Splenic Conditions
The essential role of the spleen in the defense against bacterial organisms is well documented. King and Schumacher first described the susceptibility of splenectomized infants to infection in 1952.1 The immunologic role of the spleen led pediatric surgeons to initiate a nonoperative approach to splenic injuries in children which has evolved into the preferred method for treating children and also adults.2 Currently, the primary role of splenic surgery is in the management of hematologic disorders. The most significant change in management has been the introduction of laparoscopic splenectomy in adults by Delaitre and Maignien3 and subsequently in children by Tulman and Holcomb.4
Embryology, Anatomy and Physiology
The splenic primordium develops as a mesenchymal bulge in the dorsal mesogastrium between the stomach and the pancreas, initially observed at the 8–10 mm embryo stage. A true epithelium is noted at the 10–12 mm stage as sinusoids communicate with capillaries. The spleen produces white and red cells by the fourth month of fetal life, although this function ceases later in gestation. The anatomic arrangement of the spleen is consistent with the various functions of the spleen. The splenic artery branches into segmental vessels, which further branch into trabecular arteries. After further bifurcations, small arteries enter the white pulp, which is composed of lymphocytes and macrophages arranged as a germinal center around the central artery. The central artery delivers particulate material into the white pulp, an arrangement that may facilitate antibody formation in response to particulate antigens.5–8 The red pulp consists of the endothelial cords of Billroth, which receive the blood after it passes through the white pulp. The red pulp destroys old and defective cells. The spleen also removes Howell–Jolly bodies (nuclear remnants), Heinz bodies (denatured hemoglobin), and Pappenheimer bodies (iron granules). These particles are noted on peripheral smear after splenectomy. The immune response occurs in the white pulp as antigens come in contact with macrophages and helper T-cells. T-cells initiate cytokine synthesis, and activated T-cells circulate to modulate the response. A humoral response occurs as macrophages and helper T-cells come in contact with antigens.9
Splenic function also involves removal of particulate matter as well as production of nonspecific opsonins, which further activate the complement system. In addition, the spleen serves as a biologic filter. If little antibody is available for opsonization of bacteria, the spleen assumes a greater role. This may be a factor in the age-related differences in postsplenectomy infections in young children who lack an adequate antibody response.6 The spleen also serves as a reservoir for platelets and factor VIII.
Anatomic Abnormalities
Asplenia and Polysplenia
Asplenia is often noted with complex congenital heart disease as well as bilateral ‘right-sidedness’ such as bilateral three-lobed lungs and right-sided stomach and central liver.10 Intestinal malrotation has also been observed with asplenia.11 These infants are at risk for overwhelming infection and should receive antibiotics for prophylaxis.
Polysplenia usually consists of a cluster of very small splenic masses and is often found with biliary atresia. Other associated conditions include a preduodenal portal vein, situs inversus, malrotation, and cardiac defects.10 These children have adequate splenic immune function.
Wandering Spleen
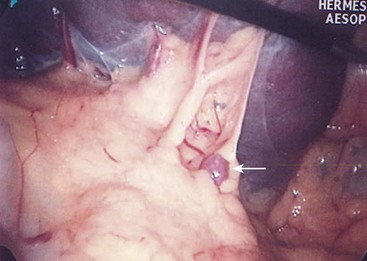
This condition is characterized by a lack of ligamentous attachments to the diaphragm, colon, and retroperitoneum, resulting in a mobile spleen. This is likely due to failure of development of the splenic ligaments from the dorsal mesentery.12 Children can present with an abdominal mass and episodic pain, but also with torsion and infarction.13,14 Pancreatitis has also been noted as a presenting sign.15 Splenopexy is the preferred method of treatment and can be performed with placement of the spleen into a mesh basket, suture splenopexy, colonic displacement with gastropexy, placement in an omental basket, or placement in an extraperitoneal pocket.16–20 The laparoscopic approach is the preferred technique, and the use of an absorbable or nonabsorbable mesh with fixation in the left upper quadrant is demonstrated in Figure 47-1.21,22 Placement of the spleen in an extraperitoneal pocket is seen in Figure 47-2. Torsion with infarction requires splenectomy. Cases of chronic torsion have also been reported with massive splenomegaly which may necessitate splenectomy.23
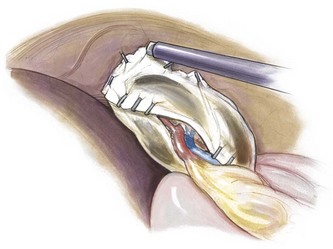
FIGURE 47-1 Laparoscopic splenopexy with placement of the spleen between two sheets of absorbable mesh with fixation in the left upper quadrant. (© IUSM Visual Media.)
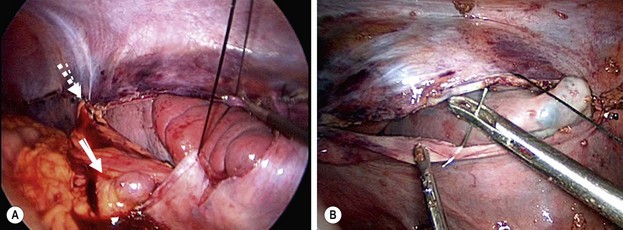
FIGURE 47-2 (A) The upper pole of the spleen was placed in the retroperitoneal pouch, and the upper aspect of the pouch (dotted arrow) was closed with interrupted sutures. Note the splenic vessels (solid arrow) coursing into the spleen. A generous opening was left in the pouch for these vessels so that the vessels would not be compressed by closure of the pouch. (B) One of the interrupted silk sutures is being placed to approximate the peritoneal flaps over the spleen. At this point, most of the spleen has been placed into the extraperitoneal pouch. (From Upadhyaya P, St. Peter SD, Holcomb GW III. Laparoscopic splenopexy and cystectomy for an enlarged wandering spleen and splenic cyst. J Pediatr Surg 2007;42:E23–7. Reprinted with permission.)
Accessory Spleens
Accessory spleens have been noted in 15–30% of children, with a large series noting a 19% rate.21 Accessory spleens likely originate from mesenchymal remnants that fail to fuse with the main splenic mass, with most (75%) located near the splenic hilum (Fig. 47-3). Other locations that must be evaluated during surgery include the lesser sac along the splenic vessels, omentum, and retroperitoneum. Eighty-six per cent of accessory spleens are single, 11% have two, and 3% have three or more.24,25 A missed accessory spleen at the time of planned total splenectomy can lead to recurrence of the primary disease process, which in cases of immune thrombocytopenic purpura (ITP) is early and with hereditary spherocytosis (HS) is later.26–28
Splenic Gonadal Fusion
This condition in which the left gonad and the spleen are attached is a result of early fusion between the two structures prior to descent of the testes.29 The remnant can be a continuous band (see Fig 50-6) or discontinuous with splenic tissue attached to the gonad. A splenic remnant has also been noted in the left scrotum as an accessory splenic remnant type of abnormality.30
Splenic Cysts
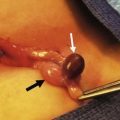
Cysts of the spleen are most frequently primary splenic cysts containing an epithelial lining and are also referred to as epithelial or epidermoid cysts (Fig. 47-4). Post-traumatic pseudocysts are occasionally seen. Inclusion of surface mesothelium into the splenic parenchyma is the most likely etiology of epithelial cysts. They may present with symptoms related to their size with gastric compression or pain, an abdominal mass, rupture, or infection with abscess.31,32 Simple cysts less than 5 cm can be observed, but cysts that are enlarging, symptomatic, or larger than 5 cm require treatment. Most symptomatic cysts are larger than 8 cm.31 Percutaneous aspiration and sclerosis utilizing alcohol or other agents have been reported with variable success.33,34
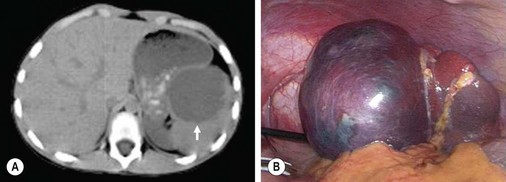
FIGURE 47-4 (A) A large epithelial splenic cyst (arrow) is seen on the computed tomography scan. (B) At laparoscopy, the large cyst (seen in A) is seen to occupy most of the spleen.
