CHAPTER 36 Spine Infections: An Algorithmic Approach
CHILDHOOD DISCITIS
Pathogenesis, etiology, and natural history
The natural history of childhood discitis varies from neonates/infants to young children to teenagers.1
Neonate/infant and young children discitis
A high proportion of negative disc cultures in infants and toddlers has prompted some authors to state that childhood discitis may be either inflammatory or infective. Infective childhood discitis usually results from hematogenous seeding of organisms. The intervertebral disc is avascular even in infants.2 It has been postulated that pediatric spine infections commence in the microarterioles of the vertebrae3 because the nutrient arteries have been demonstrated to be a more direct route for the spread of infection to the vertebral column than its venous drainage.3 This theory proposes that spinal infection in infants and toddlers starts in a very similar way to the development of metaphyseal osteomyelitis in long bone infection. Terminal arterioles arising from the circumferential vessels fed from the extraperichondrial arterial plexus and from nutrient metaphyseal arteries penetrate the hyaline cartilage endplates of the vertebral bodies and terminate adjacent to the intervertebral disc in neonates up to 1 year of age.2,4,5
Venous drainage follows the same route. Blood-borne bacteria can be delivered directly to the intervertebral disc during bacteremia.6 The pediatric host cannot mount a response to invading organisms within an avascular disc, allowing the organisms to multiply unimpeded. Pyogenic bacteria release proteolytic enzymes, leading to destruction of the intervertebral disc. Spread of infection to the vertebral body in infants is limited by the cartilage-capped endplates. However, with further progression, the hyaline cartilage-capped endplates may be destroyed, allowing the invading organisms direct access to the vertebral body.7 Infants have widespread anastomotic connections between intraosseous arterioles within the vertebral body.8 Disappearance of these anastomoses later in childhood increases the risk of vertebral bone necrosis and subsequent osteomyelitis through microthrombosis. Vertebral osteomyelitis is present in 25% of cases. Spread to the epidural space is rare in young children but has been reported in previously well males.9
Clinical presentation – history and clinical features
Childhood discitis is uncommon. The clinical presentation can be subdivided into three distinct age groups – neonates/infants, young children, and early teenagers. Infants and young children present acutely with malaise and a limp or refusal to bear weight on one leg.10 Brown et al.11 report an insidious onset of symptoms in young children with typical late presentation. Viral or bacterial infections often precede childhood discitis. A history of recent mild spinal trauma is sometimes elicited. Seventy-eight percent of cases involve the lumbar spine.12 Fifty percent to 57% of young children present with backache.10,13 Teenagers present with spinal and occasionally abdominal pain.14
The commonest clinical signs are trunk stiffness and a loss of the normal lumbar lordosis.11 Paravertebral muscle spasm and hamstring tightness are common. Some children may present with a totally rigid lumbar spine. Spinal tenderness is easier to detect in children old enough to communicate verbally. A minority (28%) of children with discitis are febrile (>37.9°C).12 If the disease progresses to spondylodiscitis (which includes vertebral osteomyelitis), up to 79% have an elevated temperature.12 Neurological deficit in this age group is rare.
ADULT PYOGENIC SPONDYLODISCITIS
Pathogenesis, etiology, and natural history
Spondylodiscitis may complicate generalized septicemia or result from a distant focus such as vegetation of a heart valve or florid skin infection. Blood-borne adult pyogenic spondylodiscitis originates in the endplate of the vertebra (rather than the intervertebral disc), most likely in the capillary loop or postcapillary venous channels, spreading secondarily to the intervertebral disc. Pyogenic bacteria secrete proteolytic enzymes, causing necrosis of bone and intervertebral disc.
Individuals at risk include those affected by:
Infection of males has a slight preponderance,15 with a male:female ratio ranging up to 1.8:1. If the patient has been previously well and the organism is indolent, the spondylodiscitis can be arrested at an early stage with nonoperative management. If the patient has been previously unwell and the organism is more virulent, the natural history of spondylodiscitis is of worsening local sepsis, sometimes spreading paravertebrally.
Infection may spread to form a psoas abscess and extend to its insertion at the lesser trochanter of the proximal femur. Epidural spread also occurs. The spinal cord or cauda equina may be compressed by an enlarging abscess, usually at one spinal level. The microvascular circulation from the anterior spinal artery to the spinal cord/cauda equina is also disturbed by the presence of microvascular thrombosis as a result of persisting adjacent infection.16
Prolonged immobilization with osteopenia and progressive vertebral osteomyelitis with bone destruction predispose to pathological fracture. The resultant angular kyphosis or posteriorly displaced bone fragments into the spinal canal may contribute to neurological compromise. Mortality has been reported in 10–16% of adults with pyogenic spondylodiscitis.17–19
Clinical presentation – history and clinical features
Patients typically present with insidious onset of unremitting spinal pain and loss of spinal movement. The clinical presentation can be classified into acute, subacute and chronic, depending on the virulence of the organism and the ability of the affected individual to mount a response.20 A history of malaise, anorexia, fevers, weight loss, and night or resting spinal pain is common. Most have a diminished range of movement and mild tenderness over the spinous process of the affected vertebra early in the disease.
With more-advanced spinal infection, neurological deficit is present in 29–51% of spondylodiscitis.17,18,21–23 Two-thirds of patients with paralysis from spinal infection have central cord syndrome and one-third have anterior cord syndrome.21 Neurological deficit is most common at the cervical spine level and least common at the lumbar level.
Factors associated with neurological deficit include diabetes mellitus, advancing age, steroid therapy, organ transplantation, chronic inflammatory conditions, and intravenous drug abuse.18
ADULT PYOGENIC DISCITIS
Pathogenesis, etiology, and natural history
True adult pyogenic discitis usually follows surgical intervention. The incidence following discography is 0.5–1% and following any type of spinal procedure 0.4–4%.24 It has been reported after every type of spinal procedure including laminectomy, discectomy, arthrodesis, discography, chemonucleolysis, myelography. and lumbar puncture. Discitis complicates discectomy in 0.4–2.8% of patients.24,25
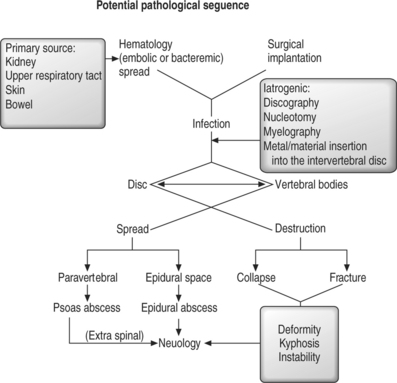
Microdiscectomy using a microscope was reported to reduce the rate of discitis24 but others have found no difference in infection rate.26 It is probably due to direct inoculation of the organism into the avascular intervertebral disc although some believe that it results from aseptic inflammation.24 During the stage of suppuration, the invading organisms multiply and a surrounding inflammatory response is mounted by the host. In a similar manner to spondylodiscitis, the bacteria secrete proteolytic enzymes, leading to necrosis of the intervertebral disc and endplates. Again, the amount of disc destruction depends on the virulence of the invading organism and the resistance of the host. In a previously well patient, the discitis may be locally contained with little treatment, when the invading organism is indolent. However, the infection may spread to the vertebral endplates and beyond if the organism is virulent or in an immunocompromised individual. Should this occur, the pathogenesis and natural history of discitis is similar to that of adult pyogenic spondylodiscitis (Fig. 36.1).
Clinical presentation – history and clinical features
Pain is expected following a surgical procedure or injection.24 The clinical course of postprocedural discitis is often reflected by early relief of symptoms due to the surgical procedure, typically followed by recurrence of similar spinal pain 1–4 weeks after the procedure (range, 2 days to 10 weeks).27 The constant, throbbing pain that subsequently develops is often out of proportion to the clinical picture. Unlike postoperative deep wound infection, the surgical skin incision/scar is normal in more than 90% of cases.24 Most patients with postprocedural discitis have only a very mildly elevated temperature. They eventually develop malaise, anorexia and weight loss. Clinically, patients develop marked paravertebral muscle spasm and stiffness of the affected spine. Fewer than 15% of patients develop a new or worsening neurological deficit from the spread of infection.24,28
POSTOPERATIVE DEEP WOUND INFECTION
Pathogenesis, etiology, and natural history
Early wound complications of spine surgery
Early postoperative infections occur less frequently following discectomy (0.5–1%) than instrumented posterior spine arthrodesis (up to 12.9%).29 Laminectomy alone has been reported to result in a 1.5% wound infection rate. When arthrodesis is added, the risk increases 50%, and with the addition of instrumentation it increases 100%.29
The preoperative nutritional status of the patient is an important factor in the incidence of wound dehiscence and subsequent infection.30–32 Generally, patients have a better chance of uncomplicated postoperative wound healing with a plasma albumin level of greater than 3.5 g/dL and a total lymphocyte count of greater than 2000 cell/mm3.12
Preoperative radiation increases the risk of early wound complication in patients with spinal tumours.29,32 Risk factors for early wound complications include prolonged intraoperative time (>4 hours), massive blood loss, blood transfusions, large wound hematomas, posterior rather than anterior approach, and large numbers of operating theater personnel.33
Staphylococcus aureus accounts for approximately 60% of all infections. Other organisms implicated in primary and postprocedural pyogenic spinal infections are shown in Table 36.1. Patients with immune deficiency are susceptible to the rarer fungal organisms including those shown in Table 36.2.
