CHAPTER 26 SPINE: SPINAL CORD INJURY, BLUNT AND PENETRATING, NEUROGENIC AND SPINAL SHOCK
In the acute setting, spinal cord injury (SCI) represents a complex management issue, with optimal patient care depending on the smooth execution of diagnostic and therapeutic interventions, involving several different disciplines within the medical field. These include emergency medical system (EMS) personnel, emergency department (ED) staff, radiologists, orthopedic and neurological surgeons, intensivists, and physiotherapists. Of these, the immediate interventions employed within hours of injury often dictate the overall prognosis, and provide the patient with the best opportunity to improve long-term functional outcome. For this reason, adequate spinal immobilization, prompt diagnosis, and early consultation of the appropriate surgical service are measures that every ED physician should strive to incorporate into the evaluation each individual trauma patient. This chapter focuses on the epidemiology, classification, and management of SCI, as well as the complications that are typically encountered in both the acute and chronic spine-injured patient. It is through a healthy understanding of the diagnostic and therapeutic guidelines and recommendations that the morbidity and mortality associated with SCI can continue to trend downward, as it has consistently over the past 30 years.
INCIDENCE
Spinal cord injury as a whole most often afflicts young men between the ages of 16 and 30. The mean age is 29.7 years, and there is an 82% male gender bias, likely reflecting the greater tendency of young males to engage in risk-related activities.1–3 Injury most often occurs in the warmer summer months, especially during the weekend, with 53% of all SCI occurring between Friday and Sunday.4 With regard to specific etiologies of SCI, results often vary from one trauma center to the next, based largely on the socioeconomic setting of the respective institution. Vehicle trauma is common to both rural and urban healthcare centers as the leading cause of SCI. Prevention programs, including mandatory seatbelt laws, coupled with evolving innovations in automobile safety have served to drastically reduce the overall proportion of SCI attributable to motor vehicle accidents in recent years. In one 10-year period between 1980 and 1990, the overall proportion of SCI caused by vehicle trauma decreased from 47.2% to 38.1%.2 In rural centers, falls account for the second highest cause of SCI. Whereas in urban centers, violence has rivaled vehicle trauma as the leading cause of SCI. Specifically, gunshot wounds (GSW) from handguns represents approximately 90% of all SCI resulting from violence in urban centers, with an over-representation of minorities in these specific cases. Sports-related injuries continue to play a significant role in the overall incidence of SCI, regardless of the socioeconomic setting. In a study focusing on sports-related SCI out of the University of Alabama, Birmingham, the following sports contributed most to SCI in descending order: diving/surfing, football, winter sports, gymnastics, wrestling, and horseback riding.5 With the ever-increasing geriatric population, and youth violence on the rise, it is likely that falls and penetrating SCI will represent an increasing proportion of total SCI cases in years to come.
SCI represents a major economic burden on the healthcare industry for a variety of reasons. At the core of the problem is the fact that SCI patients not only represent an acute management challenge, with the average first year postinjury costs ranging from $123,000 to $417,000 depending on the neurologic level of injury, but they also represent a chronic financial burden, when long-term direct and indirect costs are factored in.2,6 Whereas direct costs are absorbed as a direct result of the injury, including rehospitalizations, nursing home care, durable equipment, and attendant care, indirect costs are more esoteric, and include loss of future wages, fringe benefits, and productivity. While it is indeed a triumph of modern medicine and critical care that greater than 95% of SCI patients survive their initial hospitalization, with an overall lifespan now approaching that of the average citizen, these factors have only further contributed to the escalating direct and indirect costs associated with the life-long care for SCI patients.1,2 The young average age of SCI patients at the onset of injury contributes further to the economic impact of SCI, with the estimated lifetime direct and indirect costs in excess of $2.5 million for high cervical injury patients (injury between C1 and C4).2
MECHANISM OF INJURY
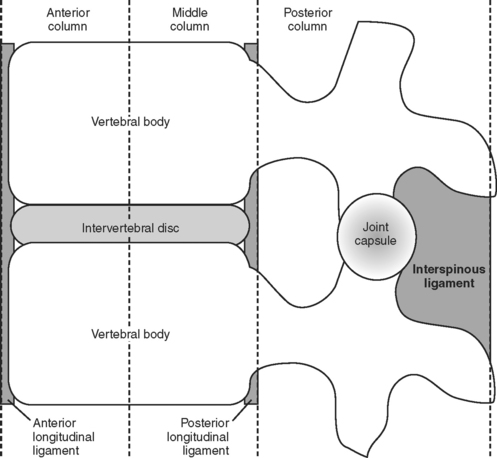
First described by Denis,7 the three-column model of spinal anatomy divides the spine into three distinct longitudinally-oriented anatomical columns (Figure 1). The anterior column includes the anterior longitudinal ligament (ALL), the anterior half of the vertebral body, and the intervertebral disc. The middle column consists of the posterior half of the vertebral body/intervertebral disc and posterior longitudinal ligament (PLL). Finally, the posterior column represents all bony/ligamentous elements posterior to the PLL (pedicles, lamina, spinous process, ligamentum flavum, and the interspinous ligament). By definition, SCI resulting in disruption of at least two of these three columns is considered an unstable injury. This somewhat simplistic representation of spinal anatomy serves to provide a mental framework for appreciating spine biomechanics and the potential injuries that may result from various blunt and penetrating forces to the spinal column.
Biomechanics of the Spine
Prior to delving into potential mechanisms of SCI, a healthy understanding of the biomechanics of the spine is imperative. As is frequently the case, there is often a certain degree of discrepancy between the actual level of deforming injury to the spine, and the resultant radiographic findings and neurologic deficits associated with the injury. Multiple mechanisms are often simultaneously involved in producing SCI. In their simplest forms, the four types of injurious forces that may be imparted to the intact spinal column are (1) flexion and extension (deflexion) injuries, (2) vertical compression and longitudinal distraction trauma, (3) rotational injuries, and (4) injuries with combined mechanisms.1,6
Regarding flexion-extension injuries, the spinal cord is often damaged by compression, transverse/longitudinal shear, torsion, and rotational forces. These injuries typically involve the cervical spine, and often result in disk protrusion, and/or interspinous/anterior column/posterior column ligamentous tears. When an associated disk protrusion and/or subluxation is present, there is a high incidence of concomitant local cord damage with mainly central cord necrosis and hemorrhage seen. In children under the age of 8, extreme hyperflexion injuries are often associated with complete cord transection, secondary to the physiologic high cervical ligamentous laxity normally found in the pediatric population.8–10
Hyperextension (retroflexion) injuries most often result in damage to the spinal cord at the C5–C6 level, as extension is maximal at this specific level from a biomechanical perspective. These injuries are often associated with bony dislocation, ventral fracture-dislocation, avulsion of articular processes, disruption/displacement of the intervertebral disks, and disruption of the anterior longitudinal ligament/posterior longitudinal ligament, all of which result in significant compromise of the anteroposterior diameter of the spinal canal and subsequent central cord lesions.9
Compression and longitudinal distraction injuries are most often seen in the setting of vertical stress to the spinal column secondary to the falls on the head, buttocks, or neck. They also often occur with an acute increase in axial load forces during a motor vehicle accident (MVA). Radiographically, these injuries are typically characterized by vertebral body flattening, end-plate fractures, and acute disk herniations. Often, there is associated retropulsion of bony fragments/disk material into the spinal canal, and varying degrees of resultant cord compression. When the mechanism of injury involves a fall, the majority of these injuries occur at the thoracolumbar junction, the most mobile segment of the spinal column. Conversely, the lower cervical spine is more often involved in cases where a vertical axial load is imparted the spinal column.
