CHAPTER 52 SPLENIC INJURIES
The spleen has had a prominent role in medical theory and practice throughout history. The Greeks and Romans believed that the spleen played a role in filtering the humors of the body, mirroring some of our modern concepts. During the middle of the last millennium, the Thuggee was a cult that worshipped Kali, a Hindu goddess of destruction. The members were professional assassins, and the act of murder for pay was an act of worship for their goddess. They were most famous for their use of the noose, but also targeted the left upper quadrant where, often fragile and swollen from malaria, the spleen lay. A well-placed blow leading to splenic rupture and bleeding in the absence of available transfusion and modern surgery might well prove fatal.
INCIDENCE AND MECHANISM OF INJURY
The spleen is listed, along with the liver, as either the first or second most commonly injured solid viscus in the abdomen after blunt trauma. Because splenic injuries have a tendency to demonstrate themselves clinically more often than do hepatic injuries, splenic injury was listed as the most commonly injured intra-abdominal solid viscus before the advent of computed tomography (CT) scanning. After the advent of CT scanning and our ability to better diagnose clinically silent intra-abdominal injuries, it became apparent that the liver is also commonly injured and some series now list hepatic injuries as more common than splenic injuries. One large, multi-institutional study showed a 2.6% incidence of splenic injuries (6308 of 227,656 patients) for all patients evaluated for trauma, with splenic injury confirmed by either laparotomy or computed tomography.1
For penetrating trauma, retrospective reviews of two large centers, Grady Memorial Hospital and Ben Taub General Hospital, showed the incidence of splenic injury from abdominal gunshot wounds to be 7%–9%, far less than for the hollow organs and the liver.2,3 Injuries to the spleen from stab wounds are even more infrequent.
Splenic bleeding can also occur on a delayed basis, a phenomenon of obvious importance in patients treated nonoperatively. The incidence of delayed bleeding, leading to failure of nonoperative therapy, varies depending on the grade of the injury. The failure rate of nonoperative management in aggregate for a large multi-institutional study was 10.6%, but varied from 4.8% for grade I injuries to 75% for grade V injuries.1 One hypothesis for the pathophysiology leading to delayed rupture and bleeding is that as subcapsular clot breaks down several days after injury into its component parts, the number of osmotically active particles in the area increases and draws more fluid into the area of injury. The resultant increase in size of the area may then rupture the capsule, leading to renewed bleeding. Even without being trapped under the capsule of the spleen, the inflammation and fibrinolysis in and around the healing injury and clot may weaken the clot enough to result in renewed hemorrhage.
DIAGNOSIS
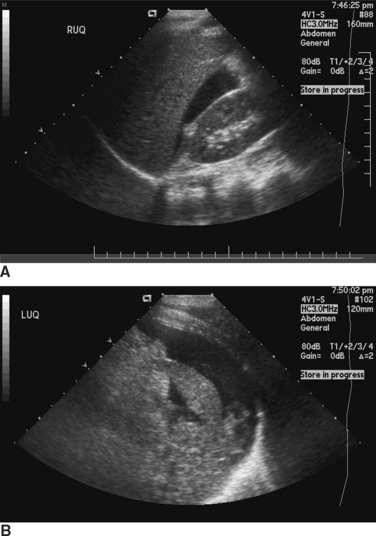
Ultrasound of the abdomen for free fluid, the so-called FAST (focused assessment with sonography for trauma) examination, is being used increasingly as a means of diagnosing hemoperitoneum in blunt trauma patients. Like DPL, it is most useful in unstable patients. Also, as with peritoneal lavage, the ability of ultrasound to determine exactly what is bleeding in the peritoneal cavity is limited. Attempts to image specific organ injuries using ultrasound have met with limited success. The most common method of using FAST examinations is for detection of intraperitoneal fluid and as a determinant of the need for either further imaging of the abdomen or for emergency surgery (Figure 1).
