CHAPTER 62 Spinal Stenosis
Pathophysiology, Clinical Diagnosis, and Differential Diagnosis
Spinal stenosis is one of the most common conditions in the elderly. It is defined as a narrowing of the spinal canal. The term stenosis is derived from the Greek word for narrow, which is “stenos.” The first description of this condition is attributed to Antoine Portal in 1803. Verbiest1–10 is credited with coining the term spinal stenosis and the associated narrowing of the spinal canal as its potential cause. Kirkaldy-Willis11–15 subsequently described the degenerative cascade in the lumbar spine as the cause for the altered anatomy and pathophysiology in spinal stenosis.
The term spinal stenosis refers to an anatomic diagnosis that increases with age and can occur in asymptomatic individuals.16,17 The exact reason for why some with this condition have debilitating symptoms while others have no symptoms is not well understood. These differences in presentation may be related to the different abilities of individuals to compensate for the anatomic changes that have occurred. When symptoms do present, they usually occur on the basis of the location of neural compression. Patients with central canal stenosis typically present with neurogenic claudication, whereas those with lateral recess and foraminal stenosis present with radicular pain. Patients with significant symptoms that do not respond to conservative treatment often elect surgical treatment. In fact, in adults older than 65, spinal stenosis is the most common reason to undergo lumbar spine surgery.18,19
Anatomy
In order to understand how spinal stenosis causes symptoms, we must first have a good understanding of the normal anatomy of the lumbar spine. The spinal canal’s anterior border is formed by the vertebral body, the disc, and the posterior longitudinal ligament. The lateral border is formed by the pedicles, the lateral ligamentum flavum, and the neural foramen. The posterior border is formed by the facet joints, lamina, and ligamentum flavum. The shape of the spinal canal may be circular, oval, or trefoil (Fig. 62–1). The circular and oval canal shapes provide the most space for the neural elements centrally and in the lateral recess. The trefoil canal has the smallest cross-sectional area.20 It is present in 15% of the individuals and predisposes these individuals to lateral recess stenosis.
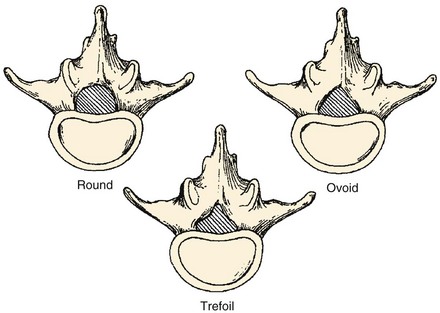
FIGURE 62–1 The three typical shapes of the spinal canal: Trefoil canals have the smallest cross-sectional area.
(From Hilibrand AS, Rand N: Degenerative lumbar stenosis: Diagnosis and management. J Am Assoc Orthop Surg 7:239-248, 1999.)
Disc
The Kirkaldy-Willis21 theory explains how these changes progress over time. This theory is based on viewing the spine as a tripod with the disc and the two facet joints making up the three legs. This analogy makes it easier to understand how alteration in one joint can alter the others. The initial stage in the degenerative cascade is circumferential tearing of the annulus, which progresses to radial tears. This, along with the biochemical changes in the disc described previously, leads to further degeneration of the disc and disc height loss. Altered disc structure and disc height loss lead to bulging of the disc and the posterior longitudinal ligament. This causes narrowing of the spinal canal and potential neural impingement. The lost disc height also leads to buckling of the ligamentum flavum and settling of the facet joints. The facet joints subsequently deteriorate and form osteophytes, which further narrows the spinal canal. The altered structure, motion, and biomechanics then lead to additional disc deterioration, which propagates the cycle of degeneration.
Intervertebral Foramen
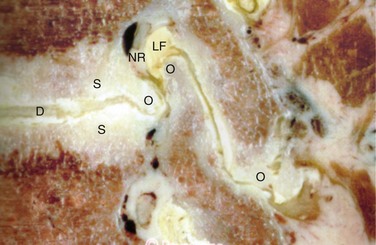
The anterior boundary of the intervertebral foramen is made up of the posterior wall of the vertebral body and the disc. The posterior boundary is made up of the lateral aspect of the facet joint and the ligamentum flavum. Superior and inferior boundaries are formed by the pedicles of the vertebral bodies corresponding to that segment. The foramen is typically larger than the ganglion and the nerve that it contains. The additional space is occupied by fat and loose areolar tissue that can accommodate for motion. With degeneration, hypertrophy of the facets can cause posterior compression of the neural elements (Fig. 62–2). Anterior compression of the neural elements usually arises from endplate osteophytes or foraminal disc herniations. Decrease in disc height with degeneration can cause a decrease in the foraminal height and neural compression. This type of vertical or up-down foraminal stenosis is important to recognize because a posterior decompression alone may not significantly improve the vertical compression and may result in persistent symptoms after surgery.