Similar to compression/longitudinal distraction injuries, rotational injuries of the spine most often involve the thoracolumbar junction and upper lumbar spine. By definition, they may involve all parts of the vertebral body, including the pedicles, articulating facets, and ligamentous complex. These injuries often result in unilateral or bilateral dislocation, or stable/unstable fracture dislocation due to interlocking of the vertebral bodies and distraction of the intervertebral disks.9,11
Mechanism of Injury
Spinal cord trauma is a broad term describing an injurious event that results in disruption of the functional and/or anatomic integrity of the spinal cord at a particular level(s). Although isolated lumbar spine injuries represent a significant proportion of SCI, the majority of debilitating SCI involve trauma to the cervical and thoracic spine. It is for this reason that the focus of this discussion will be injury of the spine from the cervical spine down to the thoracolumbar junction. With respect to the general mechanism of injury, all SCI can be categorized in one of three subgroups: (1) direct (penetrating) SCI, (2) indirect (blunt) SCI, or (3) combined direct/indirect SCI. Depending on the underlying mechanism of injury involved, the pathogenesis of SCI can be further subclassified. Primary traumatic lesions are due to direct mechanical disruption of the cord parenchyma, typically occurring at the time of the original injury. Secondary traumatic (reactive) lesions do not develop directly from the injurious stimulus, but instead evolve as a consequence of injury-related factors, including the development of edema, ischemia, improper immobilization with secondary mechanical injury, and other biochemical disorders. Finally, common to both primary and secondary traumatic lesions is the potential for delayed neurologic sequelae, including scar formation, secondary degeneration, or regenerative phenomena.9
Penetrating Spinal Cord Injury
Historically, the management of penetrating spinal cord injury (PSI) was a task relegated mainly to military physicians, as the majority of these injuries occurred in the setting of active combat. Unfortunately, the emergence of violence as a leading cause of SCI in many urban centers over the past 20 years, has served to underscore the importance of emergency room physician familiarity with the acute management of these injuries in civilian practice. In general, PSI can be classified as either gunshot wound–related (GSW-PSI) or lacerating (non-GSW) PSI. Both types of PSI most commonly involve the thoracic spine, and seldom involve more than one vertebral segment.12 Depending on the series, 52%–57% of all PSI results in complete neurological deficit (no sacral motor/sensory sparing), although lacerating PSI often results in incomplete neurologic injury.13–15 Although by definition, PSI implies direct dural/parenchymal disruption, in some cases the pathogenesis of PSI resembles that of a concussive model. This has been demonstrated in numerous military-based studies in which normal-appearing dura was encountered at the time of laminectomy in numerous cases.16–19
Blunt Spinal Cord Injury
In clinical practice, closed injuries of the spine are typically the most frequent type of SCI encountered. They often occur in the setting of MVA, industrial accidents, falls, and sport-related activities. In these cases, underlying injury to the spinal cord may occur in the presence/absence of concomitant soft tissue injuries, including fracture dislocation and subluxation of the spine. Biomechanics and mechanism of injury play a central role in the type and extent of spinal cord injury incurred. A thorough understanding of the potential types of mechanical forces distributed throughout the spine at the time of injury is paramount in order to be able to anticipate the evolution of secondary reactive lesions following the initial insult. In general, closed SCI can be distilled down into two broad categories. The first involves indirect cord injury arising from blunt trauma without space-occupying or penetrating lesions within the spinal canal. This type of injury is frequently observed in cases where the mechanism of injury involves longitudinal shearing/distraction, flexion, rotation, rotation-flexion, and/or posteroanterior acceleration.9 The second type of closed SCI involves direct cord injury secondary to blunt or penetrating forces resulting in canal compromise from a variety of space-occupying lesions. These include bony/ligamentous damage, fracture dislocation, or subluxation. It is important to note that SCI is rarely confined to the anatomic point of impact. In approximately 15% of SCI cases, lesions are observed at multiple levels due to both primary and secondary traumatic changes.20
SEVERITY/GRADING OF SPINAL CORD INJURIES
The neurologic level of injury (NLI) is a specific term that refers to the most caudal spinal cord level at which normal motor/sensory function persists following SCI. Although some degree of correlation often exists between the anatomic and neurologic level of injury, this relationship is not always consistent. Several factors including the spinal segment involved, and the underlying mechanism of injury, contribute to the ultimate NLI, once secondary traumatic lesions have manifested. Overall, according to the American Spine Injury Association (ASIA) database, approximately 53% of SCI patients are tetraplegic, 46% are paraplegic, and the remaining 1% experience complete recovery by the time they are discharged from the hospital. The classification of SCI can be further categorized as complete or incomplete injury, referring to the absence/presence of sacral motor/sensory sparing, respectively. The most common neurologic category is incomplete tetraplegia (31.2%), followed by complete paraplegia (26%), complete tetraplegia (21.9%), and incomplete paraplegia (20%).1,2
Neurological and Functional Outcome Scales