Marsupialization is commonly performed but has been associated with a high recurrence rate if an adequate amount of cyst wall is not removed (Fig. 47-5).35 In addition, a high recurrence rate with laparoscopic partial excision has also been observed.36,37 However, others have had good success with this technique, and many also recommend partial splenectomy associated with cyst resection.38,39 Our group has reported good results with partial splenectomy, emphasizing resection of a margin of normal spleen so that the cut surfaces cannot oppose which might lead to recurrence.21 Other techniques involve lining the cyst with Surgicel (Ethicon, Inc., Somerville, NJ) and omentopexy.40
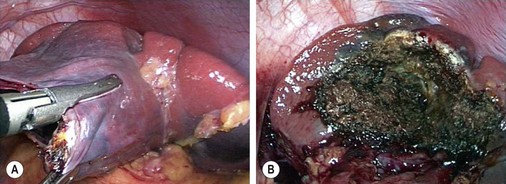
FIGURE 47-5 (A) The wall of the large epithelial splenic cyst seen in Figure 47-4 is being excised. (B) The cyst was marsupialized and the remnant lining of the cyst was ablated with the argon beam coagulator.
Indications for Splenectomy
Hereditary Spherocytosis
HS, an autosomal dominant condition, is the most common inherited red cell disorder among Northern European descendants, with approximately 25% of affected children representing new mutations. Defects in red cell proteins ankyrin or spectrin result in poorly deformable spherocytes. Most affected children have anemia, an elevated reticulocyte count, and a mild elevation in bilirubin concentration. The degree of hemolysis can vary, with some only having a mild anemia. Spherocytes on peripheral smear along with a positive osmotic fragility test confirms the diagnosis. Affected children may develop an aplastic crisis associated with parvovirus B19 infection with suppression of bone marrow red cell production and ongoing splenic red cell destruction.41 Splenectomy is usually performed for moderate-to-severe anemia. If possible, splenectomy is delayed until 5 to 6 years of age to decrease the likelihood of overwhelming postsplenectomy infection (OPSI). Splenomegaly is common in these patients. Gallstones are also often found, and an ultrasound evaluation of the gallbladder should be performed before splenectomy. The presence of gallstones in children undergoing splenectomy has been noted in 27% of those younger than age 10 years compared with 56% in children 10 years of age or older.21
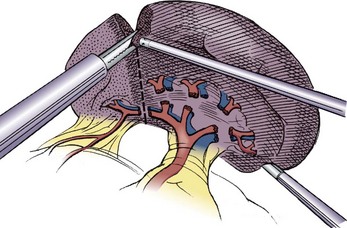
Partial splenectomy is an attractive alternative to total splenectomy in an attempt to remove enough spleen to alleviate the anemia while preserving adequate spleen to prevent OPSI (Fig. 47-6). This may be particularly useful in young children requiring splenectomy, but the long-term results are not known.
Immune Thrombocytopenic Purpura
ITP occurs due to antiplatelet autoantibodies which subsequently are destroyed in the spleen. In most children, it is primary (idiopathic), whereas in some it may be secondary to lupus, human immunodeficiency virus, malignancy, or hepatitis C infection. Most children (80%) have acute ITP that resolves with simple observation or medical management. Most treatment plans target a decrease in platelet destruction. Management includes corticosteroids, which may have their effect by inhibiting the reticuloendothelial binding of platelet/antibody complexes; intravenous immunoglobulin (IVIG), which inhibits the Fc receptor binding of platelets by macrophages; or Rho(D) immunoglobulin in Rh-positive children, which bind red cells that then saturate the splenic receptors, allowing the platelets to avoid destruction.
Response to corticosteroids, IVIG, or both, have been thought to be excellent predictors of successful outcome with splenectomy.42,43 However, two recent studies have failed to confirm this prior assumption. In a study of 19 children, Wood et al. failed to identify a positive correlation between preoperative medical treatment and response to splenectomy. In fact, they found an inverse relationship between the preoperative steroid response and response to splenectomy, with all nonresponders to steroids being complete responders to splenectomy.44 In a similar study from our institution, 31 of 37 (84%) children with ITP had complete response to splenectomy, and 6 (16%) had partial response.45 Of the eight with no response to steroids, six had a complete response and two had a partial response to splenectomy which suggests that failure to respond to steroids should not preclude splenectomy as definitive therapy.
Some children with ITP fail to respond to medical treatment, whereas others develop relapse when the treatment is stopped. In some of these patients, further therapy may include rituximab, a monoclonal antibody against CD20-positive B-cells. This depletes the B-cells and is somewhat of a ‘medical splenectomy.’ Other treatment modalities include azathioprine, cyclophosphamide, danazol, and mycophenolate mofetil.46 Platelet production may be decreased in ITP. Thus, the use of thrombopoietic agents has emerged46 and successful therapy with agents such as romiplostim (AMG 531, Amgen Inc., Thousand Oaks, CA) has been reported.47 A recent follow-up report in adults noted that those treated with romiplostim (by weekly continuous infusion) had a higher rate of platelet response and a lower incidence of splenectomy than those treated with standard medical therapy.48
Children with thrombocytopenia for longer than six months are considered to have chronic ITP and are candidates for splenectomy. The response to splenectomy has been excellent in children who have responded to medical management. In adults, the response is not as high. Accessory spleens or residual splenic tissue has been identified in up to 50% of adult patients with ITP, thus emphasizing both the importance of accessory spleen detection at exploration as well as the need to avoid parenchymal disruption.26
Sickle Cell Disease
A recent report of 53 children with SCD less than 4 years of age at the time of splenectomy found that 5.7% died during the 15 year study with one (1.8%) dying of OPSI.49 The authors concluded that it is reasonable to perform a splenectomy around age 2 years if a child with SCD has had a serious episode of sequestration.