Table 36.1 Other Organisms Implicated in Primary and Postprocedural Pyogenic Spinal Infections
| Actinomyces | Aerobacter |
| Bacteroides | Brucella |
| Enterobacter | Escherichia coli |
| Klebsiella | Proteus |
| Pseudomonas | Salmonella |
| Serratia | Streptococcus |
Table 36.2 Rarer Fungal Organisms
| Aspergillus | Candida |
| Coccidioides | Cryptococcus |
| Histoplasma | Nocardia |
Superficial wound infection may extend to involve deeper tissues, including the intervertebral disc, following spinal surgery. Prolonged surgical time may result in excess wound edema or ischemic/necrotic wound edges from prolonged skin edge retraction. Both complications allow virulent bacteria or normal skin flora to enter the wound. Skin flora of low virulence including Acinetobacter baumani, Peptiostreptococcus, Corynebacterium, coagulase-negative Staphylococcus, and Propionibacterium acnes have been cultured from postoperative surgical wounds in elective orthopedic surgery.34 Although S. aureus and methicillin-resistant S. aureus (MRSA) are the commonest hospital-based organisms responsible for early deep wound infection, some wound infections involve more than one organism and include indolent types of bacteria from normal skin flora.
Late infection and late hematogenous seeding
The range of organisms isolated from late infection of the spine following instrumented spine arthrodesis is similar to that of early wound infection. Embolization or bacteremia from the bladder, kidney, respiratory tract, skin, or bowel may result in hematogenous seeding in spinal instrumentation some time after surgery. Late infection can also follow intravenous drug abuse.35 The bacteria remain dormant for a period of time and secrete a glycocalyx which promotes their adherence to the metal of the spinal instrumentation and shields the organisms from lymphocytes and antibiotics.36–38 When the host develops an intercurrent illness and natural immunity is depressed, late spinal infection may develop. It is manifest as an initial suppurative phase with abscess formation, followed by reactive granulation tissue when the host mounts a response to the infection. Pyogenic bacteria secrete proteolytic enzymes, leading to necrosis of surrounding soft tissue and bone. Symptoms have usually developed by this stage and the patient may develop a painful swelling about the spinal instrumentation. Should the infection remain undiagnosed, a sinus may develop. Superficial drainage to the skin is not common in late pyogenic infection.
Sterile inflammation
Hematoma formation and sterile bursae are frequently evident between prominent metal and the skin in thin patients with posteriorly instrumented spinal arthrodesis. Corrosion and metal fretting with release of particulate metal from micromotion between coupled spinal instrumentation results in a sterile inflammatory response, even after solid arthrodesis.39,40 In most cases, cultures of the fluid and tissue adjacent to the metal implants are negative. Where bacteria can be cultured from the interface membrane, it is hypothesized that the metal particles and resultant inflammation may potentiate late infection due to activation of indolent organisms or hematological embolization.
Clinical presentation – history and clinical features
Deep spinal wound infection can be manifest in three clinical scenarios – early, delayed, and late. Early deep wound infection may follow a superficial wound infection from anterior or posterior spine surgery. The incidence of erythema, cellulitis, wound dehiscence, or purulent discharge before the onset of deep wound infection is up to 93%.29 Less than a one-third are noted to have a temperature of greater than 37.5°C at diagnosis. Delayed and late deep wound infections are reportedly due to or associated with intraoperative inoculation of indolent bacteria/fungi in the presence of metal fretting or from true late hematogenous seeding in spinal instrumentation. When intraoperative inoculation of indolent organisms occurs, some early wound erythema is often noted.29,41,42 Diagnosis is often made by exclusion. Clinical symptoms include spinal pain, malaise, anorexia, and subjective swelling within the spinal wound.43
EPIDURAL SPACE INFECTION
Pathogenesis, etiology, and natural history
Epidural infection is relatively rare but the incidence may be increasing due to a greater number of spinal procedures, epidural catheterization for pain control, intravenous drug abuse, and immunocompromised patients.23,44 Although they are distributed circumferentially around the spinal cord/cauda equina, anterior epidural space infection is more likely to be associated with spondylodiscitis than a posterior infective focus.
Posterior foci usually result from hematogenous spread and are associated with frequent venous puncture for steroid or antiinflammatory injection and acupuncture.45 Epidural space infections are more common in the thoracic and lumbar spines, and tend to spread rapidly, often spanning a number of spinal segments at the time of diagnosis. Neurological compromise and even paralysis can occur early in the course of the infection, being manifest in days rather than in weeks, as is sometimes the case in spondylodiscitis. A combination of neural compression and microvascular thrombosis of the vessels of the spinal cord are thought to be responsible. Neurological deficit has been described in 19–80% of cases.23,45 Rarely, other infections occur within the spinal canal, including subdural abscess and spinal cord abscess.
Clinical presentation – history and clinical features
Epidural infections affect patients of all ages (including children) and may present with signs of overwhelming infection including septicemia, bleeding diathesis, abrupt onset of paraplegia, and even adult respiratory distress syndrome with minimal overt signs of spinal infection. In contrast to spondylodiscitis, high fevers and rigors are noted early in the course of the infection. Affected individuals often have severe constitutional symptoms of malaise and anorexia. Neck rigidity is often seen in cervical epidural space infection. Meningeal irritation and radicular pain are often present. Classically, neurologic deficit is evident within 7–10 days of the onset of infection. Progressive neurologic deficits are present in 19–37% of cases,23,46 and are commoner in the thoracic spine (60% of these cases) than the cervical spine (33.3% of cases),46 although McHenry et al. report a higher incidence from cervical spine infection.18
PRIMARY AND POSTPROCEDURAL FACET JOINT INFECTION
Pathogenesis, etiology, and natural history
Even though the capsule of the lumbar facet joint has anterior perforations, paraspinal and intradural extension of the abscess is exceedingly rare.47,48 The sepsis is contained within the joint in most cases and causes localized spinal pain until drained. Severe neurological sequelae have not been reported in joint sepsis following facet joint infection.
Clinical presentation – history and clinical features
Suppurative facet joint arthritis is rare with less than 50 reported cases.47 The clinical features can mimic spondylodiscitis. It is a very rare complication following facet joint injection with only three cases reported to date.49 Because facet joint injection is gaining popularity in the therapeutic management of low back pain in Western medicine, an increase in incidence should be expected. Most patients undergoing this procedure have localized spinal pain with or without radiation. Often, the patient will have immediate benefit of pain relief while the local anaesthetic is effective. Postinjection suppurative arthritis is difficult to diagnose in the early stages because it is recognized that under normal circumstances, it may take up to 10 days for locally injected steroids to have a dampening effect on the pain. Pain localization usually occurs only when the infection is established. Patients with undiagnosed postinjection suppurative facet joint arthritis continue to deteriorate, with severe unremitting back pain.
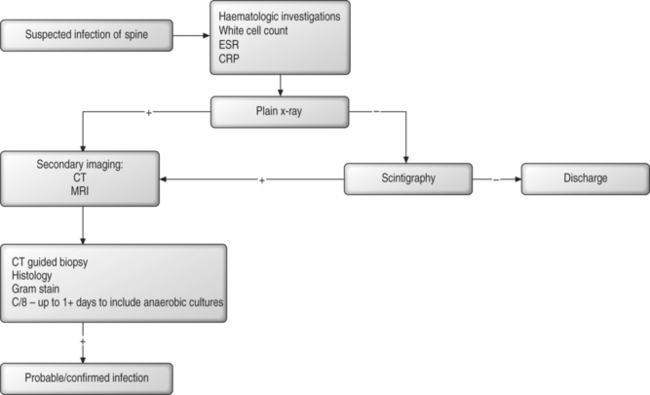
DIAGNOSIS
There is no single diagnostic test for spinal infection.
Plain radiology
Childhood discitis
In the earliest stages of infection, loss of cervical or lumbar lordosis is seen on lateral plain radiographs.7 A reduced disc height and erosion of adjacent vertebral endplates is present in up to 76% childhood discitis of 2 weeks duration.7,12 Long-standing infection leads to scalloping of the vertebral endplates. Late angular kyphosis may develop from destruction of either the vertebral body or the growth plates or both. The differential diagnosis of childhood discitis includes vertebra plana secondary to eosinophilic granuloma, bone tumors, and Scheuermann’s kyphosis in adolescents. Plain radiographs of the spine can distinguish among these conditions at presentation in most cases.