Cauda Equina
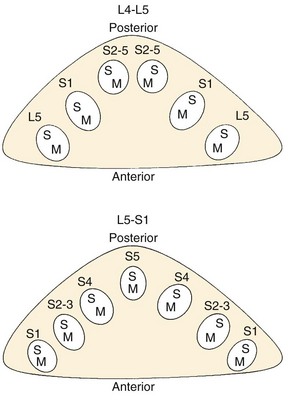
The thecal sac lies in the spinal canal and gives rise to nerve roots at each segment. The nerve root initially courses along the medial aspect of the pedicle and then progresses laterally, inferior to the pedicle in the neural foramen. The nerve roots within the cauda equina are arranged in a predictable pattern within the thecal sac (Fig. 62–3). Cross section of the thecal sac demonstrates the most caudal roots to be present in a central and posterior position. The more cephalad roots are located sequentially more lateral and anterior. At each level, the motor fibers of a root are anterior and medial to the larger sensory component. Dorsal root ganglia exist at every level and can be intraspinal or intraforaminal. A variety of clinical presentations arise on the basis of the anatomic location of neural compression.
Classification
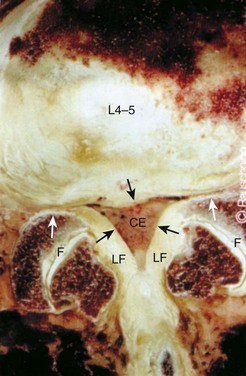
Stenosis can be anatomically classified as central, lateral recess, and foraminal on the basis of the location of neural compression. With aging, central canal stenosis occurs as degenerative changes progress. As the axial height of the disc and facet joints decreases, the disc bulges into the spinal canal. The central canal is further narrowed by posterior impingement from enlarged facets and the hypertrophied ligamentum flavum (Fig. 62–4). Hypertrophy of the soft tissues is responsible for 40% of spinal stenosis.22 With extension, the hypertrophied ligamentum buckles centrally into the canal and worsens the central stenosis. This explains why patients with stenosis typically report worsening of their symptoms in extension.
Lateral recess stenosis typically results from posterior disc protrusion in combination with some superior articular facet hypertrophy. Lateral recess stenosis can present with lumbar radiculopathy, and incidence of lateral recess stenosis ranges from 8% to 11%.23–25 These patients present with pain or neurologic symptoms in a dermatomal distribution on the basis of the nerve that is compressed in the lateral recess.
Foraminal stenosis causes compression of the exiting nerve root and ganglion and also leads to lumbar radiculopathy. Foraminal stenosis occurs most commonly in the lower lumbar spine with the fifth lumbar nerve root being the most commonly involved. Foraminal stenosis can occur from loss of disc height, vertebral endplate osteophytes, facet osteophytes, spondylolisthesis, and disc herniations. Like central canal stenosis, foraminal stenosis is worse in extension and thus exacerbating and alleviating factors for symptoms from foraminal compression are similar to those from central canal stenosis.26
Spinal stenosis can also be classified on the basis of the etiology, which can be congenital, acquired, or both. Congenital stenosis is present as a normal variant in the population and is part of certain conditions such as dwarfism. In these conditions, patients have short pedicles that are closer together than the normal lumbar spine. In congenital stenosis, few degenerative changes are sufficient to cause neural compression and symptoms. As one would expect, congenital stenosis becomes symptomatic much earlier in life and patients usually become symptomatic in the fourth decade. Acquired stenosis can be caused by trauma, neoplasms, and infection, along with other causes listed in Box 62–1.
