200 Splanchnic Ischemia
 Anatomy, Physiology, and Pathophysiology
Anatomy, Physiology, and Pathophysiology
Collateral Circulation
Numerous collaterals may exist or develop. Buhler’s arc contains embryonic remnants of vessels connecting the CA and SMA in the region of the pancreas head and duodenal bulb. Riolan’s artery or marginal artery of Drummond connect the SMA and the IMA. The bowel plexuses also form a large collateral network. Still, even with this large collateral reserve, the superficial layers of the mucosa are very susceptible to the development of ischemia. This susceptibility is due to the countercurrent arteriovenous exchange of oxygen that starts at the base of the villus; when blood flow rate is low, oxygen may be depleted before the villus tip is reached.1–3
Regulation Of Blood Flow
Vasoconstrictors
Catecholamines have different effects on the splanchnic blood flow; α1-adrenergic receptor stimulation leads to vasoconstriction, whereas β2-adrenergic receptor stimulation leads to vasodilatation. The relation between the renin-angiotensin axis and splanchnic perfusion is less uniform, although angiotensin II is a key splanchnic vasoconstrictor during low flow.4 The main splanchnic vasoconstrictor is endothelin (ET)-1.5–6 Two main ET-1 receptor types are have been described: ETA and ETB. Activation of ETA, which is expressed in the mucosa, submucosa, and muscularis of the bowel wall, leads to long-lasting vasoconstriction and plays an important and early role in the negative effects of shock on the integrity of the GI tract.5,7
Vasodilators
The main splanchnic vasodilators are nitric oxide (NO) and prostaglandins. NO has paradoxical effects on gastrointestinal perfusion and mucosal integrity. Normally, low levels of NO are produced by the endothelium to sustain perfusion by promoting local vasodilatation. In pathologic circumstances like circulatory shock or sepsis, a large amount of NO is produced and acts as free radical, similar to oxygen free radicals, and is extremely toxic. In an animal model of hemorrhagic shock, inhibition of NO production is indeed beneficial.8 Locally formed prostaglandins act as mucosal vasodilators, especially during low-flow states or following mucosal injury. Inhibition of cyclooxygenase—for example, with nonsteroidal antiinflammatory drugs (NSAIDs)—diminishes this vasodilatory response and renders the GI mucosa more susceptible to the effects of circulatory shock.9
Low-Flow Conditions
All the above receptors and messengers act to balance perfusion to metabolic demands on a moment-to-moment basis. During circulatory shock, blood flow distribution changes due to constriction and dilatation of different vascular beds. When circulating volume is decreased, relative blood flow to the heart increases and brain perfusion is maintained, but perfusion of skeletal muscles, skin, and gut are reduced. Splanchnic vasoconstriction occurs early and profoundly,10 even before systemic hemodynamic instability arises.11 Splanchnic vasoconstriction can be triggered by different shock states, the direct effects of vasoactive medications, or nicotine and cocaine abuse. GI ischemia occurs only when blood flow is reduced to less than 50% of the basal rate.12–14
During splanchnic hypoperfusion, blood flow within the bowel wall is unevenly distributed among the different layers. In general, the mucosa is protected at the expense of the serosal layers.15 Still, the surface of the mucosa is the most vulnerable area for ischemia, owing to countercurrent diffusional shunting of oxygen. Even within the mucosal layer, blood flow is unevenly distributed. Thus mismatches between metabolic demands and oxygen delivery are caused by several microcirculatory disturbances and shunting.16–18 The patchy distribution of flow when global perfusion is compromised can be observed among different villi as well as within individual villi. These phenomena help explain why, in some studies, mucosal blood flow measurements are within the normal range despite evidence of mucosal ischemia; for early detection of ischemia, flow measurements alone will never suffice. This combination of ischemia despite normal vessel anatomy has given rise to the term NOMI, nonocclusive mesenteric ischemia.
Ischemic Damage
The Ischemic Phase
The immediate effect of reduced oxygen utilization is adenosine triphosphate (ATP) depletion. One of the consequences of ATP depletion is derangements in the tight junctions between adjacent enterocytes, leading to formation of “cracks in the mucosal lining.” Also, key membrane-bound pumps are deprived of energy, and as a consequence, electrolytes and water enter the cells, which swell and, if the process continues, eventually die. Both mechanisms lead to reduced intestinal epithelial barrier function and bacteria moving across the bowel wall from the lumen into the systemic compartment (bacterial translocation).19 During cellular hypoxia, the enzyme, xanthine dehydrogenase, is converted to xanthine oxidase (XO), which is harmless at this stage, because XO needs oxygen as a substrate. Finally, tissue necrosis triggers an inflammatory response, resulting in cytokine release. The effects of the ischemic phase alone are localized and can remain clinically undetected for many hours (closed compartment). The condition sometimes is silent until reperfusion initiates a systemic inflammatory response or transmural gangrene occurs.
Local Effects of Reperfusion
After flow is restored—for example, as a result of the partial dissolution of an embolus—oxygen enters the ischemic tissue. In a reaction catalyzed by XO, oxygen forms reactive oxygen species (ROS) that can damage proteins and DNA.20 The damage to mucosa, blood vessels, and submucosal tissues is not only intensified but spreads to adjacent regions as well by diffusion of the small ROS molecules. Locally present ROS scavengers including glutathione, catalase, and superoxide dismutase, can neutralize ROS, but their efficacy is limited.
Systemic Effects of Reperfusion
Reperfusion delivers toxic products including XO, proinflammatory cytokines, and activated neutrophils into the systemic circulation.21 In animal studies, liver and lung damage have been attributed to activated neutrophils coming from reperfused ischemic bowel.20 Therefore, reperfusion leads to amplification and spreading of the ischemic damage.
 Diagnostic Methods
Diagnostic Methods
Computed Tomography Angiography
CT angiography (CTA), including arterial and venous phase with maximum slice thickness of 1 mm, followed by three-dimensional reconstruction of the vessel anatomy is increasingly used in ICU patients. It has the advantage of minimal invasiveness, very accurate vessel visualization, and additional information on bowel pathology or perfusion. It has recently been reported as an accurate diagnostic test for NOMI. The early introduction of multidetector CT (MDCT) in the decision tree of NOMI treatment, followed by efficient treatment, was safe and suggested to improve mortality.22
Inspection Of The Mucosa And Serosa
With endoscopy, mucosal ischemia can be easily detected; it develops only during malperfusion at a stage where the serosal side is still normal.14 Endoscopy is mostly used to diagnose ischemic colitis after aortic surgery. Endoscopic appearance may be difficult to interpret, especially with imperfectly rinsed bowel; therefore, preparation immediately before endoscopy by enema using 2 to 4 L of water is advisable. Differentiation of ischemic colitis from inflammatory bowel disease can be difficult, and preferably, biopsies should be taken. During the first days, ischemic colitis closely resembles ulcerative colitis; later it may be indistinguishable from Crohn’s disease. Endoscopy cannot distinguish between mucosal and transmural ischemia or gangrene. The latter, irreversible stage can be detected only by inspecting the serosal side of the bowel. Therefore, laparoscopy or laparotomy is indicated when transmural ischemia is suspected.