Numerous classification schemes have been devised to describe patients with SCI over the years. Generally speaking, two types of assessment scales exist, neurological examination scales and functional outcome scales. It is now generally accepted that the most meaningful description of SCI in the acute setting occurs when a neurological assessment tool is applied in conjunction with a functional outcome assessment scheme, in order to provide perspective on the significance of any neurological recovery on the day-to-day life of SCI patients. The first standardized neurological assessment scale for SCI was proposed by Frankel and associates in 1969.21 In this scheme, a five-grade scale (A to E) is employed to discriminate SCI patients on the basis of differing degrees of motor/sensory function preserved after their injury. Frankel grade A patients are those with complete motor and sensory lesions. Grade B patients have sensory only functions below the level of injury. Grade C patients have some degree of motor and sensory function below the level of injury, but their retained/recovered motor function is useless. Grade D patients have useful, but abnormal, motor function below the level of injury. And grade E patients are fortunate enough to experience complete motor/sensory recovery prior to discharge from the hospital. The main deficiencies involving the Frankel scale proved to be the difficulty involved in discerning grade C from grade D patients, as well as the relatively poor interobserver reliability with practical application of the scale. Despite these shortcomings, the Frankel scale provided an important classification framework from which several contemporary classification schemes have been derived. In fact, the ASIA impairment scale, largely regarded as the most studied and useful of the SCI neurological classification schemes, is essentially a permutation of the original Frankel scale, in which objective parameters are provided to better assess the significance of retained motor function between grade C and grade D patients.22
Analogous to the Frankel scale as a neurologic examination tool in SCI is the Functional Independence Measure (FIM) as a functional outcome scale. The FIM is an 18-item, seven-level scale designed to assess the severity of patient disability, estimate the burden of care, and to prognosticate on medical rehabilitation and overall functional outcome. Specifically, the FIM complements neurological assessment by providing scores for activities of grooming, bathing, eating, dressing the upper body, dressing the lower body, and toileting.22
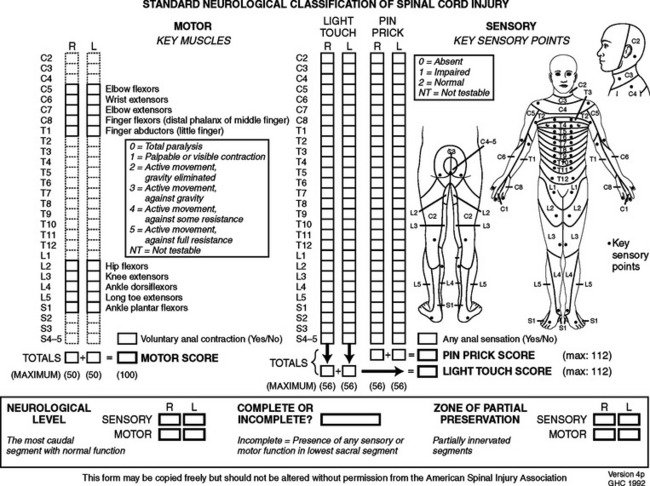
Concomitant assessment of both the neurological and functional deficits in acute SCI is imperative in assessing the impact of injury on the patient as a whole. Additionally, linkage of these independent scales allows clinicians to specifically evaluate whether therapeutic interventions resulting in improvement of the gross neurological assessment score also result in enhanced functional recovery for the patient. It is on the basis of functional outcome that the overall significance of various therapeutic interventions can be truly assessed. Omission of such functional outcome assessment in the National Acute Spinal Cord Injury Study (NASCIS) I and II clinical trials assessing the benefit of acute methylprednisolone (MP) therapy in acute SCI patients is often cited as a critical shortcoming of the design study, rendering the interpretation of improved neurological outcome scores essentially impossible. A recent meta-analysis of the current literature regarding classification schemes for SCI prompted the Section on Disorders of the Spine and Peripheral Nerves of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) to recommend the ASIA standards for neurological and functional classification of SCI as the preferred neurological examination for clinicians involved in the evaluation and management of acute SCI.22 This recommendation was based largely on the finding that the ASIA scale provided the greatest discrimination in grouping subjects with SCI into mixed-injured categories, with a relatively high degree of interobserver reliability. Similar to the other SCI classification schemes, the ASIA scale has undergone several revisions since its inception in 1984. Currently, the scale now consists of several components, including the ASIA impairment scale, the ASIA motor index score, the ASIA sensory score, and the FIM (Figure 2).
Spinal Cord Syndromes
Several spinal cord syndromes have been described in the setting of acute SCI. Central cord syndrome typically occurs with a cervical region injury leading to greater weakness in the upper limbs than the lower limbs, associated with sacral sparing. Brown-Sequard syndrome is classically seen in the setting of penetrating SCI resulting in a hemisection lesion of the cord. It is typically associated with a relatively greater ipsilateral proprioceptive and motor loss, with contralateral loss of sensitivity to pain and temperature below the NLI. Conversely, anterior cord syndrome is associated with a lesion causing variable loss of motor function and sensitivity to pain and temperature, while posterior tracts including proprioception are spared. Conus medullaris syndrome is associated with injury to the sacral cord and lumbar nerve roots, leading to areflexic bladder, bowel, and lower extremity, while sacral segments may occasionally demonstrate preserved reflexes. Finally, cauda equine syndrome is due to injury involving the lumbosacral nerve roots within the spinal canal, resulting in areflexic bladder, bowel, and lower extremities. Similar to the various brainstem vascular syndromes (e.g., Wallenberg’s syndrome), spinal cord syndromes often do not present with the classic textbook constellation of signs/symptoms. However, as is the case with brainstem vascular syndromes, an understanding of the various spinal cord syndromes serves to provide a rough neuroanatomical framework regarding the complex structural organization intrinsic to the spinal cord.
DIAGNOSIS
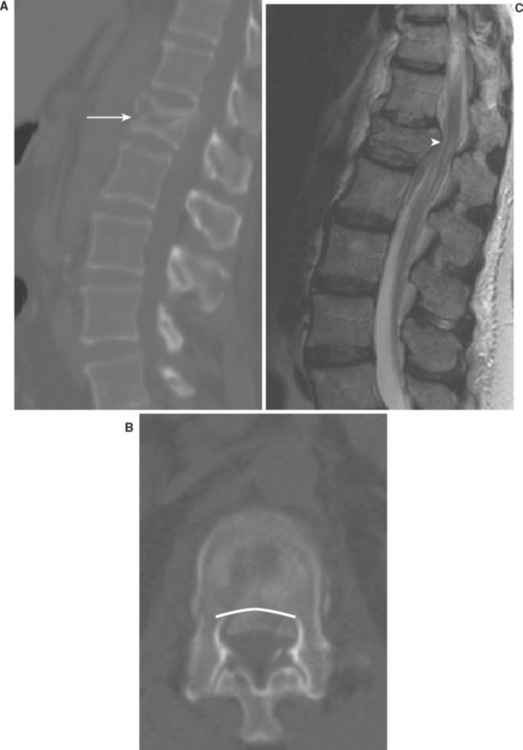
The organization of the central nervous system provides the physician with the opportunity to localize traumatic lesions to the spinal cord with a relatively high degree of accuracy, based on careful neurologic examination alone. However, adequate presurgical care, as well as optimal surgical planning, are both heavily dependent upon accurate imaging of the spine. For decades, plain roentgenograms of the spine have been an invaluable localization tool in SCI for several reasons. First, unlike other more sophisticated imaging modalities, the technology and resources required are not typically a limiting factor. Second, the portability of x-ray technology and the relative ease of acquisition provide the physician the opportunity to rapidly attain important anatomic information, even under the most hectic conditions. Lastly, plain films of the spine can provide a whole host of information regarding underlying SCI, including the presence/absence of fractures, subluxation/dislocation, and spinal canal patency (Figure 3). Although soft tissue structures are not well-visualized with standard x-ray technology, malalignment/angulation of the spine detected on a plain film may hint to underlying acute ligamentous or disk injury.