Pyogenic spondylodiscitis
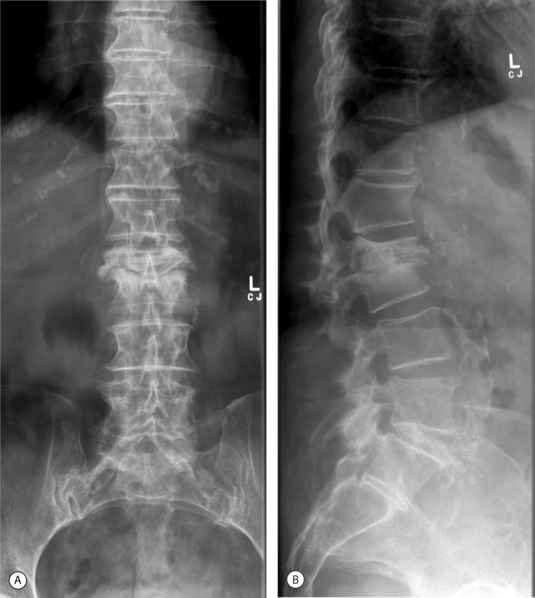
Depending on the nature of the invading organisms and the general condition of the patient, plain radiological changes appear from 2 weeks to 3 months after the onset of infection.50 Progressive narrowing of the disc space and irregularity and loss of the sharp, straight outline of the adjacent vertebral endplates is demonstrated better on lateral radiographs.51 Subchondral endplate lysis or defects may follow. Evidence of bone repair is manifest by hypertrophic or sclerotic bone formation adjacent to the vertebral endplate. With progression of the disease, paravertebral soft tissue swelling is evident on anteroposterior (AP) radiographs. A psoas abscess may form, changing the profile of the psoas shadow on AP radiographs. If gas is demonstrated in the soft tissues, anaerobic bacteria are usually responsible. With progressive osteomyelitis, bone destruction of the vertebral bodies may cause a pathological fracture,50 acute angular kyphosis or, less commonly, scoliosis. Pyogenic spondylodiscitis may heal by late bony or fibrous ankylosis (Fig. 36.2).
Axial and spiral CT
Pyogenic spondylodiscitis
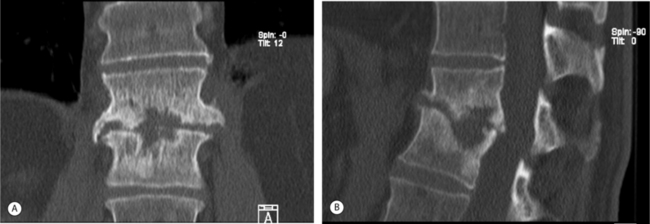
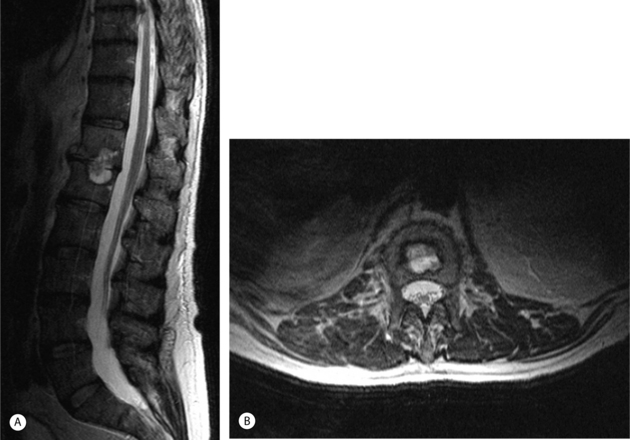
Multiplanar (spiral) CT is the investigation of choice to demonstrate the presence and extent of paraspinal/psoas abscesses, because MRI sagittal plane sequences are usually limited laterally to the tips of the transverse processes. Spiral CT imaging has the added advantage of providing the clinician with a 3-D view of the vertebral bodies of the infected spine.50,51 CT-guided percutaneous spinal biopsy allows for precise direction and localization of the infected disc or paravertebral abscess. MR imaging is superior in differentiating among epidural blood, pus, or tumor (Fig. 36.3).
Nuclear medicine (scintigraphy)
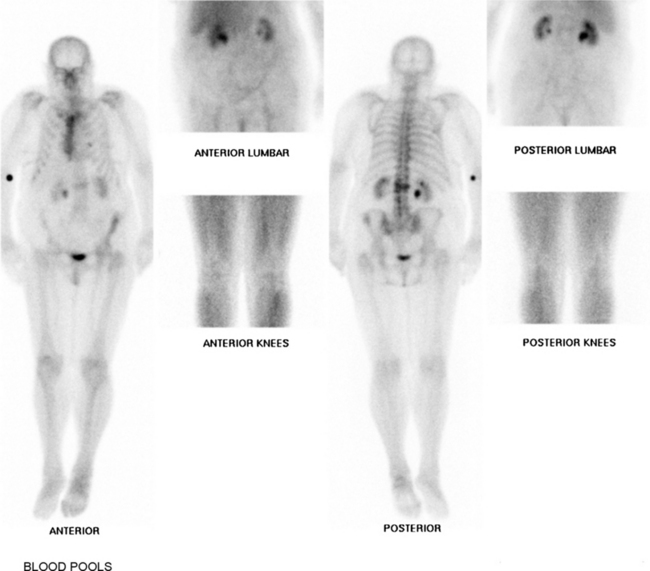
Gallium is an analog of ferritin which is secreted by leukocytes. In111-WBC scans have a low sensitivity (17%).50,51 Ga-67 SPECT images are accurate in diagnosing spinal osteomyelitis in up to 91% of cases. Combined accuracy of Technetium 99 and Gallium 67 citrate scans is as high as 94%.50 Technetium 99 scanning is recommended in very young children when discitis is suspected and exact localization is difficult from history and clinical examination. The scan appearance may show a high probability of infection as early as 3–5 days after clinical symptoms develop.52,53 During the healing phase of infection, the Gallium-67 scan may become negative although the Technetium-99 scan remains positive.54 For this reason, Gallium-67 scan alone has been recommended for follow-up studies of disc space infections.55 The authors’ preferred recommendation is follow-up MRI with gadolinium, except when general anesthesia is required for children. Under these circumstances, follow-up Technetium-99 and Gallium-67 scans are both considered (Fig. 36.4)
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the investigation of choice in assessment of early spondylodiscitis.50 It allows for multiplanar imaging. It has a specificity of up to 94% in distinguishing among discitis, vertebral osteomyelitis, epidural, paraspinal and prevertebral abscess, transverse myelitis, and spinal cord or intradural suppuration with a sensitivity of up to 97%,56,57 when gadolinium is added for enhancement. Early infection can be detected on T1-weighted sequences, in which decreased signal intensity in the intervertebral disc and the adjacent vertebral bodies from edema is evident. The intranuclear cleft is nearly always demonstrable (94%)51 in normal discs and is not visible in discitis. Signal hyperintensity of the disc on T2-weighted images, combined with loss of disc height, is useful in identifying the infected disc. After discography or facet joint blocks, injected fluids demonstrate low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences, occasionally mimicking purulent fluid. T2-weighted images demonstrate increased signal intensity within the bone marrow of adjacent vertebral endplates in spondylodiscitis due to inflammatory changes. Axial plane gradient echo sequences are useful in distinguishing between normal and infected vertebral bodies. Fat suppression techniques are useful for differentiating inflammatory areas in the vertebral bodies from fatty marrow in older individuals. Fat suppression also distinguishes between epidural suppuration and epidural fat. Epidural abscesses demonstrate high signal on T2-weighted sequences and may be isointense with cerebrospinal fluid (CSF). Proton density-weighted sequences demonstrate a darker image for CSF than pus. Gadolinium enhances images when tissue is hypervascular, and can help distinguish infection from scar tissue, tumor, CSF, and chronic degenerative changes. Enhancement of the disc, vertebral endplates, and bone marrow on postgadolinium sequences is highly suggestive of pyogenic spondylodiscitis.50,58,59 Gadolinium is especially useful when the spinal cord or dural sac is compressed by abscess, distinguishing pus from surrounding edema fluid.50 The signal from the spinal cord may be hyperintense under compression but acute changes are felt to be reversible. Persisting spinal cord hyperintensity usually reflects cord ischemia or myelomalacic change.60
One favored protocol for MRI of cervical spine infections involves:60
MRI is more difficult to interpret following surgery which involves bone grafting. Also, postoperative enhancement of the uninfected disc is common.50 In the first few days after surgery, paravertebral edema and slight enhancement of the bone graft–vertebra interface is evident on T2-weighted sequences. By 4 weeks postsurgery, a noninfected bone graft and adjacent vertebrae enhance irregularly. The bone graft normally demonstrates high signal intensity on T2-weighted sequences for 1 year following surgery. This enhancement eventually reduces as the bone graft incorporates into the adjacent vertebral bodies. When infection intervenes, the bone graft usually displays a hyperintense, uniform enhancement. Stainless steel causes more artifact than titanium on all sequences, and its use may preclude MRI as a useful tool for diagnosing postoperative infection.