Laboratory Tests
In general, serologic tests are of limited use for ischemia detection. Classical parameters like leukocyte count and arterial lactate level are of limited value because they lack both sensitivity and specificity. The most promising serologic markers include intestinal fatty acid binding protein (IFABP), D-lactate, ischemia modified albumine, and glutathione S-transferase (GST),23 but clinical data are sparse.24–26
PCO2 Measurement (Tonometry)
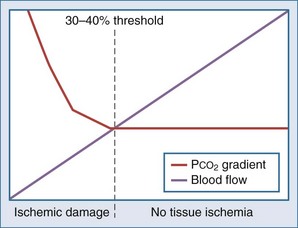
Intraluminal measurement of PCO2 has been shown to detect ischemia, irrespective of flow or metabolism. This extra CO2 is released during ischemia as protons accumulating during anaerobic glycolysis are buffered by tissue bicarbonate. Because CO2 is a small molecule, intraluminal CO2 increases within minutes of increased mucosal CO2. The relationship between CO2 and ischemia was first described in 1979 in heart and skeletal muscles27–28 and in 1982 for the stomach.29 The technique was subsequently popularized by Fiddian-Green and thereafter marketed as tonometry (Figure 200-1, A). He also introduced the term pHi, indicating mucosal acidosis using luminal CO2 and arterial bicarbonate in the Henderson-Hasselbalch equation. Unfortunately, the company making the equipment has decided to stop production, although alternative measurement techniques have been described (see Figure 200-1, B).30–31 Whatever its future, tonometry has demonstrated the important role splanchnic ischemia plays in critical care patients. Moreover, it has enabled us to select patients who could benefit from treatment of splanchnic stenoses.32–34 In the abundance of diagnostics allowing for vessel anatomy assessment, intraluminal PCO2 measurement is the only well-validated test for actual ischemia. An increased intraluminal-to–arterial PCO2 gradient is indicative of ischemia. In the stomach, the normal gastric-arterial PCO2 gradient is below 0.9 kPa (7 mm Hg)35; in the jejunum, the threshold is 1.4 kPa.36 That an increased PCO2 gradient does not relate to changes in perfusion per se can be concluded from studies where the gradient only increases as soon as the splanchnic blood flow decreased to below 30% to 40% of baseline14,35 (Figure 200-2).
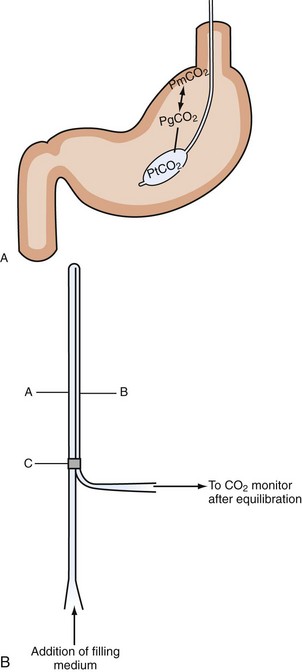
Figure 200-1 Intraluminal PCO2 measurement techniques. A, Tonometry.113 PCO2 can be measured from a specialized balloon-tipped catheter placed in stomach or small or large bowel. Because CO2 diffuses rapidly over different membranes, mucosal PCO2 (PmPCO2) will equal gastric lumen PCO2 (PgCO2). Because the balloon is CO2 permeable as well, balloon PCO2 reflects mucosal values. This balloon PCO2 is measured from air aspirated and inflated automatically into the balloon using a modified capnograph, the Tonocap (Datex-Engström). B, Balloonless intraluminal PCO2 measurement.31 PCO2 is measured using a balloonless catheter, where air flows via a tube which is CO2 permeable only at the intragastrically placed tip and connected with a capnograph on the sampling site.
PCO2 Measurement in the Intensive Care Unit
Because splanchnic ischemia is one of the earliest events in circulatory stress and typically begins at a stage when all other systemic parameters remain within the normal range, it has been referred to as “the canary of the body.”37 Like the canary that was once used in coal mines to detect toxic levels of mine gas, PCO2 measurement may be a good, inexpensive, and relatively early warning of impending trouble.38
Despite its good track record for ischemia detection, PCO2 measurement has not been widely used, either in the ICU or in GI or vascular medicine. Several reasons can be identified for this lack of success. First, saline-based PCO2 measurement was initially laborious, time-consuming, and error prone. Second, many methodological issues clouded the studies in the first years. These included the need for acid suppression and errors introduced by food intake. Third, there was a lack of evidence that tonometry-based ischemia detection led to therapeutic interventions that improved outcome. The first two issues have been properly addressed and resolved by using air-based PCO2 measurement (Tonocap device), potent acid suppression, and use of standardized meals during testing.39–40 Despite its unique properties in the assessment of ischemia, only studies in trauma patients showed an advantage over standard monitoring.41–42 A recent comparative study in septic patients failed to show a survival advantage in patients where resuscitation was aimed at normalization of tonometry, compared to standard systemic parameters.43
Outside the ICU, the situation is different. As a functional test to detect ischemia in the stomach and small bowel, the gold standard is measurement of PCO2 during submaximal exercise, with a 78% sensitivity and 92% specificity.32 Using this exercise test, we could select patients with single-vessel stenosis for treatment and follow-up.34 It enabled us to investigate the entire spectrum of splanchnic stenotic disease from asymptomatic stenoses, to single and multivessel stenoses with ischemic complaints, and finally imminent bowel infarction.44 Measurement of an increased PCO2 after a meal in patients with symptomatic splanchnic stenosis was first shown in 1991.45 Subsequent investigations using gastric PCO2 measurement after a test meal showed variable results,46–47 probably owing to buffering and dilution effects of the test meals.48 With standardized test meals and acid suppression by proton pump inhibitors, the diagnostic accuracy of PCO2 measurement in the stomach and small bowel for detection of ischemia improved considerably.40 Having used this test in over 400 cases, three patterns emerged. First, the normal baseline is below 8 kPa and varies at least 1 kPa. Second, after a liquid meal, the gastric and small-bowel PCO2 did not increase above 10.6 kPa in nonischemic individuals. Third, increased PCO2 levels during the night are quite common and are probably related to buffering effects from duodenogastric reflux. An imminent bowel infarction is characterized by an increased PCO2 for several hours, often above 15 kPa. Also, a suppressed and invariably low PCO2 without the normal variation was seen in patients with an imminent infarction (paper in preparation).