Within the past 10–15 years, the increased availability of computerized tomography (CT) in most trauma centers has served to revolutionize the diagnosis of SCI in the acute setting. In many centers, CT has supplanted plain x-ray as the acute imaging modality of choice for the evaluation of the spine in trauma patients, due largely to the improved accessibility of this technology. CT is superior to plain x-ray in many respects. It provides an outstanding view of the osseous structures, unparalleled by any other imaging modality. Additionally, the recent advent of three-dimensional reconstruction software now provides the added benefit of viewing the relevant anatomy in the axial, coronal, and sagittal planes (Figure 4). For these reasons, CT is generally considered to be able to detect vertebral fractures with greater sensitivity/specificity than plain x-ray alone. Although far inferior to magnetic resonance imaging (MRI) technology with regard to the associated soft-tissue anatomy, CT can provide some resolution of soft tissue, including the presence of paraspinous hematoma, which further raises the likelihood of underlying unstable ligamentous injury. Combined with myelography, CT technology can provide a view of intracanal anatomy that approaches MRI specificity in diagnosing space-occupying lesions of the spinal canal. Unfortunately, the technical burden and logistics involved with myelography on polytrauma patients has limited its applications for assessment of the acute spine-injured patient.
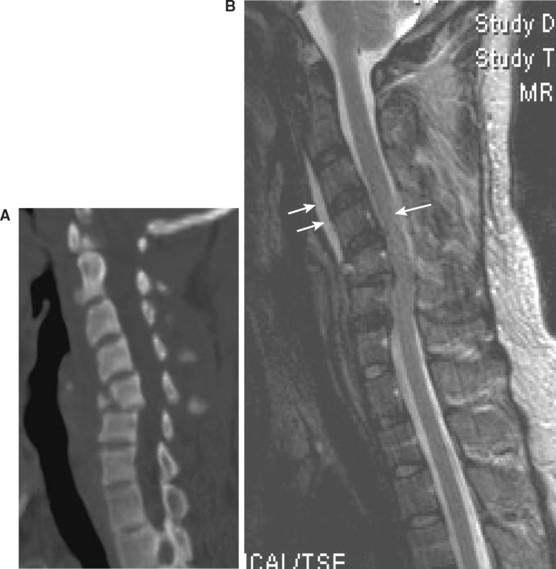
Since its inception as a medically applicable imaging modality by Raymond Damadian in 1971, MRI technology has continuously evolved into the diagnostic tool that it is today. This evolution in technology has been mirrored by a concomitant increase in its availability. Although inferior to CT technology in terms of resolution of bony architecture, MRI undoubtedly provides the most information regarding injury to soft tissue structures, particularly the spinal cord itself. In particular, T2 sequencing provides valuable information regarding the actual NLI, once secondary traumatic lesions, such as cord edema, have manifested (Figure 5). Most importantly, active mechanical compression of the spinal cord and direct spinal cord parenchymal injury can be directly ascertained by MRI, thus guiding the overall time table for surgical decompression/stabilization.
MANAGEMENT OF ACUTE SPINAL CORD INJURY
The optimal management of acute SCI represents a daunting task, requiring a well-executed multidisciplinary effort in order to provide patients with the greatest chance for meaningful neurological recovery. In the broadest sense, the management of acute SCI can be fractionated into several important phases, beginning with care rendered by emergency medical service (EMS) personnel in the field, and culminating with intensive spinal cord rehabilitation following the acute hospitalization. In between, careful orchestration of patient care between emergency department (ED) personnel, radiologists, surgeons, intensivists, and physiotherapists, all play an equally important role in the overall prognosis and outcome of SCI patients. In general, the phases of management in SCI include (1) prehospital care, (2) acute ED evaluation/care, (3) postacute care, and (4) posthospital care/rehabilitation. Certain aspects of care rendered during a particular phase of treatment may overlap with subsequent phases of management. For instance, pharmacotherapy initiated during acute evaluation in the ED often continues into the postacute phase of care. Additionally, certain treatment options, such as surgery, may be offered at different points of care (e.g., acute vs. subacute surgical intervention).
Prehospital Care
The main tenet of prehospital care of SCI has remained rapid and effective spinal immobilization, in order to prevent further neurological deterioration secondary to pathological motion of the unstable spine. It is estimated that approximately 3%–25% of SCIs occur after the initial traumatic insult, either during transport, or early in the course of management.22 For this reason, it is recommended that complete spine immobilization utilizing a rigid cervical-spine collar with supportive blocks on a rigid backboard with straps, be performed on all trauma patients in which the underlying mechanism of injury suggests potential underlying SCI. The judicious use of spine immobilizers must be tempered with their rapid discontinuation once definitive evaluation and treatment has been rendered, given concern over immobilization device-associated morbidity. Specifically, elevated intracranial pressure (ICP), development of pressure sores, significant patient discomfort/distress, falls, and increased risk of aspiration have all been associated with spinal immobilization devices.23,24 Once a patient is safely extricated and immobilized, timely transport to a regional medical center capable of evaluating and treating acute SCI is paramount.
Acute Emergency Department Evaluation/Management
Depending on the institution, evaluation of acute SCI lies within the realm of either neurological or orthopedic surgery. In many regards, the most important aspect of the surgeon’s involvement actually begins in the setting of the ED. It is the surgeon’s responsibility to rapidly process several diverse pieces of information including the neurological examination findings, radiographic findings, and the presence of other detracting injuries, in order to arrive at the major branch point in the early management of the spine-injured patient: acute surgical intervention versus conservative management with the potential for delayed surgical intervention. The decision to intervene surgically is typically made on a case-by-case basis, as each case represents a unique set of circumstances to consider and diagnostic/therapeutic obstacles to overcome. Generally speaking, the decision to operate in the acute setting is heavily influenced by the specific type of injury radiographically present, the patient’s neurological exam findings, and the overall stability of the patient vis-a-vis other potentially immediately life-threatening traumatic injuries. Polytrauma in the SCI patient is common, with the following injuries occurring with decreasing frequency: fractures of the trunk (17.2%), long-bone fractures (13.9%), head and facial trauma (13.8%), pneumothorax and chest injury (8.8%), and abdominal injury (8.6%).1 In these cases, surgery should be delayed until the patient is first adequately resuscitated, including treatment of immediately life-threatening injuries, maintenance of adequate blood pressure parameters, and the employment of cervical traction and corticosteroid therapy if deemed appropriate.