Deformity and Instability
The static changes that we discussed thus far can be worsened by dynamic factors such as segmental instability. Instability typically arises from degenerative changes and can be in the form of translational or rotational abnormality. Translational abnormality is found most commonly in women as a degenerative anterolisthesis of L4 on L5.27 The attachment of the iliolumbar ligaments to the L5 level may act as a restraining force and cause more relative motion at L4-5. The more sagittally oriented facet joints between the fourth and fifth lumbar vertebrae can be an additional predisposing factor for instability at this level.28 Because the lamina and the spinous process typically project inferior to the vertebral body, the amount of room available between the inferior aspect of the L4 lamina and the posterior superior aspect of L5 is substantially decreased. This anterior translation of the L4 posterior elements, along with hypertrophy of the facets and the ligamentum flavum, leads to central and lateral recess stenosis. Foraminal stenosis can also occur in this setting with collapse of the disc space, disc herniation, endplate osteophytes, or facet hypertrophy. With scoliosis, lateral subluxation and rotational instability can cause altered biomechanics that leads to degeneration. The altered anatomy can also be a cause of narrowing of the central canal, lateral recess, and foraminal regions. Degenerative changes superimposed on abnormal anatomy lead to stenosis in these patients.
Pathophysiology
The term spinal stenosis describes the anatomic narrowing of the spinal canal. How does spinal stenosis result in pain and altered neurologic function? A number of cadaver and animal studies have attempted to elucidate the mechanism of these symptoms. Schonstorm evaluated the changes in nerve pressure that occur as the spinal canal narrows.29 In his human cadaver study, the thecal sac constriction of 45% or more led to an increased pressure in the nerve roots. As the degree of compression increased, the pressure in the nerve roots increased. Delamarter and colleagues30 also demonstrated the importance of the magnitude of thecal sac compression in alteration of neural function. They noted no alteration in neurologic function when the animal’s cauda equina was constricted by 25%, whereas more than 50% compression led to motor or sensory deficits. Pedowitz and colleagues31 demonstrated that the duration of compression was also an important factor in neural dysfunction.
Rydevik and colleagues32–37 demonstrated another effect of compression of the thecal sac. They noted that once pressure of more than 50 mm Hg was achieved, capillary restriction and electrophysiologic alteration occurred in the nerve roots. Even at pressures as low as 5 to 10 mm Hg, venous congestion of the intraneural microcirculation occurred. Solute transport decreased 45% across nerve root segments with the low pressure of 10 mm Hg. This suggests that low-grade sustained compression of the nerve roots could lead to vascular impairment and potential detrimental changes in the function of the nerve roots. In addition to neural compression and altered nutrition, inflammatory chemical mediators have also been shown to be a cause of pain.38–43
Natural History
Patients with congenital stenosis typically become symptomatic earlier in life. Due to congenital narrowing of the canal in these patients, significant stenosis is present at multiple levels even with little degenerative change.44 Patients with degenerative stenosis present later in life during their 60s and have far more advanced degeneration in their spine. Females are more commonly affected with stenosis and the L4-5 level is the most common segment involved.45
Multiple studies have looked at the short-term and long-term results of nonoperative treatment of patients with lumbar stenosis.46–53 These studies show that a significant number of patients respond favorably to nonoperative treatment. Some patients, however, do not improve and some even worsen. Johnsson and colleagues reviewed the results of 32 patients who declined to have surgery at a 4-year follow-up period.54 They noted that 70% of patients were unchanged, while 15% were the same and 15% worsened.
Recently, prospective studies have reported short-term and long-term results of nonoperative and operative treatment. The Maine Lumbar Spine prospective observational study reported 8- to 10-year follow-up results on 97 patients.55 They noted that a large number of patients (39%) that had initially elected nonsurgical treatment subsequently elected to undergo surgery. Of the patients who continued nonoperative treatment, most had stable symptoms. Miyamoto and colleagues reported prospective results of nonsurgical treatment in 120 patients.56 Sixteen percent of the patients required surgical treatment during the follow-up period of 5 years. Of the nonsurgically treated patients, 53% of patients reported no hindrance during the activities of daily living. No sudden neurologic deterioration was reported. Roughly 23% of patients had worsened but did not elect to undergo surgical intervention.
The Finnish Lumbar Spinal Research Group reported the results of a randomized controlled trial in 2007.57 They randomized 94 patients with mild to moderate stenosis into either the surgical group or nonoperative group. At the 2-year follow-up, patients noted improvement of symptoms in both groups; however, the outcome of patients undergoing surgical treatment was significantly better. The most recent prospective study to evaluate patients with lumbar stenosis is the SPORT study.58 This study reported prospective outcomes of 634 patients at 2-year follow-up. Patients undergoing surgical treatment had better outcomes than those who underwent nonsurgical treatment. Patients in the nonsurgical treatment group showed small improvements in most outcome measures. It should be noted that no disastrous neurologic deterioration was noted with nonoperative treatment.
