CHAPTER 12 Spine Imaging
Techniques
Plain Films
Routine plain films are universally available and inexpensive, but are limited by an inability to visualize directly neural structures and nerve root or cord compression. The presence of degenerative changes within the cervical and lumbar spine has been shown to be age related and equally present in asymptomatic and symptomatic individuals.1 By the 5th decade of life, 25% of asymptomatic patients have degenerative changes in the intervertebral disc spaces. By the 7th decade, 75% have degenerative changes. Routine spine radiographs are of little value in determining the degree and clinical severity of cervical or lumbar degenerative disc disease.
Orthogonal conventional radiography is the first line of evaluation in an instrumented postoperative patient, and plain radiographs are usually obtained at 6 weeks and 3, 6, and 12 months postoperatively.2 Regardless of which fusion approach is taken, the presence or absence of demonstrable motion or evidence of hardware failure or loosening is a key factor in the evaluation. In the case of posterolateral fusion, arthrodesis is deemed successful if follow-up radiographs show continuity in the fusion mass between the cephalad and the caudal transverse processes. Instrumented interbody fusion is considered fused if:
Pseudarthrosis or failure of fusion is indicated by progressive loss of disc height, vertebral displacement, broken or loose hardware, and loss of position of the implant or resorption of the bone graft. Flexion and extension views are useful for assessing stability or functional fusion, but the central x-ray beam should pass through the same area in both views.3
Myelography
The diagnosis of extradural neural compression by myelography is inferred indirectly by changes in the contour of normal contrast agent–filled thecal sac and root sleeves rather than by direct visualization of the lesion.4 Multiple water-soluble agents are available that provide excellent contrast and lower rates of side effects, such as iohexol (Omnipaque) and iopamidol (Isovue). Current water-soluble agents are associated with less toxicity, and their absorption through the theca and arachnoid villi makes their removal unnecessary.5 Newer nonionic water-soluble agents generally produce mild side effects, although significant adverse reactions can still rarely occur, such as hallucinations, confusion, or seizures.
The major disadvantage of myelography is its invasive nature and lack of diagnostic specificity.6 The use of less toxic second-generation, water-soluble nonionic agents has obviated the need for overnight hospitalization after the procedure. Routine postprocedural monitoring of 2 to 4 hours is usually sufficient. The technique of myelography involves instillation of the contrast agent through either lumbar puncture (midline or oblique approaches) or lateral C1-2 puncture. Adequate visualization depends on pooling of sufficient contrast agent in the region of interest to provide enough electron density to stop the x-ray beam. Absence of a significant cervical lordosis can make it difficult to concentrate the dye in the cervical region, resulting in dilution of the dye and suboptimal image quality.7 Dilution of contrast agent also occurs when attempting to visualize more than one spine region, such as lumbar and cervical. The plain film image quality of the second region studied invariably is markedly diminished (although most of these cases are diagnostically adequate by CT myelography).
Accuracy rates for water-soluble nonionic cervical myelography in the diagnosis of nerve root compression range from 67% to 92%.1,6,8,9 In a study of 53 patients with surgical confirmation of pathologic entities, myelography was associated with no false-positive findings and a 15% false-negative rate for an overall accuracy of 85%.8 Because the diagnosis of extradural neural compression is inferred indirectly by changes in the contour of the contrast agent–filled subarachnoid space, the exact nature of the compressing lesion may be uncertain. Central indentation of the dye column at the level of the disc space may be due to either compression by the disc itself or compression by a marginal osteophyte. Similarly, incomplete filling of a nerve root sleeve may be due to either a lateral disc herniation or foraminal narrowing; the distinction is sometimes difficult by myelography. There is currently almost no role for conventional myelography alone, without postmyelographic CT. The exception is the presence of stainless steel spinal implants, where CT image quality is degraded by the presence of the spinal instrumentation.
Computed Tomography
CT permits direct visualization of potential neural compressing structures and provides better visualization of lateral pathology, such as foraminal stenosis.10–12 An important benefit from a surgical perspective is the ability of CT to distinguish neural compression owing to soft tissue from compression owing to bone.9,11,13,14 CT is still limited compared with MRI in visualization of the neural structures below a complete myelographic block. What often appears as a complete myelographic block may permit passage, however, of enough contrast agent past the block to allow CT myelographic distinction.
Disadvantages of CT include radiation exposure, the effects of partial volume averaging, the time involved in performing multiple thin (1.5 to 3 mm) sections over multiple vertebral bodies and intervening discs, streak artifacts in the cervical spine caused by the dense bone of the shoulder girdle, and changes in configuration of the spine that occur between successive motion segments.15 Many of the limitations can be obviated by obtaining multiple thin sections (1.5 to 3 mm) with the gantry tilted to permit imaging parallel to the plane of the disc. Further accuracy is obtained by routinely imaging the spine by CT after the introduction of water-soluble contrast agents (intrathecal contrast medium–enhanced CT).
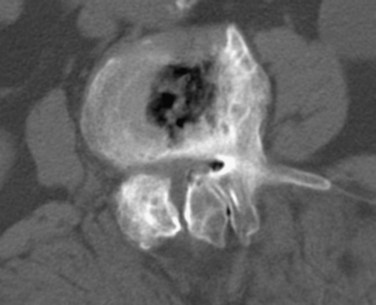
Reported accuracy rates for CT range from 72% to 91%.6,9,11,14 Agreement rates between contrast medium–enhanced CT and myelography have been reported to range from 75% to 96%.11,14 When a discrepancy exists between myelographic and CT findings, postcontrast CT is invariably the more accurate study (Fig. 12–1). New multirow detector technology is becoming available that allows for extremely rapid thin-slice acquisitions over long body segments. With this new technology, contiguous 3-mm slices can be obtained from L1 to S1 in less than 30 seconds. The acquisition of isotropic voxels allows for multiplanar reformation of the CT data with no loss in spatial resolution.
Magnetic Resonance Imaging
For most patients who present for evaluation of suspected degenerative disease, spin-echo (SE) T1-weighted and fast spin-echo (FSE) T2-weighted sagittal images and T1-weighted axial images suffice. This examination can be completed in approximately 20 minutes. If contrast between the disc and cerebrospinal fluid (CSF) is inadequate on axial images, FSE T2-weighted axial study may be useful. If there is a history of prior low back surgery, gadolinium-based intravenous contrast medium is administered, and T1-weighted sagittal and axial images are included. Patients with possible vertebral osteomyelitis can undergo this routine study. If the study shows an area that suggests a disc space infection, post–gadolinium-enhanced T1-weighted sagittal and axial sequences are often very helpful in defining disease extent and in characterizing epidural inflammatory disease.16–24
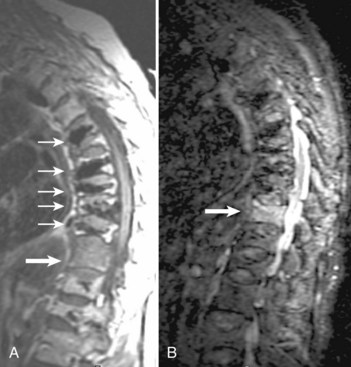
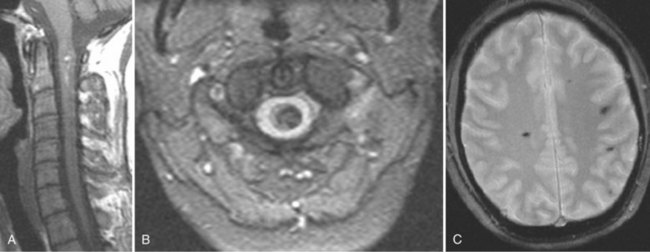
For axially oriented images, low flip angle, two-dimensional or three-dimensional, gradient-echo (GE) sequences producing “myelographic” contrast are a reasonable baseline standard of comparison, acknowledging that these sequences were developed for detecting disc herniations and are not the “gold standard” for detecting intramedullary cord lesions.17,25,26 Short tau inversion recovery (STIR) has shown a high sensitivity for musculoskeletal pathology (Fig. 12–2).27–29 STIR has been favorably compared with T1-weighted and T2-weighted FSE, conventional SE, and fat-saturated FSE in the detection of vertebral metastatic disease.30–32 STIR may also be used for intramedullary cord lesions.
For disc disease, bright CSF-type images are preferred because of the problem of visualizing low signal intensity ligaments or osteophytes against the dark CSF images on T1-weighted images.33–37 The major problem of two-dimensional MRI techniques for cervical disease is the failure to identify foraminal disease accurately owing to long echo times, relatively thick image slices (3 to 5 mm), and the inability to view the course of the exiting nerve roots in planes other than axial.38 Three-dimensional imaging allows an increase in signal-to-noise ratio over two-dimensional imaging with thin contiguous slices with a more accurate slice thickness that can be obtained without the problem of crosstalk.39,40
Artifacts
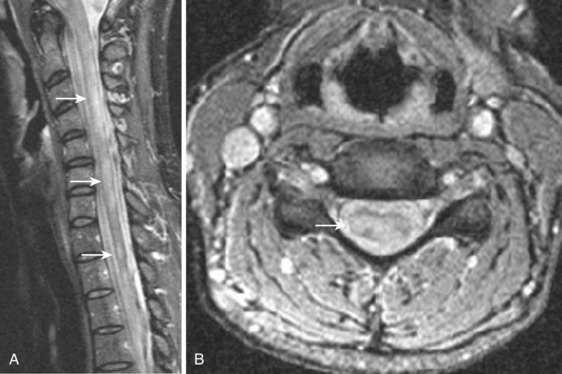
MRI studies may be severely compromised in the presence of spinal instrumentation, and there can be potential safety and biologic considerations (Fig. 12–3). There are many strategies one can employ to reduce susceptibility artifacts on MRI, including the use of SE techniques, especially FSE variants over GE; larger fields of view; higher readout bandwidths; smaller voxel sizes; and appropriate geometric orientation of the frequency-encoded direction in relationship to metallic objects. Geometric orientation is especially important in the case of pedicle screws.41 There is less apparent widening of the short axis of screws when the direction of the frequency-encoded gradient is parallel, as opposed to perpendicular to the long axis of the screw.
Spinal Angiography
Spinal angiography is extremely useful for spinal vascular malformations for the delineation of the vascular supply and for therapeutic treatment.42,43 Spinal angiography is also used in the pretherapeutic workup of suspected vascular neoplasms involving the vertebral bodies, posterior elements, and spinal canal and is coupled with preoperative or palliative embolization. Spinal angiography should address three areas for the surgeon or interventionalist: (1) the exact location and configuration of the lesion, (2) vascularity of the lesion including feeding and draining vessels, and (3) regional vascular anatomy.44
Spinal vascular malformations are a very heterogeneous group of lesions that have had a wide variety of classification schemes applied to them. One common classification system is from Anson and Spetzler,45 who classify them as types 1 to 4:
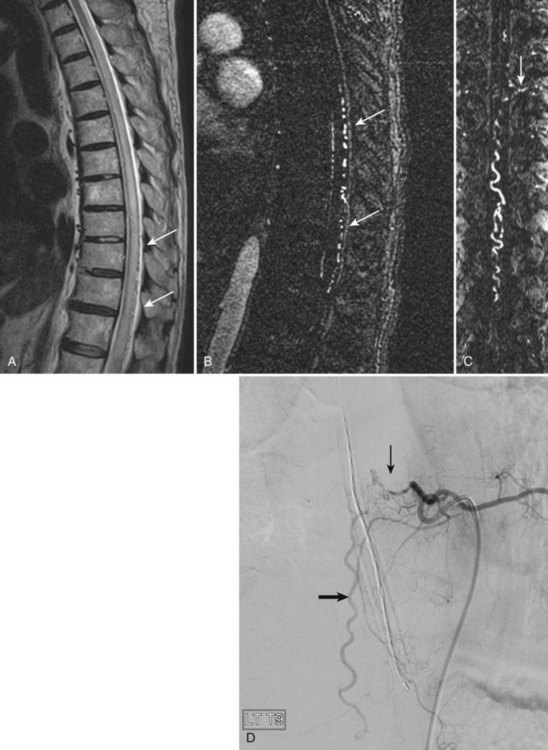
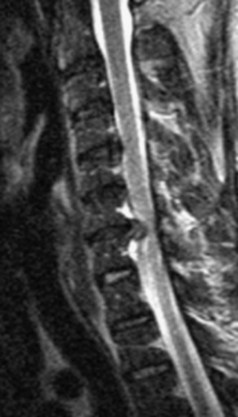
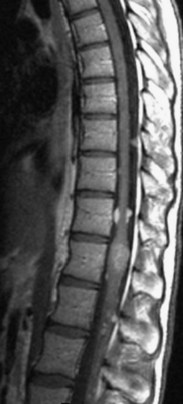
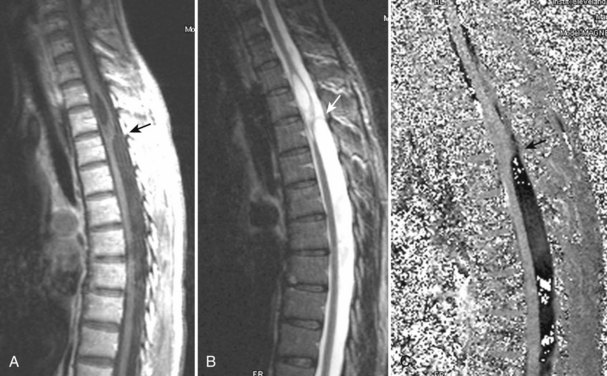
The most common spinal vascular lesion is a dural arteriovenous fistula (Fig. 12–4). These lesions are thought to be acquired and are particularly present in the thoracic and lower lumbar spine. Spinal dural arteriovenous fistulas are more common in men (3.4 : 1) older than 60 years. There is often a delay from symptom onset to time of diagnosis, averaging 27 months. Clinical findings include weakness (55%), a progressive clinical course (100%), and a myelopathy on examination (84%). The nidus of the fistula is most often located between T6 and T12 and in the sacrum and intracranially in 8% to 9% each. In 1977, Kendall and Logue46 definitively identified the site of the arteriovenous shunting within the root sleeve. The symptoms are a result of intramedullary edema and ischemia secondary to increased venous backpressure within the varicose coronal veins. Gilbertson and colleagues47,48 identified increased signal intensity on T2-weighted images within the cord as the most sensitive imaging finding in spinal dural fistula.