 Clinical Presentations of Splanchnic Ischemia
Clinical Presentations of Splanchnic Ischemia
Splanchnic vascular disorders encompass a spectrum of acute and chronic occlusive, nonocclusive, and aneurysmal disorders affecting the vessels of the abdominal viscera. A classification of ischemic disorders can be made depending on vessel anatomy and ischemia (Figure 200-3). Acute splanchnic ischemia can be caused by arterial embolism, arterial and venous thrombosis, arterial stenoses, or NOMI. For the intensivist, NOMI is the most common problem and will be discussed first. The discussion on occlusive ischemia will focus on the different and often underappreciated clinical presentations, diagnostic problems, and treatment issues, with special emphasis on ICU care.
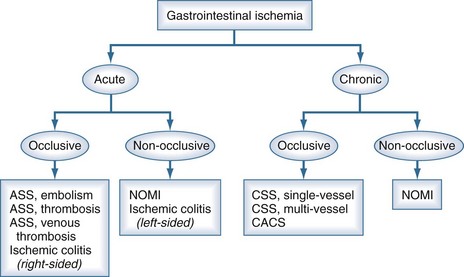
Figure 200-3 Classification of the spectrum of gastrointestinal vascular disease and ischemia.
(Adapted from Kolkman JJ, Bargeman M, Huisman AB, Geelkerken RH. Diagnosis and management of splanchnic ischemia. World J Gastroenterol 2008;14:7309-20.)
Nonocclusive Mesenteric Ischemia
Critically Ill Patients and Major Operations
In gastroenterology and surgery, NOMI is probably a rare disorder that can lead to ischemic colitis49 or acute splanchnic infarction.50 It can also lead to chronic complaints comparable to chronic splanchnic ischemia related to vascular spasm. Treatment with vasodilators has been successful in the majority of patients, and the condition has been referred to as abdominal migraine.51 In many cases, NOMI is reportedly caused by drugs, especially digoxin, or underlying cardiovascular and renal diseases.
NOMI is the end result of the physiologic response to a decreased intravascular blood volume. Early and profound splanchnic vasoconstriction accompanies many major operations, may lead to splanchnic ischemia, and is then associated with an adverse prognosis.52–53 Similarly, in acute pancreatitis, gastric mucosal ischemia was associated with a worse outcome.54 The relevance of this finding was reinforced in a recent randomized study evaluating the effects of probiotics in acute pancreatitis. In this study that investigated the potential beneficial effects of supplementing early feeding with probiotics, the mortality in the probiotic group was significantly higher and was especially associated with bowel infarction.55
It has been suggested that NOMI could play a key role in the pathogenesis of multiple organ failure syndrome (MODS). For example, endotoxinemia can cause mucosal microcirculatory disturbances directly, contributing to hypovolemia-induced vasoconstriction,56 and increased gut-derived cytokine and endotoxin levels have been detected in patients with this syndrome.57–58
Hemodialysis Patients
In hemodialysis patients, NOMI is quite common59 and may lead to bowel infarction in 2%, with a 45% mortality rate.60 The incidence of this complication has been reported in 0.5% to 0.9% of these patients,60–62 in whom NOMI has been associated with hypotension, often during hemodialysis. Close monitoring and prevention of hypotension are crucial to avoid this problem.60
Medications
Many drugs have been implicated as causative agents in NOMI, especially digoxin. NSAIDs affect the integrity of the GI mucus and bicarbonate layer and reduce mucosal perfusion. α-Adrenergic agents like epinephrine and dopamine reduce GI perfusion, and β-adrenergic agents like dobutamine and dopexamine tend to sustain mucosal perfusion.63–65 The clinical importance of these differences is probably very small, because recent comparative studies failed to show differences in mortality between norepinephrine plus dobutamine versus epinephrine,66 and norepinephrine versus dopamine.67
Occlusive Ischemia
The incidence of asymptomatic splanchnic stenoses, so-called chronic splanchnic disease, ranges between 8% and 70% in populations with other manifestations of atherosclerotic disease and is comparable to the incidence of carotid atherosclerosis. Nevertheless, the incidence of symptomatic occlusive splanchnic ischemia, or chronic splanchnic syndrome, is relatively rare, being only 4 to 5 cases per 100,000 inhabitants yearly.68 The incidence of acute splanchnic ischemia is relatively low but increases sharply with age. In a recent autopsy study, it was shown that 1.2% of all deaths in patients over the age of 80 was attributable to acute splanchnic ischemia.69 The diagnosis was suspected in a minority of patients.70
Etiology
External compression by the arcuate ligament of the diaphragm is the predominant cause of single-vessel CA stenosis in young adults. Atherosclerosis is the main cause of single-vessel SMA or IMA occlusive disease and multivessel disease. The latter is defined as stenoses or occlusions in more than one main splanchnic artery. Information on the natural history of splanchnic artery occlusive disease is scarce. Using serial duplex ultrasound, it was demonstrated that visceral artery atherosclerotic stenoses progress in approximately 20% of patients per year. This progression of lesions is especially important in multivessel chronic splanchnic disease, which carries a considerable risk for acute splanchnic infarction.71
Chronic Splanchnic Syndrome (Single-Vessel Disease)
It has long been debated whether patients with a single splanchnic vessel stenosis developed symptoms. In 1972, Szilagyi suggested that “no patient had ever been proven, on scientific grounds, to have an abnormality of intestinal structure or function which was caused by extraluminal compression of the coeliac artery, or supposed relief from the operation could be anything other than a placebo effect.”72 Recently we have shown in patients with typical complaints of ischemia, an abnormal function test and an eccentric respiratory-dependent stenosis of the CA; resolution of symptoms was seen in 89% after open or endoscopic release of the CA.73 In another study, we demonstrated that disappearance of symptoms was associated with a normalized function test.34 Because these patients are normally in good health, they will rarely be admitted to the ICU.
Chronic Splanchnic Syndrome (Multivessel Disease)
Untreated, progressive multivessel splanchnic syndrome may result in bowel infarction.33 Because the diagnosis is usually made in a late stage, the time frame for treatment may be limited. In our experience, patients with multivessel stenoses and clinical indications of an imminent bowel infarction should be treated within days. These clinical indicators encompass ulcerations in stomach duodenum or right-sided colon during endoscopy, abdominal pain not associated with eating, and complete incapability of eating. When a bowel infarction finally occurs, it may remain clinically silent for several hours or even days as long as the necrotic segment remains without perfusion.14 With reperfusion or perforation of gangrenous bowel, MODS develops rapidly, and death usually ensues within days.