The recently published North American Spine Society evidence-based guidelines for diagnosis and treatment of lumbar stenosis provide some tangible conclusions from these studies.59 They state that in one third to one half of patients with mild to moderate stenosis, the natural history is favorable. Unfortunately, predicting which patients with stenosis will worsen over time is impossible. In some studies symptomatic patients with severe stenosis did poorly over time, but overall there is not sufficient evidence to draw any conclusions. What is known is that rapid or catastrophic deterioration is rare in patients with spinal stenosis. Knowing this can be helpful in guiding treatment and evaluating these patients. When a patient with spinal stenosis has rapidly worsening neurologic status, other causes of neurologic dysfunction should be investigated.
Clinical Presentation
Patients with lumbar spinal stenosis most commonly present with leg pain.22 This leg pain presents as either neurogenic claudication or radicular leg pain. Patients with neurogenic claudication report a feeling of pain, heaviness, numbness, cramping, burning, or weakness. The symptoms typically start from the back or the buttocks and bilaterally radiate down below the knees. One lower extremity may be worse than the other; however, both legs are typically involved. Symptoms usually do not follow a dermatomal pattern and are usually related to activities. These abnormal sensations are typically worse with extension of the lumbar spine during walking or standing for a prolonged time. Some report worsening weakness if they keep walking. They may note ankle dorsiflexion weakness that is typically described as feet slapping or even falling as they attempt to keep walking. Walking downhill is more challenging for these patients as the lumbar spine is extended while going downhill. Most describe a set distance they can walk before the symptoms become disabling. As the stenosis worsens, this distance typically decreases, further disrupting the daily life and function of these patients. Relief of symptoms typically comes from flexing the lumbar spine by leaning forward, sitting, or lying down. As discussed earlier, the degree of stenosis decreases as the lumbar spine is flexed and patients naturally learn to position themselves in a posture that minimizes discomfort and maximizes function. Keeping this in mind, it is easy to understand why these patients typically lean forward on a grocery cart and have an easier time riding a bike, walking uphill, or driving while sitting in a car.
Low back pain is also a common complaint in patients with stenosis. Although most patients note the radiation of this pain into their legs, some present without leg pain or note radiation of the pain only into their buttocks. Exacerbating and alleviating factors for claudicatory low back pain are similar to those for the leg pain. Spondylotic change with or without spondylolisthesis is a common finding in this patient population and often the cause for low back pain. Patients with symptoms in both the low back and leg have a greater disability than those who have symptoms only in one location.60
Severe neurologic symptoms such as bowel and bladder incontinence or profound weakness are uncommon in patients with stenosis. Urinary dysfunction is a common complaint in this elderly population and can be present in 50% to 80% of patients.61,62 Because various causes of urinary dysfunction such as preexisting stress incontinence, urinary tract infections, and prostatic hypertrophy are common in this population, a careful history can help exclude these common causes. The factors more commonly noted in patients with neurogenic bladder dysfunction are perianal sensory disturbance, longer duration of symptoms, and higher mean residual volumes on urodynamic studies.63
In addition to obtaining a history specific to the patient’s pain and neurologic symptoms, it is important to obtain a comprehensive medical history. A patient’s report of preexisting peripheral arterial occlusive disease, hip arthritis, multiple sclerosis, or neuropathy would substantially alter what symptoms are attributed to the stenosis. Similarly, obtaining an overall picture of the medical comorbidities and physiologic condition will also shed light on the ability of the patient to safely undergo any invasive procedures. Knowing the patient’s other medical conditions can also help identify patients who may be at risk for inferior outcomes. Cardiovascular comorbidities, depression, and disorders influencing walking ability have all been noted to be preoperative predictors of poor postoperative outcomes.64
Physical Examination
A good physical examination of patients with lumbar spinal stenosis should start with observation. Often these patients will be sitting flexed forward on a chair in the examination room. While standing and ambulating, stenosis patients still often flex their trunk forward to decrease their symptoms. This may also be noticed when checking their range of motion as a decrease in the active lumbar extension. Reproduction of the patient’s usual symptoms by prolonged lumbar extension can also be helpful in confirming the diagnosis. Neurologic examination is often normal in spite of long-standing debilitating symptoms. Lateral recess stenosis is more commonly responsible for neurologic changes.65 When motor weakness or sensory deficit is present, it is most often in the L5 distribution. A frequent neurologic finding is an asymmetrical deep tendon reflex at the patellar or Achilles tendon. A symmetric decrease in the reflexes is more indicative of age-related changes. Nerve root tension signs are usually not present. Changes in neurologic examination may become more obvious after stressing the patient’s neurologic system. This can be accomplished by asking the patient to walk until he or she experiences significant symptoms. Re-examination at this point may reveal changes in motor, sensory, or reflex examination that were not detected before the stress.