Although imaging, in particular MRI, has become a mainstay for the evaluation of vascular malformations, spinal angiography remains a crucial technique for precise definition of the type of lesion, the overall morphology, the flow characteristics, and the identification of specific feeding vessels.49 Selective intercostal or lumbar injection using digital subtraction angiography typically uses 2 to 4 mL of nonionic contrast agent per injection, diluted with heparinized saline. Arterial and delayed venous imaging may be necessary to appreciate fully the venous drainage of the vascular pathology, particularly in arteriovenous malformations and dural fistulas. Arterial films allow examination of abnormal blush or arteriovenous shunting. The normal vascular supply to the cord, in particular, the artery of Adamkiewicz, should be defined. In addition to the usual general complications of angiography, embolization to the anterior spinal artery could occur after angiography, which may lead to an ascending paralysis. Generally, given the small catheters used, nonionic contrast medium, and an improved speed of the examination with digital subtraction angiography, complications are rare.
Spinal Angiography Using Magnetic Resonance Imaging and Computed Tomography
Technologic advances have allowed high-resolution, high-contrast discrimination imaging for evaluation of the spinal arteries, with the goal of minimizing the need for conventional catheter angiography for identification of spinal vascular disease.50–52 The size of the anterior spinal artery (0.2 to 0.8 mm) and the close approximation of the spinal veins necessitate a sophisticated MRI sequence with bolus gadolinium–based intravenous contrast medium administration. Although various techniques may be used, the three main requirements are a large field of view, high spatial resolution, and high temporal resolution.
The large field of view should be 30 to 50 cm, which would allow visualization of the mid-thoracic and lower thoracic spine and the upper lumbar spine covering the major sources of the anterior spinal artery (70% arise from T8-L1). The high spatial resolution is required because of the small target vessel size and should employ a voxel size on the order of 1 mm or less. Temporal resolution is required to try to separate the anterior spinal artery from the adjacent vein, which is larger (0.4 to 1.5 mm). Simply trying to define the artery and vein based on morphology is extremely difficult. Temporal resolution is often in a tradeoff with spatial resolution for MRI, and time frames vary with the particular sequence and hardware, but is generally 40 to 60 seconds per acquisition to 2 to 4 minutes. Mull and colleagues52 in a series of 34 patients showed that contrast-enhanced spinal magnetic resonance angiography (MRA) could reliably detect or exclude spinal cord arteriovenous abnormalities, with a 100% predictive value. The main arterial feeder can be reliably defined by MRA, but small secondary feeders may be missed. The main reasons for obtaining MRA would be for primary identification of a vascular abnormality and to pinpoint the likely site of a feeder for conventional catheter angiography.
CT angiography can also define normal and abnormal spinal vasculature.53,54 The technique requires a multidetector row CT scanner (generally ≥16) and 1-mm section thickness. Given the tremendous speed of current CT scanners, the thoracic and upper lumbar spine can be covered in 30 seconds. As with MRA, the examination relies on a bolus of intravenous contrast medium and precise triggering of the contrast bolus at its maximum density within the thoracic aorta. Contrast injection rates are on the order of 2 to 3 mL/sec for a total of 50 to 75 mL. Considerable postprocessing is required to segment the target vessel and to connect the intraspinal vessel with the appropriate intercostal vessel to define the arterial side of the vasculature. CT angiography does not have the ability to separate out the artery and vein based on temporal resolution, as does MRA.
Discography
Discography was originally conceived as a morphologic study of disc herniation but then morphed into a useful but limited test relying on pain provocation through disc pressurization.55,56 Although discography can accurately define disc degeneration, this procedure is now seen as a physiologic evaluation of the disc consisting of volumetric, manometric, radiographic, and pain provocative challenge.57,58 This procedure remains quite controversial; it has enthusiastic supporters and detractors and has generated a voluminous literature. Some authors see discography as helpful in identifying internal disc disruption and in verifying painful disc levels before surgery (particularly fusion), whereas others see it as unproven and of questionable benefit.59–66
Discography is an invasive procedure and is not performed as a screening technique. Discography is most accurate when the diagnosis of discogenic pain is probable based on appropriate history, physical examination, and imaging.56 This test is always limited in sensitivity and specificity owing to the subjective report of pain type and location by the patient. According to Tehranzadeh and others,67–69 indications for discography include the following:
Generally, small-gauge needles (22-gauge) are placed with fluoroscopic guidance into the nucleus pulposus of one or more discs. With proper placement of the needles confirmed under fluoroscopy, contrast medium is injected into the nucleus pulposus centrally. A normal disc takes 1 to 2 mL of contrast agent. A normal disc is painless, with the contrast agent remaining centrally within the nucleus pulposus. Abnormal discs are associated with pain on injection, which simulates the patient’s symptoms and which may or may not be referred to the legs. Videotaping of the patient’s pain response and the fluoroscopic display may be performed.66 After injection of contrast agent, anteroposterior and lateral plain radiographs are obtained, followed by axial CT images through the level of the discograms. Different grading of radial pairs can be performed accurately only on the axial CT projections. The main complication for this invasive technique is a disc space infection. The main risk of discitis is 0.1% to 0.2%.70–72 A prophylactic broad-spectrum antibiotic is often used.
Magnetic Resonance Imaging Safety
The specific and important aspects of MRI safety are widely available on multiple websites, and the interested reader is referred to them for detailed answers.73–75 One more recent aspect of MRI safety that is perhaps less widely recognized outside of radiology is nephrogenic systemic fibrosis (NSF), previously called nephrogenic fibrosing dermopathy. NSF is a systemic disorder of widespread fibrosis that has been tied to prior administration of gadolinium-based contrast agents in the setting of renal disease. The incidence of NSF in the setting of severe renal dysfunction is approximately 1% to 7% after exposure to gadolinium-based contrast material. The U.S. Food and Drug Administration (FDA) has asked manufacturers to include a new boxed warning on the product labeling of all gadolinium-based contrast agents that are used to enhance the quality of MRI. The warning states that patients with severe kidney insufficiency who receive gadolinium-based agents are at risk for developing NSF, a debilitating and potentially fatal disease.74 Also, patients just before or just after liver transplantation and patients with chronic liver disease are at risk for developing NSF if they are experiencing kidney insufficiency of any severity.
The risk of a patient developing NSF may be minimized by the following steps:76–80
Degenerative Disc Disease
Multiple authors suggest that an imaging study is indicated in the evaluation of a patient with sciatica when (1) true radicular symptoms are present, (2) there is objective evidence of nerve root irritation on physical examination (i.e., positive straight-leg raise test), and (3) the patient has failed “conservative management” of 4 to 6 weeks’ duration.81–83 Earlier imaging is considered appropriate if clinical features raise concern regarding malignant or infectious causes or if neurologic findings worsen during observation. These recommendations are based on several studies of successful nonoperative treatment of sciatica.84–89 Imaging is recommended only for the remaining minority of patients with persistent signs and symptoms who are believed to be surgical candidates or in whom diagnostic uncertainty remains.
In addition to these observed changes within the degenerating disc, vertebral marrow signal abnormalities adjacent to the degenerating disc are common.90 Type I endplate change manifests as decreased marrow signal paralleling the endplates on T1-weighted images and increased signal on T2-weighted images. These changes reflect replacement of normal fatty marrow with fibrovascular marrow, which has greater water content. Type II endplate changes are slightly more common than type I changes, showing increased signal on T1-weighted images and isointense to slightly increased signal on T2-weighted images. Histologically, these changes correlate with fatty marrow replacement. These changes may be preceded by type I changes, and often these changes exist in combination at the same level or different levels. Type III endplate changes show decreased marrow signal on T1-weighted and T2-weighted images, a finding that correlates with endplate sclerosis seen radiographically.90
Fissures (tears) of the anulus fibrosus can also be visualized with MRI. They appear as small areas of increased signal on T2-weighted images and can enhance after contrast agent administration, presumably secondary to the ingrowth of granulation tissue into the fissure as a consequence of healing.91 Three types of annular fissures have been described, depending on their orientation relative to the concentric annular fibers.92 The high frequency of annular fissures seen in association with large disc bulges challenges the concept that the anulus fibrosis is intact in bulging discs but ruptured in herniated discs. The clinical significance of annular fissures is unknown. In patients without nerve root compression, back pain may be secondary to irritation of the nerve endings in the peripheral anulus either from scar tissue within an annular fissure or from a disc herniation; this is what is referred to as discogenic pain. Although this concept is often used to ascribe clinical significance to these lesions, many asymptomatic patients harbor annular fissures.