Acute Splanchnic Syndrome
Acute splanchnic ischemia is defined as sudden cessation of splanchnic mucosal perfusion. It should be considered in patients presenting with acute severe abdominal pain where no obvious diagnosis is found. In elderly patients, acute splanchnic ischemia can present with unexplained confusion. Classically the severity of pain is out of proportion to the almost normal physical findings. If untreated, acute splanchnic ischemia ultimately results in bowel necrosis within 6 to 8 hours. In 75% of patients with acute splanchnic artery occlusion, an embolus in the SMA is present. The prognosis depends on the cause of the infarction and ranges from approximately 32% for venous thrombosis and 54% for arterial embolism to 70% to 80% for acute arterial thrombosis and nonocclusive ischemia. The overall survival after acute splanchnic ischemia has improved over the past 4 decades.74 The Mayo Clinic’s 2002 vision, “the contemporary management of acute splanchnic ischemia with revascularization with open surgical techniques, resection of nonviable bowel, and liberal use of second-look procedures results in early survival of two thirds of the patients with embolism and thrombosis,” is still valid.75
Ischemic Colitis
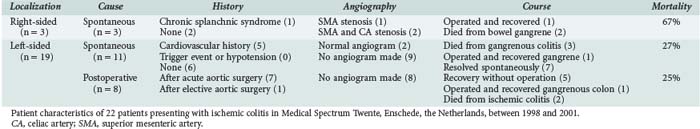
Ischemic colitis is a well-defined disease. It is a nonocclusive disorder in most cases, and angiograms are almost invariably normal.49–50 Still, most cases of spontaneous ischemic colitis are not preceded by shock states as has been suggested; most cases are found as a result of unexpected findings at endoscopy performed to evaluate patients because of abdominal cramps, diarrhea, or blood loss (Table 200-1). Because the course of spontaneous left-sided ischemic colitis is benign, patients rarely come to the attention of the intensivist.
In contrast, ischemic colitis following aortic surgery is frequently seen in the ICU. It was observed in 20% to 27% of patients after conventional open repair of ruptured abdominal aortic aneurysm and was associated with an overall mortality rate of 48%.76–79 After elective aortic surgery, sigmoid ischemia is reported in less than 2% of patients.80 The main factors inducing postoperative left-sided ischemic colitis, therefore, seem to be preoperative shock, massive blood loss, and persisting hemodynamic instability. In these patients, the IMA is usually already occluded or surgically ligated, so ischemic colitis may be partially occlusive in nature.77 With the introduction of endovascular stent placement for the elective management of abdominal aortic aneurysms (AAA) or the treatment of acute ruptured aneurysms, mortality rate, ICU stay, and incidence of ischemic colitis after AAA repair has decreased dramatically.81 Still, when patients remain unstable for more than 48 hours after aortic repair, a sigmoidoscopy is indicated.
Left- Versus Right-Sided Ischemic Colitis
An important clinical distinction should be made between left-sided ischemic colitis (discussed earlier) and right-sided ischemic colitis.82–83 The latter was associated with an adverse prognosis, increased surgery rates, and increased mortality.83 In many cases, right-sided ischemic colitis is a symptom of severe compromised SMA flow and consequently acute splanchnic infarction. To improve the prognosis, this entity requires immediate treatment. Our preference would be to perform an urgent CTA. Because the time between onset of acute complete small-bowel ischemia and irreversible gangrene is only 6 to 8 hours, this investigation should not be postponed. It can be used to rule out other pathology, guide an appropriate revascularization, and avoid a “blind” laparotomy. Recent studies have indicated that CT assessment of morphology and diameter of the SMA could be used to positively diagnose NOMI.22
 Treatment
Treatment
Nonocclusive Mesenteric Ischemia
The key factors for successful treatment of NOMI include:
The first step towards successful treatment is early detection of mucosal ischemia. Only intraluminal PCO2 measurement has proven accuracy for the detection of early ischemia.49 Using gastric PCO2 measurement as an endpoint for fluid resuscitation, rapid optimization of intravascular volume could be achieved.84–86 Still, the results of resuscitation trials aimed at normalizing luminal PCO2 showed conflicting results, with positive effects in trauma patients41–42 but no different from standard monitoring in septic patients.43 An alternative might be the use of CTA, although the experience is still limited in severe cases.87–88
Medication
In patients with a high probability of mucosal ischemia, avoidance of epinephrine and dopamine makes sense.89–90 Still, recent studies failed to show a difference between various catecholamines for resuscitation following fluid correction.66–67 Treatment with angiotensin-converting enzyme (ACE) inhibitors has been effective in animal studies91 but only in one of two clinical studies.92–93 In recent studies, administration of prostaglandin E1 was reported to improve outcome in case series of severe NOMI.
Feeding
Early institution of enteral nutrition may improve perfusion, in addition to providing salutary immunologic and nutritional effects. The mechanisms responsible for mucosal vasodilatation due to enteric nutrition are autoregulatory responses driven by the metabolic demands associated with absorption of food in the lumen.94 However, in extreme low or no-flow states, enteral nutrition can be very harmful and provoke infarction, and it should therefore be used cautiously.95 This mechanism may explain the high rate of bowel infarction in the aforementioned probiotic pancreatitis study where a rapid institution of high-volume feeding was protocol.55 Treatment of reperfusion damage is a promising but clinically unproven approach. Several new compounds96–98 and established drugs including N-acetylcysteine99 and vitamin E100 have been used in animal models to reduced ischemia/reperfusion-induced damage. The best known ROS scavenger, N-acetylcysteine, increases intracellular glutathione levels and increases NO release,101 leading to vasodilatation of small blood vessels. In some studies, administration of N-acetylcysteine early in sepsis was associated with improved hemodynamic parameters102 and splanchnic ischemia.103 However, data from clinical studies are still lacking.
Occlusive Ischemia: Splanchnic Syndrome
Single-Vessel Chronic Splanchnic Syndrome
The majority of these patients have no comorbidities, and the operative course is usually uneventful.33 These patients are rarely encountered by the intensivist.
Multivessel Chronic Splanchnic Syndrome
Preoperative Workup
Many of these patients have severe comorbidities and have lost a considerable amount of weight, often more than 15 kg resulting in a BMI below the normal range. Still, attempts to correct the nutritional deficit preoperatively is not without risk. In patients with critical stenoses and minimal blood flow to the bowel, feeding may induce acute bowel infarction. Even parenteral nutrition is not without risk, because it can provoke liver and bowel ischemia. This is explained by the increased energy expenditure from metabolizing nutrients in the liver, which has severely compromised perfusion because flow from the portal vein as well as from the hepatic artery is impaired because of occlusive disease involving the CA and SMA. Moreover, the increased hepatic blood flow causes an intramesenteric steal, with blood shunting from the bowel to the liver, thereby causing bowel ischemia as well. Using tonometry, these patients showed extreme increases in gastric and jejunal PCO2 for several hours following polymeric feeding.44 In general, patients with critical stenoses should be treated by revascularization as soon as possible; feeding should be delayed until restoration of blood flow has been achieved.
Revascularization
There are many potential treatment options in these patients, partly because solid clinical evidence to prefer one above the other is lacking104 (Table 200-2). Restoration of blood flow can be achieved with three different treatment strategies: operative antegrade or retrograde revascularization or percutaneous endovascular antegrade revascularization.
