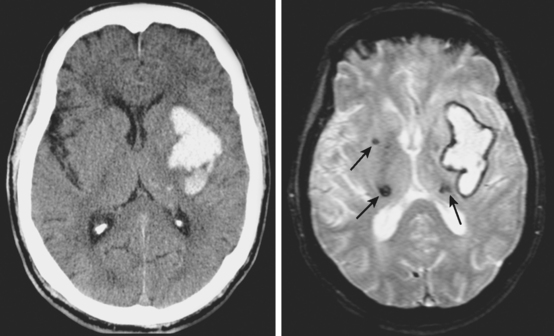
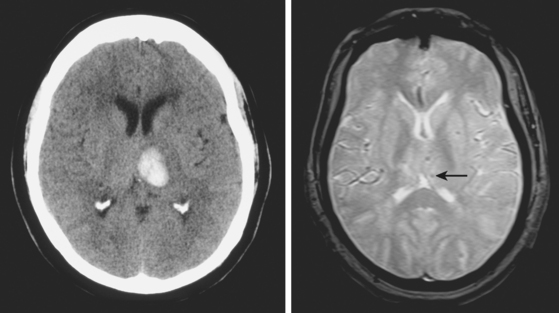
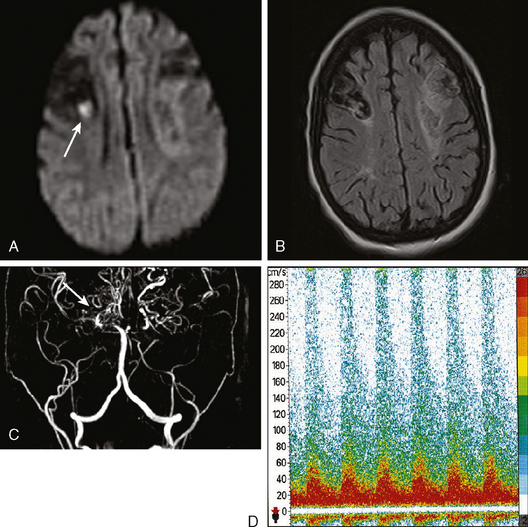
MICROHEMORRHAGES
Susceptibility-weighted MR sequences, particularly T2*-weighted GRE, enable us to recognize asymptomatic microscopic hemorrhages with enormous accuracy (Figures 10-30 and 10-31). Microhemorrhages are seen as small areas of signal loss due to the magnetic susceptibility effect caused by deposits of hemosiderin.65 These lesions have become the focus of extensive research, and knowledge about their significance and implications is growing rapidly. The current status of such knowledge can be briefly summarized as follows:
1 Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1-5.
2 Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783-1787.
3 Wijdicks EF, Rabinstein AA. Absolutely no hope? Some ambiguity of futility of care in devastating acute stroke. Crit Care Med. 2004;32:2332-2342.
4 Hemphill JCIII, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891-897.
5 Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304-1305.
6 Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized MRI stroke protocol: comparison with CT in hyperacute intracerebral hemorrhage. Stroke. 1999;30:765-768.
7 Broderick JP, Adams HPJr, Barsan W, Feinberg W, Feldmann E, Grotta J, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905-915.
8 Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823-1830.
9 Fiebach JB, Schellinger PD, Gass A, Kucinski T, Siebler M, Villringer A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke. 2004;35:502-506.
10 Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. 2007 Update. A guideline from the American Heart Association, American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001-2023.
11 Kidwell CS, Saver JL, Mattiello J, Warach S, Liebeskind DS, Starkman S, et al. Diffusion-perfusion MR evaluation of perihematomal injury in hyperacute intracerebral hemorrhage. Neurology. 2001;57:1611-1617.
12 Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52:266-272.
13 Schellinger PD, Fiebach JB, Hoffmann K, Becker K, Orakcioglu B, Kollmar R, et al. Stroke MRI in intracerebral hemorrhage: is there a perihemorrhagic penumbra? Stroke. 2003;34:1674-1679.
14 Herweh C, Juttler E, Schellinger PD, Klotz E, Jenetzky E, Orakcioglu B, et al. Evidence against a perihemorrhagic penumbra provided by perfusion computed tomography. Stroke. 2007;38:2941-2947.
15 Pascual AM, Lopez-Mut JV, Benlloch V, Chamarro R, Soler J, Lainez MJ. Perfusion-weighted magnetic resonance imaging in acute intracerebral hemorrhage at baseline and during the 1st and 2nd week: a longitudinal study. Cerebrovasc Dis. 2007;23:6-13.