Gaucher Disease
Gaucher disease is characterized by a deficiency of the enzyme β-glucocerebrosidase, resulting in excessive glucocerebroside in the macrophages of the spleen, liver, bone marrow, and lungs. Splenomegaly may be severe, and both partial and total splenectomy have been utilized to alleviate the symptomatic hypersplenism and to decrease destruction of the red blood cells, leukocytes, and platelets.50 However, massive bleeding and death has been reported several months postoperatively in a child with Gaucher disease who underwent partial splenectomy.51
Splenectomy
Open Splenectomy
The open technique through a left upper quadrant incision is usually reserved for massive splenomegaly. The initial division of the splenorenal, splenocolic, and splenophrenic ligaments allows the spleen to be mobilized from the left upper quadrant and out of the abdominal cavity. The short gastric vessels are divided initially, followed by the hilar vessels. A careful search must be undertaken for accessory spleens. A lateral muscle-splitting approach has been reported with a 2.7-day length of stay which is comparable to some early laparoscopic series but longer than more recent series.21,52,53
Laparoscopic Splenectomy
Laparoscopic splenectomy has evolved over the past 15 to 20 years to become the preferred approach for splenectomy.3,4 Less pain, shorter hospitalization, faster return to regular activities, and smaller scars are the main advantages over open splenectomy. However, it is associated with a longer operative time and can be difficult in patients with splenomegaly. The main technical advances have been the result of smaller instrumentation and advanced energy sources such as the Harmonic Scalpel (Ethicon Endosurgery Inc, Cincinnati, OH) and Ligasure (Valley Lab, Tyco Healthcare Group, Boulder, CO). At our institution, we primarily utilize the Ligasure because it allows division of vessels up to 7 mm.
The most significant initial concern for the laparoscopic approach was related to accessory spleen detection. However, in adult studies and comparison pediatric series, similar rates of accessory spleen detection at laparoscopic and open splenectomy have been noted.26,27,53–56 For the laparoscopic operation, most surgeons utilize a lateral approach with slight elevation of the left flank. One technique to improve the ease of port placement is to have the patient’s left side initially elevated approximately 45° rather than the true lateral position. The operating table is then tilted to the patient’s left to achieve a flat position for the port placement and then is tilted to the patient’s right to achieve a lateral position for the procedure (Fig. 47-7). The surgeon and assistant stand on the patient’s right. In young children and patients with small spleens, the upper midline instruments (3 mm) can be inserted without cannulas because these instruments are not removed during the procedure (Fig. 47-8). The first assistant holds the two upper midline instruments to provide elevation of the spleen and traction on surrounding tissues. The surgeon holds the camera (5 mm, 30–45° telescope) in the left hand and the energy source in the right hand.
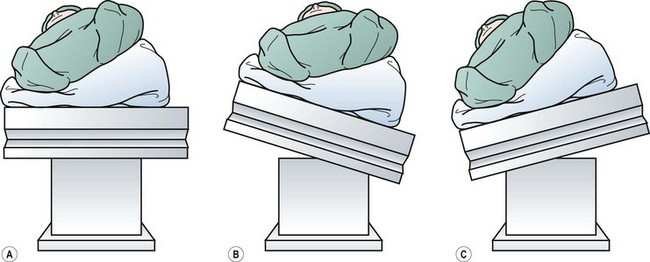
FIGURE 47-7 (A) Initial positioning of the patient before any movement of the table. (B) The table has been tilted to the patient’s left to obtain a near supine position of the patient for port placement. (C) The table is then rotated back to the patient’s right to achieve a right lateral decubitus position for the operation. (From Rescorla FJ. Laparoscopic splenectomy. In: Holcomb GW III, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 121–6. Reprinted with permission.)
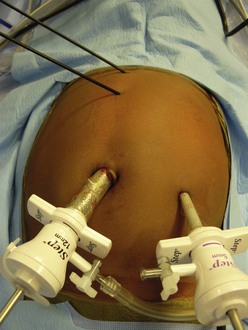
FIGURE 47-8 Placement in a child of 3 mm midline instruments without the use of cannulas. The umbilical port is 12 mm, and the left lower quadrant port is 5 mm.
Initially, the splenocolic ligament is divided with the energy device, allowing the splenic flexure to fall away from the spleen. The inferior portion of the gastrosplenic ligament is divided, and the surgeon works in a cephalad direction dividing the short gastric vessels and opening the lesser sac (Fig. 47-9). The most superior short gastric vessels are often very short, and care must be taken to avoid injury to the stomach or diaphragm. This is often the most difficult part of the procedure. The lesser sac should be inspected for the presence of accessory spleens. The splenophrenic ligament is divided to fully mobilize the upper pole. At this point, the hilum can be approached. It is often easiest to divide the splenorenal ligament because this allows the option to use the endovascular stapler on the hilum. In the early era of laparoscopic splenectomy, clips were applied to individual hilar vessels with division using endoscopic scissors. Most surgeons now use an endoscopic stapler which has the disadvantage of requiring a 12 mm port (Fig. 47-10). Proximity of the hilum and pancreas is the main issue in terms of positioning the stapler. Pancreatitis and pancreatic fistula have been reported, although many reports have confirmed the safety of this technique.57–60 The Ligasure has limited lateral thermal spread (<2 mm) and is relatively easy to use for dissection and ligation of the hilar vessels.61
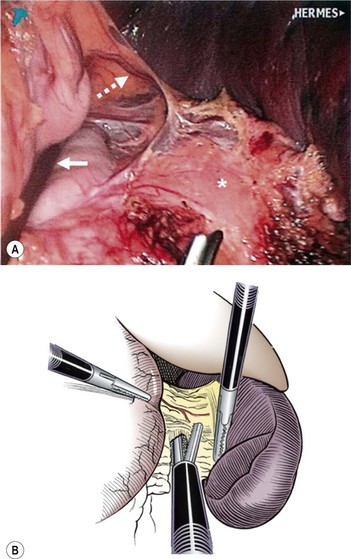
FIGURE 47-9 After division of the short gastric vessels using the ultrasonic scalpel, the lesser sac is entered. In the operative photograph, the stomach is being retracted by the assistant’s instrument (solid arrow). The ultrasonic scalpel, at the bottom of the photograph, is approaching one of the intact short gastric vessels (dotted arrow). The pancreas is marked with an asterisk. (From Rescorla FJ. Laparoscopic splenectomy. In: Holcomb GW III, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 121–6. Reprinted with permission.)
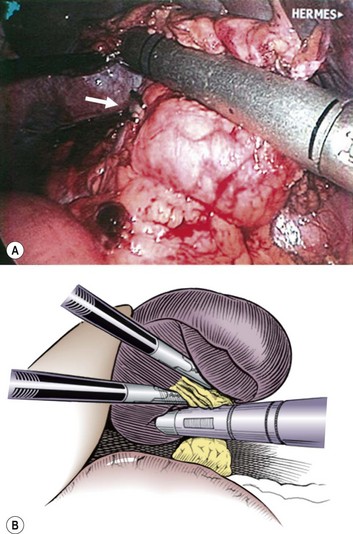
FIGURE 47-10 Once the spleen has been mobilized and is attached only through the hilar vessels, the camera is rotated to the left lower quadrant port and the stapler is introduced through the umbilical port. It is then placed across the hilar vessels, taking care not to incorporate a portion of the pancreas in the tissue to be divided. In the operative photograph, note that the splenic artery has been ligated with clips (arrow) before hilar division because the spleen was extremely large. (From Rescorla FJ. Laparoscopic splenectomy. In: Holcomb GW III, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 121–6. Reprinted with permission.)
One study comparing the endoscopic stapler to the Ligasure demonstrated safety and efficiency of both with a lower blood loss and conversion rate with the Ligasure.62 We utilize the Ligasure to ligate and divide the individual hilar vessels, usually with one application on the pancreas side without division and a second cautery toward the spleen with division.
