Diagnosis best made on portal venous phase images due to heterogeneous arterial phase enhancement
• Chronic findings on CECT
• MR findings: Low signal on T1WI, heterogeneous high signal on T2WI, and hypoenhancing on T1WI C+ images
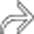 . While sickle cell patients can develop a small, calcified autoinfarcted spleen, the spleen may be enlarged in the early stages of the disease.
. While sickle cell patients can develop a small, calcified autoinfarcted spleen, the spleen may be enlarged in the early stages of the disease.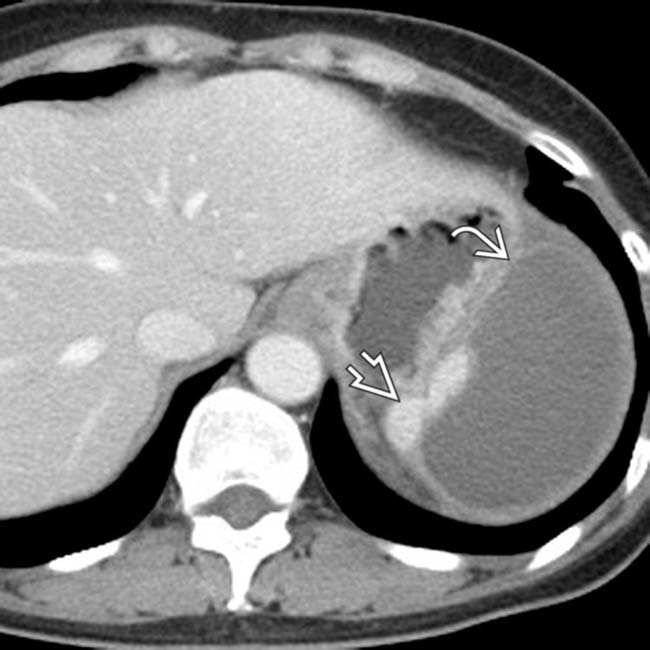
 . Notice the peripheral enhancement (rim sign)
. Notice the peripheral enhancement (rim sign)  at the margins of the infarct as a result of preserved flow through capsular vessels.
at the margins of the infarct as a result of preserved flow through capsular vessels.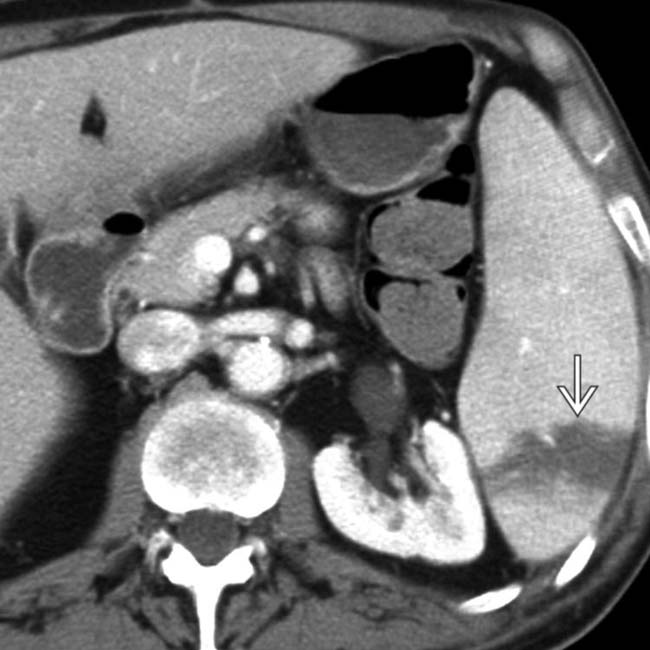
 .
.
 as the source of the arterial embolus to the spleen. Embolic disease is likely the most common cause of splenic infarcts in older patients.
as the source of the arterial embolus to the spleen. Embolic disease is likely the most common cause of splenic infarcts in older patients.IMAGING
General Features
CT Findings
• CECT
 Acute findings
Acute findings
 Acute findings
Acute findings
– Diagnosis best made on portal venous phase images: Heterogeneous enhancement during arterial phase (due to differential enhancement of red and white pulp) makes identification of subtle infarcts difficult
– Global: Complete nonenhancement of spleen
DIFFERENTIAL DIAGNOSIS
Splenic Laceration
Splenic Cyst
• Nonneoplastic cysts divided into primary “true” epithelial cysts and secondary “false” cysts (no epithelial lining)
PATHOLOGY
General Features
CLINICAL ISSUES
Presentation
Demographics
• Epidemiology
 Many different causes, but 2 most common causes are
Many different causes, but 2 most common causes are
 Many different causes, but 2 most common causes are
Many different causes, but 2 most common causes are
 in the pelvis.