Some conditions are difficult to distinguish from spondylodiscitis on MRI. Severely degenerative discs with acute fibrovascular infiltration demonstrate decreased signal on T1-weighted images and increased signal on T2-weighted sequences in the subchondral endplates. These degenerate discs, however, demonstrate reduced T2-weighted disc signals in the less hydrated nucleus pulposus. Inflammatory spinal conditions have similar signal changes to infection on all MRI sequences. They include ankylosing spondylitis, rheumatoid arthritis, sarcoid disease, gout, and calcium pyrophosphate crystal deposition. Gouty lesions (very rare) are usually sharply delineated and are associated with punched-out lesions of the endplate. If a spinal infection is less vascular, for example a granuloma, then MRI may not distinguish the infection from tumors which rarely cross a disc space (multiple myeloma and lymphoma).60 In these cases, there is an indication to perform spinal biopsy (Figs 36.5, 36.6).
Laboratory studies
Leukocytosis is usually indicative of infection but can often be absent or minimal in patients with pyogenic vertebral osteomyelitis. Elevated leukocyte counts are evident in 13–60% of cases.46 Elevation of erythrocyte sedimentation rate (ESR) is sometimes minimal in spine infection, but a vast majority of patients with discitis demonstrate a raised ESR at some stage.24 The sensitivity for using ESR as a postprocedural index of infection ranges 73–100%.24,46 The specificity ranges 38–62%.24 The ESR peaks 4 days after surgery and returns to normal levels between 14 and 42 days after surgery, depending on the extent of surgery and the amount of spinal instrumentation.24
C-reactive protein (CRP) is nearly always elevated when inflammation or infection occurs and is an excellent indicator of the degree of infection/inflammation. The sensitivity for using CRP as a postprocedural index of infection is 64–100%;24 the specificity is 62–96%.24 CRP levels demonstrate a faster return to normal following surgery compared to ESR.24 CRP levels fall with the leukocyte count. The magnitude of spinal surgery/instrumentation also determines how quickly the CRP level returns to normal. It is recommended that both ESR and CRP be serially monitored to gauge the efficiency of treatment of spinal infections.24 Blood cultures are reported to be positive in 25% of spinal infections61 in adults and up to 50% in childhood discitis,62 if taken at the time of a rigor.
Diagnostic procedures
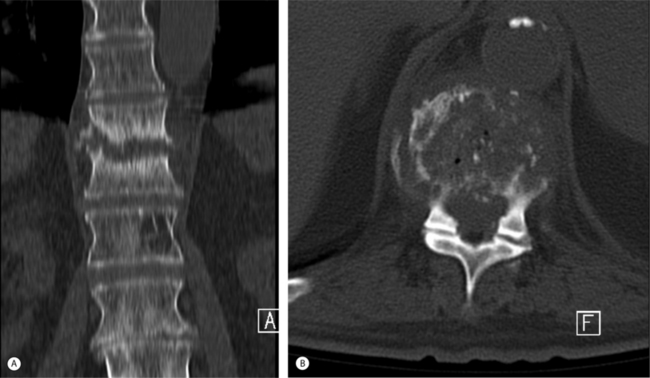
CT-guided percutaneous biopsy accurately predicts the invading organism in 37% of childhood discitis7 and 60–70% of adult cases.62 Cultures may be negative if the patient has been previously administered antibiotics. Histological examination will exclude tumors and foreign body granulomas. Gram stain and acid-fast stain as well as cultures for aerobic, anaerobic, fungal, and TB cultures of fresh material will maximize the chances of an accurate diagnosis. Bacterial cultures should be maintained for a minimum 10 days to detect indolent organisms. The volume of material obtained reflects the degree of accuracy in diagnosis. A tissue core obtained through a Craig biopsy needle or a TruCut (Baxter Travenol) needle generally provides more material than a fine needle aspiration, unless forced aspiration is applied as the needle is withdrawn. An open surgical biopsy has a higher yield for the confirmation of a positive bacterial culture62 and is obligated if CT-guided aspiration fails to grow an organism and the patient does not respond to antistaphylococcal antibiotics (Fig. 36.7)
MANAGEMENT
Nonoperative
The management of spinal infection changed in the 1940s and 1950s with the introduction of penicillin and other antibiotics. The traditional treatment prior to this was rest in bed and immobilization. Today, treatment of individual spinal infections depends on the nature of the invading organism, the host resistance, and the stage of spinal infection at the time of diagnosis (acute, subacute, chronic). Ideal nonoperative management involves establishing the diagnosis, isolating the causative organism, providing or restoring nutrition to the host to fight the infection, maintaining spinal stability, eliminating the invading organism initially with intravenous antibiotics, and detecting early neurological deficit. Broadly, definitive antibiotic medication management commences after procuring positive cultures and sensitivities of the invading organisms. The duration of antibiotic therapy is variable. For pyogenic infections, intravenous antibiotics are continued empirically for approximately 6 weeks. The patient’s reaction to the infection is monitored by serial white cell count, ESR, and C-reactive protein. Oral antibiotics are generally ceased when the ESR has returned to less than 20.63 Failure of nonoperative treatment is high in immunocompromised patients −18/57 HIV positive and 42/57 intravenous drug abusers.64
Specific infections
Childhood discitis
Childhood discitis can usually be treated nonoperatively. In the past, no difference has been reported in the outcome for infants receiving antibiotics and those treated with bed rest alone.65–69 Immunocompetent infants who are systemically well are more likely to respond to this management. However, prolonged or recurrent symptoms can occur in children not initially receiving intravenous antibiotics.70 For this reason, intravenous antibiotics are now recommended for initial management.2,5,11,71,72
Recommendations for the ideal length of an intravenous antibiotic course range from 1 to 8 weeks.5,6,11,12 Generally, intravenous antibiotics are administered for up to 2 weeks followed by oral antibiotics for up to 6 weeks. Response to antibiotic treatment is monitored by weekly white cell count, ESR, and C-reactive protein. Patients should be followed up for at least 12–18 months after resolution of spinal pain and malaise. The indications for repeat radiological assessment include a recurrence of symptoms or failure of the ESR and CRP to return to normal.
Narrowing of the disc space and vertebral endplate sclerosis are expected;5 however, 20% infants and 30% older children demonstrate spontaneous fusion.10,66 If an infant develops vertebral osteomyelitis, an extreme form of angular kyphosis may develop.1 As bone necrosis is rare in infantile discitis, the only explanation is that the vertebral growth plates are destroyed as a result of the infection. The indications for considering surgical debridement of the disc/vertebrae are continuing septicemia during an antibiotic course or progressive neurological deficit.11,73
An updated algorithm for investigating/managing childhood discitis is:
Pyogenic discitis and spondylodiscitis
Ninety percent patients treated nonoperatively are pain free and 75% have a bony or short fibrous union within 2 years of the onset of infection.28 However, longer-term outcomes in larger series portray a poorer outcome, with up to 75% having severe chronic spinal pain and an inability to work.18
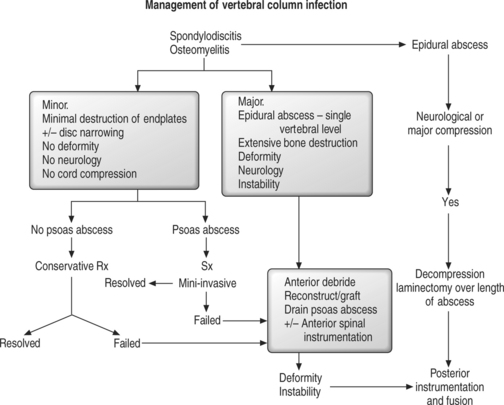
Indications for surgical debridement are failure of nonoperative treatment manifest by continuing sepsis, development of a neurological deficit, or increasing/significant spinal deformity. Hodgson and Stock popularized anterior debridement and bone grafting for spinal tuberculosis in 1960 following publication of successful outcomes in terms of spinal stability and alignment.74 Subsequently, others reported successful outcomes following anterior debridement and bone grafting for pyogenic and fungal infections.17,21,75,76 A costotransversectomy approach to the thoracic spine may be indicated for unilateral abscess drainage or in a fragile patient. Thoracic endoscopically assisted abscess drainage or spinal debridement may be indicated in unwell or older patients with comorbidities. However, mixed outcomes from minimally invasive techniques for spinal debridement have been reported with an increased complication rate and conversion to an open procedure.24 Minimally invasive techniques may have an impact on surgical debridement for spine infection in the future.