The Endocatch II (Covidien, Norwalk, CT) can be inserted through a 15 mm umbilical port with the telescope moved to the left lower quadrant site. If a 12 mm port has been placed in the umbilicus, it can be removed and the bag can be introduced directly into the abdominal cavity. This bag has a 13 cm diameter opening with a 23 cm depth and can accommodate most spleens in children. A smaller bag can be used for smaller spleens. The neck of the bag is delivered through the umbilical incision, and either a finger or ring forceps is introduced to disrupt the capsule and morcellate the spleen (Fig. 47-11). Use of an ultrasonic morcellator and liposuction has been reported, but are not routinely used.63
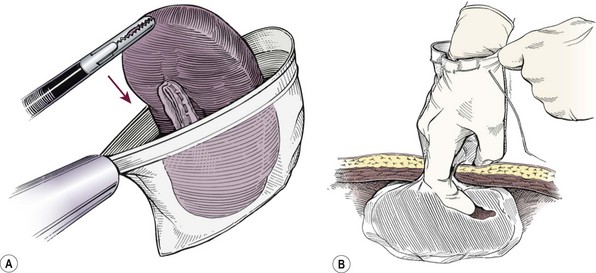
FIGURE 47-11 (A) After complete mobilization, the spleen is dropped into an endoscopic retrieval bag. (B) The neck of the bag is then exteriorized through the umbilicus, and the surgeon’s finger is used to fracture the splenic capsule. A combination of ring forceps and the surgeon’s finger is then used to remove the splenic fragments. (From Rescorla FJ. Laparoscopic splenectomy. In: Holcomb GW III, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 121–6. Reprinted with permission.)
These basic techniques are also utilized for treatment of splenic cysts and a wandering spleen. The laparoscopic approach is usually not applicable for traumatic splenic injuries because children who require operation are usually very unstable and unstable conditions require rapid control of bleeding. However, one report described laparoscopic splenectomy after splenic artery embolization in a 15-year-old girl with a traumatic injury.64
Single-port access splenectomy has been reported utilizing either a multi-instrument port or with separate umbilical fascial incisions after raising skin flaps.65–68 A standard laparoscopic splenectomy can be performed although some surgeons have recommended avoiding large patients with splenomegaly, and some have extended the umbilical incision to remove the spleen intact which appears to diminish the benefit of the laparoscopic approach.65,66 Several authors have utilized standard laparoscopic instruments without the use of roticulating graspers.67,68
Partial Splenectomy
Concerns about postsplenectomy infection have led to the concept of partial splenectomy. This is primarily utilized in children with HS but has also been utilized with Gaucher disease, hypersplenism with cystic fibrosis, splenic hamartomas, and splenic cysts.50,69,70 Partial splenectomy generally involves removal of 85–95% of the enlarged spleen with preservation of approximately 25% of the normal splenic size. In this approach, the remnant spleen is supplied by one or two short gastric vessels with division of all of the hilar vessels (see Fig. 47-6). An alternative approach, depending on the anatomy of the segmental vessels, is to leave the uppermost segmental vessel as the only blood supply. Although historically most partial splenectomies have been performed through an open technique, the laparoscopic approach is gaining popularity.21,71,72
The splenic parenchyma can be divided with a stapler, cautery, or energy device. Our group has reported 12 laparoscopic partial splenectomies.21 In all cases, a combination of Ligasure and cautery were utilized, often with a topical agent placed over the cut surface. After devascularization, a clear line of demarcation is usually noted. We divide the spleen 1 cm onto the ischemic side to minimize bleeding. We currently use cautery to divide the splenic parenchyma and then apply a topical hemostatic agent and omentum to the exposed parenchyma. The upper splenorenal and splenophrenic ligaments are left to avoid torsion. Another report of seven laparoscopic partial splenectomies described using the ultrasonic scalpel for parenchymal division and complete mobilization of the spleen.73 The authors then performed a splenopexy to prevent torsion. We have occasionally sutured the remaining parenchyma to the lateral abdominal wall superiorly near the diaphragm when the vascular anatomy required preservation of the lower pole vasculature rather than the upper pole.
Several series have noted an increase in red cell half-life, higher hemoglobin levels, and lower reticulocyte and bilirubin levels after partial splenectomy for spherocytosis.71,74 The lack of Howell–Jolly bodies on peripheral smear and a decreased number of pitted red cells is evidence of preservation of splenic phagocytic function. Normal levels of IgM- and IgG-specific antibody titers after Streptococcus pneumoniae have also been observed. Splenic regeneration occurs in all patients although the degree of regrowth does not correlate with hemolysis.72 The development of cholelithiasis after partial splenectomy varies from 7–22%, indicating ongoing hemolysis. Moreover, the need for subsequent total splenectomy has been reported.71,75 Rice and colleagues have popularized this procedure and summarized their experience in 29 children undergoing partial splenectomy.72 They noted decreased transfusion requirements, elimination of splenic sequestration, higher hematocrits, and lower reticulocyte counts and bilirubin levels. A recent multi-institutional study of 62 children with HS undergoing partial splenectomy noted a mean hemoglobin increase of 3.0 ± 1.4 g/dL and reticulocyte count decrease of 6.6 ± 6.6% at one year.76 An alternative technique has been described that leaves a 10 cm3 remnant as a small cylinder supplied by one vessel.77
Another recent report of nine children undergoing laparoscopic partial splenectomy (10–30% remnant) observed improved hemoglobin levels (12.9 g/dL vs 10.9 g/dL) and decreased reticulocyte counts.78 A second multicenter series of 11 partial splenectomies noted no need for subsequent total splenectomy with an average follow-up of 19 months (range, 4–53 months).73 A recent comparative study found that laparoscopic partial splenectomy patients had more pain and a longer hospitalization than open partial splenectomy.79 We have also noted an increased hospitalization with laparoscopic partial splenectomy (2.3 days) compared with an open operation (1.2 days).80
Complications and Controversies with Laparoscopic Splenectomy
Accessory Spleen Detection
The identification of residual splenic function in up to 50% of selected adults after laparoscopic splenectomy has led to concern about the ability to adequately detect accessory spleens at laparoscopy.26 Parenchymal injury at the time of laparoscopic splenectomy has also been associated with a higher rate of residual splenic function.26,27 Although preoperative computed tomography and splenic scintigraphy have been used to identify accessory spleens preoperatively, most surgeons have abandoned this approach.81
There are reports of equivalent detection rates of accessory spleens with laparoscopy.26,82 In reviewing several comparative pediatric series, accessory spleens were found in 20.3% of patients undergoing an open splenectomy and 20.2% at laparoscopy.53–56 Our report of over 200 laparoscopic splenectomies noted accessory spleens in 19%, and follow-up evaluation of over 300 laparoscopic splenectomies has confirmed this rate.21 Laparoscopic removal of missed accessory spleens has been reported with good success; however, the efficacy of accessory splenectomy in improving ITP ranges from 26–75%.83,84
Splenomegaly
Splenomegaly in children is usually due to HS. The optimal management of children with splenomegaly is controversial as most articles come from the adult literature. The benefits of the laparoscopic technique have been demonstrated in adults with spleens between 15–25 cm.85 Hand-assisted procedures have been utilized in adults with massive splenomegaly (700 g to >3 kg). In one report, a 7 cm right subcostal incision was used for hand access and also the retrieval site for a bowel bag with the spleen.86 In this report, the conversion rate was only 2.3%; however, 5% required reoperation for bleeding. The hand-assisted laparoscopic technique compared to an open approach in patients with splenomegaly (>20 cm) has resulted in less blood loss and shorter length of stay.87
A recent study in adults with massive splenomegaly (craniocaudad length ≥17 cm or weight ≥600 g) and supramassive splenomegaly (craniocaudad length ≥22 cm or weight ≥1600 g) comparing laparoscopic to open splenectomy found lower blood loss and shorter length of stay with the laparoscopic approach.88 This was accomplished at the expense of longer operative times and a 25% conversion rate, most frequently due to uncontrolled bleeding. Malignancy was the cause of the massive splenomegaly in the supramassive group, which is rare in children. The authors concluded that the laparoscopic approach was better than the open operation for massive splenomegaly, and at least comparable to hand-assisted laparoscopic splenectomy.