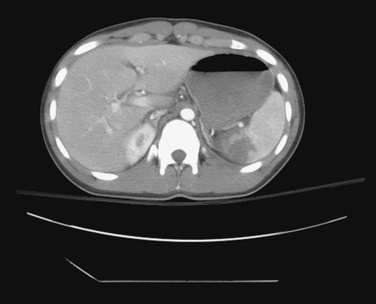
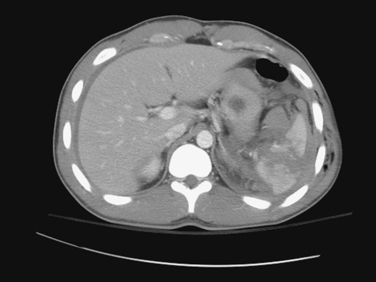
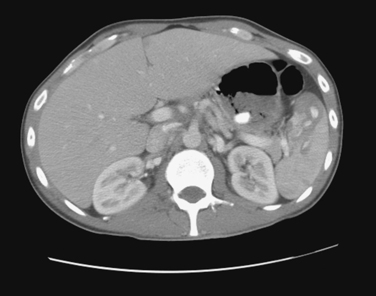
CT of the abdomen is the dominant means of nonoperative diagnosis of splenic injury (Figures 2, 3, and 4). Patients are sent either directly for abdominal CT scanning after initial resuscitation or are screened by abdominal ultrasonography as reasonable candidates for subsequent CT. When abdominal CT scanning is done, intravenous contrast is quite helpful in diagnosis; oral contrast is less helpful and does not measurably increase the sensitivity of CT for splenic injury detection.
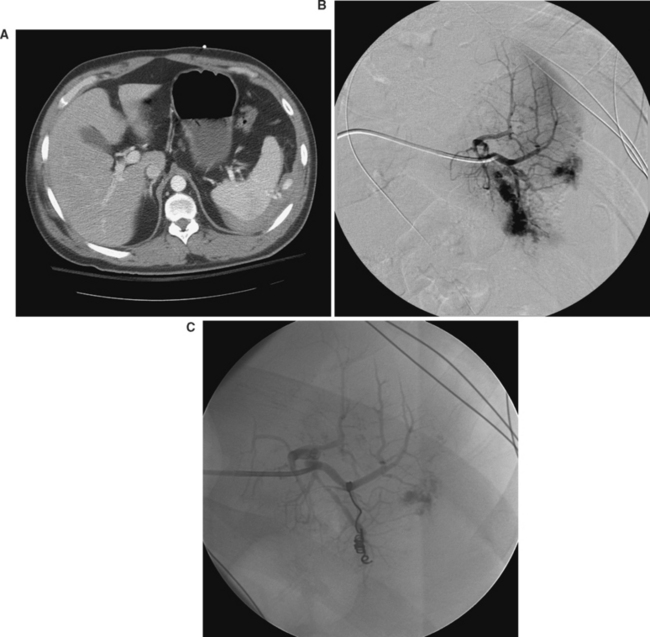
A CT finding in the spleen that has received a great deal of attention is the presence in the disrupted splenic parenchyma of a “blush,” or hyperdense area with a collection of contrast in it. When present, a blush is thought to represent ongoing bleeding with active extravasation of contrast. There is reasonably convincing evidence that the presence of a blush correlates with an increased likelihood of continued or delayed bleeding from the splenic parenchyma. Such a finding therefore has important implications with respect to either operative intervention or the use of angiographic splenic embolization to stop ongoing bleeding (Figure 5).
Anatomic Location of Injury and Injury Grading: American Association for the Surgery of Trauma Organ Injury Scale
A number of different grading systems have been devised to quantify the degree of injury to the spleen. These systems have been created based both on the computed tomographic appearance of ruptured spleens as well as the intraoperative appearance of the spleen. The best known splenic grading system is the one created by the American Association for the Surgery of Trauma (AAST) (Table 1).4 Implicit in the AAST grading system are the perhaps fairly obvious concepts that grade increases with an increase in either the length or depth of parenchymal injury, injury to the hilum, or injuries to multiple areas of the spleen.