Surgical Intervention
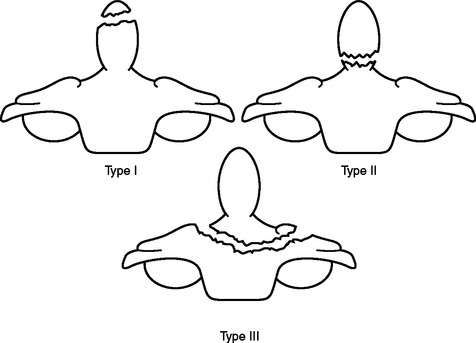
Traumatic atlanto-occipital (C1-occipital) dislocation injuries may be best treated with internal fixation and arthrodesis utilizing any variety of craniocervical fusion techniques. Although traction and external immobilization have been used to successfully treat a subset of these injuries, transient or permanent neurological worsening, or delayed instability, have been observed more often in these cases than in cases with surgical stabilization. Occipital condyle fractures may be most optimally treated with external cervical immobilization. Management of isolated fractures of the atlas (C1) is typically dictated by the specific atlas fracture present. It is recommended that all isolated atlas fractures with preservation of the transverse ligament be treated with immobilization alone. Whereas isolated atlas fractures occurring in the context of transverse ligament disruption may be treated with either external cervical immobilization alone, or with surgical fixation. The rule of Spence states that in the setting of atlas fractures, when the sum of the displacement of the lateral masses of C1 on C2 exceeds 8 mm on plain open-mouth x-ray, the likelihood of underlying transverse ligament disruption is especially high, and may require an MRI for further assessment. Management of fractures of the axis (C2) similarly depends on the type of fracture present. Type I (tip of dens), type II (base of dens), and type III (involvement of dens with extension into the body of C2) odontoid fractures may all be initially managed conservatively with external immobilization (Figure 6). However, surgical fixation should be entertained in cases of type II/III odontoid fractures where the dens is displaced greater than 5 mm, there is comminution of the dens fracture (type IIA), or where fracture alignment is difficult to maintain with external immobilization alone. Additionally, initial surgical fixation should be considered in patients over age 50, with type II odontoid fractures, given the relatively high rate of non-union in this subset of patients. Hangman’s fractures of C2 (traumatic spondylolisthesis of the axis) may also be managed with external immobilization in most cases, although it is important to balance these considerations with the morbidities associated with HALO vest use. Surgical stabilization should be considered in cases of severe C2–C3 angulation, disruption of the C2–C3 disk space, or inability to maintain alignment with external immobilization. Isolated fractures of the axis body should be treated with external immobilization alone. The management of combination fractures of the atlas and axis should be based primarily on the specific characteristics of the underlying axis fracture. The majority of C1–C2 combination fractures may be treated with external immobilization, with the exception of C1-type II odontoid combination fractures with an atlanto-dens interval of 5 mm or more, and C1-Hangman’s combination fracture with C2–C3 angulation of 11 degrees or more. The surgical technique employed must be modified to accommodate for any loss of integrity of the ring of the atlas.
Thoracolumbar Fractures
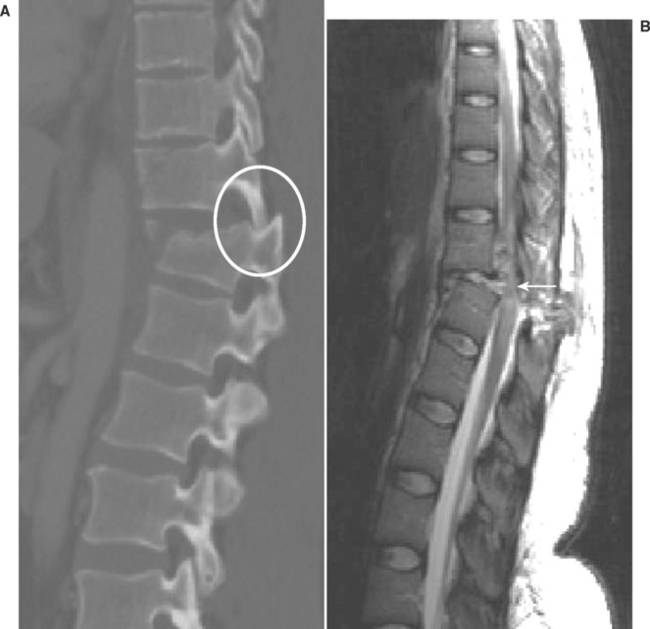
The three-column model of spine anatomy is particularly helpful in evaluating injuries to the thoracic and lumbar spine. In general, there are four main types of blunt injury that can be imparted to the thoracolumbar spine: (1) compression fractures, (2) burst fractures, (3) flexion distractions, and (4) fracture dislocations. Compression fractures are characterized by intact middle/posterior columns, with compression and loss of height of the anterior column. Conversely, burst fractures typically involve both the anterior and middle columns, and are most often generated by pure axial load injuries. They most commonly occur between T10 and L2, and, by definition, are associated with retropulsion of bone/ligament complex into the spinal canal, often resulting in significant cord compression (Figure 7). Flexion distraction injuries typically involve horizontal fractures that can extend exclusively through bone (type I), through bone and ligament (type II), and through the disk, facet capsule, and the interspinous ligament (type III). These injuries may be missed by conventional axial CT, due to the horizontal orientation of the fractures. Fracture dislocations are typically caused by shear forces, are highly unstable injuries, and are often associated with significant canal compromise (Figure 8).
Optimal Timing of Surgical Intervention in Spinal Cord Injuries
The role of surgical decompression in acute SCI remains a topic of considerable debate. Whereas experimental data involving early surgical decompression in animal models has consistently demonstrated enhanced neurological recovery, these results have proven difficult to extrapolate back to actual SCI patients, mainly due to the lack of clinical trials needed to demonstrate definitive and unequivocal benefit of acute surgical decompression in SCI.25–27 The majority of studies on surgical decompression in the literature represent retrospective case series with historical controls. Generally speaking, most studies comparing decompressive surgery with conservative management actually fail to definitively demonstrate improved outcome with surgery. Interestingly, in a recent meta-analysis by La Rosa et al.,28 early surgical decompression performed within 24 hours of injury resulted in statistically significant neurological improvement when compared to both delayed surgical intervention (more than 24 hours postinjury) and conservative management, particularly in patients with initial incomplete injury. Despite the lack of irrefutable supportive evidence, early surgical decompression remains the recommended treatment option in patients with acute cord compression.