Conversion to Open Splenectomy
Splenomegaly and bleeding are the main reasons for conversion and has been reported in 1.3–2.8% of patients.54,81,89,90 A relatively recent multicenter report of 159 laparoscopic splenectomies found a conversion rate of 5% with bleeding the most common reason.91 This high conversion rate may have been due to surgeon experience as these data came from four centers over a ten-year time period. The largest pediatric series documented a 1.7% rate.21 Higher conversion rates have been noted with larger spleens, less surgeon experience, and obese patients.92 A large multicenter Italian registry study of 676 patients undergoing laparoscopic splenectomy found that body mass index and hematologic malignancy were independent predictors for intraoperative complications and conversion to an open splenectomy.93 Their overall conversion rate was 5.8%. Complications occurred in 17% with bleeding in 4.5% and splenic/portal vein thrombosis in 2.1%.
Operative Time
The laparoscopic approach has uniformly been associated with somewhat longer operative times than open splenectomy.53–56 A large series over 10 years noted a decrease in operative time from a mean of 110 ± 36 minutes in the early period to 86 ± 35 minutes in the later period as experience was gained.21 This operative time is compared with 83 minutes for open splenectomy in a previous report from the same institution.56
Postoperative Hospitalization
Comparative studies in children have consistently demonstrated a lower duration of hospitalization with the laparoscopic approach compared with the open technique, ranging from 2.5–4.9 days for the open operation and 1.3–3.6 days for the laparoscopic approach.21,54,55 Our series of over 200 cases noted the postoperative hospitalization to vary by diagnosis with HS at 1.23 days, ITP at 1.20 days, and SCD at 2.37 days.21 An Italian single-institution series of 33 children (2000–2005) undergoing splenectomy (19 laparoscopic, 14 open) noted a shorter hospitalization (5.5 ± 2.9 versus 8.7 ± 4.8) in the laparoscopic cohort but still considerably longer than contemporary studies in the USA.94 The increased length of stay in patients with SCD is secondary to the higher incidence of complications in this subgroup of patients, such as acute chest syndrome. Other retrospective series have confirmed the role of laparoscopy in children with SCD, noting a shorter hospitalization with the laparoscopic approach without an increase in postoperative pain or acute chest syndrome compared with open splenectomy.95,96 Splenomegaly itself, which is common in HS, does not appear to affect the postoperative hospitalization. An unreported benefit of the shorter hospitalization after each operation is the effect on the parent’s return to work. One study noted children return to full activity faster with laparoscopy compared with open splenectomy.97
Complications
Most pediatric series have had relatively few complications with the laparoscopic technique. A meta-analysis of adult and pediatric studies reported between 1991 and 2002 noted a complication rate of 15.5% for laparoscopic and 26.6% for open splenectomy (p < 0.0001).98 The laparoscopic group had fewer pulmonary, wound, and infectious complications but more hemorrhagic complications when conversions for bleeding were included. A large adult comparative study noted equivalent grade I and II complications (minor, potentially life threatening) but higher grade III and IV (residual or lasting disability, death) with the open approach compared with laparoscopy.99 Symptomatic splenosis has been reported in ITP due to rupture of an Endocatch II bag with thrombocytopenia occurring 13 months after the initial laparoscopic splenectomy.100 Our review of over 200 pediatric laparoscopic splenectomies found an overall complication rate of 11% with no wound infections or deaths.21 Postoperative ileus, acute chest syndrome, and bleeding requiring transfusion were the most common complications. The complication rate was 22% among the children with SCD and only 8.3% among those with other conditions (p = 0.0083). This report documented one (0.5%) symptomatic portal vein thrombosis.
Splenic vein or portal vein thrombosis is a rare complication after splenectomy but may be more common than is clinically appreciated, and is particularly common in patients with splenomegaly.101,102 A small adult study identified a 50% incidence of portal or splenic vein thrombosis, with splenic weight the strongest predictor of thrombosis.103 Another prospective study in adults identified an incidence of 4.79%, and noted spleen weight over 650 g and a platelet count over 650,000/mm3 to be associated with this complication.104 A retrospective evaluation of splenic weight and portal vein thrombosis noted rates of 14%, 40%, 45%, and 71% for weights 700 g–1 kg, 1–2 kg, 2–3 kg, and >3 kg, respectively.86 Despite early anticoagulation, 13% of the entire group progressed to complete thrombosis. A prospective series in children noted an incidence of 5.88% with the same risk factors.105 Of the patients with total splenic vein thrombosis, over 50% were symptomatic with fever and abdominal pain, emphasizing the need to evaluate for this condition in patients with these symptoms. Most authors recommend treatment with antithrombotic and antiplatelet therapy.
Postsplenectomy Sepsis
OPSI was initially reported by King and Shumacker in a group of five infants undergoing splenectomy for HS.1 The actual risk of OPSI is difficult to determine because most of the data was accumulated before the routine use of preoperative vaccinations. Data from the 1990s note the rate of OPSI between 0.13–8.1% in children and adolescents less than 15 years of age compared with 0.28–1.9% in adults.106–108 Most authors quote the current OPSI rate between 3.5–4.4%.75,107,108 Some have also noted a higher mortality in children younger than 4 years of age (8.1%) compared with a lower rate (3.3%) in older children.106–108 Most deaths occur within four years of splenectomy.109,110 The causative organism is likely Streptococcus pneumoniae which has been found in 50–90% of all infections.111,112 Pneumococcus is responsible for 60% of all fatal infections, followed by Haemophilus influenzae, meningococcus, and Group A Streptococcus.113 Vaccinations have been shown to decrease the risk of bacteremia.113,114
A single-institution study compared two time periods.107 The first one was prior to immunizations and antibiotics. The second era (with a 70% immunization rate and 100% antibiotic prophylaxis rate) noted a decrease in OPSI (6% to 3.8%) and mortality (3.9% to 0.9%). This study found age to be important as the rate of infection was 13.8% for those children younger than 6 years of age compared with 0.5% in older children. Although older studies noted mortality rates of 50% with OPSI, more recent studies have a rate of around 10%.107,115,116
Routine immunization against S. pneumoniae, H. influenzae, and meningococcus as well as postoperative antibiotics prophylaxis (usually penicillin) is recommended by most authors. Although the optimal length of antibiotic prophylaxis is unclear, the highest rate of OPSI appears to occur within the first 2 years. An adult study of sepsis in patients splenectomized as children noted an estimated frequency of late postsplenectomy infections of 0.69 and deaths at 0.46 per 1,000 patient years.117 Of interest, one of the surviving patients had low antibody levels against pneumococcal serotypes despite pneumococcal meningitis and subsequent vaccinations, suggesting that nonresponders may be at increased risk for OPSI.