It is useful to review physical examination findings found in some studies to get a better idea of their frequency. Amundsen and colleagues66 prospectively evaluated the clinical and radiographic features of 100 patients with symptomatic spinal stenosis. They reported a motor weakness in 23% and sensory deficit in 51%. In the 2007 randomized controlled trial of 94 stenosis patients from the Finnish Lumbar Spinal Research Group, 22% of patients had an L5 motor weakness and 19% had a sensory deficit. Straight leg raise test was positive in 3% of the patients. In the recent SPORT study, asymmetric reflexes were noted in 26%, motor weakness was noted in 28%, and sensory deficit was noted in 29%.
Diagnostic Studies
Radiography
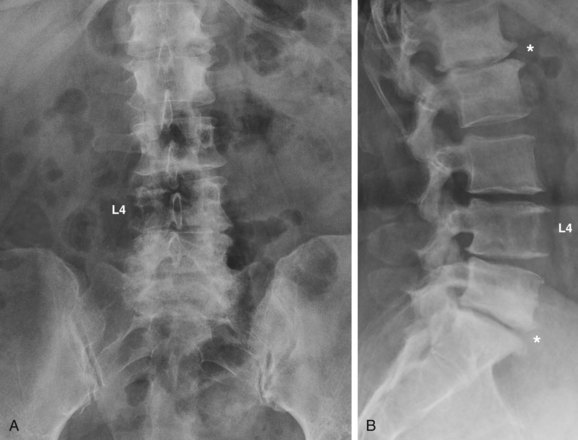
The diagnostic testing of patients with spinal stenosis often starts with plain radiographs. In addition to the anteroposterior and lateral radiographs, flexion and extension lateral views should be considered. Most patients suspected of stenosis are elderly and thus likely demonstrate a variety of spondylotic changes on the radiographs. Particular attention should be paid to diagnosing scoliosis and spondylolisthesis in addition to any dynamic instability that can be detected on the flexion-extension views. If scoliosis is noted, long cassette scoliosis films would be helpful in evaluating the full extent of the deformity in both the coronal and sagittal planes. Narrowing of the neural foramen and inferred narrowing of the spinal canal from the location and extent of degenerated structures should be evaluated. Ossification of ligamentous structures, ankylosis of the spine, erosion of the disc space, or any abnormal appearance of the bony structures should be assessed (Fig. 62–5). It should be kept in mind that even severe degenerative changes can be seen in asymptomatic patients.67
Computed Tomography with and Without Myelography
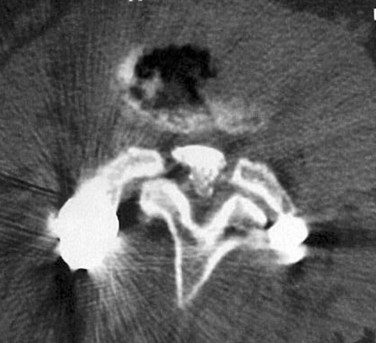
Prior to the common availability of magnetic resonance imaging (MRI), a computed tomography (CT) scan was the study of choice for visualizing pathologic anatomy in the axial plane. Because a significant portion of the stenosis comes from soft tissue pathology, visualization of the soft tissues is the top priority in axial imaging. A CT scan is a poor modality for detailed analysis of the soft tissue pathology (Fig. 62–6). A meta-analysis demonstrated that the sensitivity of a CT scan in detecting spinal stenosis ranges from 70% to 100%.68
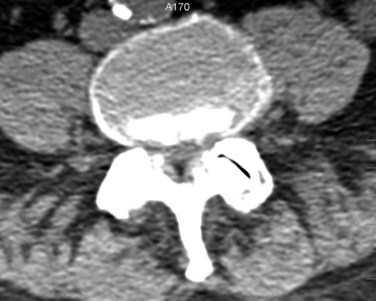
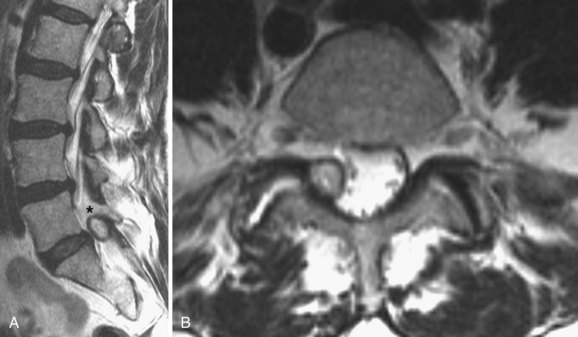
FIGURE 62–6 Soft tissue window of a computed tomography scan through the L4-5 level demonstrating stenosis. Please note the facet and ligamentum hypertrophy better seen on the magnetic resonance imaging of this patient in Figures 62-9 and 62-10.