Surgery in Penetrating Spinal Cord Injuries
Similar to early surgical intervention for blunt (nonpenetrating) SCI, the value of early surgical intervention in PSI remains debatable. Retrospective analyses of PSI typically do not demonstrate any improved outcome with early surgical intervention. Additionally, the rate of complication tends to be higher in PSI patients that have been treated with laminectomy in the acute setting.12 For this reason, early surgical intervention in the setting of acute PSI is only recommended as a treatment option in cases associated with progressive neurological deterioration. However, open wounds or wounds with suspicion of CSF leakage may require surgical debridement/exploration and dural repair.
Nonoperative Acute Interventions
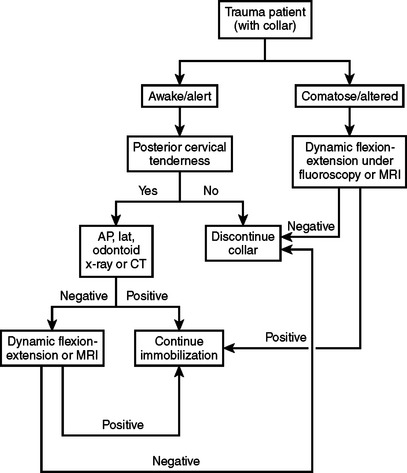
Concomitant with the assessment for potential acute surgical intervention, several other interventions may be implemented during the acute phase of SCI, while the patient is still in the ED. These include closed reduction of C-spine dislocation injuries, pharmacotherapeutic intervention, and correction/prevention of hypotension. Awake patients with isolated cervical fracture dislocation injuries should undergo early closed reduction with cranio-cervical traction, in order to restore the anatomical alignment of the spine. The overall rates of transient and permanent neurological complication associated with closed reduction are 2%–4% and 1%, respectively.22 If the patient is alert and can be examined, then the risk of reduction/traction without a prior MRI is low. Therefore, external cervical reduction should be performed as soon as possible, after the diagnosis has been made radiographically. Prereduction MRI examination is recommended only in cases when the patient cannot be examined during the reduction. The presence of a significant disc herniation under these conditions represents a relative indication for open ventral decompression prior to reduction. Additionally, MRI is recommended for patients who fail initial attempts at closed reduction. An algorithm for the clearance of the cervical spine in a trauma patient is presented in Figure 9.
Pharmacotherapy and Spinal Cord Injury
Unlike the case of closed reduction for cervical dislocation injuries, the role of pharmacotherapy in the treatment of acute SCI has been less definitive. Of the various agents available for treatment of acute SCI, methylprednisolone (MP) has, by far, been the most extensively studied. After initial enthusiasm over the potential benefit of early corticosteroid (MP) therapy in acute SCI, recent studies and intense scrutiny of the study parameters and data interpretation employed in the original NASCIS clinical trials have served to weaken the argument that MP therapy is associated with a meaningful improvement in functional outcome of SCI patients. The main criticisms of the NASCIS trials have been related to the determination of optimal timing of therapy, the method of motor assessment, and the apparent lack of correlation between motor recovery scores and functional outcome measures. These shortcomings, coupled with the inability to demonstrate any clear therapeutic benefit of MP therapy on outcome of SCI in several independent studies, have prompted the AANS and CNS to recommend MP therapy as a treatment option only, which should only be undertaken with the knowledge that evidence suggesting harmful side effects is more consistent than any suggestion of clinical benefit.22 Harmful side effects of MP therapy include pneumonia, sepsis, hyperglycemia, gastrointestinal ulcers/bleeding, and avascular necrosis of the femoral head, all of which have been observed to occur at significantly higher rates in NASCIS II/III protocol MP-treated SCI patients.29–31 GM-1 ganglioside has similarly been implicated in enhancement of neurological recovery in acute SCI. However, available medical evidence does not support such a benefit, and thus, the administration of GM-1 ganglioside in the setting of acute SCI is recommended only as a potential treatment option at this time. Nimodipine, naloxone, and tirilazad mesylate have also been implicated as potential neuroprotectants following SCI, and are currently the focus of ongoing clinical trials.30,32–34
Subacute Management of SCI
Following initial resuscitation and acute surgical intervention, SCI patients then enter the subacute phase of their initial hospitalization. Review of the literature suggests that severe SCI patients are susceptible to life-threatening cardiovascular instability and respiratory insufficiency in the first 7–14 days postinjury.22 This is particularly true for severe cervical SCI patients. For this reason, it is strongly recommended that patients with severe cervical level SCI be managed in an intensive care unit (ICU) setting, and that cardiac, hemodynamic, and respiratory monitoring devices be employed in their care to detect any cardiopulmonary dysfunction. Even in otherwise stable patients, maintenance of MAP parameters with continuous infusion of pressor agents often necessitates ICU nursing care. While in the ICU, as well as afterward on the wards, SCI patients benefit from prompt evaluation by physical and occupational therapy (PT/OT) services, and early participation in associated rehabilitation activities. Care should be taken to prevent the development of pressure ulcers and extremity contractures, as these factors negatively impact neurological recovery potential, as well as outcome in general. Once the patient has been transferred out of the ICU setting, priority should be placed on timely transfer to a qualified rehabilitation center, in order to provide SCI patients with the greatest opportunity to regain neurological function and improve their overall functional outcome.