References
1. King, H, Shumacker, HB, Jr. Splenic studies: I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952; 136:239–242.
2. Douglas, GJ, Simpson, JS. The conservative management of splenic trauma. J Pediatr Surg. 1971; 6:565–570.
3. Delaitre, B, Maignien, B. Splenectomy by the coelioscopic approach: Report of a case. Presse Med. 1991; 20:2263.
4. Tulman, S, Holcomb, GW, III., Karamanoukian, HL, et al. Pediatric laparoscopic splenectomy. J Pediatr Surg. 1993; 26:689–692.
5. Nossal, GJV, Austin, CM, Pye, J, et al. Antigens in immunity: XII. Antigen trapping in the spleen. Int Arch Allergy Appl Immunol. 1966; 29:368–383.
6. Pearson, HA, The spleen and disturbances of splenic function. Hematology of Infancy and Childhood. 5th ed. Nathan, DG, Orkin, SH, eds. Hematology of Infancy and Childhood; vol. II. WB Saunders, Philadelphia, 1998:1051–1068.
7. Rowley, DA. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950; 64:289.
8. Rowley, DA. The formation of circulating antibody in the splenectomized human being following intravenous injection of heterologous erythrocytes. J Immunol. 1950; 65:515.
9. Meyer, AA. Spleen. In: Greenfield LJ, Mulholland MW, Oldham KT, et al, eds. Surgery. 2nd ed. Philadelphia: Lippincott-Raven; 1997:1262–1281.
10. Anderson, C, Devine, WA, Anderson, RH, et al. Abnormalities of the spleen in relation to congenital malformations of the heart: A survey of necropsy findings in children. Br Heart J. 1990; 63:122–128.
11. Tu, ST, Wu, JM, Yeh, BH, et al. Intestinal obstruction in asplenia syndrome: Report of three cases. Acta Paediatr Sin. 1994; 35:70–77.
12. Skandalakis, LJ, Gray, SW, Ricketts, R, et al. The spleen. In: Skandalakis JE, Gray SW, eds. Embryology for Surgeons. 2nd ed. Baltimore: Williams & Wilkins; 1994:334–365.
13. Greig, JD, Sweet, EM, Drainer, IK. Splenic torsion in a wandering spleen, presenting as an acute abdominal mass. J Pediatr Surg. 1994; 29:571–572.
14. Schmidt, SP, Andrews, HG, White, JJ. The splenic snood: An improved approach for the management of the wandering spleen. J Pediatr Surg. 1992; 27:1043–1044.
15. Lebron, R, Self, M, Mangram, A, et al. Wandering spleen presenting as recurrent pancreatitis. J Soc Laparoendosc Surg. 2008; 12:310–313.
16. Allen, KB, Andrews, G. Pediatric wandering spleen—the case for splenopexy: Review of 35 reported cases in the literature. J Pediatr Surg. 1989; 24:432–435.
17. Lacreuse, I, Moog, R, Kauffmann, I, et al. Laparoscopic splenopexy for wandering spleen in a child. J Laparoendosc Adv Surg Tech. 2007; 17:255–257.
18. Caracicolo, F, Bonatti, PC, Castruci, G, et al. Wandering spleen: Treatment with colonic displacement. J R Coll Surg Edinb. 1986; 31:242–244.
19. Stringel, G, Soucy, P, Mercer, S. Torsion of the wondering spleen: Splenectomy or splenopexy? J Pediatr Surg. 1982; 17:373–375.
20. Upadhyaya, P, St Peter, SD, Holcomb, GW, III. Laparoscopic splenopexy and cystectomy for an enlarged wandering spleen and splenic cyst. J Pediatr Surg. 2007; 42:E23–E27.
21. Rescorla, FJ, West, KW, Engum, SA, et al. Laparoscopic splenic procedures in children: Experience in 231 children. Ann Surg. 2007; 246:683–688.
22. Palanivelu, C, Rangarajan, M, Senthilkumar, DR, et al. Laparoscopic mesh splenopexy (sandwich technique) for wandering spleen. J Soc Laparoendosc Surg. 2007; 11:246–251.
23. Misawa, T, Yoshida, K, Shiba, H, et al. Wandering spleen with chronic torsion. Am J Surg. 2008; 195:504–505.
24. Halpert, B, Alden, ZA. Accessory spleens in or at the tail of the pancreas. Arch Pathol. 1964; 77:652.
25. Halpert, B, Gyorkey, F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol. 1959; 32:165.
26. Gigot, JF, Jamar, F, Ferrant, A, et al. Inadequate detection of accessory spleens and splenosis with laparoscopic splenectomy: A shortcoming of the laparoscopic approach in hematologic diseases. Surg Endosc. 1998; 12:101–106.
27. Targarona, EM, Espert, JJ, Balagué, C, et al. Residual splenic function after laparoscopic splenectomy: A clinical concern. Arch Surg. 1998; 133:56–60.
28. Crawford, DL, Pickens, PV, Moore, JT. Hypertrophied splenic remnants in hereditary spherocytosis. Contemp Surg. 1998; 53:103–104.
29. Putschar, WGJ, Manion, WC. Splenic-gonadal fusion. Am J Pathol. 1986; 32:15.
30. Emmett, JM, Dreyfuss, ML. Accessory spleen in the scrotum: Review of literature on ectopic spleens and their associated surgical significance. Ann Surg. 1943; 117:754.
31. Tsakayannis, DE, Mitchell, K, Kozakewich, HPW, et al. Splenic preservation in the management of splenic epidermoid cysts in children. J Pediatr Surg. 1995; 30:1468–1470.
32. Rathaus, V, Zissin, R, Goldberg, E. Spontaneous rupture of an epidermoid cyst of the spleen: Preoperative ultrasonographic diagnosis. J Clin Ultrasound. 1991; 19:235–237.
33. Akhan, O, Baykan, Z, Oguzkurta, L, et al. Percutaneous treatment of a congenital splenic cyst with alcohol: A new therapeutic approach. Eur Radiol. 1997; 7:1067–1070.
34. Moir, C, Guttman, F, Jequier, S, et al. Splenic cysts: Aspiration, sclerosis, or resection. J Pediatr Surg. 1989; 24:646–648.
35. Morgenstern, L. Nonparasitic splenic cysts: Pathogenesis, classification, and treatment. J Am Coll Surg. 2002; 194:306–314.
36. Fisher, JC, Gurung, B, Cowles, RA. Recurrence after laparoscopic excision of nonparasitic splenic cysts. J Pediatr Surg. 2008; 43:1644–1648.
37. Schier, F, Waag, KL, Ure, B. Laparoscopic unroofing of splenic cysts results in a high rate of recurrences. J Pediatr Surg. 2007; 42:1860–1863.
38. Fan, H, Zhang, D, Zhao, X, et al. Laparoscopic partial splenectomy for large splenic epidermoid cyst. Chin Med J. 2011; 124:1751–1753.
39. Keckler, SJ, Peter, SD, Tsao, K, et al. Laparoscopic excision of splenic cysts: A comparison to the open approach. Eur J Pediatr Surg. 2010; 20:287–289.
40. McColl, RJ, Hochman, DJ, Sample, C. Laparoscopic management of splenic cysts: Marsupialization, cavity lining with surgicel and omentectomy to prevent recurrence. Surg Laparosc Endosc Percutan Tech. 2007; 17:455–458.