16 Siddique MS, Fernandes HM, Wooldridge TD, Fenwick JD, Slomka P, Mendelow AD. Reversible ischemia around intracerebral hemorrhage: a single-photon emission computerized tomography study. J Neurosurg. 2002;96:736-741.
17 Gebel JMJr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2631-2635.
18 Gebel JMJr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636-2641.
19 Kase CS, Caplan LR. Clinical features at different sites. In: Kase CS, Caplan LR, editors. Intracranial Hemorrhage. Boston: Butterworth Heinemann; 2005:305-307.
20 Kase CS, Mohr JP, Caplan LR. Intracerebral hemorrhage. In: Mohr JP, Choi DW, Grotta JC, Weir B, Wolf PA, editors. Stroke. Pathophysiology, diagnosis, and management. Philadelphia: Churchill-Livingstone; 2005:327-376.
21 Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29:1352-1357.
22 Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Hier DB, et al. Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival. Ann Neurol. 1991;29:658-663.
23 Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617-621.
24 Chung CS, Caplan LR, Yamamoto Y, Chang HM, Lee SJ, Song HJ, et al. Striatocapsular haemorrhage. Brain. 2000;123:1850-1862.
25 Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387-397.
26 Stein RW, Kase CS, Hier DB, Caplan LR, Mohr JP, Hemmati M, et al. Caudate hemorrhage. Neurology. 1984;34:1549-1554.
27 Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43:1175-1180.
28 Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441-1446.
29 Steiner I, Gomori JM, Melamed E. The prognostic value of the CT scan in conservatively treated patients with intracerebral hematoma. Stroke. 1984;15:279-282.
30 Helweg-Larsen S, Sommer W, Strange P, Lester J, Boysen G. Prognosis for patients treated conservatively for spontaneous intracerebral hematomas. Stroke. 1984;15:1045-1048.
31 Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167-1173.
32 Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175-1181.
33 Rabinstein AA, Atkinson JL, Wijdicks EF. Emergency craniotomy in patients worsening due to expanded cerebral hematoma: to what purpose? Neurology. 2002;58:1367-1372.
34 Rabinstein AA, Wijdicks EF. Surgery for intracerebral hematoma: the search for the elusive right candidate. Rev Neurol Dis. 2006;3:163-172.
35 Mori S, Sadoshima S, Ibayashi S, Fujishima M, Iino K. Impact of thalamic hematoma on six-month mortality and motor and cognitive functional outcome. Stroke. 1995;26:620-626.
36 Kwak R, Kadoya S, Suzuki T. Factors affecting the prognosis in thalamic hemorrhage. Stroke. 1983;14:493-500.
37 Weisberg LA. Thalamic hemorrhage: clinical-CT correlations. Neurology. 1986;36:1382-1386.
38 Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology. 1990;40:616-619.
39 Chung CS, Caplan LR, Han W, Pessin MS, Lee KH, Kim JM. Thalamic haemorrhage. Brain. 1996;119:1873-1886.
40 Fisher CM. The pathologic and clinical aspects of thalamic hemorrhage. Trans Am Neurol Assoc. 1959;84:56-59.
41 Choi D, Sudarsky L, Schachter S, Biber M, Burke P. Medial thalamic hemorrhage with amnesia. Arch Neurol. 1983;40:611-613.
42 Hankey GJ, Stewart-Wynne EG. Amnesia following thalamic hemorrhage. Another stroke syndrome. Stroke. 1988;19:776-778.
43 Hirose G, Kosoegawa H, Saeki M, Kitagawa Y, Oda R, Kanda S, et al. The syndrome of posterior thalamic hemorrhage. Neurology. 1985;35:998-1002.
44 Rabinstein AA, Tisch SH, McClelland RL, Wijdicks EF. Cause is the main predictor of outcome in patients with pontine hemorrhage. Cerebrovasc Dis. 2004;17:66-71.
45 Murata Y, Yamaguchi S, Kajikawa H, Yamamura K, Sumioka S, Nakamura S. Relationship between the clinical manifestations, computed tomographic findings and the outcome in 80 patients with primary pontine hemorrhage. J Neurol Sci. 1999;167:107-111.
46 Wessels T, Moller-Hartmann W, Noth J, Klotzsch C. CT findings and clinical features as markers for patient outcome in primary pontine hemorrhage. AJNR Am J Neuroradiol. 2004;25:257-260.
47 Wijdicks EF, St Louis E. Clinical profiles predictive of outcome in pontine hemorrhage. Neurology. 1997;49:1342-1346.
48 Chung CS, Park CH. Primary pontine hemorrhage: a new CT classification. Neurology. 1992;42:830-834.
49 Ott KH, Kase CS, Ojemann RG, Mohr JP. Cerebellar hemorrhage: diagnosis and treatment. A review of 56 cases. Arch Neurol. 1974;31:160-167.