Anterior surgery involves thorough debridement of the disc and vertebral bodies back to normal bleeding bone and anterior reconstruction with bone graft. Tricortical iliac crest or middle third of fibula bone grafting is recommended in adults. Rib graft is useful in children. Vascularized rib and iliac crest grafts unite to the host spine faster than free bone grafts.77 Disadvantages of using vascularized bone grafts are the protracted surgical time and the number of theater personnel required. Fresh frozen cortical allografts are indicated for a large defect created by spinal debridement. Harvesting a very large piece of tricortical iliac crest may create donor site pain, although back-filling the defect with a bone graft substitute is now popular as it may reduce future donor site pain. Fresh frozen cortical allografts should be supplemented with autogenous cancellous bone graft.
Recently, successful outcomes have been reported following anterior reconstruction using titanium cages or mesh filled with autogenous cancellous bone graft.78,79 Posterior spinal instrumentation is recommended following extensive anterior debridement, to correct/minimize kyphosis and to allow early patient ambulation.80,81 Posterior stabilization is often delayed following anterior debridement if medical, hematological, and nutritional interventions are thought to be helpful in optimizing the patient’s recovery, if initial surgery is protracted, or if the patient is not robust enough to continue the procedure.
Limited data on the outcomes following simultaneous debridement and anterior instrumentation in the thoracic and lumbar spines are available.78,82–84 Both patients in one report had complications,78 one subsequently dying of sepsis and the other developing recurrent osteomyelitis and subsequent angular kyphosis. Anterior debridement and instrumentation has better results if performed in the cervical spine.85–87 Outcomes following all types of surgery are satisfactory in 79%18 and depend on the absence of residual neurological deficits.
Mortality from spondylodiscitis treated with or without surgery ranges from 3% in patients who are neurologically intact17 to 16%15,17,18 who are neurologically impaired. Advancing age, neurological deficit, immune incompetence, delay in diagnosis, and the presence of diabetes mellitus are the commonest factors identified causing mortality.15,17,18 McHenry et al.18 reported mortality in five of seven neurologically impaired patients in 253 cases of spondylodiscitis. The other two remained significantly disabled. The recovery of neurological function following surgical debridement varies according to whether the deficit is complete or incomplete prior to surgery. Carragee reported that of 33 patients with neurological deficits, nine developed a complete spinal cord lesion of whom four recovered completely and three incompletely.17 Of 21 patients with incomplete spinal cord lesions, only nine recovered completely. Additionally, new postoperative neurological deficits developed in three patients, all subsequently resolving completely.17 Others have reported similar percentages of postoperative recovery of neurological function.15 McHenry et al. reported outcomes of 253 infected spines with a median age of 60 years. Neurological improvement or recovery was reported in 86 of 109 patients treated surgically.18 When neurological decompression was completed via an anterior approach, 83% had a satisfactory outcome, and when completed via a posterior approach, 57% had a satisfactory outcome (p =0.067).11 Carragee reported 8% of surviving patients having chronic severe spinal pain at minimum 2 years follow-up following treatment for vertebral osteomyelitis.17 Causes of chronic spinal pain included pseudarthrosis, kyphosis at the level of the infection, and rheumatoid arthritis in other spinal joints. Hadjipavlou et al. reported residual spinal pain in 64% of those treated nonoperatively versus 26% following surgical debridement and arthrodesis.46 Spontaneous bony ankylosis occurred in 35%.46
McHenry et al.18 reported relapse of infection in up to 14% with a mean follow-up of 6.5 years, with 75% of relapses occurring within 12 months of the initial infection. Factors associated with relapse include inadequate surgical debridement or drainage of a sinus, suboptimal antibiotic therapy, the presence of gross vertebral destruction, and recurrent bacteremia. The presence of a relapse of spinal pain is regarded as a red flag sign of relapse of the spinal infection.18 About one-half of the surviving patients return to work and reasonably normal activities of daily living following treatment for spondylodiscitis (Fig. 36.8).88
Epidural abscess/infection
Staphylococcus is the usual cause. MRI confirmation is critical for early diagnosis and treatment. Nonoperative management is preferred only if the patient is not septicemic and has no neurological deficit (rare), if the patient is not expected to survive surgery, or if complete spinal cord paralysis has been present for 48 hours.89 Urgent surgical drainage results in the best neurological recovery.9,23,45,89 Hadjipavlou et al.46 reported 100% neurological recovery following paraparesis. If epidural granulation tissue rather than pus was found, the neurological recovery was also 100%.46 However, recovery of neurological deficit was evident in only 18% of all cases including complete paralysis following surgery. Single-level epidural abscess anterior to the spinal cord/cauda equina is best treated with corpectomy/abscess debridement followed by strut bone grafting and stabilization by anterior instrumentation. Multiple-level epidural abscess noted posterior to the neural elements can be treated by either multilevel lamino-foramenotomy or contiguous laminectomy, followed by clinical assessment of spinal stability.
Supplementary posterolateral bone grafting is only required for multiple-level contiguous laminectomy. Posterior instrumentation is required only infrequently if deformity is present/imminent. Mortality has not recently been reported following childhood epidural abscess;9 however, morbidity from neurological deficit is 18%.9
Mortality in adults ranges from 5% to 23%.9,90 The presence of a low platelet count of less than 100 × 109/L is associated with a poorer outcome.45
Postoperative spinal infections
Generally, it is recommended that spinal instrumentation be removed if the patient exhibits uncontrollable local or general sepsis or if the posterior arthrodesis is radiologically united when wound debridement is undertaken.91–93 Recommendations for the management of early postoperative spine infection include thorough wound debridement with up to 9 liters pulsatile lavage irrigation and parenteral antibiotics.27 Satisfactory outcomes have been reported when the instrumentation is left in situ, if debrided back to healthy viable tissue. If necrotic tissue is present, repeat wound debridement is recommended and delayed primary closure is inevitable.29,42 The use of continuous irrigation with antibiotic-laden fluid for a period of 5 days postoperatively combined with primary wound closure is successful.91,93 Antibiotic impregnated polymethylmethacrylate beads are beneficial when repeated surgical debridement is required for severe spinal infection.94 If severe vertebral osteomyelitis complicates late infection, it can only be effectively treated by a ‘radical’ debridement of a pedicle screw tract/other vertebral element, back to normal bleeding bone. A large defect in the pedicle and vertebral body may sometimes result from debridement. Supplementary autogenous bone grafting of the large defect should be beneficial. If the spinal infection is severe, delayed primary closure is advantageous.29 If the posterior bone graft is not sound when debridement for infection is undertaken, stability of the potentially unstable spine may be regained through the use of external orthoses and/or delayed spinal instrumentation through a clean surgical field. Simultaneous debridement/exchange posterior instrumentation for spinal infection has not been reported.
Facet joint infection
Because the diagnosis is invariably delayed, it is difficult to eradicate by nonoperative means. Good results are reported following surgical joint debridement.48 Noninstrumented posterolateral arthrodesis is indicated following extensive facet joint debridement or when the contralateral facet joint is subluxed on preoperative imaging.
PROPHYLAXIS
Postoperative spinal infection
Postprocedural spine infection rates range from 0.7% to 11.9%.29 Risk factors for iatrogenic infection include postoperative bowel and bladder continence, posterior approach, procedure for resection of tumor, morbid obesity (BMI greater than 35),95 arthrodesis and the use of spinal instrumentation.29,33,96,97 Using prophylactic gentamicin or cephalosporin reduces postdiscectomy wound infections by 100%, from 2% to 1%.98,99 A recent meta-analysis confirms the efficacy of prophylactic antibiotics in spine surgery.100 The British Society for Anti-Microbial Chemotherapy Working Party recommends a first- or second-generation cephalosporin for adequate infection prophylaxis.101 Exceptions include patients who are allergic to these antibiotics or are known to be colonized or infected with MRSA. These patients should receive vancomycin or teicoplanin plus gentamicin. Single doses at induction of anesthesia are adequate unless the procedure extends beyond 4 hours, in which case 50% of the initial dosage of intraoperative antibiotics should be administered every 4 hours.
Although there are no reports regarding the ideal duration of postoperative prophylactic antibiotic administration, there is no demonstrable benefit from continuing intravenous antibiotics longer than 24 hours.102
Prophylaxis following interventional procedures
Interventional procedures on intervertebral discs uncommonly result in discitis (lumbar discography,103 chemodiscolysis with chymopapain, nucleodiscectomy (ONIK), and laser discectomy.104 A 1% infection rate follows cervical discography.25,105 Septic arthritis of a facet joint following a diagnostic facet joint injection is exceedingly rare.49 It is recommended that patients undergoing percutaneous procedures have a single dose of antibiotics at the time of intervention. Choices of antibiotics are the same as those for open procedures.