TABLE 200-2 Summary of Treatment Options in Splanchnic Ischemia
| NOMI |
Antegrade multivessel autologous revascularization yields excellent long-term results with regard to patency and clinical response.105–106 The downside of this approach is that it uses a supraceliac aortic clamp technique, resulting in at least 15 to 20 minutes of ischemia affecting the bowel, legs, and kidneys. In older patients and in patients in poor clinical condition, this approach is quite risky, as the hemodynamic instability and other adverse effects of lower-body reperfusion may not be well tolerated. Antegrade multivessel autologous revascularization should not been attempted in patients with a body mass index (BMI) below 19.5 kg/m2, with confined life expectancy, or with relevant comorbidity.
Endovascular treatment by percutaneous transluminal angioplasty (PTA) with stent placement can be performed either via the femoral artery in the groin or the brachial artery. The former approach is suboptimal for proper positioning of the stent at the origin of the CA or SMA if either of these vessels makes a sharp angle with the aorta. The brachial artery approach includes a risk of 10% to 15% of local complications, including median nerve damage, hemorrhage, and pseudoaneurysm formation. If antegrade endovascular revascularization is not appropriate, retrograde endovascular recanalization (the hybrid procedure) of the SMA is a worthwhile alternative (Figure 200-4). After a small supra-umbilical laparotomy, the outflow of the SMA is controlled. Retrograde, a 5F sheet is introduced in the SMA, and under manual and fluoroscopic visualization, endovascular connection between the SMA outflow and the aorta is achieved. Thereafter, PTA and stenting of the occluded trajectory of the SMA could be performed.107
Endovascular repair is recommended in patients with limited life expectancy, high cardiopulmonary risk, cachexia, or hostile abdomen. Open repair is still considered the preferred option for patients who are relatively young and otherwise fit for surgical repair.108
Acute Splanchnic Syndrome
In the surgical management of acute splanchnic syndrome with bowel gangrene, many recommend not resecting intestine with marginal viability at the initial procedure, but performing a routine “second look” procedure 24 hours after the original operation and resecting additional intestine at this time if necessary. This approach is advocated to save as much bowel length as possible. However, many of these patients eventually die from MODS, presumably related to ischemia/reperfusion-induced inflammation related to areas of bowel with borderline ischemia. We currently prefer an alternative approach with initial restoration of blood flow followed by removal of all nonvital bowel. In our experience, this reduces the postoperative problems but results in more patients with short bowel syndrome. Initially these patients will be dependent on parenteral nutrition. With intestinal adaptation, which may take up to 1 year, most can resume enteral nutrition. Restoration of complete but adjusted109 enteral nutrition can be expected in patients with remaining small-bowel length of greater than 50 cm with an intact ileocecal valve, or between 50 and 100 cm without an ileocecal valve.110 The quality of life of these patients is relatively good and comparable to hemodialysis patients.111 In future, small-bowel transplants may become an option, with a current 1-year transplant survival of 60% (source: International Intestinal Transplant Registry). Therefore, in patients who seem otherwise in relatively good health, with nearby complete necrotic bowel but without clear involvement of the stomach, duodenum, liver, and pancreas, revascularization and resection treatment should at least be considered.
Acute-on-Chronic Splanchnic Ischemia
The course of patients with multivessel chronic splanchnic syndrome is initially stable or slowly progressive. Ultimately, these patients become severely cachectic. The end stage of the disease is rapidly progressive. Bowel infarction develops in up to 30% of patients after 1 year and 60% after 4 years of follow-up.71 The prognosis for these patients is very poor; mortality is 80% once bowel infarction develops.50,74 Therefore, symptomatic patients with severe bowel pain and weight loss and multivessel disease should be analyzed and treated in a matter of days to weeks. During the time leading up to the revascularization procedure, maintenance of adequate intravascular volume is essential.
Emergency revascularization is the main goal of treatment. Angiography can be useful for stenting of the CA or SMA and eventually removing an SMA embolus. In cases of NOMI, papaverine (30-60 mg/h for a maximum of 4 hours) or prostaglandin E1 (bolus 0.020 mg, then 0.060 mg/h for up to 72 hours) can be administered by selective SMA catheterization to diminish arterial spasm.112 CTA or splanchnic angiography is essential to provide the surgeon guidelines for revascularization during laparotomy.
 Postoperative Care
Postoperative Care
Ischemic Colitis
In most cases, left-sided ischemic colitis resolves spontaneously with only fluid resuscitation and antibiotics; endoscopic bowel decompression should be considered if the colon is markedly dilated. Surgery is restricted to patients with transmural irreversible ischemia, but occurrence of these complications is associated with a poor prognosis. Some experts advocate routine repetitive sigmoidoscopy to evaluate high-risk patients after acute aortic surgery, especially in those with severe preoperative shock or requiring large volumes of intravenous fluids.76 Repeated coloscopy should also be considered in patients post aortic surgery who have persistent hemodynamic instability lasting more than 48 hours. At endoscopy, ischemic colitis is graded in 4 categories: grade 0, normal mucosa; grade 1, mucosal edema; grade 2, deep mucosal ulcers; grade 3, gangrene. Grade 3 and progressive grade 2 ischemia are indications for laparotomy and subsequently subtotal colon resection. Angiography is rarely indicated in these patients. Treatment is aggressive fluid resuscitation, antibiotics, and bowel decompression if indicated.
Key Points
Hamilton-Davies C, Mythen N, Salmon LB, Jacobson D, Shukla A, Webb AR. Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometer. Intensive Care Med. 1997;23:276-281.
Knichwitz G, Rotker J, Mollhoff T, Richter KD, Brussel T. Continuous intramucosal PCO2 measurement allows the early detection of intestinal malperfusion. Crit Care Med. 1998;26:1550-1557.
Kolkman JJ, Otte JA, Groeneveld AB. Gastrointestinal luminal PCO2 tonometry: an update on physiology, methodology and clinical applications. Br J Anaesth. 2000;84:74-86.
MacDonald PH. Ischaemic colitis. Baillieres Best Pract Res Clin Gastroenterol. 2002;16:51-61.
van Bockel JH, Geelkerken RH, Wasser MN. Chronic splanchnic ischaemia. Best Pract Res Clin Gastroenterol. 2001;15:99-119.
van Petersen AS, Kolkman JJ, Beuk RJ, Huisman AB, Doelman C, Geelkerken RH. Open or percutaneous revascularization for chronic splanchnic syndrome. J Vasc Surg. 2010;51:1309-1316.
1 Haglund U, Hulten L, Ahren C, Lundgren O. Mucosal lesions in the human small intestine in shock. Gut. 1975;16:979-984.
2 Bustamante SA, Jodal M, Nilsson NJ, Lundgren O. Evidence for a countercurrent exchanger in the intestinal villi of suckling swine. Acta Physiol Scand. 1989;137:207-213.
3 Haglund U. Gut ischaemia. Gut. 1994;35:S73-S76.
4 Reilly PM, Bulkley GB. Vasoactive mediators and splanchnic perfusion. Crit Care Med. 1993;21:55-68.
5 Burgener D, Laesser M, Treggiari-Venzi M, et al. Endothelin-1 blockade corrects mesenteric hypoperfusion in a porcine low cardiac output model. Crit Care Med. 2001;29:1615-1620.