Table 1 American Association for the Surgery of Trauma Organ Injury Scaling: Splenic Injury Grading
| Gradea | Injury Type | Description of Injury |
|---|---|---|
| I | Hematoma | Subcapsular, <10% surface area |
| Laceration | Capsular tear, <1 cm parenchymal depth | |
| II | Hematoma | Subcapsular, 10%–50% surface area |
| Laceration | Capsular tear, 1–3 cm parenchymal depth that does not involve a trabecular vessel | |
| III | Hematoma | Subcapsular, >50% surface area or expanding; ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma 5 cm or expanding |
| Laceration | >3-cm parenchymal depth or involving trabecular vessels | |
| IV | Laceration | Laceration involving segmental or hilar vessels producing major devascularization (>25% of spleen) |
| V | Laceration | Completely shattered spleen |
| Vascular | Hilar vascular injury which devascularizes spleen |
a Advance one grade for multiple injuries, up to grade III.
Adapted from Moore EE, Cogbill TH, Jurkovich GJ, et al: Organ injury scaling: spleen and liver. J Trauma, 38:323, 1995.
The CT and intraoperative appearances of a splenic injury are often different from one another. Some of these differences might be because of evolution of the injury between the time of CT scanning and operation, but it is also likely that CT scanning is imperfect in describing the pathologic anatomy of a splenic rupture. Splenic injury scores based on CT scans can both overestimate and underestimate the degree of splenic injury seen at surgery. It is possible to have a CT appearance of fairly trivial injury but at surgery find significant splenic disruption. Conversely, it is possible to see what looks like a major disruption of the spleen on CT scanning and not see the same kind of severity of injury at surgery. In general, the CT scan and associated scores tend, if anything, to underestimate the degree of splenic injury compared with what is seen at surgery.5
An important point about CT-based grading systems is that clinical outcome does not tightly correlate with the degree of injury seen on CT. Although there is a rough correlation between the grade of splenic injury seen on CT scanning and the frequency of operative intervention, exceptions are common. It is possible to have what looks like a fairly trivial injury on CT scan turn out to require delayed operative intervention. In contrast, severe looking splenic injuries on CT scan quite often follow a benign post injury course and are successfully managed nonoperatively.
MANAGEMENT
Nonoperative Management
Appropriate patient selection is the most important element of nonoperative management. Although it is certainly true that nonoperative management is possible in a large number of patients with splenic injury, emergency surgery is still sometimes necessary to stop life-threatening hemorrhage. Of paramount importance in the determination of the suitability of nonoperative management is the hemodynamic stability of the patient. Hemodynamic stability can be a somewhat illusory concept and one for which there is no consensus definition, but hypotension (systolic blood pressure <90 mmHg in an adult) is generally considered worthy of concern. Prehospital or emergency department hypotension is worrisome, and a high index of suspicion for ongoing hemorrhage should be maintained when either is present. In most instances, those patients that remain hemodynamically unstable are inappropriate candidates for abdominal CT scanning. They require either a direct trip to the operating room or, more commonly, abdominal ultrasonography or DPL to determine the presence or absence of intraperitoneal fluid and help guide the initial decision-making process.
Reported success rates for nonoperative management are 95% or higher for pediatric patients and approximately 80% or higher in adults.6,7 These high success rates can be misleading, however, in that they apply only to the group of patients in whom nonoperative management was chosen rather than all patients with splenic injury. When immediate splenectomy patients are included, the overall nonoperative management rates tend to be around 50%–60% in adult patients.1,8 It is also important to remember that these series generally do not include patients in whom the initial impetus was for nonoperative management but in whom emergency surgery was necessary when the patient got into trouble either in the emergency department or during the acquisition of CT scans. The published series of nonoperatively managed spleens generally include only patients who were stable enough to undergo CT scanning and in whom the scan showed a ruptured spleen.