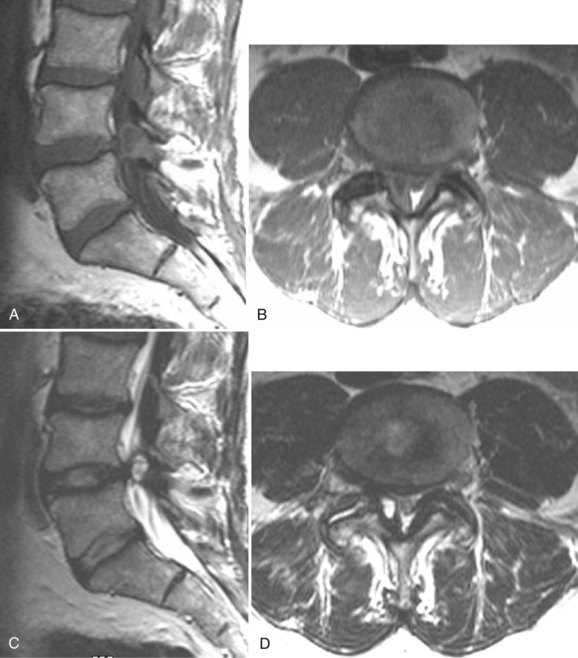
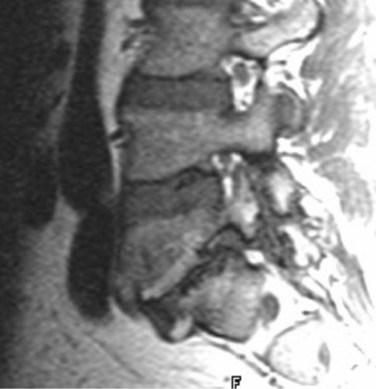
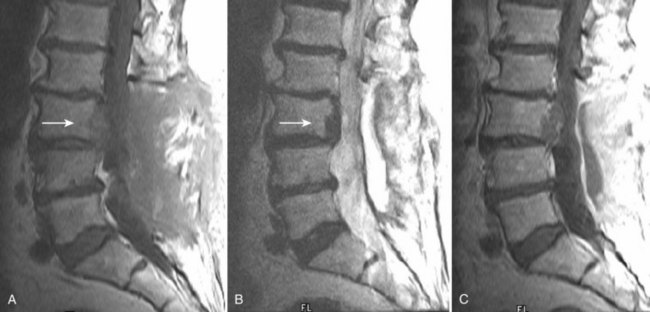
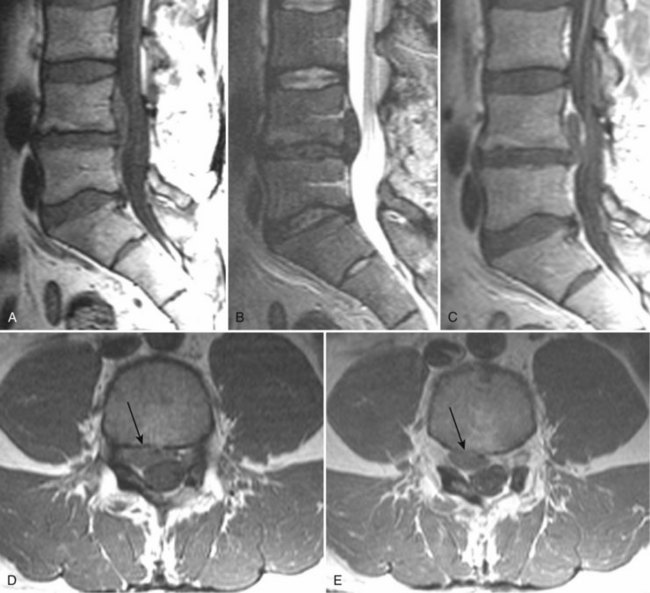
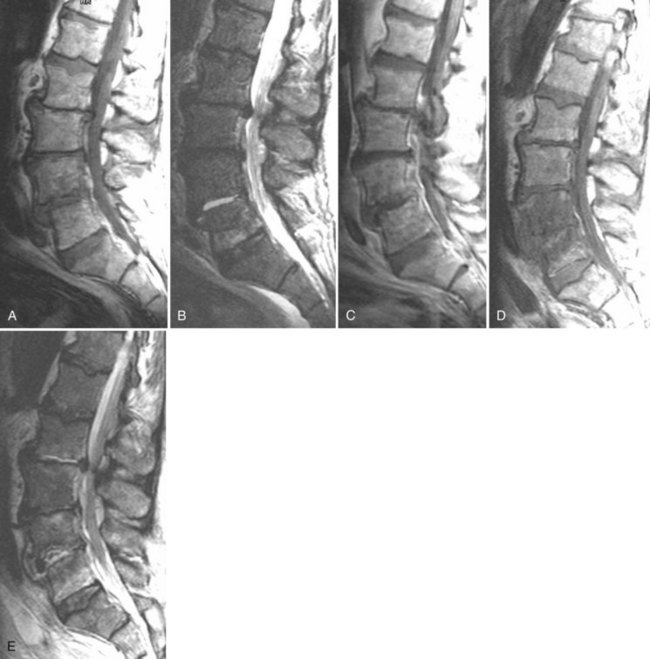
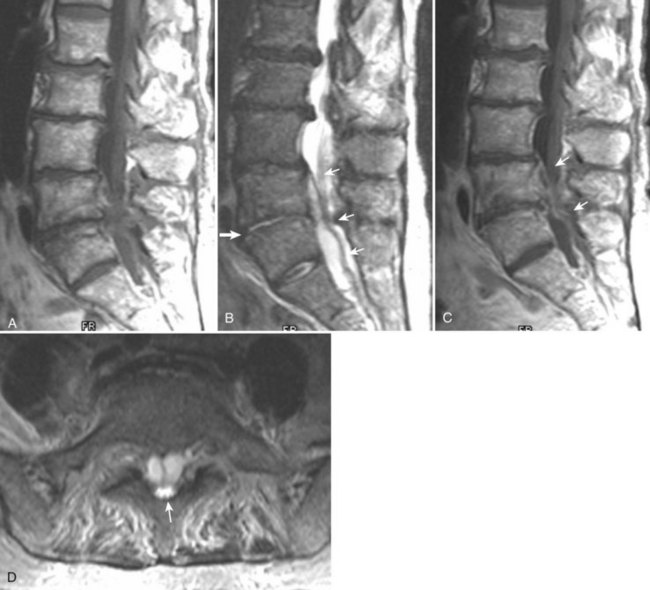
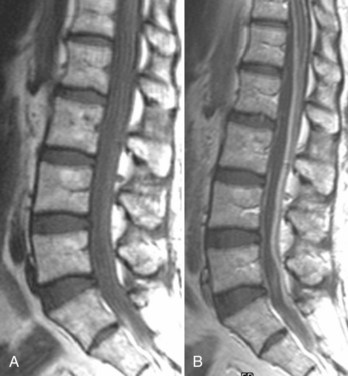
There is no universally accepted classification system describing degenerative disc disease. A multispecialty task force released recommendations for disc nomenclature spanning the orthopaedic, neurosurgical, and radiologic communities.93 This group has defined a protrusion as a herniation that maintains contact with the disc of origin by a bridge as wide as, or wider than, any diameter of the displaced material (Fig. 12–5). An extruded disc is a larger herniation where the diameter of the disc material beyond the interspace is wider than the bridge, if any, that connects it to the disc of origin (Fig. 12–6). A sequestered (free) disc fragment is an extrusion that is no longer contiguous with the parent disc. It may reside either anterior or posterior to the posterior longitudinal ligament or rarely may be intradural (Figs. 12-7 and 12-8). A free fragment may be located at the disc level or may migrate superiorly or inferiorly, often lateralized by the thin, sagittally oriented midline septum seen in the lower anterior epidural space.
Lumbar Stenosis
As an anatomic entity, spinal stenosis refers to narrowing of the central spinal canal, neural foramina, or lateral recesses. Most commonly, it is acquired secondary to degenerative disease of the intervertebral disc or facets or both, although developmentally shortened pedicles are an important component of symptomatic spinal stenosis in patients with otherwise mild degenerative changes (Figs. 12-9 and 12-10).94 Before the development of MRI, plain films and CT were used to diagnose spinal stenosis by measuring the dimensions of the bony canal. At present, such measurements are not commonly performed. These measurements do not take into account the normal anatomic variation between patients or the role of the disc and ligamentum flavum in spinal stenosis and are inaccurate predictors of clinical symptoms. MRI accurately depicts the degree and cause of thecal sac narrowing in patients with central canal stenosis. Such narrowing is most commonly due to bony and ligamentous hypertrophy.
Facet Disease
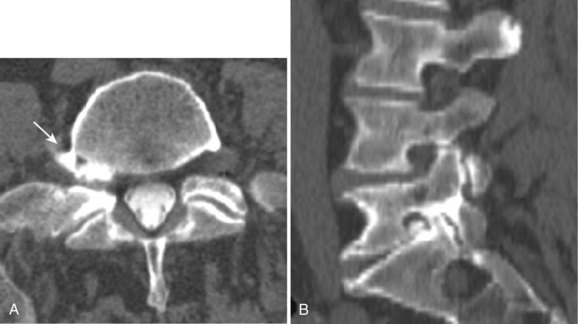
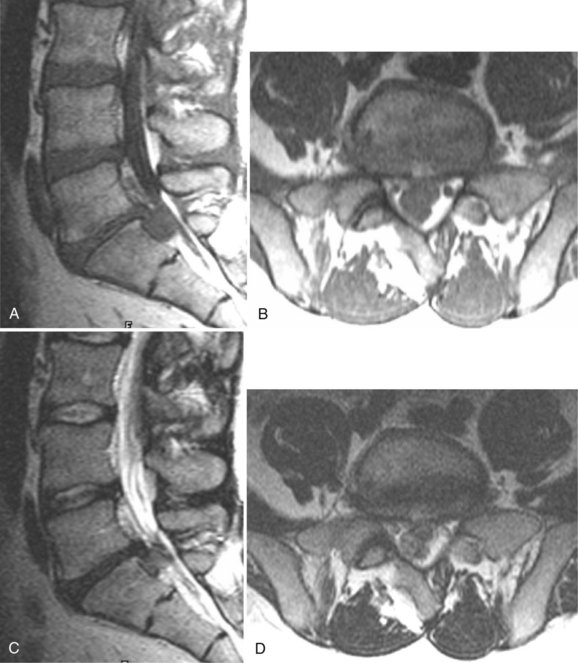
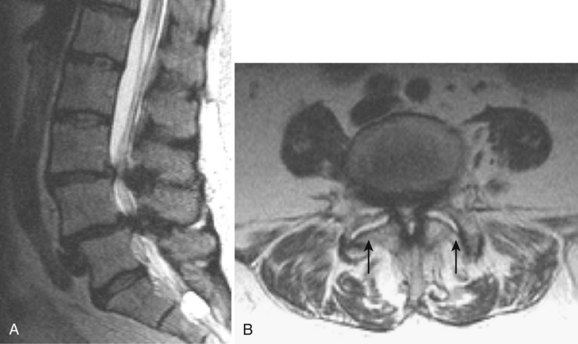
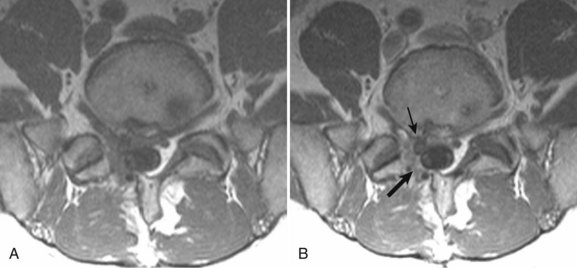
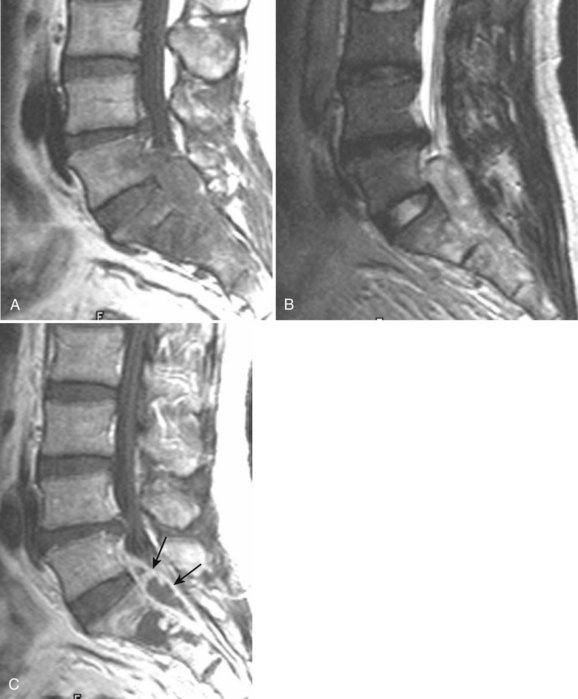
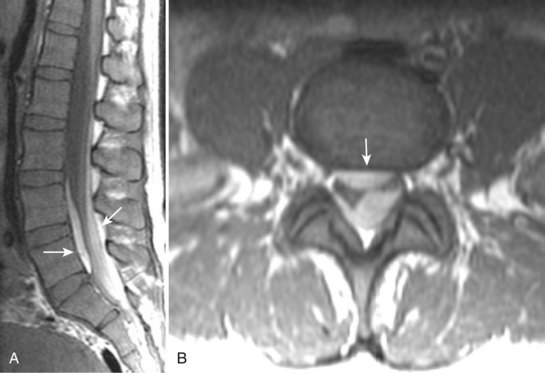
Degenerative disease of the facet joints typically occurs in combination with degenerative disc disease, although facet disease alone may be responsible for symptoms of back pain and radiculopathy. As with any synovial-lined joint, facet joints are susceptible to the development of joint space loss, subchondral sclerosis and cyst formation, osteophytosis, and subluxation. Because of the richly innervated synovium and joint capsule, these changes alone can be a source of pain, or alternatively they can contribute to nerve root impingement by causing spinal stenosis or foraminal compromise. On MRI, degenerated facets appear hypertrophied, sclerotic, and irregular. Enlarged ligamentum flavum is commonly present. Facet degeneration can lead to the formation of synovial cysts that can compress the thecal sac and roots from a posterior direction. Synovial cysts are best depicted on axial images and appear as posterolateral epidural masses adjacent to a degenerated facet, most commonly at the L4-5 level. Synovial cysts have variable signal characteristics secondary to varying cyst fluid composition and associated hemorrhage, calcification, or gas within the cyst (Fig. 12–11).95 A peripheral hypointense rim on T2-weighted images related to calcification may be seen. Intravenous contrast medium is useful in suspected cases to define better the lesion and its relationship to the adjacent facet joint and thecal sac.