MORBIDITY AND COMPLICATIONS MANAGEMENT IN SPINAL CORD INJURY
Neurogenic Shock
Of the potential complications commonly encountered in the context of acute SCI, those implicated in development of hemodynamic compromise are associated with a significant proportion of morbidity and mortality in the early management of these patients. Neurogenic shock is classically associated with the triad of hypotension, bradycardia, and core hypothermia, in the setting of acute SCI with the NLI localized to T6 and above. The loss of thoracic sympathetic outflow produces a state of unopposed predominant vagotonia, with resultant end-organ effects including decreased peripheral vascular resistance, impaired thermoregulation, and bradycardia. The diminished vascular tone, in combination with diminished cardiac output (CO) secondary to bradycardia, often results in profound hypotension that may be refractory to crystalloid/colloid resuscitation. Untreated, systemic hypotension can contribute to cord ischemia, facilitating the progression and severity of secondary traumatic lesions. It is this premise that has underscored the rationale of maintaining MAP goals in the acute SCI patient. Typically this is achieved through fluid/colloid resuscitation, the use of pressor agents, or a combination of the two.35–37 Pressors with intrinsic β1-agonist chronotropic activity, including dobutamine and dopamine, are of particular usefulness, as they address both the vasomotor and cardiogenic aspects of neurogenic shock. Persistent bradycardia may be treated with intermittent atropine, with the knowledge that atropine may exacerbate pulmonary dysfunction by thickening secretions. In general, sympathetic tone begins to return in 3–7 days.35 However, during this period of time, the SCI patient is at greatest risk for developing a cardiopulmonary complication, and thus continued ICU monitoring of these patients during the first 1–2 weeks postinjury is strongly recommended.22
Pulmonary Complications
Pulmonary complications represent a significant source of morbidity and mortality in SCI patients, in both the acute as well as the chronic setting. Injury to the cervical and midthoracic cord often results in underlying respiratory dysfunction, which, in turn, increases susceptibility to pulmonary infections. SCI above C4 often compromises phrenic nerve function, resulting in subsequent diaphragmatic dysfunction. The majority of patients with a high cervical injury thus require prolonged mechanical ventilation, often necessitating tracheostomy to avoid laryngotracheal malacia associated with prolonged intubation. Injury at lower cervical and upper thoracic levels can impair innervation to accessory muscles of respiration, including the intercostal muscles, resulting in a progressive loss of vital capacity, tidal volume, and negative inspiratory pressure. This serves to effectively reduce lung volume, create ventilation-perfusion mismatches with intrapulmonary shunting, and decrease the arterial oxygen saturation.37 Loss of the ability to produce an adequate cough and the normal sigh mechanism, leads to the accumulation of uncleared secretions, and plugging/collapsing of the terminal pulmonary segments. This serves to promote an optimal intra-alveolar environment for the development of repetitive, and in many cases fatal, pneumonias. The excessive use of analgesia and sedatives can further depress respiratory function. In order to decrease the risk of pulmonary complications, it is imperative that an aggressive pulmonary toilet regimen be immediately instituted, consisting of aerosol treatments, chest physiotherapy, intermittent positive pressure breathing (IPPB), and frequent suction in mechanically ventilated patients. Bronchoscopy has been a valuable tool for retrieval of tenacious mucus plugs in chronically debilitated patients. Additionally, early mobilization, if possible, also serves to improve overall pulmonary function.
Thromboembolism
Thromboembolic phenomena, including deep venous thrombosis (DVT) and pulmonary embolism (PE), represent a significant, and potentially fatal, complication in the SCI patient population. DeVivo and colleagues38 have documented a 500-fold increased risk of dying from a PE in the first month following SCI when compared against both age- and gender-matched controls. Although the risk of DVT and PE appears to decline proportionately with time from injury, SCI patients, especially those with complete injury, are perpetually at an elevated risk for thromboembolism due to immobility. For this reason, prophylaxis for at least 3 months following injury has been advanced as a standard of care in SCI patients.22 A variety of methods have been used to achieve this end, including the use of low-molecular-weight heparins, caval filters, rotating beds, adjusted-dose heparin, low-dose warfarin, pressure stockings, pneumatic compression stockings, and electrical stimulation.35,36 Of these, low-dose heparin, in combination with pneumatic compression stockings, represents the prophylactic regimen of choice in most centers, despite the lack of any direct evidence of synergy. Use of a prophylactic regimen has been observed to reduce the risk of DVT in SCI from 27.3%–10.3%.35 In the event of a documented DVT or PE, a 4–6 month duration of full anticoagulation is warranted. Caval filters are recommended for SCI patients who experience refractory thromboembolic events despite anticoagulation, and in patients in whom anticoagulation is contraindicated (e.g., recent or impending surgery). The diagnosis of DVT can be accomplished with duplex Doppler ultrasound with a sensitivity of approximately 90%.22 More invasive diagnostic techniques such as venography should be reserved for cases in which the index of suspicion for DVT remains high, despite a negative Doppler study. Similarly, invasive diagnostic modalities for PE such as transfemoral pulmonary angiogram should be reserved only for cases where suspicion of PE remains high, despite a negative ventilation-perfusion or spiral pulmonary CT angiogram study.
Skin Care
In the setting of neurological injury, the development of pressure ulcers is a significant source of discomfort, and represents yet another potential route of infection. The sacral prominence, femoral greater trochanters, ischial tuberosities, and heels are particularly vulnerable to ulcer formation. The incidence of pressure necrosis requiring surgical debridement within 2 years of SCI is approximately 4%.36 Prevention is paramount, and typically involves early patient mobilization, air mattresses, limited use of braces/orthotics, and aggressive skin inspection and wound care.
Post-Traumatic Syringomyelia
Typically occurring on a more chronic timetable, post-traumatic syringomyelia (PTS) has been encountered with increasing frequency in SCI over the past 20 years, likely due to a combination of factors including increased life expectancy in SCI patients, as well as the emergence of MRI technology as a highly sensitive diagnostic tool. By definition, PTS is a central cavitation of the spinal cord, typically occurring months to years after the initial injury. Although presenting symptoms vary based on the location and severity of the syrinx, persistent local or radicular pain, motor weakness, spasticity, dissociated sensory loss, autonomic dysreflexia, sphincter loss, sexual dysfunction, Horner’s syndrome, and respiratory dysfunction have all been described. Extension of cervical syrinx cavities into the medulla, termed syringobulbia, has also been described, and typically manifests with corticobulbar dysfunction. Although the exact pathognomic mechanism involved in syrinx formation has not been definitively elucidated, the prevailing hypothesis favors a progression of post-traumatic cystic myelopathy. Alteration in spinal subarachnoid cerebral spinal fluid (CSF) flow dynamics secondary to post-traumatic arachnoiditis may also play a role. The incidence of PTS ranges from 1.1% to greater than 50%, with the duration of time of injury to diagnosis of syrinx ranging from 2 months to 33 years.39 Development of symptomatic syringomyelia is a poor prognostic sign. Although several surgical procedures have been employed in the treatment of PTS, including placement of cysto-peritoneal and cysto-pleural shunts, the overall success of these interventions is generally poor.
MORTALITY
Despite significant advances made in first responder management of SCI, approximately 10%–20% of acute SCI patients do not survive to reach hospitalization, and another 3% of patients die during their acute hospitalization.3,6,14,38,40 For those patients who survive the acute hospitalization, the major cause of death is pneumonia and other respiratory complications, followed by heart disease, subsequent trauma, and septicemia. The leading causes of death among incomplete paraplegics are cancer and suicide. Suicide is also the leading cause of death in complete paraplegics, followed by heart disease. In general, the suicide rate is higher among the SCI population under the age of 25 years. In terms of life expectancy, individuals aged 20 years at the time of their injury have life expectancies of approximately 33 years as tetraplegics, 39 years as low tetraplegics, and 44 years as paraplegics.