Prevention of discitis following spinal surgery
Rhode reported success in totally preventing postoperative discitis by leaving a gentamicin-containing absorbable sponge within the disc following microdiscectomy in 1134 consecutive patients.99 His previous 508 consecutive patients had no prophylaxis and 3.7% developed discitis. Intraoperative irrigation with bacitracin-loaded saline is less effective, reducing the rate of postoperative discitis to 0.2–1.2%.43,99 The incidence of postoperative discitis following prophylactic parenteral gentamicin or cephalosporin administration varies from 0% to 0.5%.99
Experimentally, antibiotics are unable to arrest the progression of established discitis in a sheep model.106 However, the incidence of discitis in this sheep model was zero when 1 g of cefalozin was administered intravenously 30, 60, and 120 minutes prior to an intradiscal injection of 20 Staphylococcus epidermidis in 0.1 mL of Conray 280. Cefalozin was detected in the anulus fibrosus only if the antibiotic was given 30 minutes prior to the procedure and not when it was administered 60 or 120 minutes earlier.
Results are similar in human discs with the highest intradiscal levels of antibiotics being recorded 30 minutes after the parenteral administration of antibiotic.107 Antibiotics which are known to penetrate the nucleus pulposus include aminoglycosides, glycopeptides, and clindamycin.99 Those that do not penetrate the nucleus pulposus include penicillins and cephalosporins. The current recommendation for antibiotic prophylaxis is to administer a single intravenous dose of an appropriate antibiotic 30 minutes prior to the surgical procedure, unless surgery lasts longer than 4 hours.24,99 At this point, an extra dose equaling 50% of the first dose of antibiotic is recommended.101
Wound irrigation and protection
Saline irrigation has not been definitively proven to be of benefit in reducing the incidence of wound infection, although wound contamination is reduced.99 The use of iodinated occlusive sticky surgical drapes has not been demonstrated to lower deep wound infection in hip surgery.108 There are no similar reports involving spine surgery.
Wound drainage systems
Little evidence for or against use of drains is reported.99 There is probably a good indication to use drains in patients with a bleeding diathesis or when surgery is complicated by excessive bleeding to prevent possible neurological complications from an excessively large wound/epidural hematoma.
TUBERCULOUS SPINAL INFECTION
Tuberculous infection is still prevalent world-wide, accounting for about one-third of all bone and joint infections. Three and one-half million die of tuberculosis annually.109 Three percent of tuberculosis infections involve the skeleton, of which one-half involve the spine. Three-quarters of spine infections involve the thoracic region and 20% are in the lumbosacral spine. Tuberculosis is endemic in some Asian, African, and Central American countries and is more prevalent in patients infected with the human immunodeficiency virus.109
Pathogenesis and natural history
Tuberculous infections progress slowly as the organisms are often indolent. Three species of Mycobacterium are known to infect humans: M. tuberculosis, bovis, and africanum.109 They are all strict aerobic organisms and invade the lungs of humans. Miliary spread of the bacilli can occur to all organs. Bacilli invade the vertebral body through the microvenous circulation adjacent to the vertebral endplate. Spinal tuberculosis is manifest most commonly in the thoracolumbar junction which parallels the entry of the renal veins. A granuloma can form under the anterior longitudinal ligament and spread to a number of spinal segments (anterior granuloma) before detection.109 Primary granulomatous lesions have also been reported in the laminae, pedicles, facet joints, and even spinous processes–appendiceal lesions.109
The pathogenesis of tuberculous and pyogenic spondylodiscitis is different. Typically, bacilli start a slow inflammatory response with the formation of a tuberculous granuloma in the vertebral endplate. It caseates centrally and forms an abscess. The untreated abscess may extend in any direction. Paravertebral extension and swelling are common. Paravertebral and psoas abscesses may become large and eventually a ‘cold’ abscess may result. A sinus then develops, usually just below the groin or above the iliac crest and occasionally on the medial side of the thigh or even the popliteal fossa. In the neck, large abscesses are more common in children than adults, sometimes causing compression of adjacent structures.109 The abscess may also extend into the spinal canal.
Tuberculous spondylodiscitis usually results in a fibrous ankylosis. When osteomyelitis becomes established, pathological fracture of the vertebral body may eventually result in angular kyphosis, which is commonest in the thoracic spine. The spinal canal may be further compromised by sequestration of vertebra and disc or by dislocation of one spinal segment. Contiguous vertebral involvement can be evident in spinal tuberculous infection. Neurological deficits can result from neural compression or stretching from spinal fracture/angulation as well as (1) thecal compression by an epidural granuloma, (2) intramedullary or intradural tuberculous infection (rare), (3) microvascular compromise, or (4) tuberculous meningitis.109 Neurological deficits are present in 10–60% of tuberculous spondylitis at presentation.109
Clinical features
Presentations are variable, depending on the age and general condition of the patient, the delay in diagnosis, and the presence of neurological deficits. Symptoms include fever, anorexia, weight loss, and night sweats. Eventually, the patient complains of an insidious onset of spinal pain. The patient may have angular kyphosis, draining sinuses, torticollis, stridor and even dysphagia at presentation. The leukocyte count is normal and the ESR elevated above 20 mm/hour in at least 80% of patients.109 The Mantoux (tuberculin skin) test is nearly always positive. Definitive diagnosis only follows positive culture of a CT-guided needle, or occasionally open, biopsy of the spine. Positive cultures may not be evident for a number of weeks following biopsy. More recently, polymerase chain reaction (PCR) techniques have hastened diagnosis.
Surgery
Indications for surgery parallel those for pyogenic infection. Although patients with mild neurological deficit have been reported to have up to 79% improvement with chemotherapy alone, more recent reports have demonstrated up to 94% improvement when surgery is performed as early as possible, followed by chemotherapy.109 Early debridement and bone graft of all tuberculous spinal cases by an anterior approach has always been preferred in Hong Kong.74 Long-term results from this treatment are superior to nonoperative management in terms of spinal stability and alignment, with less angular kyphosis. Most recent reports have emphasized better outcomes when the anterior bone graft is combined with anterior instrumentation, although posterior instrumentation is equally effective.109,110 Whether anterior or posterior instrumentation with bone graft is used, better spinal alignment is maintained long-term following instrumentation.109 A costotransversectomy approach was preferred 50 years ago but is now used only when the patient is fragile.
FUNGAL SPINE INFECTIONS
Fungi reported to cause spine infections in humans are summarized in Table 36.2.
Aspergillus spinal osteomyelitis has been reported following organ transplantation and chemotherapy.111,112 Spinal infection is rare, with 50 reported cases113 and results in death if untreated.111 Treatment with amphotericin B of 1 mg/kg/day initially, followed by antifungal medication for 12 weeks up to 3 years is considered ideal treatment.114 The mortality rate following antifungal medication and surgical debridement is 25%114 and neurological deficits are noted in 30%.113
Only 60 cases of Candida vertebral osteomyelitis have been reported.115 Candida albicans is the commonest species involved (62%). Nearly all cases involve the lower thoracic or lumbar spine. Most cases have back pain for longer than 1 month, one-third have an elevated temperature at presentation, and about 20% have neurological deficits. Eighty-five percent of cases have a good outcome following treatment with amphotericin and surgical debridement.115
Coccidioides spine infection is uncommon and typically affects immunocompromised individuals. Spinal involvement usually follows disseminated disease in 100 reported cases.116–119 Neurological deficit is evident in 20% cases. Mortality is reported in 20% following treatment with amphotericin and surgical debridement. Long-term antifungal therapy is recommended.116
Cryptococcal spine infection is uncommon.120,121 There is difficulty radiologically differentiating cryptococcal from tuberculous spine infection. All patients succumb without antifungal medication. Fluconazole, flucytosine,120 and amphotericin B121 have recently been successful in terms of patient survival. Neurological recovery may also follow this treatment with three of five recovering neurological function completely.121
Histoplasma spondylodiscitis is rare and also difficult to separate radiologically from tuberculous infection. Ketoconazole medication and surgical debridement were successful in a single case. Another case presented with complete paralysis.122 Nocardia asteroides spondylodiscitis is rare, with 11 reported cases.123 Successful outcomes have been reported following surgical debridement and long-term antifungal medication.123
1 Eismont J, Bohlman H, Soni P, et al. Vertebral osteomyelitis in infants. J Bone Joint Surg. 1982;64B:32-35.
2 Whalen J, Parke W, Mazur J, et al. The intrinsic vasculature of developing vertebral end plates and its nutritive significance to the intervertebral discs. J Ped Orthop. 1995;15:652-660.
3 Wiley A, Trueta J. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg. 1959;41B:796-809.
4 Coventry M, Ghormley R, Kernohan J. The intervertebral disc: Its microscopic anatomy and pathology. 1: Anatomy, development and physiology. J Bone Joint Surg. 1945;27:105-112.