Instability
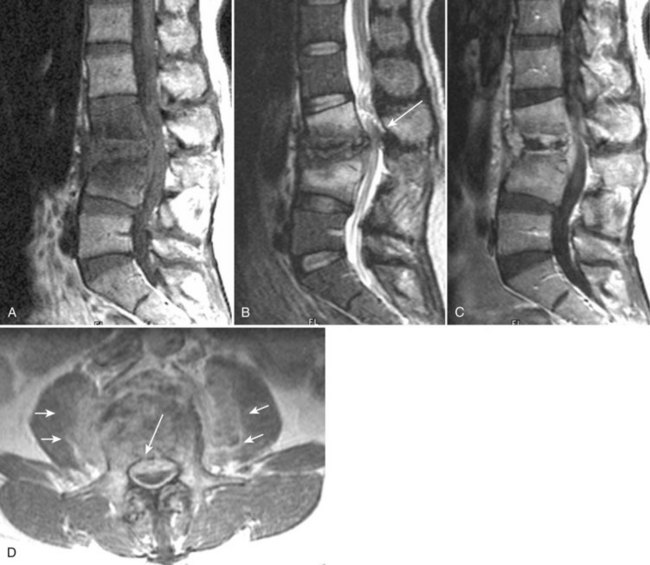
Because of its ability to obtain direct sagittal images free of overlapping structures and patient rotation, MRI is an accurate method of diagnosing spondylolisthesis. MRI is nearly always performed with the patient supine, however. In that position, a vertebra with subluxation can be normally aligned. A more accurate method of detecting listhesis is by weight-bearing lateral lumbar radiographs. The detection of spondylolysis (pars interarticularis defect without ventral slippage) by MRI can be problematic, and it is generally agreed that plain films and CT are more reliable for its diagnosis. Because MRI is being increasingly used as the first and only imaging modality in evaluating patients with low back pain and radicular symptoms, many cases of spondylolysis are imaged without the benefit of correlative plain films or CT studies.96 Using MRI, sagittal T1-weighted images are best for showing the pars interarticularis owing to their higher signal-to-noise ratio, the depiction of the pars marrow as hyperintense, and the minimal obliquity of the pars in this imaging plane (Fig. 12–12). If the pars appears normal (i.e., contiguous normal marrow signal), one can be certain that it is intact.97 The presence of abnormal pars signal is not specific for spondylolysis, however, because benign sclerosis, partial volume averaging with an adjacent degenerative facet, and osteoblastic metastases can also give this appearance.
Cervical Radiculopathy and Myelopathy
Various studies have shown that canal size is reduced in patients with cervical spondylotic myelopathy. The normal diameter of the canal from C3 to C7 is approximately 17 mm and can be decreased to 12 mm or less in cervical spondylotic myelopathy. The size that is associated with myelopathy has ranged, however, from less than 10 mm to 14 mm. Additionally, myelopathic symptoms tend to occur when the canal cross-sectional area is less than 60 mm2. The ratio of the anteroposterior canal diameter to the vertebral body diameter has been used to assess cervical stenosis. This Pavlov ratio (sometimes referred to as the Torg ratio) is normal if it is 1 or greater.98 A ratio of 0.8 or less is considered abnormal. As a ratio, however, it can be abnormal not only because of an abnormally small canal diameter (small numerator), but also because of an abnormally large vertebral body (large denominator). This ratio method also does not take into account the size of the spinal cord itself. As an isolated tool, this method is of historical interest only and is useless in evaluating cervical spinal cord compression.
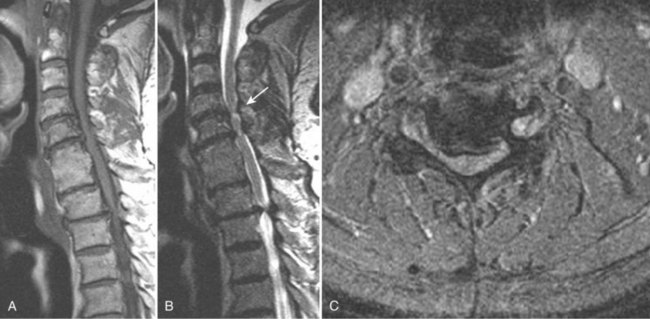
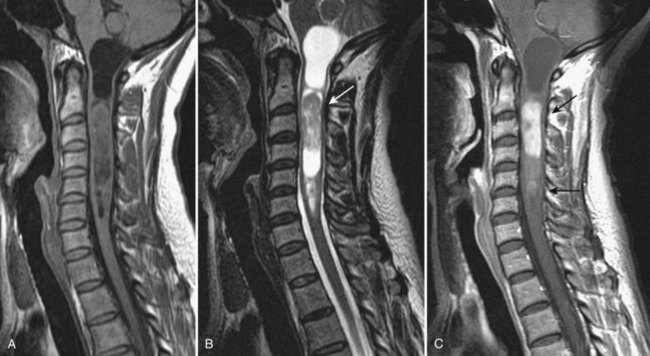
Takahashi and colleagues99 and others have described areas of increased signal intensity on T2-weighted images within the cervical cord owing to extradural compression, which variously reflects myelomalacia, gliosis, and demyelination and edema (Fig. 12–13). Patients who show areas of abnormal signal within the cord tend to have a worse clinical condition than patients with normal cord signal intensity. These abnormal signal changes can disappear or diminish after surgery to relieve the cord compression.
Ossification of Posterior Longitudinal Ligament
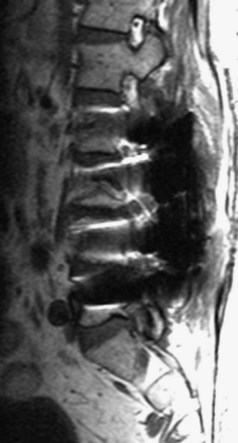
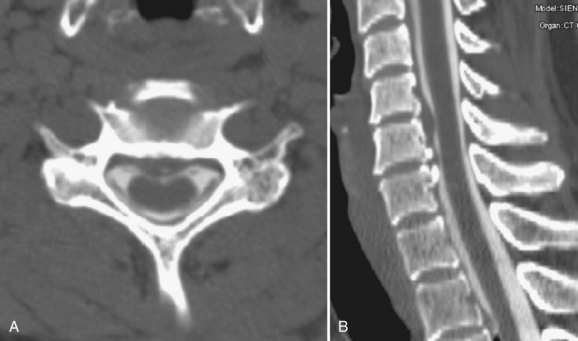
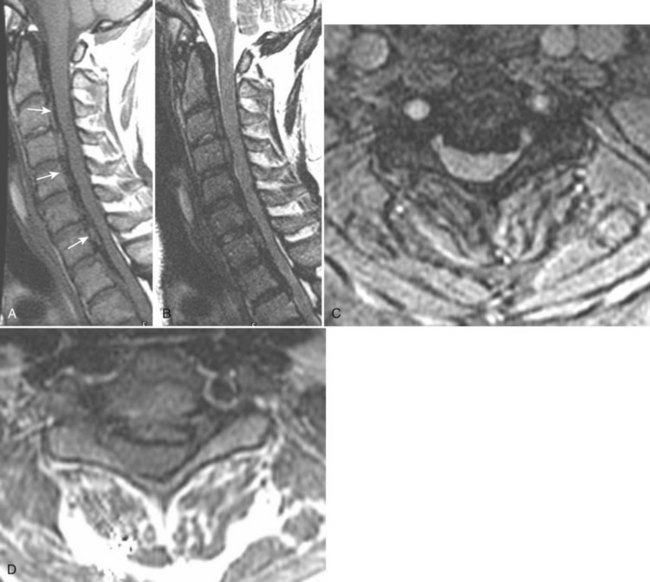
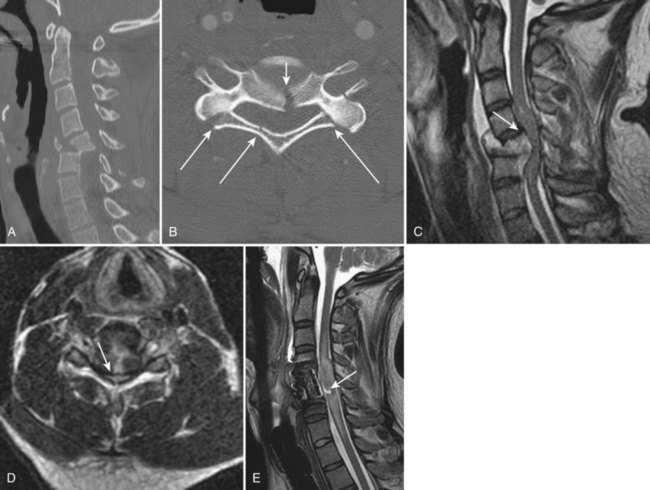
Ossification of the posterior longitudinal ligament (OPLL) begins with calcification followed by frank ossification of the posterior longitudinal ligament in the upper cervical spine (C3-4 or C4-5). It may progress inferiorly to the upper thoracic spine (Figs. 12-14 and 12-15).100 Patients tend to present in the 6th decade, are generally older than the usual patients with disc disease, and are younger than patients with cervical spondylosis. Presenting complaints include neck pain, dysesthesias, and upper and lower extremity weakness. Hirabayashi and Satomi101 divided OPLL into four types based on CT: (1) Continuous OPLL extends between vertebral bodies and crosses multiple disc spaces (27% of cases), (2) segmental OPLL is limited to the posterior vertebral body margins (39% of cases), (3) mixed OPLL is continuous and segmental (29% of cases), and (4) the remaining 5% of OPLL is restricted to the disc space level.
Postoperative Issues
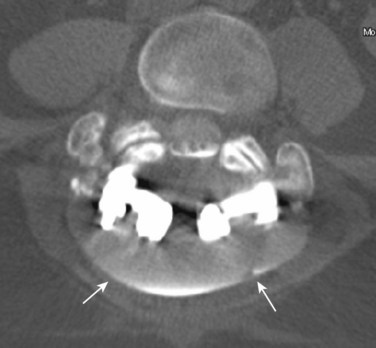
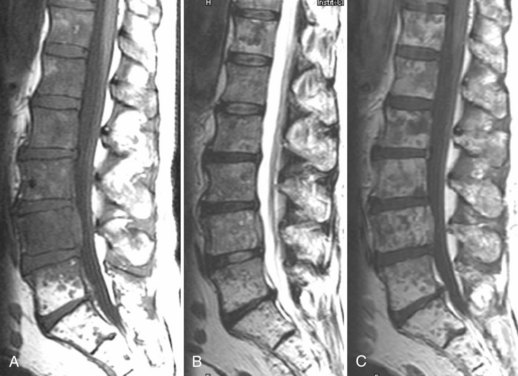
Causes of early and delayed failure of surgery are listed in Tables 12-1 and 12-2. Caution must be used in interpretation of CT, CT myelography, and MRI within the first 6 weeks after surgery owing to the large amount of tissue disruption and edema that may be present producing a mass effect on the anterior thecal sac, even in the absence of any clinical symptoms. MRI may be used in the immediate postoperative period for a more gross view of the thecal sac and epidural space, to exclude significant postoperative hemorrhage, pseudomeningocele, or disc space infection at the laminectomy site. CT myelography is also a direct way to define a pseudomeningocele and to image the spine when hardware is present (Fig. 12–16).
TABLE 12–2 Technical Causes of Delayed Recurrence of Low Back Pain or Radiculopathy
Small fluid collections are commonly seen in the posterior tissues after laminectomy. The signal intensities can vary depending on whether the collections are serous (follow CSF signal intensity) or serosanguineous (increased signal on T1-weighted images owing to hemoglobin breakdown products). The distinction between small postoperative fluid collections and infected collections cannot be made by MRI morphology or signal intensity. Acute hemorrhage typically shows isointense to increased signal in the epidural space on T1-weighted images and should show diminished signal on GE or T2-weighted images. Very acute blood collections may be isointense, however, on T1-weighted and T2-weighted images (Fig. 12–17).
Epidural Fibrosis and Disc Herniations
The use of contrast medium–enhanced MRI in the evaluation of scar versus disc has been examined by several authors, with reported accuracy rates of 96% to 100% for distinguishing scar from disc.102 Lumbar epidural fibrosis (scar) is a replacement of the normal epidural fat with postoperative fibrotic tissue, which is capable of binding the dura and nerve roots to the surrounding structures anteriorly and posteriorly. Epidural fibrosis is seen to enhance consistently immediately after injection of contrast material (Fig. 12–18). This enhancement occurs regardless of the time since surgery. Disc material does not enhance on the early postinjection images owing to its lack of vascularity (Fig. 12–19). In cases with a mixture of scar and disc material, scar enhances, and the disc material does not enhance on early postinjection images.
Selective fat suppression on T1-weighted images has been used in the evaluation of postoperative patients. Georgy103 examined 25 patients with recurrent pain after lumbar disc surgery with MRI to evaluate the usefulness of gadolinium-enhanced fat suppression imaging in patients with failed back surgery. The addition of fat suppression to enhanced T1-weighted images improved the visualization of enhancing scar in all of their cases, helped distinguish scar from recurrent herniated disc, and showed more clearly the relationship of scar to the nerve roots and thecal sac.