in the pelvis.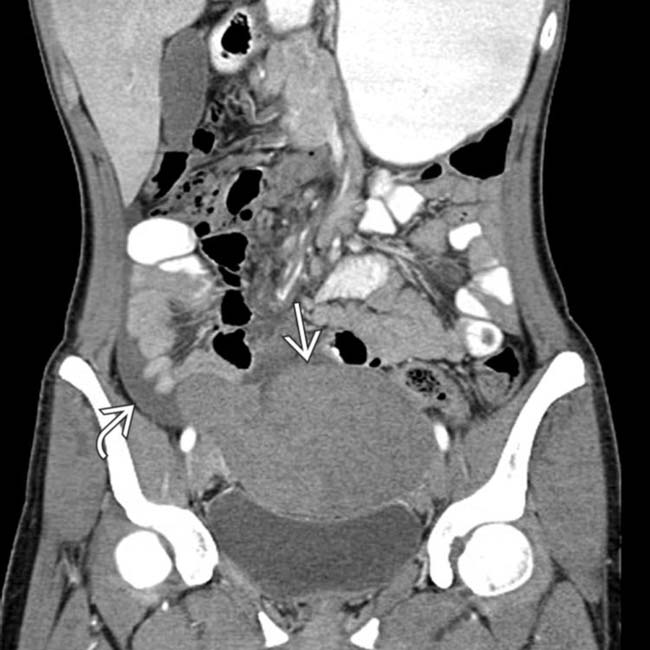
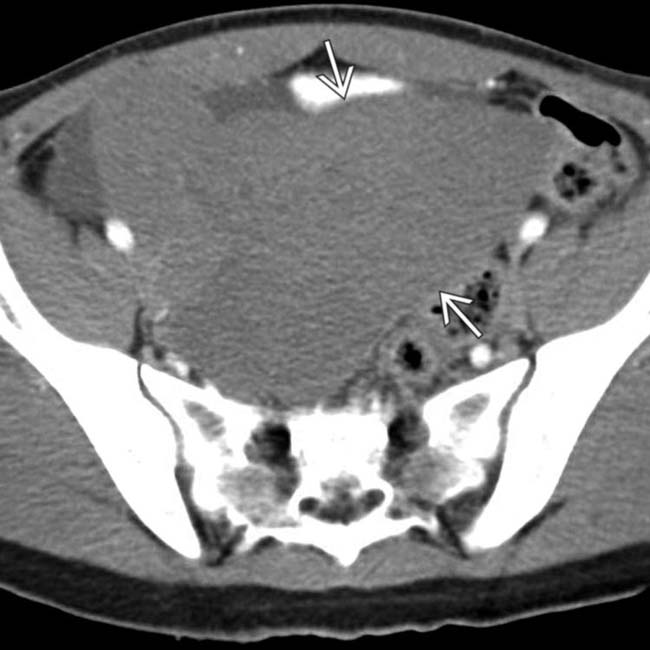
 in the pelvis with fluid in the adjacent right pelvis
in the pelvis with fluid in the adjacent right pelvis  and no spleen noted in the abdomen. This mass was found at surgery to represent torsion and infarction of a “wandering” spleen. The spleen in such cases is found in ectopic locations due to laxity or absence of the splenic ligaments.
and no spleen noted in the abdomen. This mass was found at surgery to represent torsion and infarction of a “wandering” spleen. The spleen in such cases is found in ectopic locations due to laxity or absence of the splenic ligaments.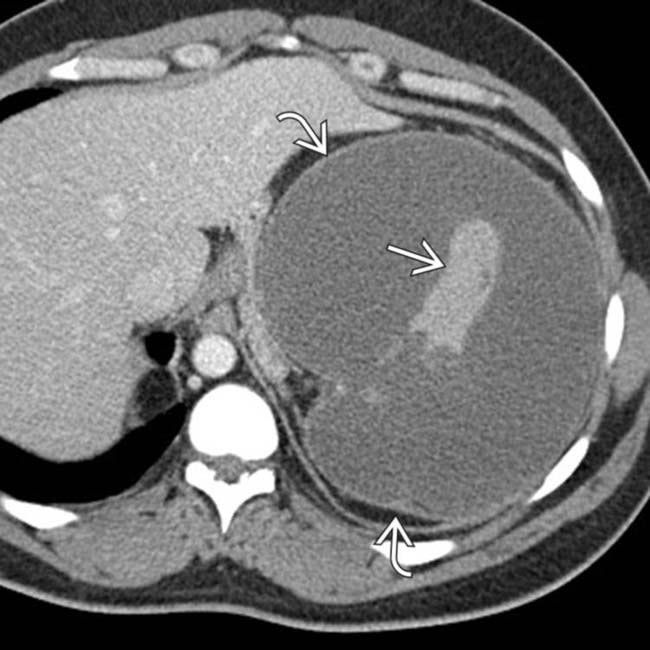
 envelops small areas of normal splenic tissue
envelops small areas of normal splenic tissue  . This was found at surgery to represent a massively infarcted spleen with contained rupture, resulting in the fluid collection.