41. Young, NS, Brown, KE. Parvovirus B19. N Engl J Med. 2004; 350:2006–2007.
42. Davis, PW, Williams, DA, Shamberger, RC. Immune thrombocytopenia: Surgical therapy and predictors of response. J Pediatr Surg. 1991; 26:407–413.
43. Holt, D, Brown, J, Terrill, K, et al. Response to intravenous immunoglobulin predicts splenectomy response in children with immune thrombocytopenic purpura. Pediatrics. 2003; 111:87–89.
44. Hood, JH, Partrick, DA, Hays, T, et al. Predicting response to splenectomy in children with immune thrombocytopenic purpura. J Pediatr Surg. 2010; 45:140–144.
45. Hollander, LL, Leys, CM, Weil, BR, et al. Predictive value of response to steroid therapy on response to splenectomy in children with immune thrombocytopenic purpura. Surgery. 2011; 150:643–648.
46. Bromberg, ME. Immune thrombocytopenic purpura-the changing therapeutic landscape. N Engl J Med. 2006; 355:1643–1645.
47. Bussel, JB, Kluter, DJ, Phil, D, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006; 355:1672–1681.
48. Kuter, DJ, Phil, D, Rummel, M, et al. Romiplostim or standard care in patients with immune thrombocytopenia. NEJM. 2010; 363:1889–1961.
49. Lesher, AP, Kalpatthi, R, Glenn, JB, et al. Outcome of splenectomy in children younger than 4 years with sickle cell disease. J Pediatr Surg. 2009; 44:1134–1138.
50. Fonkalsrud, EW, Philippart, M, Feig, S. Ninety-five percent splenectomy for massive splenomegaly: A new surgical approach. J Pediatr Surg. 1990; 25:267–269.
51. Holcomb, GW, III., Greene, HL. Fetal intra-abdominal hemorrhage following partial splenectomy for Gaucher’s disease. J Pediatr Surg. 1993; 18:1572–1574.
52. Geiger, JD, Dinh, VV, Teitelbaum, DH, et al. The lateral approach for open splenectomy. J Pediatr Surg. 1998; 33:1153–1156.
53. Farah, RA, Rogers, ZR, Thompson, RW, et al. Comparison of laparoscopic and open splenectomy in children with hematologic disorders. J Pediatr. 1997; 131:41–46.
54. Minkes, RK, Lagzdins, M, Langer, JC. Laparoscopic versus open splenectomy in children. J Pediatr Surg. 2000; 35:699–701.
55. Reddy, VS, Phan, HH, O’Neill, JA, et al. Laparoscopic versus open splenectomy in the pediatric population: A contemporary single-center experience. Am Surg. 2001; 67:859–863.
56. Rescorla, FJ, Breitfeld, PP, West, KW, et al. A case-controlled comparison of open laparoscopic splenectomy in children. Surgery. 1996; 124:670–676.
57. Misawa, T, Yoshida, K, Iida, T, et al. Minimizing intraoperative bleeding using a vessel sealing system and splenic hilum hanging maneuver in laparoscopic splenectomy. J Hepatobiliary Pancreat Surg. 2009; 16:786–791.
58. Gelmini, R, Romano, F, Quaranta, N, et al. Sutureless and stapleless laparoscopic splenectomy using radiofrequency: LigaSure device. Surg Endosc. 2006; 20:991–994.
59. Kennedy, JS, Stranahan, PL, Taylor, KD, et al. High burst strength, feedback controlled bipolar vessel sealing. Surg Endosc. 1998; 12:876–878.
60. Vecchio, R, Marchese, S, Swehli, E, et al. Splenic hilum management during laparoscopic splenectomy. J Laparoendosc Adv Surg Tech. 2011; 21:717–720.
61. Machado, NO, Kindy, NA, Chopra, PJ. Laparoscopic splenectomy using LigaSure. JSLS. 2010; 14:547–552.
62. Romano, F, Gelmini, R, Caprotti, R, et al. Laparoscopic splenectomy: Ligasure versus EndoGIA: A comparative study. J Laparoendosc Adv Surg Tech. 2007; 17:763–767.
63. Lai, PB, Leung, KL, Ho, WS, et al. The use of liposucker for spleen retrieval after laparoscopic splenectomy. Surg Laparosc Endosc Percutan Tech. 2000; 10:39–40.
64. Ransom, KJ, Kavic, MS. Laparoscopic splenectomy following embolization for blunt trauma. J Soc Laparoendosc Surg. 2008; 12:202–205.
65. Malladi, P, Hungness, E, Nagle, A. Single access laparoscopic splenectomy. JSLS. 2009; 13:601–604.
66. Rottman, SJ, Podolsky, ER, Kim, E, et al. Single port access (SPA) splenectomy. JSLS. 2010; 14:48–52.
67. Hansen, EN, Muensterer, OJ. Single incision laparoscopic splenectomy in a 5-year-old with hereditary spherocytosis. JSLS. 2010; 14:286–288.
68. Colon, MJ, Telem, D, Chan, E, et al. Laparoendoscopic single site (LESS) splenectomy with a conventional laparoscope and instruments. JSLS. 2011; 15:384–386.
69. Thalhammer, GH, Eber, E, Uranus, S, et al. Partial splenectomy in cystic fibrosis patients with hypersplenism. Arch Dis Child. 2003; 88:143–146.
70. Havlik, RJ, Touloukian, RJ, Markowitz, RI, et al. Partial splenectomy for symptomatic splenic hamartoma. J Pediatr Surg. 1990; 25:1273–1275.
71. Rice, HE, Oldham, KT, Hillery, CA, et al. Clinical and hematologic benefits of partial splenectomy for congenital hemolytic anemias in children. Ann Surg. 2003; 237:281–288.
72. Diesen, DL, Zimmerman, SA, Thornburg, CD, et al. Partial splenectomy for children with congenital hemolytic anemia and massive splenomegaly. J Pediatr Surg. 2008; 43:466–472.
73. Héry, G, Becmeur, F, Méfat, L, et al. Laparoscopic partial splenectomy: Indications and results of a multicenter retrospective study. Surg Endosc. 2008; 22:45–49.
74. Bader-Meunier, B, Gauthier, F, Archambaud, F, et al. Long-term evaluation of the beneficial effect of subtotal splenectomy for management of hereditary spherocytosis. Blood. 2001; 97:399–403.
75. de Buys Roessingh, AS, de Lagausie, P, Rohrlich, P, et al. Follow-up of partial splenectomy in children with hereditary spherocytosis. J Pediatr Surg. 2002; 37:1459–1463.
76. Buesing, KL, Tracy, ET, Kiernan, C, et al. Partial splenectomy for hereditary spherocytosis: A multi-institutional review. J Pediatr Surg. 2011; 46:178–183.
77. Stoehr, GA, Stauffer, UG, Eber, SW. Near-total splenectomy: A new technique for the management of hereditary spherocytosis. Ann Surg. 2005; 241:40–47.
78. Slater, BJ, Chan, FP, Davis, K, et al. Institutional experience with laparoscopic partial splenectomy for hereditary spherocytosis. J Pediatr Surg. 2010; 45:1682–1686.
79. Morinis, J, Dutta, S, Blanchette, V, et al. Laparoscopic partial vs. total splenectomy in children with hereditary spherocytosis. J Pediatr Surg. 2008; 43:1649–1652.