For patients who are stable enough to undergo CT scanning and in whom a ruptured spleen is seen, nonoperative management is reasonable if they continue to remain stable. In addition to vital signs, one of the other commonly followed parameters in such patients is the hematocrit. A common practice is to determine a cut-off value below which the hematocrit will not be allowed to fall. If the hematocrit drops to that level or below, operative intervention is undertaken. Such an approach works best if there are no associated injuries; when other injuries are present, it can be difficult to know if the spleen is continuing to bleed or if the fall in hematocrit is secondary to bleeding from sites other than the spleen. When contemplating transfusion in patients with splenic injury who are being managed nonoperatively, it should be kept in mind that there is increasingly convincing evidence that transfusion has harmful immunologic effects and is an independent predictor of poor outcome after trauma.9
There is some evidence that older patients (>55 years old) might have a worse prognosis with respect to nonoperative management than do younger patients, but there are other reports concluding that outcomes are the same in older versus younger patients.10,11 Although the evidence in this area is mixed, a relatively recent large multicenter study showed that older patients are more likely to fail nonoperative management, and older patients undergoing nonoperative therapy would likely benefit from earlier conversion to invasive therapy if their condition worsens.12
The optimal hospital length of stay is also poorly defined, and there is a variety of practice in this regard. A large multiinstitutional study showed that most failures of nonoperative management occur within the first 6–8 days after injury.1 Our institutional approach is to keep patients in the hospital for an arbitrary 7 days, picking up the vast majority of delayed bleeding episodes during the inpatient stay.
Transcatheter Embolization
Embolization of bleeding areas in a ruptured spleen can be an important adjunct to successful nonoperative management. As with nonoperative management in general, patient selection is of paramount importance. Hemodynamic stability is a prerequisite, in that the patient has to be able to tolerate a possibly lengthy diagnostic and therapeutic procedure. There are no consensus guidelines at present for when angiography and embolization are indicated,13–16 but current trends generally reserve catheter-based interventions for higher grade injuries as well as those with CT evidence of a contrast blush, pseudoaneurysm, arteriovenous fistula, or active extravasation, regardless of the grade of injury.
Operative Management
The first step in mobilization of the spleen is to cut the lateral attachments of the spleen, the splenophrenic and splenorenal ligaments. This step should be started with sharp dissection and can then be continued with a combination of blunt and sharp dissection. The lateral and superior attachments should be cut to near the level of the esophageal hiatus. Cutting the lateral attachments is sometimes facilitated by putting a finger or clamp underneath them and then bluntly developing the underlying plane before dividing the peritoneum. In large patients and in those with a spleen that is very posterior, it may be necessary to do some of the sharp dissection by feel.
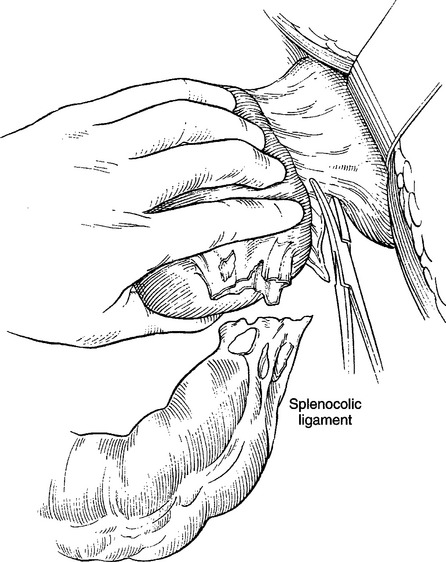
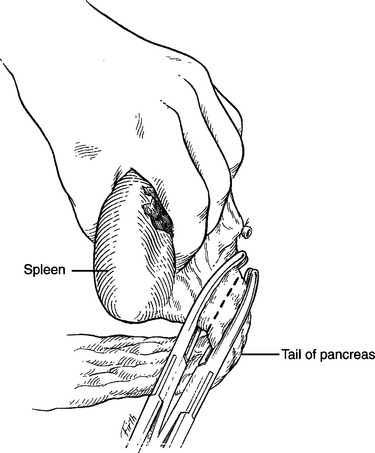
The final step necessary for full mobilization of the spleen is division of the splenocolic ligamentous attachment between the lower pole of the spleen and the distal transverse colon and splenic flexure (Figure 6). During division of both the short gastric vessels and the splenocolic ligament, bleeding from the spleen can be controlled using digital compression of the hilum. If the patient is exsanguinating and the bleeding is massive, a clamp can be placed on the hilum during the later steps of mobilization. Mass clamping should only be done in extreme circumstances, however, because it increases the chances of injury to the tail of the pancreas (Figure 7).