. This was found at surgery to represent a massively infarcted spleen with contained rupture, resulting in the fluid collection.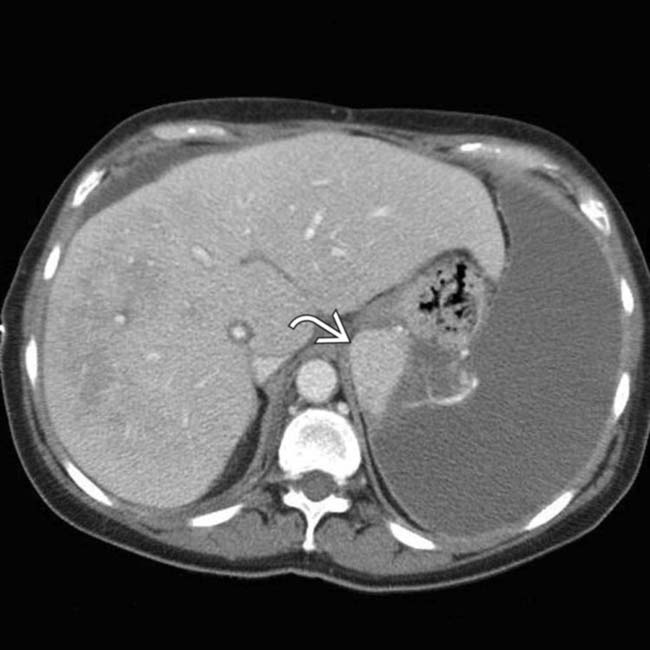
 . Massive acute infarction is often not desired in splenic embolotherapy, as patients can develop infections of infarcted tissue.
. Massive acute infarction is often not desired in splenic embolotherapy, as patients can develop infections of infarcted tissue.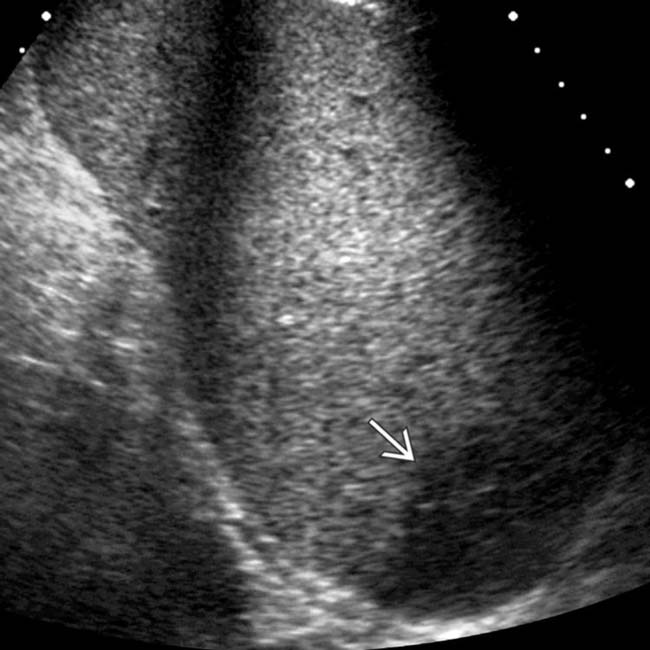
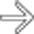 in the lower pole of the spleen, consistent with infarction. The patient was placed on analgesics and recovered uneventfully.
in the lower pole of the spleen, consistent with infarction. The patient was placed on analgesics and recovered uneventfully.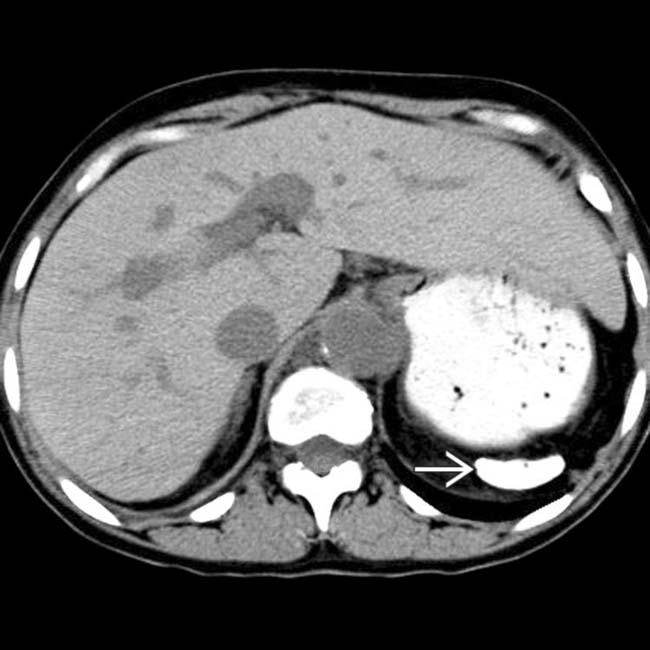
 , and nonfunctional, sometimes termed “autosplenectomy.”