Stenosis
Infection
Radionuclides most commonly used for detecting inflammatory changes of the spine are technetium 99m (99mTc) phosphate complexes, gallium (67Ga) citrate, and indium-111 (111In)–labeled white blood cells. Although scintigraphy with 99mTc and 67Ga compounds is sensitive to infection, it is also nonspecific. Healing fractures, degenerative arthritis, sterile inflammatory reactions, tumors, and loosened prosthetic devices can show increased uptake.108–110
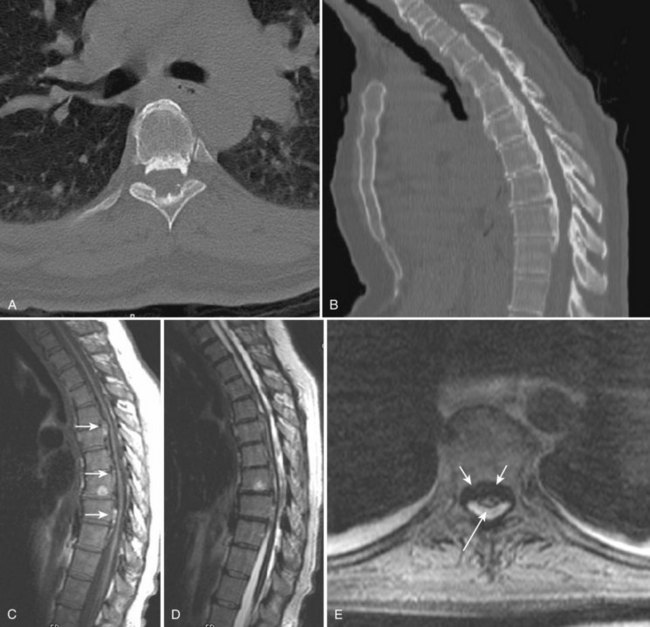
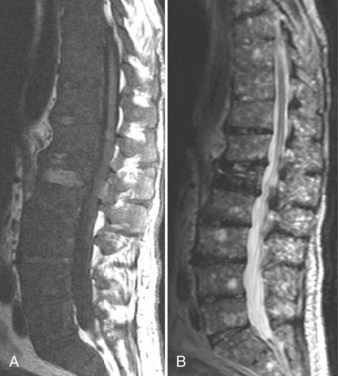
111In has several advantages compared with other radionuclides, including higher target-to-background ratios, better image quality (compared with 67Ga), and more intense uptake by abscesses. Its main disadvantage is its accumulation within any inflammatory lesion, whether infectious or not.111 The radionuclide study also takes time to perform—hours to days. CT has played a minor diagnostic role in cases with bony or soft tissue components and is not considered a mainstay for the diagnosis of disc space infection.112,113 In appropriate situations, the sensitivity of MRI for detecting vertebral osteomyelitis seems to exceed the sensitivity of plain films and CT, and it approaches or equals the sensitivity of radionuclide studies (Figs. 12-20 and 12-21).114,115
On T2-weighted images, the normal intervertebral disc usually shows increased signal intensity within its central portion that is bisected by a thin horizontal line of decreased signal, termed the intranuclear cleft. After the age of 30 years, the cleft is almost a constant feature of normal intervertebral discs. Disc space infections on MRI typically produce confluent decreased signal intensity of the adjacent vertebral bodies and the involved intervertebral disc space on T1-weighted images compared with the normal vertebral body marrow. A poorly defined endplate margin exists between the disc and adjacent vertebral bodies. T2-weighted images show increased signal intensity of the vertebral bodies adjacent to the involved disc and an abnormal morphology and increased signal intensity from the disc itself, with absence of the normal intranuclear cleft. These MRI findings are much more typical of pyogenic than of tuberculous spondylitis.116 In a comparative study of patients with suspected vertebral osteomyelitis, MRI had a sensitivity of 96%, a specificity of 92%, and an overall accuracy of 94%.114 Scintigraphy with 67Ga and 99mTc bone scintigraphy had a sensitivity of 90%, specificity of 100%, and accuracy of 94% when combined. In this study, MRI was as accurate and sensitive as radionuclide scanning for the detection of osteomyelitis.
Dagirmanjian and colleagues117 investigated the sensitivity of MRI findings for vertebral osteomyelitis. They considered the “classic” MRI changes of vertebral osteomyelitis to include decreased signal of disc and adjacent vertebral bodies on T1-weighted images, increased nonanatomic signal of the disc on T2-weighted images, increased signal of the adjacent vertebral bodies on T2-weighted images, and enhancement of the disc and adjacent vertebral bodies. These investigators found 95% of disc space infection levels had typical T1-weighted vertebral body changes, and 90% had increased nonanatomic signal of the disc on T2-weighted images. Only 54% of the abnormal levels showed increased signal of the vertebral bodies on T2-weighted images, however. Although 84% of patients showed the typical T1-weighted vertebral body and T1-weighted and T2-weighted disc changes, only 49% of cases showed the typical T1-weighted and T2-weighted vertebral body and disc findings as originally described. T1-weighted vertebral body, disc, and endplate changes and T2-weighted disc changes are the most reliable findings of disc space infection and vertebral osteomyelitis. In the initial stages of vertebral osteomyelitis, when the disc space is not yet involved, it may be difficult to exclude neoplastic disease or compression fracture from the differential diagnosis using only MRI. Follow-up studies are usually necessary to define the nature of the lesion further.
Boden and colleagues118 suggested that in the postoperative spine the triad of intervertebral disc space enhancement, annular enhancement, and vertebral body enhancement leads to the diagnosis of disc space infection, with the appropriate laboratory findings, such as an elevated sedimentation rate. There is a group of normal postoperative patients, however, with anulus enhancement (at the surgical site), intervertebral disc enhancement, and vertebral endplate enhancement without evidence of disc space infection. In these cases, the intervertebral disc enhancement is typically seen as thin bands paralleling the adjacent endplates, and the vertebral body enhancement is associated with type I degenerative endplate changes. This pattern should be distinguished from the amorphous enhancement seen within the intervertebral disc with disc space infection.
Staphylococcus aureus is the organism most commonly associated with vertebral osteomyelitis and epidural abscess, accounting for approximately 60% of the cases (Fig. 12–22). S. aureus is ubiquitous, tends to form abscesses, and can infect compromised and normal hosts. Other gram-positive cocci account for approximately 13% of cases, and gram-negative organisms account for approximately 15%. Clinical acute symptoms classically include back pain, fever, obtundation in severe cases, and neurologic deficits. Chronic cases may have less pain and no elevated temperature. Rankin and Flothow119 described the classic course of epidural abscess in four stages: spinal ache, root pain, weakness, and paralysis. Acute deterioration from spinal epidural abscess remains unpredictable, however. Patients may present with abrupt paraplegia and anesthesia. The cause for this precipitous course is unknown, but it is thought to be related to a vascular mechanism (e.g., epidural thrombosis and thrombophlebitis, venous infarction).120,121
The primary diagnostic modality in the evaluation of epidural abscess is MRI. MRI is as sensitive as CT myelography for diagnosing epidural infection, but it also allows the exclusion of other entities, such as herniation, syrinx, tumor, and cord infarction.122,123 MRI of epidural abscess shows a soft tissue mass in the epidural space with tapered edges and an associated mass effect on the thecal sac and cord. The epidural masses are usually isointense to the cord on T1-weighted images and of increased signal on T2-weighted images. Post and colleagues124,125 recommended that in ambiguous cases either CT myelography or contrast medium–enhanced MRI is necessary for full elucidation of the abscess (Fig. 12–23).
The patterns of MRI contrast medium enhancement of epidural abscess include (1) diffuse and homogeneous, (2) heterogeneous, and (3) thin peripheral. Post and colleagues125 found that enhancement was a very useful adjunct for identifying the extent of a lesion when the plain MR image was equivocal, for showing activity of an infection, and for directing needle biopsy and follow-up treatment. Successful therapy should cause a progressive decrease in enhancement of the paraspinal soft tissues, disc, and vertebral bodies.
Tumors
Intramedullary Lesions
The most common intramedullary neoplasms are gliomas, principally astrocytomas and ependymomas. Ependymomas are cited as the most frequent intramedullary tumors in adults (Fig. 12–24). Although ependymomas may involve any portion of the cord, they most commonly involve the conus medullaris and filum terminale and are the most common primary tumor of the lower spinal cord. Patients with these tumors present in the 4th to 5th decades, often with back pain.126,127 A typical appearance would be an intradural extramedullary mass involving the filum terminale and cauda equina, although it can appear as fusiform enlargement of the cord itself.128 Cervical intramedullary tumors may be seen in patients with neurofibromatosis type 2 (Fig. 12–25). These tumors typically enhance and may have intratumoral cysts. The myxopapillary subtype is particularly common in the lumbosacral region, typically appearing as a large, intensely enhancing mass spanning several vertebral levels. In most cases, the tumors appear as intradural extramedullary lesions because of their bulky exophytic growth, which fills the spinal canal. Overall signal intensities are nonspecific, but because of their highly vascular nature, ependymomas often show areas of T2-weighted shortening secondary to the presence of hemosiderin and ferritin, which is strongly suggestive of the diagnosis.129 They also may manifest as subarachnoid hemorrhage.130
Hemangioblastomas are unusual cord tumors and usually manifest in the 3rd to 4th decades of life. They are frequently multiple and seen in association with von Hippel–Lindau disease.131–136 These lesions most often manifest as dorsal intramedullary masses containing a nodule that enhances, although these vary between the amount of cyst component and solid component. There may be extensive widening of the cord showing increased signal intensity on T2-weighted images related to cord edema, extending several segments away from the nidus itself. Occasionally, metastatic disease may manifest as an intramedullary enhancing mass. Carcinoma of the lung and breast are the most common, with melanoma, lymphoma, and renal cell carcinoma also being reported.137,138 Spread of intracranial neoplasms such as ependymoma and glioma may also seed the leptomeninges and produce direct involvement of the cord.139–141 Benign intramedullary tumors are uncommon, but cavernous angiomas can occur in the cord with typical signal characteristics of speckled increased and decreased signal on T1-weighted images and evidence of hemosiderin deposition on T2-weighted images (Fig. 12–26).
The various causes of inflammatory myelopathies include multiple sclerosis, postviral demyelinating disease, viral infection, pyogenic infection, and granulomatous disease. The archetypal inflammatory lesion is multiple sclerosis (Fig. 12–27). The spinal cord is the site of much clinical involvement in patients with multiple sclerosis; however, imaging of the spinal cord has always been subordinate to brain imaging in radiologic investigations of multiple sclerosis. Because some of the clinical disease activity in multiple sclerosis is related to the spinal cord, it is important to correlate cord disease activity to gain further insights into the nature of disability in these patients and to correlate objective improvements in brain and cord lesion burden with changes in clinical disability scoring. Most focal plaques are less than two vertebral body lengths in size, occupy less than half the cross-sectional diameter of the cord, and are characteristically peripherally located with respect to a transverse, cross-sectional reference. Of spinal cord multiple sclerosis lesions, 60% to 75% are present in the cervical region, and more than half of multiple sclerosis patients with cord plaques have multiple plaques. Of patients with cord plaques, 90% have intracranial multiple sclerosis plaques.
Intradural Extramedullary Lesions
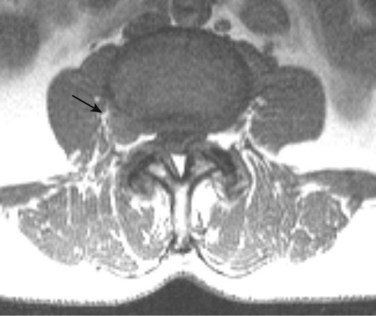
Intradural extramedullary neoplasms constitute the largest single group of primary spine neoplasms, accounting for approximately 55% of all primary spine tumors. Most of these tumors are benign, with nerve sheath tumors and meningiomas representing the most common lesions.141 Nerve sheath tumors are the most common intraspinal tumors and are divided histologically into two types: schwannomas (i.e., neuromas, neurinomas, neurilemmomas) and neurofibromas (Fig. 12–28). Solitary schwannomas constitute most intraspinal nerve sheath tumors, whereas neurofibromas are almost always associated with neurofibromatosis type 1. Patients with neurofibromatosis type 2 more commonly have multiple schwannomas rather than neurofibromas, however.142 Isolated nerve sheath tumors can arise anywhere in the spine.143
Meningiomas most commonly occur in the thoracic spine.144 As is the case intracranially, there is a female sex predilection, and these lesions occur in a slightly older age group than nerve sheath tumors. Most are entirely intradural and typically are isointense to the neural elements on T1-weighted and T2-weighted images. Meningiomas enhance intensely after gadolinium–diethylenetetraminepentaacetic acid (DTPA) administration, which may allow demonstration of the typical broad dural base.145,146
The last category of intradural extramedullary lesions is the so-called leptomeningeal pattern, which includes leptomeningeal metastatic disease, inflammation, and benign granulomatous processes such as sarcoid and tuberculosis (Fig. 12–29).147 The list of tumors that may seed the CSF is long, but the most common offenders are cranial ependymomas, glioblastomas, and medulloblastomas (especially in pediatric patients). Additional malignancies that can spread less commonly are ependymoma, pineoblastoma, germinoma, and retinoblastoma. Lesions outside the central nervous system that are capable of spreading along the leptomeninges include carcinoma of the lung and breast, lymphoma, leukemia, and melanoma. Administration of contrast material with T1-weighted images is mandatory and shows a linear and nodular enhancement pattern along the leptomeninges. The overall sensitivity of MRI examinations is low in patients with proven histologic evidence of neoplastic seeding, so examination of the CSF remains the “gold standard.”