After the spleen has been fully mobilized, it is possible to inspect it in its entirety. It is also possible to examine the posterior aspect of the body and tail of the pancreas. It is helpful after mobilization to pack the splenic fossa to tamponade any minor bleeding and also to help keep the spleen and distal pancreas elevated into the field. During this packing maneuver, the left adrenal gland can be inspected and the left hemidiaphragm re-examined.
MORBIDITY AND COMPLICATIONS OF MANAGEMENT
There are also potential complications of transcatheter therapy. A failed embolization with persistent bleeding is the most common problem. Arterial injuries may occur during vascular access. Necrotic spleen, either from injury or from embolization, can evolve into a splenic abscess. Finally, missed injury remains a concern for this set of patients.15
Although commonly mentioned, overwhelming postsplenectomy sepsis is a rare entity. The actual rate at which overwhelming sepsis in asplenic patients occurs is unknown, but one estimate is a 0.026 lifetime risk for adults and a 0.052 lifetime risk for children.17 Pneumococcus and meningococcus are the most common pathogens, and protection against H. influenzae may also be helpful. Given the extremely low incidence of overwhelming postsplenectomy sepsis, it is difficult to prove the efficacy of vaccination. Nevertheless, vaccination has become the standard of care in patients who have had splenectomy.
MORTALITY
Mortality from splenic injury alone should be fairly low given careful selection and monitoring of nonoperative management and the definitively curative nature of splenectomy. In a study of a national database involving nearly fifteen thousand patients with splenic injury, the mortality was approximately 1%–3%.18 Much of the mortality in patients with splenic injury results from associated injuries, especially head injury.
CONCLUSIONS AND ALGORITHM:
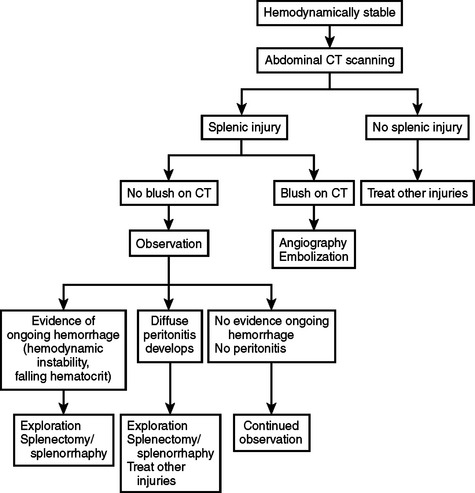
Patients with abdominal trauma and possible splenic injury should be managed initially with the ABC’s of initial trauma resuscitation (Figure 8). If hemodynamically unstable, ultrasound or diagnostic peritoneal lavage should be done to determine if there is intraperitoneal hemorrhage. If the patient remains hemodynamically unstable and there is intraperitoneal hemorrhage, the patient should be explored. If splenic injury is found, splenectomy should be done if the splenic injury is of high grade or the patient has severe associated injuries and/or hemodynamic instability (Figure 9).
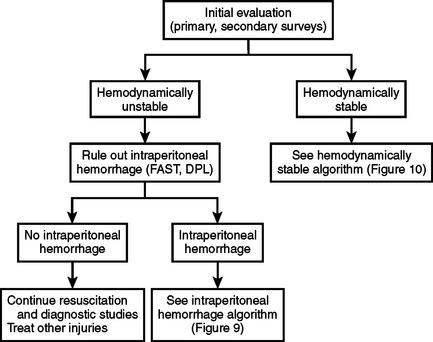
Figure 8 Algorithm for initial evaluation of patients with abdominal trauma and possible splenic injury.