, and nonfunctional, sometimes termed “autosplenectomy.”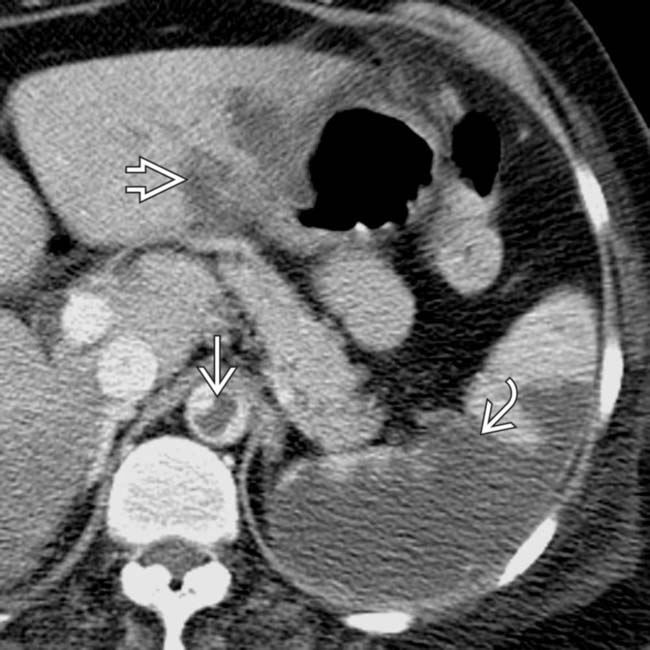
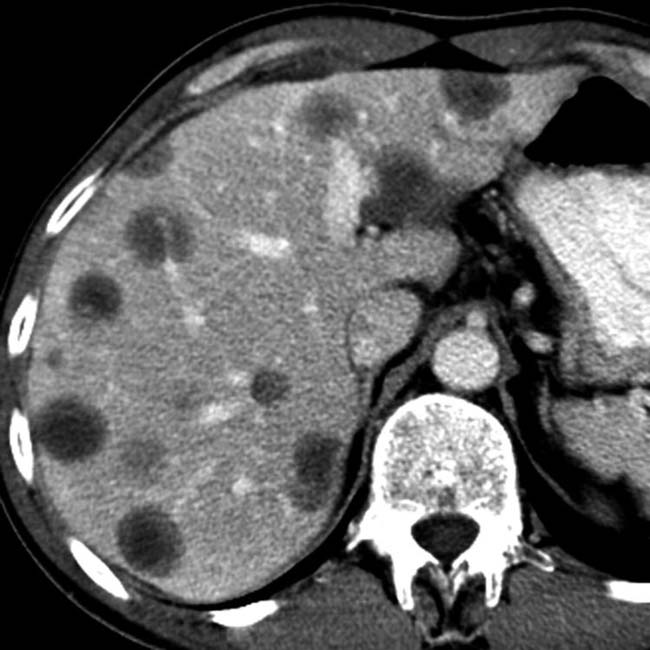
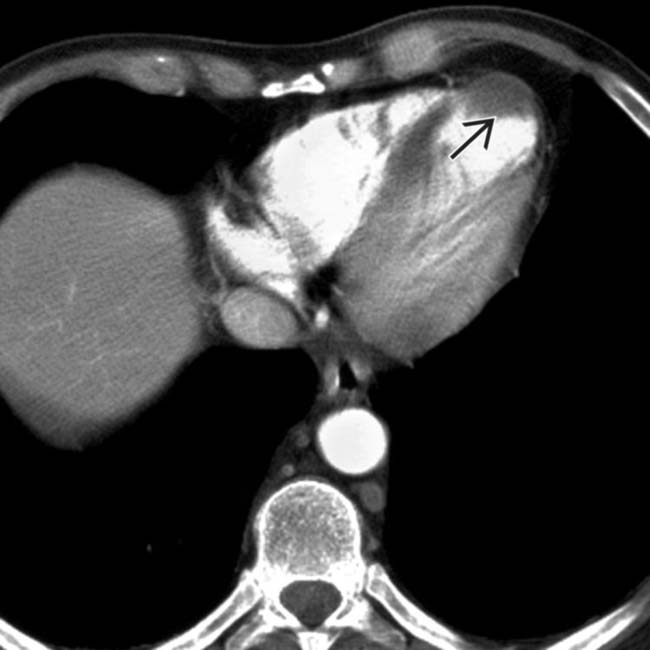
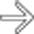
 and an extensive area of nonenhancement due to splenic infarction
and an extensive area of nonenhancement due to splenic infarction 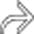 . Note the left lobe hepatic infarction
. Note the left lobe hepatic infarction  .