5 Song K, Ogden J, Ganey T, et al. Contiguous discitis and osteomyelitis in children. J Ped Orthop. 1997;17:470-477.
6 Glazer P, Hu S. Pediatric spinal infections. Orthop Clin North Am. 1996;27:111-123.
7 Early S, Kay R, Tolo V. Childhood diskitis. J Am Academy Orthop Surg. 2003;6:413-420.
8 Ratcliffe J. Anatomic basis for the pathogenesis and radiologic features of vertebral osteomyelitis and its differentiation from childhood discitis: A micro-arteriographic investigation. Acta Radiol Diagn. 1985;26:137-141.
9 Auletta J, John C. Spinal epidural abscesses in children: A 15-year experience and review of the literature. Clin Inf Diseases. 2001;32:9-16.
10 Wenger D, Bobechko W, Gilday D. The spectrum of intervertebral disc-space infection in children. J Bone Joint Surg. 1978;60A:100-108.
11 Brown R, Hussain M, McHugh K, et al. Discitis in young children. J Bone Joint Surg. 2001;83B:106-111.
12 Fernandez M, Carrol C, Baker C. Discitis and vertebral osteomyelitis in children: An 18-year review. Paediatrics. 2000;105:1299-1304.
13 Dich V, Nelson J, Haltalin K. Osteomyelitis in infants and children: a review of 63 cases. Am J Dis Child. 1982;129:1273-1278.
14 Crawford A, Kucharzyk D, Ruda R, et al. Diskitis in children. Clin Orthop. 1991;266:70-79.
15 Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Gotenborg, Sweden: A retrospective study of patients during 1990–95. Scand J Infect Dis. 2001;33:527-532.
16 Feldenzer J, McKeever P, Schaberg D, et al. The pathogenesis of spinal epidural space abscess: microangiographic studies in an experimental model. J Neurosurg. 1988;69:110-114.
17 Carragee E. Pyogenic vertebral osteomyelitis. J Bone Joint Surg. 1997;79A:874-880.
18 McHenry M, Easley K, Locker G. Vertebral osteomyelitis: Long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342-1350.
19 Waldvogel F, Vasey H. Osteomyelitis: the past decade. New Engl J Med. 1980;303:360.
20 Kulowski J. Pyogenic osteomyelitis of the spine. An analysis and discussion of 102 cases. J Bone Joint Surg. 1936;18:343-364.
21 Eismont F, Bohlman H, Soni P, et al. Pyogenic and fungal osteomyelitis with paralysis. J Bone Joint Surg. 1983;65A:19-29.
22 Frederickson B, Yuan H, Olans R. Management and outcome of pyogenic vertebral osteomyelitis. Clin Orthop. 1978;131:160-167.
23 Anand S, Maini L, Agarwal A, et al. Spinal epidural abscess – a report of six cases. Int Orthop. 1999;23:175-177.
24 Silber J, Anderson D, Vaccaro A, et al. Management of postprocedural discitis. Spine J. 2002;2:279-287.
25 Guyer R, Ohnmeiss D, Mason S, et al. Complications of cervical discography: findings in a large series. J Spinal Disord. 1997;2:95-101.
26 Tronnier V, Schneider R, Kunz U, et al. Postoperative spondylodiscitis: results of a prospective study about the aetiology of spondylodiscitis after operation for lumbar disc herniation. Acta Neurochir. 1992;117:149-152.
27 Weinberg J, Silber J. Infections of the spine: What the orthopedist needs to know. Am J Orthoped. 2004;1:13-17.
28 Rawlings C, Wilkins R, Gallis H, et al. Postoperative intervertebral disc space infection. Neurosurgery. 1983;13:371-375.
29 Weinstein M, McCabe J, Cammisa F. Postoperative spinal wound infection: A review of 2391 consecutive index procedures. J Spinal Dis. 2000;13:422-426.
30 Klein J, Garfin S. Nutritional status in the patient with spinal infection. Orthop Clin N America. 1996;27:33-36.
31 Theiss S, Lonstein J, Winter R. Wound infections in reconstructive spine surgery. Orthop Clin N Am. 1996;27:105-110.
32 McPhee IB, Williams R, Swanson C. Factors influencing wound healing after surgery for metastatic disease of the spine. Spine. 1998;23:726-732.
33 Massie J, Heller J, Abitbol J, et al. Postoperative posterior spinal wound infections. Clin Orthop. 1992;284:99-108.
34 Dietz F, Koontz F, Round E, et al. The importance of positive bacterial cultures of specimens obtained during clean orthopaedic operations. J Bone Joint Surg. 1991;73A:1200-1207.
35 Heggeness M, Esses S, Errico T, et al. Late infection of spinal instrumentation by hematogenous seeding. Spine. 1993;18:492-496.
36 Viola R, King S, Adler S, et al. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine. 1997;22:2444-2451.
37 Gristina A, Costerton J. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg. 1985;67A:264-273.
38 Richards B. Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg. 1995;77A:524-529.
39 Dubousset J, Shufflebarger H, Wenger D. Late ‘infection’ with CD instrumentation. Orthop Trans. 1994;18:121.
40 Hatch R, Sturm P, Wellborn C. Late complication after single-rod instrumentation. Spine. 1998;13:1503-1505.
41 Ido K, Shimizu K, Nakayama Y, et al. Suction irrigation for deep wound infection after spinal instrumentation: a case study. Eur Spine J. 1996;5:345-349.
42 Picada R, Lonstein J, Denis F, et al. Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation: Incidence and management. J Spinal Dis. 2000;13:42-54.
43 Bose B. Delayed infection after instrumented spine surgery: case reports and review of the literature. Spine J. 2003;3:394-399.
44 Nussbaum E, Rigamonti D, Standiford H, et al. Spinal epidural abscess: a report of 40 cases and review. Surg Neurol. 1992;38:225-231.
45 Tang H, Lin H, Liu Y, et al. Spinal epidural abscess – experience with 46 patients and evaluation of prognostic factors. J Infection. 2002;45:76-81.
46 Hadjipavlou A, Mader J, Necessary J, et al. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668-1679.
47 Coscia M, Trammell T. Pyogenic lumbar facet joint arthritis with intradural extension: a case report. J Spinal Dis Tech. 2002;15:526-528.
48 Doita M, Nishida K, Miyamoto H, et al. Septic arthritis of bilateral lumbar facet joints: report of a case with MRI findings in the early stage. Spine. 2003;28:E198-E202.
49 Orpen N, Birch N. Delayed presentation of septic arthritis of a lumbar facet joint after diagnostic facet joint injection. J Spinal Dis Tech. 2003;16:285-287.
50 Varma R, Lander P, Assaf A. Imaging of pyogenic infectious spondylodiskitis. Radiol Clin N Am. 2001;39:203-213.
51 Stabler A, Reiser M. Imaging of spinal infection. Radiol Clin N Am. 2001;39:115-135.
52 Love C, Patel M, Lonner B, et al. Diagnosing spinal osteomyelitis: A comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med. 2000;25:963-977.
53 McEwan L, Wong J. Nuclear medicine imaging in early vertebral osteomyelitis: still of clinical utility. Australasian Radiol. 2000;44:454-457.
54 Jayaraman S, Al-Nahhas A, Vivian G, et al. Demonstration of spinal osteomyelitis with Ga-67 citrate, Tc-99m MDP, and Tc-99m ciprofloxacin with provisionally negative results on MRI. Clin Nucl Med. 2000;25:224-226.
55 Hadjipavlou A, Cesani-Vazquez F, Villaneuva-Meyer J, et al. The effectiveness of gallium citrate Ga-67 radionuclide imaging in vertebral osteomyelitis. Am J Orthop. 1998;3:188-197.
56 Modic M, Feiglin D, Piraino D, et al. Vertebral osteomyelitis: Assessment using MR. Radiology. 1985;157:157-166.
57 Ozuna R, Delamarter R. Pyogenic vertebral osteomyelitis and postsurgical disc space infections. Orthop Clin N Am. 1996;27:87-94.
58 Boden S, Davis D, Dina T, et al. Postoperative diskitis: Distinguishing early MR imaging findings from normal postoperative disk space changes. Radiology. 1992;184:765-771.
59 Grand C, Bank W, Baleriaux D, et al. Gadolinium enhancement of vertebral endplates following lumbar disc surgery. Neuroradiology. 1993;34:503-505.
60 Ruiz A, Donovan-Post J, Sklar E, et al. MR imaging of infections of the cervical spine. MRI Clin N Am. 2000;8:561-579.
61 Weisz R, Errico T. Spinal infections. Diagnosis and treatment. Bulletin Hospital for Joint Diseases. 2000;59:40-46.
62 Tay B, Deckey J, Hu S. Spinal infections. J Am Acad Orthop Surgeons. 2002;10:188-197.
63 Collert S. Osteomyelitis of the spine. ACTA Orthopaed Scand. 1977;48:283.
64 Rezai A, Woo H, Errico T, et al. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999;5:1018-1025.
65 Menelaus M. Discitis: An inflammation affecting the intervertebral discs in children. J Bone Joint Surg. 1964;46B:16-23.