6 Kawano S, Tsuji S. Role of mucosal blood flow: a conceptional review in gastric mucosal injury and protection. J Gastroenterol Hepatol. 2000;15(Suppl):D1-D6.
7 Kaszaki J, Wolfard A, Szalay L, Boros M. Pathophysiology of ischemia-reperfusion injury. Transplant Proc. 2006;38:826-828.
8 Hua TC, Moochhala SM. Role of nitric oxide in hemorrhagic shock-induced bacterial translocation. J Surg Res. 2000;93:247-256.
9 Kawano S, Tsuji S, Sato N, Kamada T. NSAIDS and the microcirculation of the stomach. Gastroenterol Clin North Am. 1996;25:299-315.
10 Toung T, Reilly PM, Fuh KC, Ferris R, Bulkley GB. Mesenteric vasoconstriction in response to hemorrhagic shock. Shock. 2000;13:267-273.
11 Hamilton-Davies C, Mythen MG, Salmon LB, Jacobson D, Shukla A, Webb AR. Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometer. Intensive Care Med. 1997;23:276-281.
12 Dubin A, Estenssoro E, Murias G, et al. Effects of hemorrhage on gastrointestinal oxygenation. Intensive Care Med. 2001;27:1931-1936.
13 Kolkman JJ, Otte JA, Groeneveld AB. Gastrointestinal luminal PCO2 tonometry: an update on physiology, methodology and clinical applications. Br J Anaesth. 2000;84:74-86.
14 Knichwitz G, Rotker J, Mollhoff T, Richter KD, Brussel T. Continuous intramucosal PCO2 measurement allows the early detection of intestinal malperfusion. Crit Care Med. 1998;26:1550-1557.
15 van Bommel J, Trouwborst A, Schwarte L, Siegemund M, Ince C, Henny C. Intestinal and cerebral oxygenation during severe isovolemic hemodilution and subsequent hyperoxic ventilation in a pig model. Anesthesiology. 2002;97:660-670.
16 Buwalda M, Ince C. Opening the microcirculation: can vasodilators be useful in sepsis? Intensive Care Med. 2002;28:1208-1217.
17 Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369-1377.
18 Tugtekin IF, Radermacher P, Theisen M, et al. Increased ileal-mucosal-arterial PCO2 gap is associated with impaired villus microcirculation in endotoxic pigs. Intensive Care Med. 2001;27:757-766.
19 Wattanasirichaigoon S, Menconi MJ, Delude RL, Fink MP. Effect of mesenteric ischemia and reperfusion or hemorrhagic shock on intestinal mucosal permeability and ATP content in rats. Shock. 1999;12:127-133.
20 Nielsen VG, Tan S, Baird MS, McCammon AT, Parks DA. Gastric intramucosal pH and multiple organ injury: impact of ischemia- reperfusion and xanthine oxidase. Crit Care Med. 1996;24:1339-1344.
21 Syk I, Brunkwall J, Ivancev K, et al. Postoperative fever, bowel ischaemia and cytokine response to abdominal aortic aneurysm repair–a comparison between endovascular and open surgery. Eur J Vasc Endovasc Surg. 1998;15:398-405.
22 Woodhams R, Nishimaki H, Fujii K, Kakita S, Hayakawa K. Usefulness of multidetector-row CT (MDCT) for the diagnosis of non-occlusive mesenteric ischemia (NOMI): Assessment of morphology and diameter of the superior mesenteric artery (SMA) on multi-planar reconstructed (MPR) images. Eur J Radiol. 2009.
23 Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg. 2009;33:1374-1383.
24 Gollin G, Zieg PM, Cohn SM, Lieberman JM, Marks WH. Intestinal mucosal injury in critically ill surgical patients: preliminary observations. Am Surg. 1999;65:19-21.
25 Lieberman JM, Sacchettini J, Marks C, Marks WH. Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery. 1997;121:335-342.
26 Mensink PBF, Hol L, Borghuis-Koertshuis N, et al. Transient postprandial ischemia is associated with increased intestinal fatty acid binding protein in patients with chronic gastrointestinal ischemia. Eur J Gastroenterol Hepatol. 2009;21:278-282.
27 Case RB, Felix A, Wachter M, Case RB, Felix A, Wachter M. Measurement of myocardial PCO2 with a microelectrode: its relation to coronary sinus PCO2. Am J Physiol. 1979;236:H29-H34.
28 Jussila E, Niinikoski J, Inberg MV. Tissue gas tensions in the calf muscles of patients with lower limb arterial ischaemia. Scand J Thorac Cardiovasc Surg. 1979;13:77-82.
29 Fiddian-Green RG, Pittenger G, Whitehouse WMJr. Back-diffusion of CO2 and its influence on the intramural pH in gastric mucosa. J Surg Res. 1982;33:39-48.
30 Boda D, Kaszaki J, Talosi G. A new simple tool for tonometric determination of the PCO2 in the gastrointestinal tract: in vitro and in vivo validation studies. Eur J Anaesthesiol. 2006;23:680-685.
31 Palagyi P, Vimlati L, Boda K, Talosi G, Boda D. Practical experiences and in vitro and in vivo validation studies with a new gastric tonometric probe in human adult patients. J Crit Care. 2010.
32 Otte JA, Geelkerken RH, Oostveen E, Mensink PB, Huisman AB, Kolkman JJ. Clinical impact of gastric exercise tonometry on diagnosis and management of chronic gastrointestinal ischemia. Clin Gastroenterol Hepatol. 2005;3:660-666.
33 Mensink PB, van Petersen AS, Geelkerken RH, Otte JA, Huisman AB, Kolkman JJ. Clinical significance of splanchnic artery stenosis. Br J Surg. 2006;93:1377-1382.
34 Mensink PB, van Petersen AS, Kolkman JJ, Otte JA, Huisman AB, Geelkerken RH. Gastric exercise tonometry: the key investigation in patients with suspected celiac artery compression syndrome. J Vasc Surg. 2006;44:277-281.
35 Otte JA, Oostveen E, Geelkerken RH, Groeneveld AB, Kolkman JJ. Exercise induces gastric ischemia in healthy volunteers: a tonometry study. J Appl Physiol. 2001;91:866-871.
36 Otte JA, Huisman AB, Geelkerken RH, Kolkman JJ. Jejunal tonometry for the diagnosis of gastrointestinal ischemia. Feasibility, normal values and comparison of jejunal with gastric tonometry exercise testing. Eur J Gastroenterol Hepatol. 2008;20:62-67.
37 Dantzker DR. The gastrointestinal tract: the canary of the body. JAMA. 1993;270:1247-1248.
38 Marik PE. Gastric intramucosal pH- a better predictor of multiorgan dysfunction syndrome and death than oxygen-derived variables in patients with sepsis. Chest. 1993;104:225-229.
39 Mensink PB, Geelkerken RH, Huisman AB, Kuipers EJ, Kolkman JJ. Effect of various test meals on gastric and jejunal carbon dioxide: A study in healthy subjects. Scand J Gastroenterol. 2006;41:1290-1298.