.
 .
.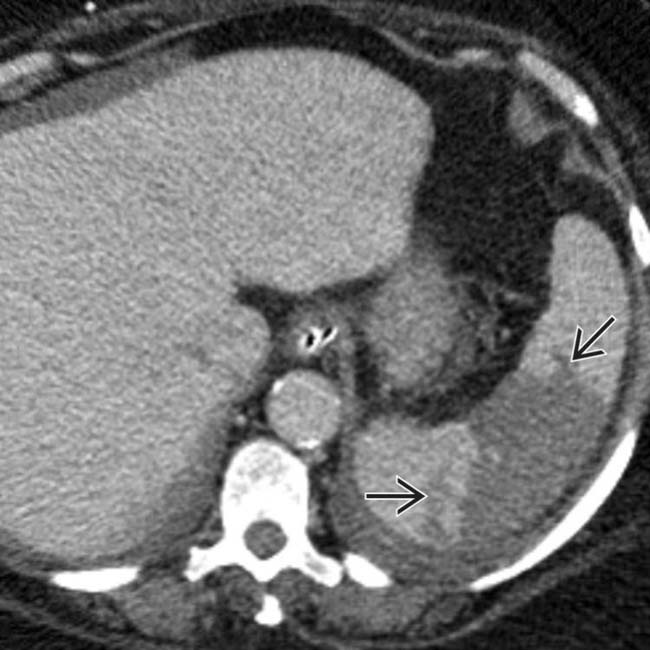
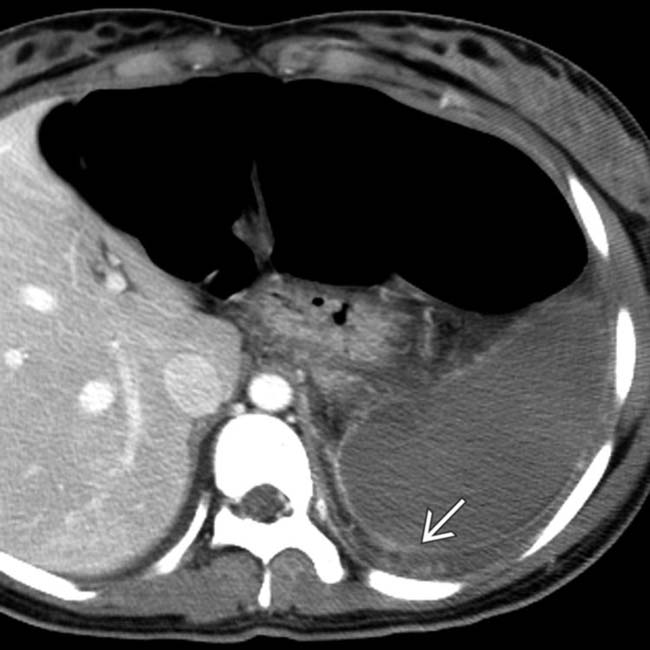
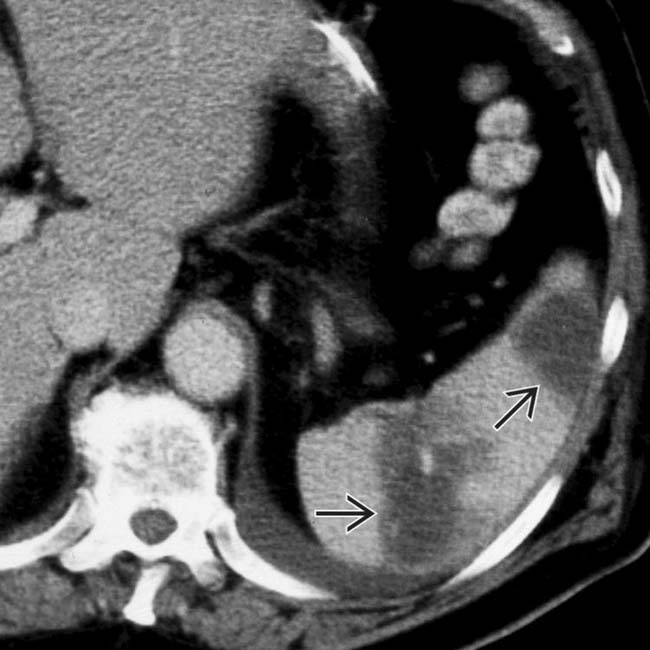
 . The absence of capsular retraction (volume loss) confirms the acute nature of the infarcts.
. The absence of capsular retraction (volume loss) confirms the acute nature of the infarcts.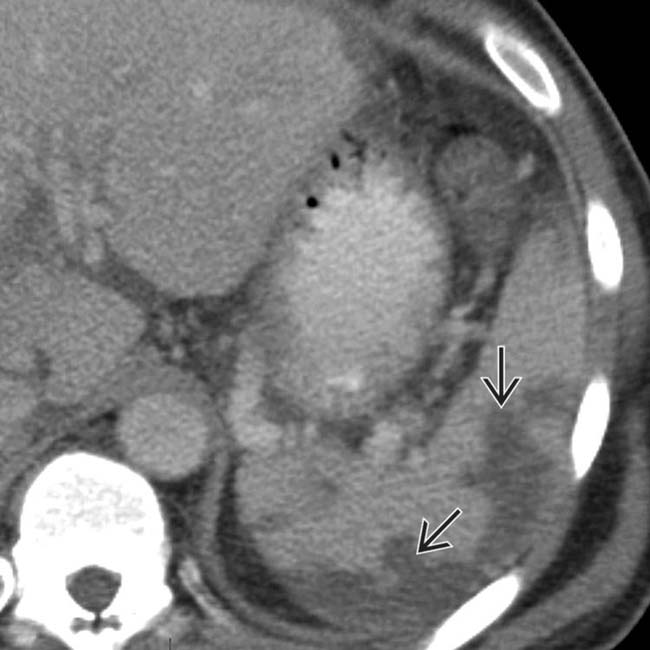
 .
.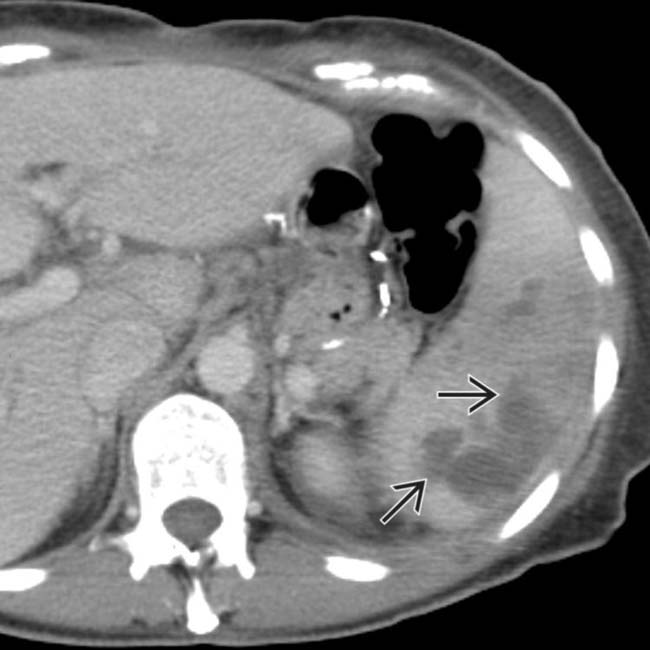
 of nonenhancing spleen, findings characteristic of acute infarction.