Extradural Lesions
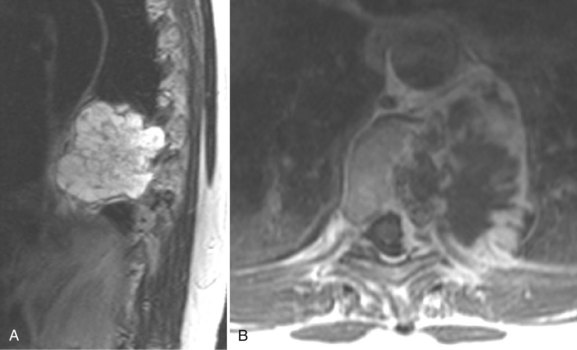
Primary and secondary tumors to the extradural space are well evaluated by MRI and CT (Fig. 12–30). Metastatic disease to the spine is the most common type of extradural tumor. Because of its high contrast sensitivity and spatial resolution, MRI is the examination of choice in the detection of osseous metastases (Fig. 12–31).148–150 Because many metastatic tumors enhance, the routine use of contrast medium–enhanced studies alone is not recommended because the distinction between metastases and normal marrow fat is diminished, occasionally to the point of masking even large lesions (Fig. 12–32). Although diffuse osseous metastases can appear as homogeneous diffuse low marrow signal on T1-weighted images, this appearance is not specific.
Cysts
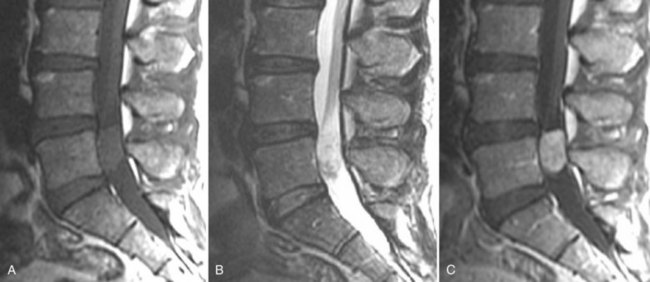
Nabors and colleagues151–153 have clarified the confusing array of terms for spinal meningeal cysts. Spinal meningeal cysts are congenital diverticula of the dural sac, root sheaths, or arachnoid that may be classified into three major groups. The first group includes extradural cysts without spinal nerve roots (type I), the second includes extradural cysts with spinal nerve roots (type II), and the third includes intradural cysts (type III) (Fig. 12–33). Type I are diverticula that maintain contact with the thecal sac by a narrow ostium. Type I cysts include extradural cysts, pouches, and diverticula and the so-called occult intrasacral meningoceles. Sacral type I cysts are found in adults and are connected to the tip of the caudal thecal sac by a pedicle. Type II meningeal cysts with contained nerve roots are extradural lesions previously called Tarlov cysts, perineural cysts, or nerve root diverticula. These are generally seen as multiple incidental lesions, but are occasionally associated with radiculopathy or incontinence. Type III meningeal cysts are intradural lesions most commonly found on the posterior subarachnoid space and have been called arachnoid diverticula or arachnoid cysts. These are lined by a single layer of normal arachnoid cells and filled with CSF.
Trauma
Studies have shown that if strict criteria are followed, patients who arrive at the emergency department with a collar in place can be clinically evaluated as to whether or not plain films are required.154,155 Patients with cervical fractures typically have at least one of the following: intoxication, neck tenderness, altered level of consciousness, or a painful injury elsewhere. Indications for CT in evaluation of the cervical spine include further evaluation of known or questioned fracture on plain films and evaluation of areas inadequately seen on plain films.156,157 Techniques vary from institution to institution, but slice thickness is generally 1.5 to 3 mm, soft tissue and bone windows, with no intravenous contrast material. The sensitivity of CT to detect fracture is 78% to 100%.158,159 CT is particularly useful in diagnosing posterior element (laminar) fractures. The use of spiral thin-section techniques (1 to 1.5 mm) with multiplanar reformats should enable sensitivity approaching 100%. Some institutions and studies use CT as the primary screening study in patients with multiple areas of trauma, bypassing plain films.160,161
MRI allows direct visualization of cord abnormalities, which cannot be identified by any other imaging modality. MRI can define intramedullary hematoma, intramedullary edema and contusion, disc herniations, ligamentous injury, and epidural hemorrhage (Fig. 12–34).162–165 Hemorrhage within the 1st week is seen as low signal on T2-weighted images related to deoxyhemoglobin. Contusion without hemorrhage is identified as high signal on T2-weighted images and as isointense or decreased signal on T1-weighted images. Ligamentous disruption is seen as loss of the usual low signal from the anterior and posterior longitudinal ligaments, with increased signal on T2-weighted images in the adjacent tissues.166,167
The most common area of traumatic involvement in the lumbar spine is the thoracolumbar junction, which acts as a fulcrum for spine motion and is susceptible to unstable traumatic injury. The thick, sagittally oriented lumbar facets minimize rotational injury, but flexion and axial loading injuries often occur. The forces may combine to produce flexion-compression injuries or the so-called burst fracture. Burst fractures are notable for instability and a predisposition for displacing fracture fragments and causing spinal cord compression.168,169 CT remains the method of choice for the detection of retropulsed bony fragments and for the demonstration of fractures of the posterior elements.170
A hyperflexion injury occurring in the lumbar spine is the seat belt or Chance fracture, which is associated with rapid deceleration motor vehicle accidents. This type of trauma produces a horizontal fracture through anterior and posterior elements.171,172 The anterior component may be through the vertebral body or through the disc itself. Although CT is more sensitive than MRI for detecting bony abnormalities, MRI is often superior for evaluating soft tissue structures. In particular, the spinal ligaments show focal discontinuity on T1-weighted images and areas of increased signal intensity on T2-weighted images.
Hemorrhage
Epidural spinal hematomas occur most frequently in elderly adults but can occur at any age.173–176 Epidural spinal hematomas are broadly classified into two groups: nonspontaneous and spontaneous. Nonspontaneous epidural spinal hematomas may result from spinal taps, spinal anesthesia, trauma, pregnancy, bleeding diathesis, anticoagulant therapy, spinal hemangiomas, vascular malformations, hypertension, and neoplasms. The history can often be revealing, yet these tumors commonly occur merely from an episode of sneezing, bending, voiding, turning in bed, or other mild trauma. Epidural spinal hematomas can be localized or can spread anywhere along the spinal column. Blood more commonly accumulates posterolaterally.
Subdural hemorrhage is capable of producing severe and irreversible neurologic deficits, and acute surgical intervention may be needed. Spinal subdural hematomas can have a typical configuration (Fig. 12–35).177–179 As opposed to epidural hematomas, which tend to be capped by fat, subdural hematomas are located within the thecal sac and are separate from the adjacent extradural fat, the vertebral bodies, and the posterior elements. Axial images are useful in defining the epidural fat surrounding the thecal sac and in defining the blood relating to the interior of the sac with subdural hematomas. These may be loculated anteriorly and posteriorly within the thecal sac. The loculation can take the form of a “Mercedes Benz” sign, showing a trefoil configuration (see Fig. 12–35B).
Key Points
1 Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology: Recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26:E93-E113.
This is “must reading” for standardization of this Tower of Babel.
2 Mehta RC, Marks MP, Hinks RS, et al. MR evaluation of vertebral metastases: T1-weighted, short-inversion-time inversion recovery, fast spin-echo, and inversion-recovery fast spin-echo sequences. AJNR Am J Neuroradiol. 1995;16:281-288.
3 Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: Assessment using MR. Radiology. 1985;157:157-166.
This classic definition of MRI changes still applies today.
4 Nabors MW, Pait TG, Byrd EB, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988;68:366-377.
A cogent classification of a confusing area is presented.
5 Russell EJ. Cervical disk disease. Radiology. 1990;177:313-325.
The author provides an excellent summary of a broad subject.
1 Hitselberger WE, Witten RM. Abnormal myelograms in asymptomatic patients. J Neurosurg. 1968;28:204-206.
2 Slone RM, McEnery KW, Bridwell KH, et al. Principles and imaging of spinal instrumentation. Radiol Clin North Am. 1995;33:189-211.
3 Hanley SD, Gun MT, Osti O, et al. Radiology of intervertebral cages in spinal surgery. Clin Radiol. 1999;54:201-206.
4 Bell GR, Modic MT. Radiology of the lumbar spine. In: Rothman RH, Simeone FA, editors. The Spine. 3rd ed. Philadelphia: WB Saunders; 1992:125-153.
5 Olsen NK, Madsen HH, Eriksen FB, et al. Intracranial iohexol-distribution following cervical myelography: Postmyelographic registration of adverse effects, psychometric assessment and electroencephalographic recording. Acta Neurol Scand. 1990;82:321-328.
6 Modic MT, Ross JS, Masaryk TJ. Imaging of degenerative disease of the cervical spine. Clin Orthop. 1989;239:109-120.
7 Fon GT, Sage MR. Computed tomography in cervical disc disease when myelography is unsatisfactory. Clin Radiol. 1984;35:47-50.
8 Coin CG. Cervical disk degeneration and herniation: Diagnosis by computerized tomography. South Med J. 1984;77:979-982.
9 Sobel DF, Barkovich AJ, Munderloh SH. Metrizamide myelography and postmyelographic computed tomography: Comparative adequacy in the cervical spine. AJNR Am J Neuroradiol. 1984;45:385-390.
10 Jahnke RW, Hart BL. Cervical stenosis, spondylosis and herniated disc disease. Radiol Clin North Am. 1991;29:777-791.
11 Landman JA, Hoffman JC, Braun IF, et al. Value of computed tomographic myelography in the recognition of cervical herniated disk. AJNR Am J Neuroradiol. 1984;5:391-394.
12 Simon JE, Lukin RR. Diskogenic disease of the cervical spine. Semin Roentgenol. 1988;23:118-124.
13 Vassilouthis J, Kalovithouris A, Papandreou A, et al. The symptomatic incompetent cervical intervertebral disk. Neurosurgery. 1989;25:232-239.
14 Nakagawa H, Okumura T, Sugiyama T, et al. Discrepancy between metrizamide CT and myelography in diagnosis of cervical disk protrusions. AJNR Am J Neuroradiol. 1983;4:604-606.
15 Dorwart RH, LaMasters DL. Applications of computed tomographic scanning of the cervical spine. Orthop Clin North Am. 1985;16:381-393.
16 Filippi M, Yousry T, Baratti C, et al. Quantitative assessment of MRI lesion load in multiple sclerosis: A comparison of conventional spin echo with fast fluid-attenuated inversion recovery. Brain. 1996;119:1349-1355.
17 Finelli DA, Hurst GC, Karaman B, et al. Use of magnetization transfer for improved contrast on gradient echo images of the cervical spine. Radiology. 1994;193:165-171.
18 Kidd D, Thorpe JW, Thompson AJ, et al. Spinal cord MRI using multi-array coils and fast spin echo: Findings in multiple sclerosis. Neurology. 1993;43:2632-2637.
19 Lyclama A, Nijeholt GJ, Barkof F, et al. Comparison of two MR sequences for the detection of multiple sclerosis lesions in the spinal cord. AJNR Am J Neuroradiol. 1996;17:1533-1538.
20 Ross JS, Ruggieri P, Tkach J, et al. Lumbar degenerative disk disease: Prospective comparison of conventional T2-weighted spin echo imaging and T2-weighted RARE. AJNR Am J Neuroradiol. 1993;14:1215-1223.
21 Rocca MA, Mastronardo G, Horsfield MA, et al. Comparison of three MR sequences for the detection of cervical cord lesions in patients with multiple sclerosis. AJNR Am J Neuroradiol. 1999;20:1710-1716.
22 Sze G, Merriam M, Oshio K, et al. Fast spin echo imaging in the evaluation of intradural disease of the spine. AJNR Am J Neuroradiol. 1992;13:1383-1392.