40 Mensink PB, Geelkerken RH, Huisman AB, Kuipers EJ, Kolkman JJ. Twenty-four hour tonometry in patients suspected of chronic gastrointestinal ischemia. Dig Dis Sci. 2008;53:133-139.
41 Ivatury RR, Simon RJ, Islam S, Fueg A, Rohman M, Stahl WM. A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg. 1996;183:145-154.
42 Ivatury RR, Simon RJ, Havriliak D, Garcia C, Greenbarg J, Stahl WM. Gastric mucosal pH and oxygen delivery and oxygen consumption indices in the assessment of adequacy of resuscitation after trauma: a prospective, randomized study. J Trauma. 1995;39:128-134.
43 Palizas F, Dubin A, Regueira T, et al. Gastric tonometry versus cardiac index as resuscitation goals in septic shock: a multicenter, randomized, controlled trial. Crit Care. 2009;13:R44.
44 Kolkman JJ, Bargeman M, Huisman AB, Geelkerken RH. Diagnosis and management of splanchnic ischemia. World J Gastroenterol. 2008;14:7309-7320.
45 Boley SJ, Brandt LJ, Veith FJ, Kosches D, Sales C. A new provocative test for chronic mesenteric ischemia. Am J Gastroenterol. 1991;86:888-891.
46 Geelkerken RH, Schultze Kool LJ, Hermans J, Zarza MT, van Bockel JH. Chronic splanchnic syndrome: tonometry as a functional test. Eur J Surgery. 1997;163:115-121.
47 Fiddian-Green RG. Provocative test for chronic mesenteric ischemia. Am J Gastroenterol. 1992;87:543.
48 Kolkman JJ, Groeneveld AB, Meuwissen SG. Effect of gastric feeding on intragastric P(CO2) tonometry in healthy volunteers. J Crit Care. 1999;14:34-38.
49 MacDonald PH. Ischaemic colitis. Bailliere’s Best Practice and Research in Clinical Gastroenterology. 2002;16:51-61.
50 Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954-968.
51 Bigirwamungu-Bargeman M, Geelkerken RH, Huisman AB, Kolkman JJ. Abdominal migraine, a new and treatable disorder mimicking functional dyspepsia. Gastroenterology. 2009;136:A-773.
52 Mythen MG, Webb AR. Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intensive Care Med. 1994;20:99-104.
53 Welch M, Douglas JT, Smyth JV, Walker MG. Systemic endotoxaemia and fibrinolysis during aortic surgery. Eur J Vasc Endovasc Surg. 1995;9:228-232.
54 Bonham MJ, Abu-Zidan FM, Simovic MO, Windsor JA. Gastric intramucosal pH predicts death in severe acute pancreatitis. Br J Surg. 1997;84:1670-1674.
55 Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
56 Nakajima Y, Baudry N, Duranteau J, Vicaut E. Microcirculation in intestinal villi: a comparison between hemorrhagic and endotoxin shock. Am J Respir Crit Care Med. 2001;164:1526-1530.
57 Soong CV, Halliday MI, Barclay GR, Hood JM, Rowlands BJ, Barros D’Sa AA. Intramucosal acidosis and systemic host responses in abdominal aortic aneurysm surgery. Crit Care Med. 1997;25:1472-1479.
58 Soong CV, Lewis HG, Halliday MI, Rowlands BJ. Intramucosal acidosis and the inflammatory response in acute pancreatitis. Am J Gastroenterol. 1999;94:2423-2429.
59 Diebel L, Kozol R, Wilson RF, Mahajan S, Abuhamdan D, Thomas D. Gastric intramucosal acidosis in patients with chronic kidney failure. Surgery. 1993;113:520-526.
60 John AS, Tuerff SD, Kerstein MD. Nonocclusive mesenteric infarction in hemodialysis patients. J Am Coll Surg. 2000;190:84-88.
61 Bender JS, Ratner LE, Magnuson TH, Zenilman ME. Acute abdomen in the hemodialysis patient population. Surgery. 1995;117:494-497.
62 Picazo M, Cuxart M, Sans R, Sarda C, Exposito E. [Mesenteric ischemia in hemodialysis patients]. Nefrologia. 2008;28:198-202.
63 Silva E, DeBacker D, Creteur J, Vincent JL. Effects of vasoactive drugs on gastric intramucosal pH [see comments]. Crit Care Med. 1998;26:1749-1758.
64 Levy B, Bollaert PE, Charpentier C, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med. 1997;23:282-287.
65 Marik PE, Mohedin M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA. 1994;272:1354-1357.
66 Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet. 2007;370:676-684.
67 De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779-789.
68 Otte JA, Geelkerken RH, Huisman AB, Kolkman JJ. Assessment of the incidence of chronic gastrointestinal ischemia after institution of a multidisciplinary working group. Gastroenterology. 1999;116:A915.
69 Acosta S, Ogren M, Sternby NH, Bergqvist D, Bjorck M. Incidence of acute thrombo-embolic occlusion of the superior mesenteric artery–a population-based study. Eur J Vasc Endovasc Surg. 2004;27:145-150.
70 Acosta S, Ogren M, Sternby NH, Bergqvist D, Bjorck M. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg. 2005;241:516-522.
71 Thomas JH, Blake K, Pierce GE, Hermreck AS, Seigel E. The clinical course of asymptomatic mesenteric arterial stenosis. J Vasc Surg. 1998;27:840-844.
72 Szilagyi DE, Rian RL, Elliott JP, Smith RF. The cardiac artery compression syndrome: does it exist? Surgery. 1972;72:849-863.
73 van Petersen AS, Vriens BH, Huisman AB, Kolkman JJ, Geelkerken RH. Retroperitoneal endoscopic release in the management of celiac artery compression syndrome. J Vasc Surg. 2009;50:140-147.
74 Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91:17-27.
75 Park WM, Gloviczki P, Cherry KJJr, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002;35:445-452.
76 Levison JA, Halpern VJ, Kline RG, Faust GR, Cohen JR. Perioperative predictors of colonic ischemia after ruptured abdominal aortic aneurysm. J Vasc Surg. 1999;29:40-45.
77 Schiedler MG, Cutler BS, Fiddian-Green RG. Sigmoid intramural pH for prediction of ischemic colitis during aortic surgery. Arch Surg. 1987;122:881-886.
78 Bjorck M, Lindberg F, Broman G, Bergqvist D. pHi monitoring of the sigmoid colon after aortoiliac surgery. A five-year prospective study. Eur J Vasc Endovasc Surg. 2000;20:273-280.
79 Bast TJ, Van Der Biezen JJ, Scherpenisse J, Eikelboom BC. Ischaemic disease of the colon and rectum after surgery for abdominal aortic aneurysm: A prospective study of the incidence and risk factors. Eur J Vasc Surg. 1990;4:253-257.