of nonenhancing spleen, findings characteristic of acute infarction.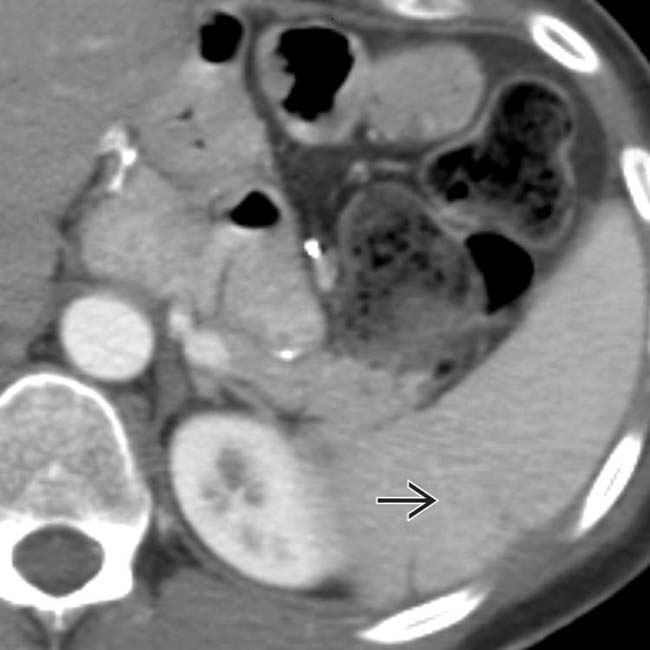
 as sequelae of infarcted tissue.
as sequelae of infarcted tissue.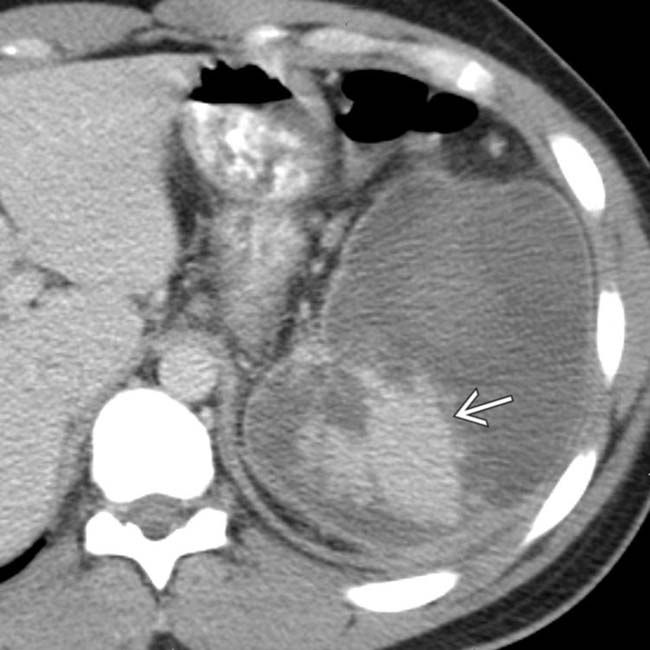
 .
.
 .
.
 .
.
 .
.
 .
.
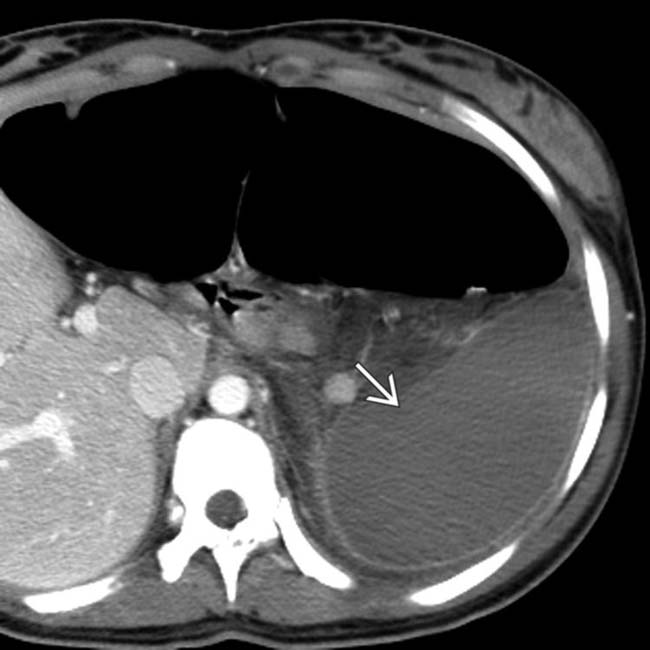
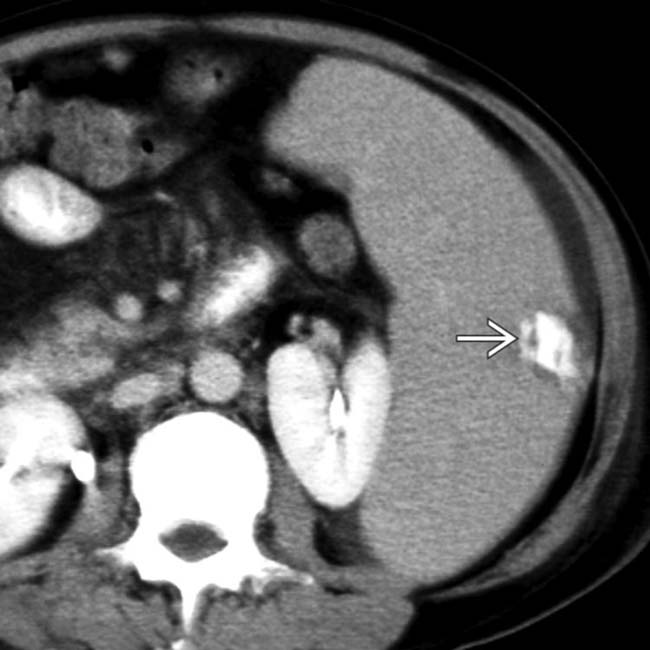
 . The absence of capsular retraction (volume loss) confirms the acute nature of the infarcts.