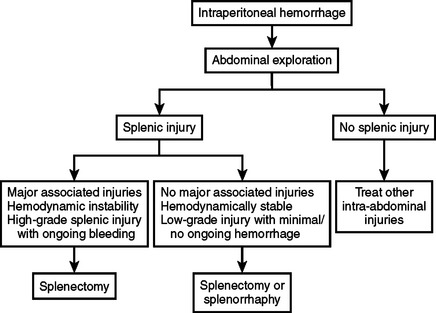
Figure 9 Algorithm for management of hemodynamically unstable patients with intraperitoneal hemorrhage.
If the patient on initial presentation is hemodynamically stable, abdominal CT scanning should be done. If there is a splenic blush on CT, angiography with embolization should be done. If there is no blush and the patient remains hemodynamically stable, a course of nonoperative management should be undertaken. If the patient develops diffuse or worsening peritonitis or shows signs of ongoing bleeding (falling hematocrit, hemodynamic instability), abdominal exploration should be done and the splenic injury managed operatively (Figure 10).
1 Peitzman AB, Heil B, Rivera L, et al. Blunt splenic injury in adults: multi-institutional study of the Eastern Association for the Surgery of Trauma. J Trauma. 2000;49:177.
2 Feliciano DV, Burch JM, Spjut-Patrinely V, et al. Abdominal gunshot wounds: an urban trauma center’s experience with 300 consecutive patients. Ann Surg. 1988;208:903.
3 Nicholas JM, Rix EP, Easley KA, et al. Changing patterns in the management of penetrating abdominal trauma: the more things change, the more they stay the same. J Trauma. 2003;55:1095.
4 Moore EE, Cogbill TH, Jurkovich GJ, et al. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38:323.
5 Shapiro MJ, Krausz C, Durham RM, et al. Overuse of splenic scoring and computed tomographic scans. J Trauma. 1999;47:651.
6 Nix JA, Costanza M, Daley BJ, et al. Outcome of the current management of splenic injuries. J Trauma. 2001;50:835.
7 Buyukunal C, Danismend N, Yeker D. Spleen-saving procedures in paediatric splenic trauma. Br J Surg. 1987;74:350.
8 Pachter HL, Hofstetter SR, Spencer FC. Evolving concepts in splenic surgery. Ann Surg. 1981;194:262.
9 Robinson WP, Ahn J, Stiffler A, et al. Blood transfusion is an independent predictor of increased mortality in nonoperatively managed blunt hepatic and splenic injuries. J Trauma. 2005;58:437.
10 Godley CD, Warren RL, Sheridan RL, et al. Nonoperative management of blunt splenic injury in adults: age over 55 years as a powerful indicator for failure. J Am Coll Surg. 1996;183:133.
11 Cocanour CS, Moore FA, Ware DN, et al. Age should not be a consideration for nonoperative management of blunt splenic injury. J Trauma. 2000;48:606.
12 Harbrecht BG, Peitzman AB, Rivera L, et al. Contribution of age and gender to outcome of blunt splenic injury in adults: multicenter study of the Eastern Association for the Surgery of Trauma. J Trauma. 2001;51:887.
13 Omert LA, Salyer D, Dunham CM, et al. Implications of the “contrast blush” finding on computed tomographic scan of the spleen in trauma. J Trauma. 2001;51:272.
14 Cloutier DR, Baird TB, Gormley P, et al. Pediatric splenic injuries with a contrast blush: successful nonoperative management without angiography and embolization. J Pediatr Surg. 2004;39:969.
15 Haan JM, Biffl W, Knudson MM, et al. Splenic embolization revisited: a multicenter review. J Trauma. 2004;56:542.
16 Haan JM, Bochicchio GV, Kramer N, Scalea TM. Nonoperative management of blunt splenic injury: a 5 year experience. J Trauma. 2005;58:492.
17 Luna GK, Delinger EP. Nonoperative observation therapy for splenic injuries: a safe therapeutic option? Am J Surg. 1987;153:462.
18 Todd SR, Arthur M, Newgard C, et al. Hospital factors associated with splenectomy for splenic injury: a national perspective. J Trauma. 2004;57:1065.