66 Spiegel P, Kengla K, Isaacson A, et al. Intervertebral disc-space inflammation in children. J Bone Joint Surg. 1972;54A:284-296.
67 Boston H, Bianco A, Rhodes K. Disk space infections in children. Clin Orth. 1975;6:953-964.
68 Fisher G, Popich G, Sullivan D, et al. Diskitis: A prospective diagnostic analysis. Pediatrics. 1978;62:543-548.
69 Ryoppy S, Jaaskelainen J, Rapola J, et al. Nonspecific diskitis in children: A nonmicrobial disease? Clin Orthop. 1993;297:95-99.
70 Ring D, Johnston C, Wenger D. Pyogenic infectious spondylitis in children: The convergence of discitis and vertebral osteomyelitis. J Ped Orthop. 1995;15:652-660.
71 Garron E, Viehweger E, Launay F, et al. Non-tuberculous spondylodiscitis in children. J Ped Orthop. 2002;22:321-328.
72 Hensinger R. Acute back pain in children. Instr Course Lect. 1995;44:111-126.
73 King H. Back pain in children. Orth Clin N Am. 1999;30:467-474.
74 Hodgson A, Stock F, Fang H, et al. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. J Bone Joint Surg. 1960;42A:295-310.
75 Cahill D, Love L, Rechtine G. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg. 1991;74:878-886.
76 Emery S, Chan D, Woodward H. Treatment of hematogenous pyogenic vertebral osteomyelitis with anterior debridement and primary bone grafting. Spine. 1989;14:284-291.
77 Hayashi A, Maruyama Y, Okajima Y, et al. Vascularized iliac bone graft based on a pedicle of upper lumbar vessels for anterior fusion of the thoracolumbar spine. Br J Plast Surg. 1994;41:425-430.
78 Hee H, Majd M, Holt R, et al. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Dis Tech. 2002;15:149-156.
79 Fayazi A, Ludwig S, Dabbah M, et al. Preliminary results of staged anterior debridement and reconstruction using titanium mesh cages in the treatment of thoracolumbar vertebral osteomyelitis. Spine J. 2004;4:388-395.
80 Moon M, Woo Y, Lee K, et al. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20:1910-1916.
81 Krodel A, Kruger A, Lohscheidt K, et al. Anterior debridement, fusion, and extrafocal stabilization in the treatment of osteomyelitis of the spine. J Spinal Dis. 1999;12:17-26.
82 Hopf C, Meurer A, Eysel PJ, et al. Operative treatment of spondylodiscitis – what is the most effective approach? Neurosurg Review. 1998;21:217-225.
83 Faraj A, Webb J. Spinal instrumentation for primary pyogenic infection: Report of 31 patients. Acta Orthop Belgica. 2000;66:242-247.
84 Askin G, Day G, McAuliffe M, et al. Spinal fixation and surgical debridement and reconstruction for the treatment of pyogenic spinal infections. J Bone Joint Surg. 2000;82B(Suppl 1):31-32.
85 Hughes J, DiGiacinto G, Sundaresan N. Anterior instrumentation in cervical osteomyelitis. Proc Cervical Spine Res Soc 1997. California.
86 Rezai A, Woo H, Errico T, et al. Contemporary management of spinal osteomyelitis. Neurosurg. 1999;44:1018-1025.
87 Przybylski G, Sharan A. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg. 2001;94S:1-7.
88 Jiminez-Mejias M, Colmenero J, Sanches-Lora F, et al. Postoperative spondylodiskitis: Etiology, clinical findings, prognosis and comparison with nonoperative pyogenic spondylodiskitis. Clin Infect Dis. 1999;29:339-345.
89 Sampath P, Rigamonti D. Spinal epidural abscess: A review of epidemiology, diagnosis and treatment. J Spinal Dis. 1999;12:89-93.
90 Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52:189-197.
91 Soultanis K, Mantelos G, Pagiatakis A, et al. Late infection in patients with scoliosis treated with spinal instrumentation. Clin Orthop. 2003;411:116-123.
92 Clark C, Shufflebarger H. Late-developing infection in instrumented idiopathic scoliosis. Spine. 1999;24:1909-1912.
93 Richards B. Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg. 1995;77A:524-529.
94 Glassman S, Dimar J, Puno R, et al. Salvage of instrumented lumbar fusions complicated by surgical wound infection. Spine. 1996;21:2163-2169.
95 Olsen M, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98(Suppl 2):140-155.
96 Roberts F, Walsh A, Wing P, et al. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine. 1998;23:366-370.
97 Capen D, Calderone R, Green A. Perioperative risk factors for wound infection after lower back fusions. Orth Clin N Am. 1996;27:83-86.
98 Mastronardi L, Tatta M. Intraoperative antibiotic prophylaxis in clean spinal surgery: a retrospective analysis in a consecutive series of 973 cases. Surg Neurol. 2004;61:129-135.
99 Rohde V, Meyer B, Schaller C, et al. Spondylodiscitis after lumbar discectomy. Spine. 1998;23:615-620.
100 Barker F. Efficiency of prophylactic therapy in spinal surgery: A meta-analysis. Neurosurgery. 2002;51:391-401.
101 Brown E, Pople I, de Louvois J, et al. Spine update: Prevention of postoperative infection in patients undergoing spinal surgery. Spine. 2004;29:938-945.
102 Dimick J, Lipsett P, Kostuik J. Spine update: antimicrobial prophylaxis in spine surgery: basic principles. Spine. 2000;25:2544-2548.
103 Fraser R, Osto O, Vernon-Roberts B. Discitis after discography. J Bone J Surg. 1987;69B:26-35.
104 Zeiger H, Zampella E. Intervertebral disc infection after lumbar chemonucleolysis: report of a case. Neurosurg. 1986;18:616-621.
105 Zeidman S, Thompson K, Ducker T. Complications of cervical discography: analysis of 4400 diagnostic disc injections. Neurosurg. 1995;37:414-417.
106 Fraser R, Osti O, Vernon-Roberts B. Iatrogenic discitis: the role of intravenous antibiotics in prevention and treatment, an experimental study. Spine. 1989;14:1025-1032.
107 Boscardin J, Ringus J, Feingold D, et al. Human intradiscal levels with cefazolin. Spine. 1992;17(Suppl):145-148.
108 Chiu K, Lau S, Fung B, et al. Plastic adhesive drapes and wound infection after hip fracture surgery. Aust NZ J Surg. 1993;63:798-801.
109 Khoo L, Mikawa K, Fessler R. A surgical revisitation of Pott distemper of the spine. Spine J. 2002;3:130-145.
110 Yilmaz C, Selek H, Gurkan I, et al. Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Joint Surg. 1999;81A:1261-1267.
111 Andriole V. Infections with Aspergillus species. Clin Inf Dis. 1993;17:S481-S486.
112 Patterson T, Miniter P, Patterson J, et al. Aspergillus antigen detection in the diagnosis of invasive aspergillosis. J Infect Dis. 1995;171:1553-1558.
113 Vinas F, King P, Diaz F. Spinal Aspergillus osteomyelitis. Clin Infect Dis. 1998;28:1223-1229.
114 Salvalaggio P, Bassetti M, Lorber M, et al. Case report: Aspergillus vertebral osteomyelitis after simultaneous kidney–pancreas transplantation. Transplant Infect Dis. 2003;5:187-189.
115 Miller D, Mejicano G. Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Inf Dis. 2001;33:523-530.
116 Wrobel C, Chappell E, Taylor W. Clinical presentation, radiological findings, and treatment results of coccidioidomycosis involving the spine: a report on 23 cases. J Neurosurg. 2001;95(1 Suppl):33-39.
117 Herron L, Kissel P, Smilovitz D. Treatment of coccidioidal spinal infection: Experience in 16 cases. J Spinal Dis. 1997;10:215-222.
118 Kushwaha V, Shaw B, Gerardi J, et al. Musculoskeletal coccidioidomycosis. A review of 25 cases. CORR. 1996;332:190-199.
119 Zeppa M, Laorr A, Greenspan A, et al. Skeletal coccidioidomycosis: imaging findings in 19 patients. Skeletal Radiol. 1996;25:337-343.
120 Cook P. Successful treatment of cryptococcal osteomyelitis and paraspinous abscess with fluconazole and flucytosine. South Med J. 2001;94:936-938.
121 Govender S, Mutasa E, Parbhoo A. Cryptococcal osteomyelitis of the spine. JBJS (Br). 1999;81:459-461.
122 N’dri Oka D, Varlet G, Kakou M, et al. Spondylodiscitis due to Histoplasma duboisii. Report of two cases and review of the literature. Neurochirurgie. 2001;47:431-434.
123 Graat H, Van Ooij A, Day G, et al. Nocardia farcinica spinal osteomyelitis. Spine. 2002;27:E253-E257.