. The absence of capsular retraction (volume loss) confirms the acute nature of the infarcts.




































































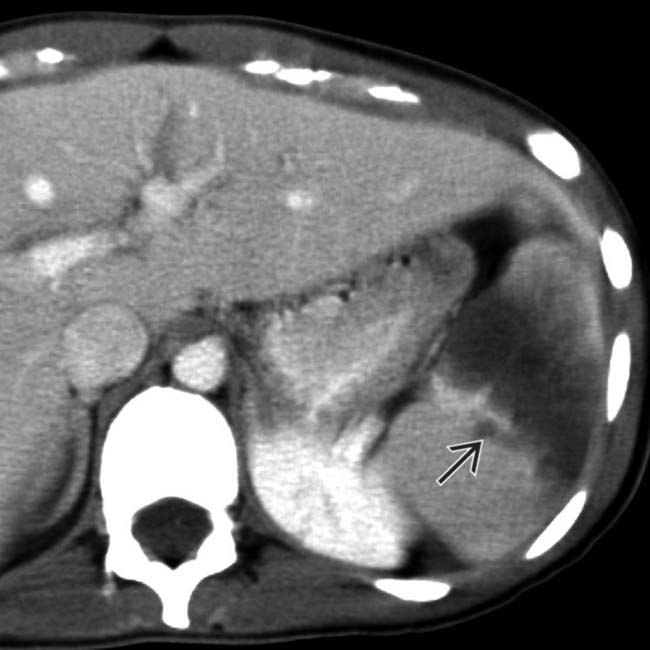
 .
.
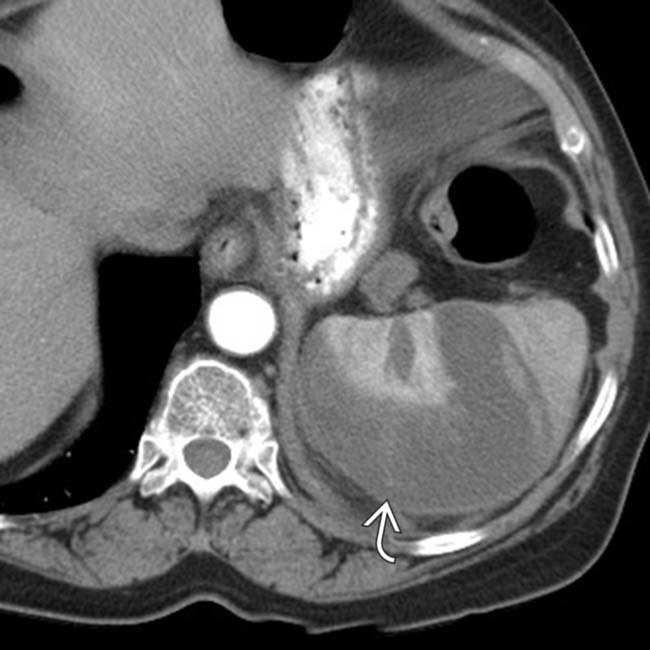
 .
.