23 Tartaglino LM, Friedman DP, Flanders AE, et al. Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology. 1995;195:725-732.
24 Thorpe JW, Halpin SF, MacManus DG, et al. A comparison between fast and conventional spin echo in the detection of multiple sclerosis lesions. Neuroradiology. 1994;36:388-392.
25 Melhem ER, Caruthers SD, Jara H. Cervical spine: Three-dimensional MR imaging with magnetization transfer prepulsed turbo field echo techniques. Radiology. 1998;207:815-821.
26 Melhem ER, Benson ML, Beauchamp NJ, et al. Cervical spondylosis: Three dimensional gradient echo MR with magnetization transfer. AJNR Am J Neuroradiol. 1996;17:705-711.
27 Dwyer AJ, Frank JA, Sank VJ, et al. Short-tau inversion-recovery pulse sequence: Analysis and initial experience in cancer imaging. Radiology. 1988;168:827-836.
28 Mehta RC, Marks MP, Hinks RS, et al. MR evaluation of vertebral metastases: T1-weighted, short-inversion-time inversion recovery, fast spin-echo, and inversion-recovery fast spin-echo sequences. AJNR Am J Neuroradiol. 1995;16:281-288.
29 Weinberger E, Shaw DW, White KS, et al. Nontraumatic pediatric musculoskeletal MR imaging: Comparison of conventional and fast-spin-echo short inversion time inversion-recovery technique. Radiology. 1995;194:721-726.
30 Hilfiker P, Zanetti M, Debatin JF, et al. Fast spin-echo inversion-recovery imaging versus fast T2-weighted spin-echo imaging in bone marrow abnormalities. Invest Radiol. 1995;30:110-114.
31 Baker LL, Goodman SB, Perkash I, et al. Benign versus pathologic compression fractures of vertebral bodies: Assessment with conventional spin-echo, chemical-shift, and STIR MR imaging. Radiology. 1990;174:495-502.
32 Jones KM, Schwartz RB, Mantello MT, et al. Fast spin-echo MR in the detection of vertebral metastases: Comparison of three sequences. AJNR Am J Neuroradiol. 1994;15:401-407.
33 Enzmann DR, Rubin JB. Cervical spine: MR imaging with a partial flip angle, gradient-refocused pulse sequence: I. General considerations and disk disease. Radiology. 1988;166:467-472.
34 Enzmann DR, Rubin JB. Cervical spine: MR imaging with a partial flip angle, gradient-refocused pulse sequence: II. Spinal cord disease. Radiology. 1988;166:473-478.
35 Hedberg MC, Drayer BP, Flom RA, et al. Gradient echo (GRASS) MR imaging in cervical radiculopathy. AJR Am J Roentgenol. 1988;150:683-689.
36 Kulkarni MV, Narayana PA, McArdle CB, et al. Cervical spine MR imaging using multislice gradient echo imaging: Comparison with cardiac gated spin echo. Magn Reson Imaging. 1988;6:517-525.
37 Tsuruda JS, Norman D, Dillon W, et al. Three-dimensional gradient-recalled MR imaging as a screening tool for the diagnosis of cervical radiculopathy. AJNR Am J Neuroradiol. 1989;10:1263-1271.
38 Russell EJ. Cervical disk disease. Radiology. 1990;177:313-325.
39 Carlson J, Crooks L, Ortendahl D, et al. Signal-to-noise ratio and section thickness in two-dimensional versus three-dimensional Fourier transform MR imaging. Radiology. 1988;166:266-270.
40 Frahm J, Haase A, Matthaei D. Rapid three-dimensional MR imaging using the FLASH technique. J Comput Assist Tomogr. 1986;10:363-368.
41 Frazzini VI, Kegetsu NJ, Johnson CE, et al. Internally stabilized spine: Optimal choice of frequency-encoding gradient direction during MR imaging minimizes susceptibility artifact from titanium vertebral body screws. Radiology. 1997;204:268-272.
42 Choi IS, Berenstein A. Surgical neuroangiography of the spine and spinal cord. Radiol Clin North Am. 1988;26:1131-1141.
43 Di Chiro G, Wener L. Angiography of the spinal cord: A review of contemporary techniques and applications. J Neurosurg. 1973;39:1-29.
44 Nelson PK, Setton A, Berenstein A. Vertebrospinal angiography in the evaluation of vertebral and spinal cord disease. Neuroimaging Clin North Am. 1996;6:589-605.
45 Anson JA, Spetzler RF. Interventional neuroradiology for spinal pathology. Clin Neurosurg. 1992;39:388-417.
46 Kendall BE, Logue V. Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology. 1977;13:181-189.
47 Gilbertson JR, Miller GM, Goldman MS, et al. Spinal dural arteriovenous fistulas: MR and myelographic findings. AJNR Am J Neuroradiol. 1995;16:2049-2057.
48 Masaryk T, Ross JS, Modic MT, et al. Radiculomeningeal vascular malformations of the spine: MR imaging. Radiology. 1987;164:845-849.
49 Merland JJ, Riche MC, Chiras J. Intraspinal extramedullary arteriovenous fistulae draining into the medullary veins. J Neuroradiol. 1980;7:271-320.
50 Backes WH, Nijenhuis RJ. Advances in spinal cord MR angiography. AJNR Am J Neuroradiol. 2008;29:619.
51 Bowen BC, Fraser K, Kochan JP, et al. Spinal dural arteriovenous fistulas: Evaluation with MR angiography. AJNR Am J Neuroradiol. 1995;16:2029.
52 Mull M, Nijenhuis RJ, Backes WH, et al. Value and limitations of contrast-enhanced MR angiography in spinal arteriovenous malformations and dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2007;28:1249.
53 Lai PH, Weng MJ, Lee KW, et al. Multidetector CT angiography in diagnosing type I and IVA spinal vascular malformations. AJNR Am J Neuroradiol. 2006;27:813.
54 Yoshioka K, Niinuma H, Ohira A, et al. MR angiography and CT angiography of the artery of Adamkiewicz: Noninvasive preoperative assessment of thoracoabdominal aortic aneurysm. RadioGraphics. 2003;23:1215.
55 Guyer RD, Ohnmeiss DD. Lumbar discography. Position statement from the North American Spine Society Diagnostic and Therapeutic Committee. Spine. 1995;20:2048-2059.
56 Saal JS. General principles of diagnostic testing as related to painful lumbar spine disorders. Spine. 2002;27:2538-2545.
57 Bernard TNJr. Don’t discard discography. Radiology. 1987;162:285.
58 Milette P, Fontaine S, Lepanto L. Differentiating lumbar disc protrusions, disc bulges, and discs with normal contour but abnormal signal intensity: Magnetic resonance imaging with discographic correlations. Spine. 1999;24:44-53.
59 Bernard TNJ. Repeat lumbar spine surgery: Factors influencing outcome. Spine. 1993;18:2196-2200.
60 Bogduk N, Modic MT. Lumbar discography. Spine. 1996;21:402-404.
61 Holt EPJ. The question of lumbar discography. J Bone Joint Surg Am. 1968;50:720-726.
62 Modic MT. Diskography: Science and the ad hoc hypothesis. AJNR Am J Neuroradiol. 2000;21:241-242.
63 Mooney V. Lumbar discography. Spine. 1996;21:1479.
64 Simmons JW, Aprill CN, Dwyer AP, et al. A reassessment of Holt’s data on: “The question of lumbar discography.”. Clin Orthop. 1988;237:120-124.
65 Smith SE, Darden BV, Rhyne AL, et al. Outcome of unoperated discogram-positive low back pain. Spine. 1995;20:1997-2000.
66 Walsh T, Weinstein J, Spratt K, et al. Lumbar discography in normal subjects. J Bone Joint Surg Am. 1990;72:1081-1088.
67 Tehranzadeh J. Discography 2000. Radiol Clin North Am. 1998;36:463-495.
68 Bosacco SJ. Lumbar discography: Redefining its role with intradiscal therapy. Orthopedics. 1986;9:399-401.
69 Simmons JW, McMillin JN, Emery SF, et al. Intradiscal steroids: A prospective double-blind clinical trial. Spine. 1992;17(Suppl):S172-S175.
70 Smith MD, Kim SS. A herniated cervical disc resulting from discography: An unusual complication. J Spinal Disord. 1990;3:392-394.
71 Schreck RI, Manion WL, Kambin P, et al. Nucleus pulposus pulmonary embolism: A case report. Spine. 1995;20:2463-2466.
72 Zeidman SM, Thompson K, Ducker TB. Complications of cervical discography: Analysis of 4400 diagnostic disc injections. Neurosurgery. 1995;37:414-417.
73 The International Center for Nephrogenic Fibrosing Dermopathy Research. Available at http://www.icnfdr.org/ Accessed January 19, 2008
74 U.S. Food and Drug Administration. Available at http://www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm Accessed January 19, 2008
75 Institute for Magnetic Resonance Safety, Education and Research. Available at http://www.imrser.org/ Accessed January 19, 2008
76 Shellock FG, Spinazzi A. MRI safety update 2008: Part I. AJR Am J Roentgenol. 2008;191:1129.
77 Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: Risk factors and incidence estimation. Radiology. 2007;243:148.
78 Kuo PH, Kanal E, Abu-Alfa AK, et al. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647.
79 Broome DR, Girguis MS, Baron PW, et al. Gadodiamide-associated nephrogenic systemic fibrosis: Why radiologists should be concerned. AJR Am J Roentgenol. 2007;188:586.
80 Prince MR, Zhang H, Morris M, et al. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology. 2008;248:807.
81 Deyo RA, Bigos SJ, Maravilla KR. Diagnostic imaging procedures for the lumbar spine. Ann Intern Med. 1989;111:865-867.
82 Long DM. Decision making in lumbar disc disease. Clin Neurosurg. 1992;39:36-51.
83 Fager CA. Identification and management of radiculopathy. Neurosurg Clin North Am. 1993;4:1-12.
84 Bell GR, Rothman RH. The conservative treatment of sciatica. Spine. 1984;9:54-56.
85 Bozzao A, Gallucci M, Masciocchi C, et al. Lumbar disc herniation: MR imaging assessment of natural history in patients treated without surgery. Radiology. 1992;185:135-141.
86 Bush K, Cowan N, Katz DE, et al. The natural history of sciatica associated with disc pathology. Spine. 1992;17:1205-1212.
87 Cowan N, Bush K, Katz D, et al. The natural history of sciatica: A prospective radiological study. Clin Radiol. 1992;46:7-12.
88 Delauche-Cavallier MC, Budet C, Laredo JD, et al. Lumbar disc herniation. Spine. 1992;17:927-933.
89 Saal JA, Saal JS. Nonoperative treatment of lumbar intervertebral disc with radiculopathy: An outcome study. Spine. 1989;14:431-437.
90 Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: Assessment of changes in vertebral body marrow with MRI. Radiology. 1988;166:193-199.
91 Ross JS, Modic MT, Masaryk TJ. Tears of the annulus fibrosus: Assessment with Gd-DTPA-enhanced MR imaging. AJNR Am J Neuroradiol. 1989;10:1251-1254.
92 Yu S, Sether LA, Ho SP, et al. Tears of the annulus fibrosus: Correlation between MR and pathologic findings in cadavers. AJNR Am J Neuroradiol. 1988;9:367-370.
93 Fardon DF, Milette PC, Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined Task Forces of the North American Spine Society, American Society of Spine Radiology and American Society of Neuroradiology. Spine. 2001;26:E93-E113.
94 Amunosen T, Weber H, Lilleas F, et al. Lumbar spinal stenosis: Clinical and radiologic features. Spine. 1995;20:1178-1186.
95 Silbergleit R, Gebarski SS, Brungerg JA, et al. Lumbar synovial cysts: Correlation of myelographic, CT, MR and pathologic findings. AJNR Am J Neuroradiol. 1990;11:777-779.
96 Ulmer JL, Elster AD, Mathews VP, et al. Distinction between degenerative and isthmic spondylolisthesis on sagittal MR images: Importance of increased anteroposterior diameter of the spinal canal (“wide canal sign”). AJR Am J Roentgenol. 1994;163:411.
97 Jinkins JR, Matthes JC, Sener RN, et al. Spondylolysis, spondylolisthesis, and associated nerve root entrapment in the lumbosacral spine: MR evaluation. AJR Am J Roentgenol. 1992;159:799.
98 Pavlov H, Torg JS, Robie B, et al. Cervical spinal stenosis: Determination with vertebral body ratio method. Radiology. 1987;164:771-775.
99 Takahashi M, Yamashita Y, Sakamoto Y, et al. Chronic cervical cord compression: Clinical significance of increased signal intensity on MR images. Radiology. 1989;173:219-224.