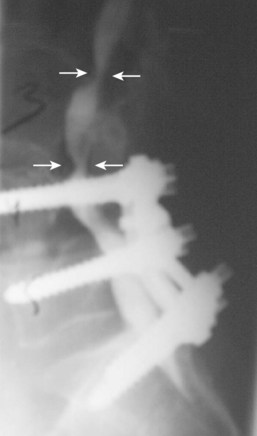
Diagnostic utility of the CT scan can be improved by combining it with myelography.69,70 The dye injected in the cerebrospinal fluid (CSF) during a myelogram provides good contrast between the thecal sac and the surrounding soft tissue and bony pathology. Preoperative complete contrast block on a CT-myelogram has been correlated with an improved surgical outcome.71 The invasiveness of the myelogram and the radiation associated with the CT are the two biggest drawbacks of this diagnostic modality. Given these limitations, patients who are unable to have an MRI, who have scoliosis, or who have previous spinal instrumentation are the most likely to undergo this study (Figs. 62-7 and 62-8). In cases where MRI findings are unclear, a CT or a CT-myelogram should be considered to gain further information about the pathology.
Magnetic Resonance Imaging
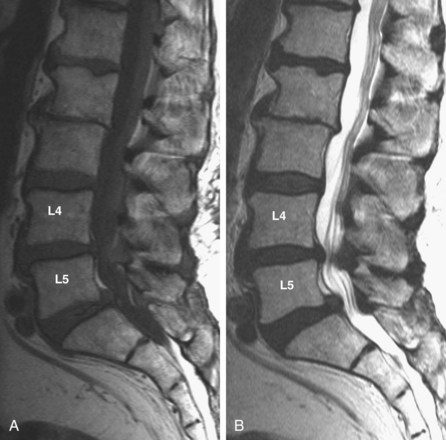
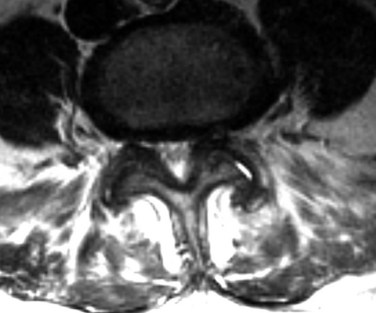
MRI is the diagnostic modality of choice in patients with stenosis. It is noninvasive and provides images in axial, coronal, and sagittal planes. MRI offers details of both the bony and soft tissue anatomy. It provides improved visualization of the soft tissue elements of the spine (Figs. 62-9 and 62-10). The degree of compression of the neural elements and the offending pathology are both easily visualized. The central canal, lateral recess, and neural foramen can all be visualized along with the degree of stenosis that is present in each of these regions. Facet arthropathy, ligamentum hypertrophy, disc bulges or herniations, and other compressive pathology such as synovial cysts are easily identified on MRI (Fig. 62–11). Foraminal stenosis is best visualized on T1-weighted sagittal images in which the nerve root and dorsal root ganglion are contrasted with the surrounding fat.
Overall, the diagnostic accuracy of MRI is similar to that of the CT-myelogram and does not have the drawbacks of ionizing radiation and contrast injection in the CSF.72–75 The reliability of MRI interpretation has also been investigated, and there appears to be significant inter-reader variability in assessing some anatomic locations.76–79 The measurement of thecal sac area, as well as the ratings of the degree of central and foraminal stenosis, show good reliability.80 The reading of the degree of lateral recess stenosis, on the other hand, shows significant variability. Although some have noted correlation between the degree of stenosis and outcomes of treatments, others have not been able to identify any such relationship.81–84
It has been theorized that supine MRIs may understate the degree of stenosis because the patients may not be in the position of the worst compression during the study. Attempts have been made to demonstrate pathology in MRI scans that is obvious only under dynamic conditions.85–89 MRIs in flexion, extension, axial loading, and upright positions have been investigated without any conclusive evidence regarding their utility. It is unclear what the results of invasive treatments will be for those patients whose stenosis can only be detected under dynamic conditions. What the existing studies do point out is that the static images on the current MRIs should be supplemented with the dynamic information available from clinical presentation, as well as flexion and extension radiographs. One study noted that supine MRIs performed with the lumbar spine extended (by having the hips and knees extended) demonstrated more thecal sac compression than the images obtained in the flexed position.90 This suggests that position of the lower extremities during the MRI should be ascertained as a routine part of the patient’s history.