80. Seims AD, Croop JM, Rescorla FJ. Efficacy of laparoscopic partial splenectomy in the management of hereditary spherocytosis: An institutional review. Poster presentation at American Society of Pediatric Hematology Oncology, Baltimore, MD, 2011.
81. Esposito, C, Schaarschmidt, K, Settimi, A, et al. Experience with laparoscopic splenectomy. J Pediatr Surg. 2001; 36:309–311.
82. Katkhouda, N, Hurwitz, MB, Rivera, RT, et al. Laparoscopic splenectomy: Outcome and efficacy in 103 consecutive patients. Ann Surg. 1998; 228:568–578.
83. Szold, A, Kamat, M, Nadu, A, et al. Laparoscopic accessory splenectomy for recurrent idiopathic thrombocytopenic purpura and hemolytic anemia. Surg Endosc. 2000; 14:761–763.
84. Choi, YU, Dominguez, EP, Sherman, V, et al. Laparoscopic accessory splenectomy for recurrent idiopathic thrombocytopenic purpura. J Soc Laparoendosc Surg. 2008; 12:314–317.
85. Feldman, LS, Demyttenaere, SV, Polyhronopoulos, GN, et al. Refining the selection criteria for laparoscopic versus open splenectomy for splenomegaly. J Laparoendosc Adv Surg Tech. 2008; 16:13–19.
86. Pietrabissa, A, Morelli, L, Peri, A, et al. Laparoscopic treatment of splenomegaly: A case for hand-assisted laparoscopic surgery. Arch Surg. 2011; 146:818–823.
87. Swanson, TW, Meneghetti, AT, Sampath, S, et al. Hand-assisted laparoscopic splenectomy versus open splenectomy for massive splenomegaly: 20-year experience at a Canadian centre. Can J Surg. 2011; 54:189–193.
88. Koshenkov, VP, Nemeth, ZH, Carter, MS. Laparoscopic splenectomy: Outcome and efficacy for massive and supramassive spleens. Am J Surg. 2012; 203:517–522.
89. Rescorla, FJ, Engum, SA, West, KW, et al. Laparoscopic splenectomy has become the gold standard in children. Am Surg. 2002; 68:297–302.
90. Terrosu, G, Baccarani, U, Bresadola, V, et al. The impact of splenic weight on laparoscopic splenectomy for splenomegaly. Surg Endosc. 2002; 16:103–107.
91. Murawski, M, Patkowski, D, Korlacki, W, et al. Laparoscopic splenectomy in children-a multicenter experience. J Pediatr Surg. 2008; 43:951–954.
92. Park, AE, Birgisson, G, Mastrangela, MJ, et al. Laparoscopic splenectomy: Outcomes and lessons learned from over 200 cases. Surgery. 2000; 128:660–666.
93. Casaccia, M, Torelli, P, Pasa, A, et al. Putative predictive parameters for the outcome of laparoscopic splenectomy. Ann Surg. 2010; 251:287–291.
94. Mattioli, G, Avanzini, S, Prato, AP, et al. Spleen surgery in pediatric age: Seven-year unicenter experience. J Laparoendosc Adv Surg Tech. 2009; 19:437–441.
95. Goers, T, Panepinto, J, DeBaun, M, et al. Laparoscopic versus open abdominal surgery in children with sickle cell disease is associated with a shorter hospital stay. Pediatr Blood Cancer. 2008; 50:603–606.
96. Alwabari, A, Parida, L, Al-Salem, AH. Laparoscopic splenectomy and/or cholecystectomy for children with sickle cell disease. Pediatr Surg Int. 2009; 25:417–421.
97. Waldhausen, JHT, Tapper, D. Is pediatric laparoscopic splenectomy safe and cost effective? Arch Surg. 1997; 132:822–824.
98. Winslow, ER, Brunt, LM. Perioperative outcomes of laparoscopic versus open splenectomy: A meta-analysis with an emphasis on complications. Surgery. 2003; 134:647–653.
99. Friedman, RL, Hiatt, JR, Korman, JL, et al. Laparoscopic or open splenectomy for hematologic disease: Which approach is superior? J Am Coll Surg. 1997; 185:49–54.
100. Lansdale, N, Marven, S, Welch, J, et al. Intra-abdominal splenosis following laparoscopic splenectomy causing recurrence in a child with chronic immune thrombocytopenic purpura. J Laparoendosc Adv Surg Tech. 2007; 17:387–391.
101. Pietrabissa, A, Moretto, C, Antonelli, G, et al. Thombosis in the portal venous system after elective laparoscopic splenectomy. Surg Endosc. 2004; 18:1140–1143.
102. Winslow, ER, Brunt, LM, Drebin, JA, et al. Portal vein thrombosis after splenectomy. Am J Surg. 2002; 184:631–636.
103. Ikeda, M, Sekimoto, M, Takiguchi, S, et al. Total splenic vein thrombosis after laparoscopic splenectomy: A possible candidate for treatment. Am J Surg. 2007; 193:21–25.
104. Stamou, KM, Toutouzas, KG, Kekis, PB, et al. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg. 2006; 141:663–669.
105. Soyer, T, Ciftci, AO, Tanyel, FC, et al. Portal vein thrombosis after splenectomy in pediatric hematologic disease: Risk factors, clinical features, and outcome. J Pediatr Surg. 2006; 41:1899–1902.
106. Lynch, AM, Kapila, R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am. 1996; 4:693–707.
107. Jugenburg, M, Haddock, G, Freedman, MH, et al. The morbidity and mortality of pediatric splenectomy: Does prophylaxis make a difference? J Pediatr Surg. 1999; 34:1064–1067.
108. Meekes, I, van der Staak, van Oostrom, C. Results of splenectomy performed on a group of 91 children. Eur J Pediatr Surg. 1995; 5:19–22.
109. Eraklis, AJ, Kewy, SV, Diamond, LK. Hazard of overwhelming infection after splenectomy in childhood. N Engl J Med. 1967; 276:1225–1229.
110. Horan, M, Colebatch, JH. Relation between splenectomy and subsequent infection. Arch Dis Child. 1962; 37:398–414.
111. Ellison, EC, Fabri, PJ. Complications of splenectomy. Surg Clin North Am. 1983; 63:1313–1330.
112. Styrt, B. Infection associated with asplenia: Risks, mechanisms, and prevention. Am J Med. 1990; 88:33–42.
113. Holdsworth, RJ, Irving, AD, Cuschieri, A. Postsplenectomy sepsis and its mortality rate: Actual versus perceived risks. Br J Surg. 1991; 78:1031–1038.
114. Ejstrud, P, Kristensen, B, Hansen, JB, et al. Risk and patterns of bacteraemia after splenectomy: A population-based study. Scand J Infect Dis. 2000; 32:521.
115. Bridgen, ML. Overwhelming post-splenectomy infection-still a problem. West J Med. 1992; 157:440–443.
116. Bridgen, ML, Pattullo, AL. Prevention and management of overwhelming postsplenectomy infection-an update. Crit Care Med. 1999; 27:836–842.
117. Eber, SW, Langendorfer, CM, Ditzig, M, et al. Frequency of very late fatal sepsis after splenectomy for hereditary spherocytosis: Impact of insufficient antibody response to pneumococcal infection. Ann Hematol. 1999; 78:524–528.