100 Epstein NE. Ossification of the posterior longitudinal ligament: Diagnosis and surgical management. Neurosurg Q. 1992;2:223-241.
101 Hirabayashi K, Satomi K. Operative procedure and results of expansive open-door laminoplasty. Spine. 1988;13:870-876.
102 Hueftle MG, Modic MT, Ross JS, et al. Lumbar spine: Postoperative MR imaging with Gd-DTPA. Radiology. 1988;167:817-824.
103 Georgy BA, Hesselink JR, Middleton MS. Fat suppression contrast-enhanced MRI in the failed back surgery syndrome: A prospective study. Neuroradiology. 1995;37:51-57.
104 Burton CV. Causes of failure of surgery on the lumbar spine: Ten-year follow-up. Mt Sinai J Med. 1991;58:183-187.
105 Djukic S, Genant HK, Helms CA, et al. Magnetic resonance imaging of the postoperative lumbar spine. Radiol Clin North Am. 1990;28:341-360.
106 Johnson CE, Sze G. Benign lumbar arachnoiditis: MR imaging with gadopentetate dimeglumine. AJR Am J Roentgenol. 1990;155:873-880.
107 Ross JS, Masaryk TJ, Modic MT, et al. MR imaging of lumbar arachnoiditis. AJNR Am J Neuroradiol. 1987;8:885-892.
108 Lisbona R, Rosenthal L. Observations on the sequential use of Tc-99m phosphate complex and Ga-67 imaging in osteomyelitis, cellulitis, and septic arthritis. Radiology. 1977;123:123-129.
109 Gelman MI, Coleman RE, Stevens PM, et al. Radiography, radionuclide imaging, and arthrography in the evaluation of total hip and knee replacement. Radiology. 1978;128:677-682.
110 Weiss PPE, Mall JC, Hoffer PB, et al. Tc-99m methylene diphosphonate bone imaging in the evaluation of total hip prosthesis. Radiology. 1979;133:727-729.
111 McAfee JG, Samin A. In-111 labeled leukocytes: A review of problems in image interpretation. Radiology. 1985;155:221-229.
112 Golimbu C, Firooznia H, Rafii M. CT of osteomyelitis of the spine. AJR Am J Roentgenol. 1984;142:159-163.
113 Jeffrey RB, Callen PW, Federle MP. Computed tomography of psoas abscesses. J Comput Assist Tomogr. 1980;4:639-641.
114 Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157-166.
115 Modic MT, Weinstein MA, Pavlicek W, et al. Nuclear magnetic resonance imaging of the spine. Radiology. 1983;148:757-762.
116 deRoos A, Van Meerten EL, Bloem JL, et al. MRI of tuberculosis spondylitis. AJR Am J Roentgenol. 1986;146:79-82.
117 Dagirmanjian A, Schils J, Modic MT. Vertebral osteomyelitis revisited. Radiology. 1993;189(Suppl P):193.
118 Boden SD, Davis DO, Dina TS, et al. Postoperative diskitis: Distinguishing early MR imaging findings from normal postoperative disk space changes. Radiology. 1992;184:765-771.
119 Rankin RM, Flothow PG. Pyogenic infection of the spinal epidural space. West J Surg Obstet Gynecol. 1946;54:320-323.
120 Baker AS, Ojemann RG, Swartz MN, et al. Spinal epidural abscess. N Engl J Med. 1975;293:463-468.
121 Browder J, Meyers R. Pyogenic infections of the spinal epidural space. Surgery. 1941;10:296-308.
122 Angtuaco EJC, McConnell JR, Chadduck WM, et al. MR imaging of spinal epidural sepsis. AJNR Am J Neuroradiol. 1987;8:879-883.
123 Tins BJ, Cassar-Pullicino VN, Lalam RK. Magnetic resonance imaging of spinal infection. Top Magn Reson Imaging. 2007;18:213-222.
124 Post MJD, Quencer RM, Montalvo BM, et al. Spinal infection: Evaluation with MR imaging and intraoperative US. Radiology. 1988;169:765-771.
125 Post MJD, Sze G, Quencer RM, et al. Gadolinium-enhanced MR in spinal infection. J Comput Assist Tomogr. 1990;14:721-729.
126 Rawlings CE, Giangaspero F, Burger PC, et al. Ependymomas: A clinicopathologic study. Surg Neurol. 1988;29:271.
127 Kahan H, Sklar EM, Post MJ, et al. MR characteristics of histopathologic subtypes of spinal ependymoma. AJNR Am J Neuroradiol. 1996;17:143-150.
128 Wippold FJII, Smirniotopoulos JG, Moran CJ, et al. MR Imaging of myxopapillary ependymoma: Findings and value of determining extent of tumor and its relation to intraspinal structures. AJR Am J Roentgenol. 1995;165:1263-1267.
129 Nemoto Y, Inove Y, Tashiro T, et al. Intramedullary spinal cord tumors: Significance of associated hemorrhage at MR imaging. Radiology. 1992;82:793.
130 Shen W-C, Ho Y-J, Lee S-K, et al. Ependymoma of the cauda equina presenting with subarachnoid hemorrhage. AJNR Am J Neuroradiol. 1993;14:399.
131 Choyke PL, Glenn GM, Walther MM, et al. von Hippel-Lindau disease: Genetic, clinical, and imaging features. Radiology. 1995;194:629-642.
132 Ho VB, Smirniotopoulos JG, Murphy FM, et al. Radiologic-pathologic correlation: Hemangioblastoma. AJNR Am J Neuroradiol. 1992;13:1343-1352.
133 Hoff DJ, Tampieri D, Just N. Imaging of spinal cord hemangioblastomas. Can Assoc Radiol J. 1993;44:377-383.
134 Murota T, Symon L. Surgical management of hemangioblastoma of the spinal cord: A report of 18 cases. Neurosurgery. 1989;25:699-707.
135 Sze G, Krol G, Zimmerman RD, et al. Intramedullary disease of the spine: Diagnosis using gadolinium-DTPA-enhanced MR imaging. AJR Am J Roentgenol. 1988;151:1193-1204.
136 Yu JS, Short MP, Schumacher J, et al. Intramedullary hemorrhage in spinal cord hemangioblastoma: Report of two cases. J Neurosurg. 1994;81:937-940.
137 Tognetti F, Lanzino G, Calbucci F. Metastases of the spinal cord from remote neoplasms: Study of five cases. Surg Neurol. 1988;30:220-227.
138 Winkelman MD, Adelstein DJ, Karlins NL. Intramedullary spinal cord metastasis: Diagnostic and therapeutic considerations. Arch Neurol. 1987;44:526-531.
139 Hamilton MG, Tranmer BI, Hagen NA. Supratentorial glioblastoma with spinal cord intramedullary metastasis. Can J Neurol Sci. 1993;20:65-68.
140 DeAngelis LM. Current diagnosis and treatment of leptomeningeal metastasis. J Neurooncol. 1998;38:245-252.
141 McCormick PC, Post KD, Stein BM. Intradural extramedullary tumors in adults. Neurosurg Clin North Am. 1990;1:591.
142 Halliday AL, Sobel RA, Martuza RL. Benign spinal nerve sheath tumors: Their occurrence sporadically and in neurofibromatosis types 1 and 2. J Neurosurg. 1991;74:248.
143 Edelhoff JC, Bates DJ, Ross JS, et al. Spinal MR findings in neurofibromatosis type 1 and 2. AJNR Am J Neuroradiol. 1992;13:1071.
144 Levy WJ, Bay J, Donn D. Spinal cord meningioma. J Neurosurg. 1982;57:804.
145 Roux FX, Nataf F, Pinaudeau M, et al. Intraspinal meningiomas: Review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996;46:458-463.
146 Sevick RJ. Cervical spine tumors. Neuroimaging Clin North Am. 1995;5:385-400.
147 Yousem DM, Patrone PM, Grossman RI. Leptomeningeal metastases: MR evaluation. J Comput Assist Tomogr. 1990;14:255.
148 Sze G, Abramson A, Krol G, et al. Gadolinium-DTPA: Malignant extradural spinal tumors. Radiology. 1988;67:217.
149 Smolen WR, Godersky JC, Knutzon RK, et al. The role of MR imaging in evaluating metastatic spinal disease. AJNR Am J Neuroradiol. 1987;8:901.
150 Carmody RF, Yang PJ, Seeley GW, et al. Spinal cord compression due to metastatic disease: Diagnosis with MR imaging versus myelography. Radiology. 1989;173:225.
151 Nabors MW, Pait TG, Byrd EB, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988;68:366-377.
152 Kronborg O. Extradural spinal cysts: A literature survey and a case of multiple extradural cysts. Dan Med Bull. 1967;14:46-48.
153 Rothman RH, Jacobs SR, Appleman W. Spinal extradural cysts: A report of five cases. Clin Orthop. 1970;71:186-192.
154 Hoffman J, Schriger D, Mower W, et al. Low risk criteria for cervical spine radiography in blunt trauma: A prospective study. Ann Emerg Med. 1992;21:1454.
155 Roberge R, Wears R, Kelly M. Selective application of cervical spine radiography in alert victims of blunt trauma: A prospective study. J Trauma. 1988;28:784.
156 Cornelius RS, Leach JL. Imaging evaluation of cervical spine trauma. Neuroimaging Clin North Am. 1995;5:451-463.
157 Schwartz ED, Flanders AE, editors. Spinal Trauma: Imaging, Diagnosis, and Management. Philadelphia: Lippincott Williams & Wilkins, 2007.
158 Kaye J, Nance E. Cervical spine trauma. Orthop Clin North Am. 1990;21:449.
159 Schleehauf K, Ross S, Civil I, et al. Computed tomography in the initial evaluation of the cervical spine. Ann Emerg Med. 1989;18:815.
160 Kirshenbaum K, Nadimpalli S, Fantus R, et al. Unsuspected upper cervical spine fractures associated with significant head trauma: Role of CT. J Emerg Med. 1990;8:183.
161 Lindsey R, Diliberti T, Doherty B, et al. Efficacy of radiographic evaluation of the cervical spine in emergency situations. South Med J. 1993;86:1253.
162 Schaefer D, Flanders A, Osterholm J, et al. Prognostic significance of magnetic resonance imaging in the acute phase of cervical spine injury. J Neurosurg. 1992;76:218.
163 Flanders AE, Spettell CM, Tartaglino LM, et al. Forecasting motor recovery after cervical spinal cord injury: Value of MR imaging. Radiology. 1996;201:649-655.
164 Kulkarni M, Bondurant F, Rose S, et al. 1.5T Magnetic resonance imaging of acute spinal trauma. RadioGraphics. 1988;8:1059.
165 Davis S, Teresi L, Bradley W, et al. Cervical spine hyperextension injuries: MR findings. Radiology. 1991;180:245.
166 Hall A, Wagle V, Raycroft J, et al. Magnetic resonance imaging in cervical spine trauma. J Trauma. 1993;34:21.
167 Silberstein M, Tress B, Hennessy O. Prevertebral swelling in cervical spine injury: Identification of ligament injury with magnetic resonance imaging. Clin Radiol. 1992;46:318.
168 Roab R. International classification of spine injuries. Paraplegia. 1972;10:78.
169 Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am. 1970;52:1534.
170 Tarr RW, Drolshagen LF, Kerner TC, et al. MR imaging of recent spinal trauma. J Comput Assist Tomogr. 1987;11:412.
171 Chance GQ. Note on a type of flexion fracture of the spine. Br J Radiol. 1948;21:452.
172 Smith WS, Kaufer H. Patterns and mechanisms of lumbar injuries associated with lap seat belts. J Bone Joint Surg Am. 1969;52:239.
173 Foo D, Rossier AB. Preoperative neurological status in predicting surgical outcome of spinal epidural hematomas. Surg Neurol. 1981;15:389-401.
174 Beatty RM, Winston KR. Spontaneous epidural hematoma. J Neurosurg. 1984;61:143-148.
175 Avrahami E, Tadmor R, Ram Z, et al. MR demonstration of spontaneous acute epidural hematoma of the thoracic spine. Neuroradiology. 1989;31:90-92.
176 Goldman P, Kulkarni M, MacDugall DJ, et al. Traumatic epidural hematoma of the cervical spine: Diagnosis with magnetic resonance imaging. Radiology. 1989;170:589-591.
177 Donovan-Post MJ, Becerra JL, Madsen PW, et al. Acute spinal subdural hematoma: MR and CT findings with pathologic correlates. AJNR Am J Neuroradiol. 1994;15:1895-1905.
178 Johnson PJ, Hahn F, McConnell J, et al. The importance of MRI findings for the diagnosis of nontraumatic lumbar subacute subdural haematomas. Acta Neurochir. 1991;113:186-188.
179 Levy JM. Spontaneous lumbar subdural hematoma. AJNR Am J Neuroradiol. 1990;11:780-781.