50 St Louis EK, Wijdicks EF, Li H. Predicting neurologic deterioration in patients with cerebellar hematomas. Neurology. 1998;51:1364-1369.
51 Kirollos RW, Tyagi AK, Ross SA, van Hille PT, Marks PV. Management of spontaneous cerebellar hematomas: a prospective treatment protocol. Neurosurgery. 2001;49:1378-1386.
52 Little JR, Tubman DE, Ethier R. Cerebellar hemorrhage in adults. Diagnosis by computerized tomography. J Neurosurg. 1978;48:575-579.
53 Salvati M, Cervoni L, Raco A, Delfini R. Spontaneous cerebellar hemorrhage: clinical remarks on 50 cases. Surg Neurol. 2001;55:156-161.
54 Taneda M, Hayakawa T, Mogami H. Primary cerebellar hemorrhage. Quadrigeminal cistern obliteration on CT scans as a predictor of outcome. J Neurosurg. 1987;67:545-552.
55 Weisberg LA. Acute cerebellar hemorrhage and CT evidence of tight posterior fossa. Neurology. 1986;36:858-860.
56 Da Pian R, Bazzan A, Pasqualin A. Surgical versus medical treatment of spontaneous posterior fossa haematomas: a cooperative study on 205 cases. Neurol Res. 1984;6:145-151.
57 van Loon J, Van Calenbergh F, Goffin J, Plets C. Controversies in the management of spontaneous cerebellar haemorrhage. A consecutive series of 49 cases and review of the literature. Acta Neurochir (Wien). 1993;122(3-4):187-193.
58 Fisher CM, Picard EH, Polak A, Dalal P, Pojemann RG. Acute hypertensive cerebellar hemorrhage: diagnosis and surgical treatment. J Nerv Ment Dis. 1965;140:38-57.
59 Yoshida S, Sasaki M, Oka H, Gotoh O, Yamada R, Sano K. Acute hypertensive cerebellar hemorrhage with signs of lower brainstem compression. Surg Neurol. 1978;10:79-83.
60 Roos YB, Hasan D, Vermeulen M. Outcome in patients with large intraventricular haemorrhages: a volumetric study. J Neurol Neurosurg Psychiatry. 1995;58:622-624.
61 Passero S, Ulivelli M, Reale F. Primary intraventricular haemorrhage in adults. Acta Neurol Scand. 2002;105:115-119.
62 Hallevy C, Ifergane G, Kordysh E, Herishanu Y. Spontaneous supratentorial intracerebral hemorrhage. Criteria for short-term functional outcome prediction. J Neurol. 2002;249:1704-1709.
63 Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767-773.
64 Naff NJ, Hanley DF, Keyl PM, Tuhrim S, Kraut M, Bederson J, et al. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54:577-583.
65 Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637-642.
66 Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology. 1996;46:1751-1754.
67 Kwa VI, Franke CL, Verbeeten BJr., Stam J. Silent intracerebral microhemorrhages in patients with ischemic stroke. Amsterdam Vascular Medicine Group. Ann Neurol. 1998;44:372-377.
68 Roob G, Lechner A, Schmidt R, Flooh E, Hartung HP, Fazekas F. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke. 2000;31:2665-2669.
69 Lee SH, Kwon SJ, Kim KS, Yoon BW, Roh JK. Topographical distribution of pontocerebellar microbleeds. AJNR Am J Neuroradiol. 2004;25:1337-1341.
70 Lee SH, Bae HJ, Kwon SJ, Kim H, Kim YH, Yoon BW, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology. 2004;62:72-76.
71 Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991-994.
72 Blitstein MK, Tung GA. MRI of cerebral microhemorrhages. AJR Am J Roentgenol. 2007;189:720-725.
73 Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. AJNR Am J Neuroradiol. 2003;24:88-96.
74 Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415-1420.
75 Jeon SB, Kang DW, Cho AH, Lee EM, Choi CG, Kwon SU, et al. Initial microbleeds at MR imaging can predict recurrent intracerebral hemorrhage. J Neurol. 2007;254:508-512.
76 Jeong SW, Jung KH, Chu K, Bae HJ, Lee SH, Roh JK. Clinical and radiologic differences between primary intracerebral hemorrhage with and without microbleeds on gradient-echo magnetic resonance images. Arch Neurol. 2004;61:905-909.
77 Fan YH, Zhang L, Lam WW, Mok VC, Wong KS. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke. 2003;34:2459-2462.
78 Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265-2275.