Electromyography, Nerve Condition Studies, and Somatosensory Evoked Potentials
Electromyography (EMG), nerve conduction studies (NCSs), and somatosensory evoked potentials (SSEPs) are not part of the routine workup of patients with spinal stenosis. EMG identifies the effect of nerve function through recording the electrical activity of muscle at rest and with stimulation. EMG identifies lower motor neuron dysfunction and does not evaluate any sensory dysfunction. Electromyographic changes have been documented in up to 80% of patients with spinal stenosis.84,91–94 EMG can be useful in differentiating chronic changes from active ongoing denervation. However, it should be kept in mind that there is a significant incidence of false-negative electromyograms in patients with spinal stenosis. This can be attributed to the fact that EMG does not measure sensory dysfunction and thus does not catch the more common abnormality in patients with neurogenic claudication. EMG also does not help differentiate symptomatic from asymptomatic patients.
NCS measures the speed with which impulses travel down an axon. NCS is useful in differentiating changes that are occurring from neuropathy versus radiculopathy. SSEPs measure the electrical transmission of sensory stimulation starting from the peripheral nerves and going through the spinal cord and brain. A lesion in the peripheral nerve will prolong the latency response, whereas lesions of the root and cord will cause changes in the waveform. SSEP is more sensitive and specific compared with an EMG, although there continue to be false negatives and false positives.95–97
Differential Diagnosis
Neurogenic claudication is the hallmark of spinal stenosis. Patients describe a variety of abnormal sensations that radiate down their legs with ambulation. Symptoms are typically relieved by forward flexion of the lumbar spine and are worse with extension. Sudden onset or severe motor weakness and bowel and bladder dysfunction in these patients should prompt evaluation of other etiologies of these symptoms such as cord compression. In spite of various attempts to create a single validated method of consistently diagnosing spinal stenosis, no single test or algorithm is available to accurately diagnose all patients with symptomatic spinal stenosis.59,98–100
Summary
Pearls
Key Points
1 Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794-810.
2 Watters WCIII, Baisden J, Gilbert TJ, et al. Degenerative lumbar spinal stenosis: an evidence based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008;8:305-310.
3 Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the Maine Lumbar Spine Study. Spine. 2005;30:936-943.
4 Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, et al. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978;3:319-328.
A classic article describing the pathogenesis of lumbar stenosis.
5 Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36:230-237.
A historical perspective on diagnosis and treatment of lumbar spinal stenosis.
1 Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36:230-237.
2 Verbiest H. Further experiences on pathologic influence of a developmental stenosis of the lumbar vertebral canal. J Bone Joint Surg Br. 1956;38:576-583.
3 Verbiest H. Spondylolisthesis: The value of radicular signs and symptoms. J Intern Coll Surg. 1963;39:461-481.
4 Verbiest H. Pathomorphic aspects of developmental lumbar stenosis. Orthop Clin North Am. 1966;6:177-196.
5 Verbiest H. Unilateral lumbo-sacral radicular symptoms due to sequestrated disc material in the spinal canal. Cesk Neurol. 1968;31:93-101.
6 Verbiest H. Neurogenic intermittent claudication in cases with absolute and relative stenosis of the lumbar vertebral canal (ASLC and RSLC), in cases with narrow lumbar intervertebral foramina, and in cases with both entities. Clin Neurosurg. 1973;20:204-214.
7 Verbiest H. Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. J Bone Joint Surg Br. 1977;59:181-188.
8 Verbiest H. The treatment of lumbar spondyloptosis or impending lumbar spondyloptosis accompanied by neurologic deficit and/or neurogenic intermittent claudication. Spine. 1979;4:68-77.