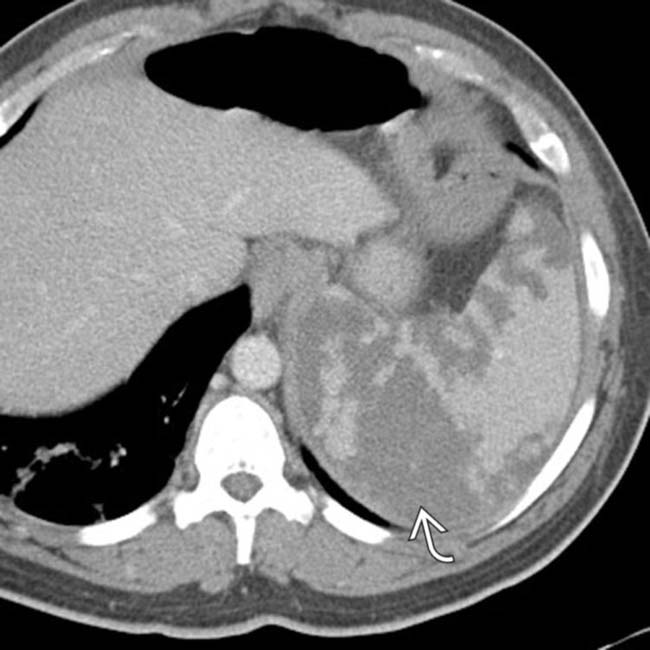
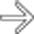
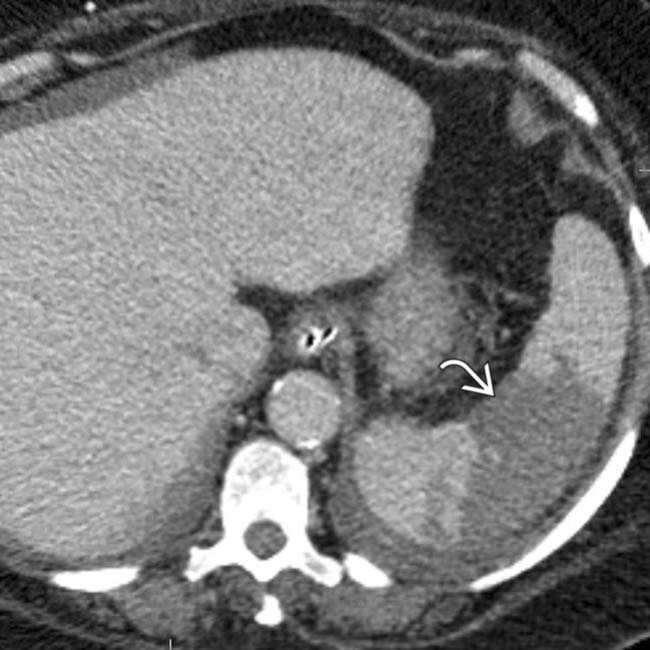
 , appearing as wedge-shaped areas of nonenhancement.
, appearing as wedge-shaped areas of nonenhancement.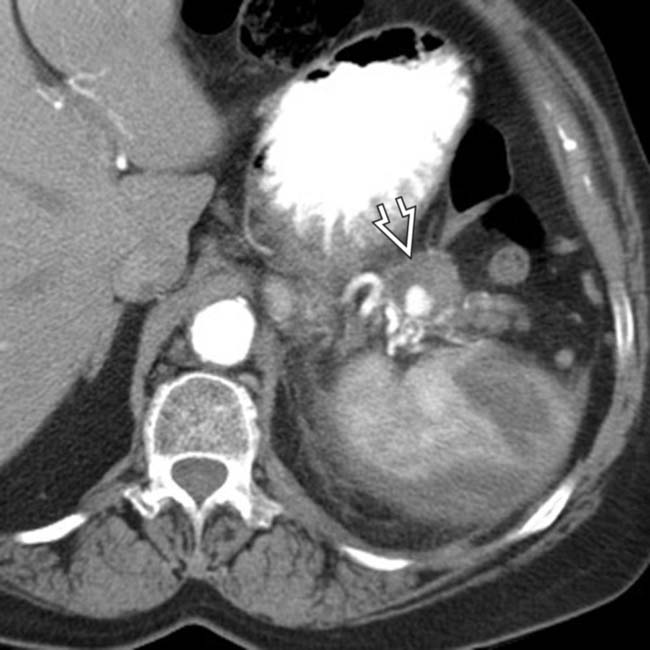
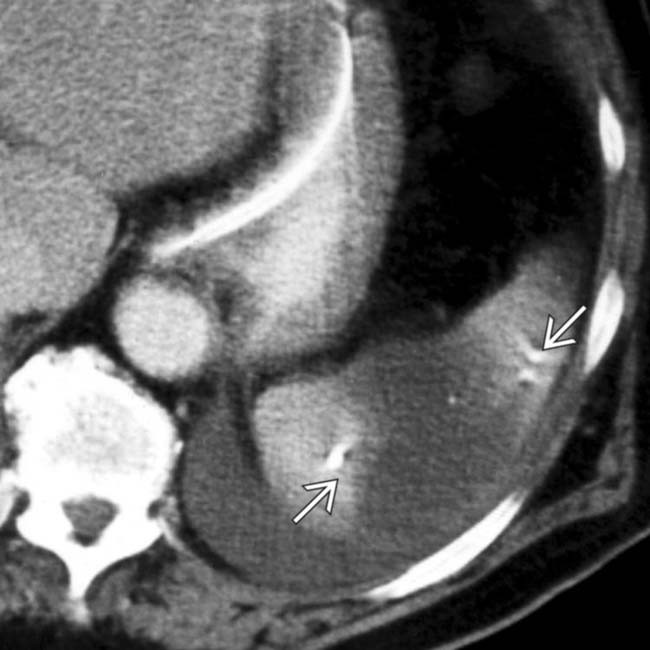
 thought to be the cause for the patient’s infarct (likely as a result of emboli from the aneurysm)
thought to be the cause for the patient’s infarct (likely as a result of emboli from the aneurysm)