80 Nakatsuka M. Assessment of gut mucosal perfusion and colonic tissue blood flow during abdominal aortic surgery with gastric tonometry and laser Doppler flowmetry. Vasc Endovascular Surg. 2002;36:193-198.
81 Miller A, Marotta M, Scordi-Bello I, Tammaro Y, Marin M, Divino C. Ischemic colitis after endovascular aortoiliac aneurysm repair: a 10-year retrospective study. Arch Surg. 2009;144:900-903.
82 Robert JH, Mentha G, Rohner A. Ischaemic colitis: two distinct patterns of severity. Gut. 1993;34:4-6.
83 Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol. 2007;102:2247-2252.
84 Silva E, De Backer D, Creteur J, Vincent JL. Effects of fluid challenge on gastric mucosal PCO(2) in septic patients. Intensive Care Med. 2004;30:423-429.
85 Miller PR, Meredith JW, Chang MC. Randomized, prospective comparison of increased preload versus inotropes in the resuscitation of trauma patients: effects on cardiopulmonary function and visceral perfusion. J Trauma. 1998;44:107-113.
86 Rittoo D, Gosling P, Bonnici C, et al. Splanchnic oxygenation in patients undergoing abdominal aortic aneurysm repair and volume expansion with eloHAES. Cardiovasc Surg. 2002;10:128-133.
87 Kamimura K, Oosaki A, Sugahara S, Mori S. Survival of three nonocclusive mesenteric ischemia patients following early diagnosis by multidetector row computed tomography and prostaglandin E1 treatment. Intern Med. 2008;47:2001-2006.
88 Mitsuyoshi A, Obama K, Shinkura N, Ito T, Zaima M. Survival in nonocclusive mesenteric ischemia: early diagnosis by multidetector row computed tomography and early treatment with continuous intravenous high-dose prostaglandin E(1). Ann Surg. 2007;246:229-235.
89 Zhou SX, Qiu HB, Huang YZ, Yang Y, Zheng RQ. Effects of norepinephrine, epinephrine, and norepinephrine-dobutamine on systemic and gastric mucosal oxygenation in septic shock. Acta Pharmacol Sin. 2002;23:654-658.
90 Yang Y, Qiu HB, Zhou SX, Tan Y, Li SQ. Comparison of norepinephrine-dobutamine to dopamine alone for splanchnic perfusion in sheep with septic shock. Acta Pharmacol Sin. 2002;23:133-137.
91 Cullen JJ, Ephgrave KS, Broadhurst KA, Booth B. Captopril decreases stress ulceration without affecting gastric perfusion during canine hemorrhagic shock. J Trauma. 1994;37:43-49.
92 Kincaid EH, Miller PR, Chang MC. Enalaprilat improves gut perfusion in critically injured patients. Shock. 1998;9:79-83.
93 Parviainen I, Rantala A, Ruokonen E, Tenhunen J, Takala J. Angiotensin converting enzyme inhibition has no effect on blood pressure and splanchnic perfusion after cardiac surgery. J Crit Care. 1998;13:73-80.
94 Kozar RA, Hu S, Hassoun HT, DeSoignie R, Moore FA. Specific intraluminal nutrients alter mucosal blood flow during gut ischemia/reperfusion. J Parenter Enteral Nutr. 2002;26:226-229.
95 Kles KA, Wallig MA, Tappenden KA. Luminal nutrients exacerbate intestinal hypoxia in the hypoperfused jejunum. J Parenter Enteral Nutr. 2001;25:246-253.
96 Dickinson E, Tuncer R, Nadler E, et al. NOX, a novel nitric oxide scavenger, reduces bacterial translocation in rats after endotoxin challenge. Am J Physiol. 1999;277:1281-1287.
97 Sun Z, Olanders K, Lasson A, et al. Effective treatment of gut barrier dysfunction using an antioxidant, a PAF inhibitor, and monoclonal antibodies against the adhesion molecule PECAM-1. J Surg Res. 2002;105:220-233.
98 Tamion F, Richard V, Bonmarchand G, et al. Reduced synthesis of inflammatory cytokines by a free radical scavenger after hemorrhagic shock in rats. Crit Care Med. 2000;28:2522-2527.
99 Cuzzocrea S, Mazzon E, Costantino G, Serraino I, De Sarro A, Caputi AP. Effects of n-acetylcysteine in a rat model of ischemia and reperfusion injury. Cardiovasc Res. 2000;47:537-548.
100 Sagach VF, Scrosati M, Fielding J, Rossoni G, Galli C, Visioli F. The water-soluble vitamin E analogue Trolox protects against ischaemia/reperfusion damage in vitro and ex vivo. A comparison with vitamin E. Pharmacol Res. 2002;45:435-439.
101 Fung HL, Chong S, Kowaluk E, Hough K, Kakemi M. Mechanisms for the pharmacologic interaction of organic nitrates with thiols. Existence of an extracellular pathway for the reversal of nitrate vascular tolerance by N-acetylcysteine. J Pharmacol Exp Ther. 1988;245:524-530.
102 Rank N, Michel C, Haertel C, et al. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med. 2000;28:3799-3807.
103 Reinhart K, Spies CD, Meier-Hellmann A, et al. N-acetylcysteine preserves oxygen consumption and gastric mucosal pH during hyperoxic ventilation. Am J Respir Crit Care Med. 1995;151:773-779.
104 van Bockel JH, Geelkerken RH, Wasser MN. Chronic splanchnic ischaemia. Best Pract Res Clin Gastroenterol. 2001;15:99-119.
105 Cho JS, Carr JA, Jacobsen G, Shepard AD, Nypaver TJ, Reddy DJ. Long-term outcome after mesenteric artery reconstruction: a 37-year experience. J Vasc Surg. 2002;35:453-460.
106 Geelkerken RH, van Bockel JH, de Roos WK, Hermans J, Terpstra JL. Chronic splanchnic syndrome, results of reconstructive surgery. Arch Surg. 1991;126:1101-1106.
107 Boelstra T, Meerwaldt R, Beuk RJ, Kolkman JJ, Huisman AB, Geelkerken RH. The hybrid procedure; retrograde endovascular splanchnic artery recanalisation for acute splanchnic syndrome. Paper in preparation.
108 van Petersen AS, Kolkman JJ, Beuk RJ, Huisman AB, Doelman C, Geelkerken RH. Open or percutaneous revascularization for chronic splanchnic syndrome. J Vasc Surg. 2010;51:1309-1316.
109 Ukleja A, Scolapio JS, Buchman AL. Nutritional management of short bowel syndrome. Semin Gastrointest Dis. 2002;13:161-168.
110 Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043-1050.
111 Jeppesen PB, Langholz E, Mortensen PB. Quality of life in patients receiving home parenteral nutrition. Gut. 1999;44:844-852.
112 Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol. 2002;12:1179-1187.
113 Kolkman JJ, Mensink PB. Non-occlusive mesenteric ischaemia: a common disorder in gastroenterology and intensive care. Best Pract Res Clin Gastroenterol. 2003;17:457-473.