CONCLUSION
Thanks in large part to marked improvements made in the firstresponder and ED management of acute SCI, overall morbidity and mortality in this patient population have substantially decreased over the past 30 years. Similarly, the implementation of aggressive prophylactic/preventative measures against many of the common complications of SCI, including thromboembolism, urinary tract infections, pulmonary infections, and pressure ulcers, have served to further improve the quality of life for these patients. With the average life span of SCI patients approaching that of the general public, the economic burden associated with lifelong treatment of these patients will undoubtedly continue to increase in the years to come. Timely diagnosis, in conjunction with the application of appropriate treatment guidelines/recommendations in the acute setting, provides patients with the best opportunity to improve functional neurological outcome. Whereas certain interventions, including spinal immobilization, closed reduction of cervical dislocation injuries, and maintenance of blood pressure parameters, have gained universal acceptance in the management of SCI, others, including the role of MP therapy and acute surgical decompression, are more widely debated and open for interpretation.
1 Zigler JE, et al. Epidemiology of spinal cord injury: a perspective on the problem. In: Levine AM, et al, editors. Spine Trauma. Philadelphia: W.B. Saunders; 1998:2-15.
2 Garland DE, et al. Epidemiology and costs of spine trauma. In: Capen DA, Haye W, editors. Comprehensive Management of Spine Trauma. St. Louis, MO: Mosby; 1998:1-5.
3 Devivo MJ, et al. Prevalence of spinal cord injury. Arch Neurol. 1980;37:707-708.
4 Stover SL, et al. Spinal Cord Injury: The Facts and Figures. Birmingham: University of Alabama, 1986.
5 Harvey C, et al. New estimates of traumatic SCI prevalence: a survey-based approach. Paraplegia. 1990;28:537-544.
6 Kurtzke JF. Epidemiology of spinal cord injury. Exp Neurol. 1975;48:163-236.
7 Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
8 Papadasiliou V. Traumatic subluxation of the cervical spine during childhood. Orthop Clin North Am. 1978;9:945-954.
9 Jellinger K. Pathology of spinal cord trauma. In: Errico TJ, Bauer RD, Waugh T, editors. Spinal Trauma. Philadelphia: Lippincott; 1991:455-495.
10 Gilles FH, et al. Infantile atlantoocipital instability. Am J Dis Child. 1979;133:30-37.
11 Adams JH, et al. Greenfield’s Neuropathology: Disease of the Spine and Spinal Cord, 4th ed., London: Edward Arnold and Company; 1984:779-812.
12 Simpson RK, et al. Penetrating spinal injuries. In: Pitts LH, Wagner FC, editors. Craniospinal Trauma. New York: Thieme Medical Publishers; 1990:197-212.
13 Stauffer ES, et al. Gunshot wounds of the spine: effects of laminectomy. J Bone Joint Surg. 1979;61:389-392.
14 Yashon D, et al. Prognosis and management of spinal cord and cauda equina bullet injuries in sixty-five civilians. J Neurosurg. 1970;32:163-170.
15 Karim NO, et al. Spontaneous migration of a bullet in the spinal subarachnoid space causing delayed radicular symptoms. Neurosurgery. 1986;18:97-100.
16 Six E, et al. Gunshot wounds to the spinal cord. South Med J. 1979;72:699-702.
17 Meirowsky AM. Penetrating spinal cord injuries. In: Coates JB, Heaton LD, Meirowsky AM, editors. Neurological Surgery of Trauma. Washington, DC: Office of the Surgeon General; 1965:257-344.
18 Haynes WG. Acute war wounds of the spinal cord. Am J Surg. 1946;72:424-433.
19 Frazier CH. Stab and gunshot wounds to the spine. In: Frazier CH, editor. Surgery of the Spine and Spinal Cord. New York: D. Appleton; 1918:457-497.
20 McCormick WF. Trauma. In: Rosenberg RN, editor. The Clinical Neuro-sciences: Neuropathology. New York: Churchill Livingstone; 1983:241.
21 Frankel HL, Hancock DO, Hyslop G, et al. The valve of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179-192.
22 Guidelines for management of acute cervical spine injury. Neurosurg Suppl. 50(3), 2002.
23 Linares HA, et al. Association between pressure sores and immobilization in the immediate post-injury period. Orthopedics. 1987;10:571-573.
24 Davies G, et al. The effect of a rigid collar on intracranial pressure. Injury. 1996;27:647-649.
25 Tarlov IM. Spinal cord compression studies III. Arch Neurol Psychiatry. 1954;71:588-597.
26 Tarlov IM, et al. Spinal cord compression studies II. Time limits for recovery after acute compression in dogs. Arch Neurol Psychiatry. 1954;71:271-290.
27 Brodkey JS, et al. Reversible spinal cord trauma in cats. Additive effects of direct pressure and ischemia. J Neurosurg. 1972;37:591-593.
28 La Rosa G, et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Nature. 2004;42(9):503-512.
29 Wing PC, et al. Risk of avascular necrosis following short-term megadose methylprednisolone treatment. Spinal Cord. 1998;36:633-636.
30 Bracken MB, et al. Effects of timing of methylprednisolone or naloxone administration on the recovery of segmental and long-tract neurological function in NASCIS 2. J Neurosurg. 1993;79:500-507.
31 Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93(Suppl 1):1-7.
32 Bracken MB. National Acute Spinal Cord Injury Study of methylpred-nisolone or naloxone. Neurosurgery. 1991;28:628-629.
33 Bracken MB, et al. A randomized, controlled trial of methylprednisolone, or naloxone in the treatment of acute spinal cord injury: results of the Second National Acute Spinal Injury Study (NASCIS-2). N Engl J Med. 1990;322:1405-1411.
34 Bracken MB, et al. Methlyprenisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow-up results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. J Neurosurg. 1998;89:699-706.
35 Schaffery C. Medical complications. In: Benzel EC, editor. Spine Surgery: Technique, Complication Avoidance, and Management. New York: Elsevier; 2005:2027-2032.
36 Rimoldi RL, et al. Immediate post-operative care. In: Levine AM, et al, editors. Spine Trauma. Philadelphia: W.B. Saunders; 1998:562-566.
37 Ruben BH. Cardiopulmonary management of spinal cord injury. In: Greenberg J, editor. Handbook of Head and Spine Trauma. New York: Marcel Dekker; 1993:497-502.
38 DeVivo MJ, et al. Cause of death for patients with spinal cord injury. Arch Intern Med. 1989;149:1761-1766.
39 Madsen PWIII, et al. Post-traumatic syringomyelia. In: Levine AM, et al, editors. Spine Trauma. Philadelphia: W.B. Saunders; 1998:608-629.
40 Bracken MS, et al. Incidence of traumatic spinal cord injury in the United States, 1970–1971. Am J Epidemiol. 1981;113:615-622.