9 Verbiest H. Stenosis of the lumbar vertebral canal and sciatica. Neurosurg Rev. 1980;3:75-89.
10 Verbiest H. Developmental stenosis of the bony lumbar vertebral canal. Acta Orthop Belg. 1987;53:373-387.
11 Kirkaldy-Willis WH, Paine KWE, Cauchoix J, et al. Lumbar spinal stenosis. Clin Orthop. 1974;99:30-50.
12 Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, et al. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978;3:319-328.
13 Kirkaldy-Willis WH. Lumbar spinal nerve entrapment. Clin Orthop. 1982;169:171-178.
14 Kirkaldy-Willis WH. The relationship of structural pathology to the nerve root. Spine. 1984;9:49.
15 Kirkaldy-Willis WH. Presidential symposium on instability of the lumbar spine. Spine. 1985;10:254-291.
16 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-imaging scans of the lumbar spine in asymptomatic subjects: A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
17 Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
18 Deyo RA, Ciol MA, Cherkin DC, et al. Lumbar spinal fusion: a cohort study of complications, reoperations, and resource use in the Medicare population. Spine. 1993;18:1463-1470.
19 Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30:1441-1445.
20 Hillabrand AS, Rand N. Degenerative lumbar stenosis: Diagnosis and management. J Am Assoc Orthop Surg. 1999;7:239-248.
21 Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of disc degeneration of the lumbar spine. Ortho Clin North Am. 1983;14(3):59-64.
22 Arbit E, Pannullo S. Lumbar stenosis: A clinical review. Clin Orthop Rel Res. 2001;384:137-143.
23 Vanderlinden R. Subarticular entrapment of the dorsal root ganglion as a cause of sciatic pain. Spine. 1984;9:19-22.
24 Kunogi J, Hasue M. Diagnosis, operative treatment of intraforaminal, extraforaminal nerve root compression. Spine. 1991;16:1312-1330.
25 Porter R, Hibbert C, Evans C. The natural history of root entrapment syndrome. Spine. 1984;9:418-421.
26 Morishita Y, Hida S, Naito M, et al. Measurement of the local pressure in the intervertebral foramen and the electrophysiologic values of the spinal nerve roots in the vertebral foramen. Spine. 2006;31:3076-3080.
27 Rosenburg NJ. Degenerative spondylolisthesis: Predisposing factors. J Bone Joint Surg Am. 1975;67:240-246.
28 Spivak JM. Degenerative lumbar spinal stenosis: Current concepts review. J Bone Joint Surg Am. 1998;80:1053-1066.
29 Schonstorm N, Bolender NF, Spengler DM, et al. Pressure changes within the cauda equine following constriction of the dural sac. An in vitro experimental study. Spine. 1984;9:604-607.
30 Delamarter RB, Bohlmann HH, Dodge LD, et al. Experimental lumbar spinal stenosis: Analysis of the cortical evoked potentials, microvasculature, and histopathology. J Bone Joint Surg Am. 1990;72:110-120.
31 Pedowitz R, Garfin S, Massie J, et al. Effects of magnitude and duration of compression on spinal nerve root conduction. Spine. 1992;17:194-199.
32 Rydevick B, Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J Plast Reconstr Surg. 1977;11:179-187.
33 Rydevick B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg. 1981;6:3-12.
34 Rydevick B, Nordborg G. Changes in nerve function and nerve fiber structure induced by acute, graded compression. J Neurol Neurosurg Psychiatry. 1981;43:1070-1082.
35 Rydevick B, Brown M, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine. 1984;9:7-15.
36 Rydevick B, Holm S, Brown MD, et al. Diffusion from the cerebrospinal fluid as a nutritional pathway for spinal nerve roots. Acta Physiol Scand. 1990;138:247-248.
37 Rydevick B, Pedowitz RA, Hargens AR, et al. Effects of acute, graded compression on spinal nerve root function and structure. An experimental study of the pig cauda equina. Spine. 1991;16:487-493.
38 Kang JD, Georgescu HI, Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271-277.
39 O’Donnel JL, O’Donnel AL. Prostaglandin E2 content in herniated lumbar disc disease. Spine. 1996;21:1653-1656.
40 Olemarker K, Nordborg C, Larsson K, et al. Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine. 1996;21:411-414.
41 Muramoto T, Atsuta Y, Iwahara T, et al. The action of prostaglandin E2 and triamcinolone acetonide on the firing activity of lumbar nerve roots. Orthopedics. 1997;21:172-175.
42 Hashizume H, Kawakami M, Nishi H, et al. Histochemical demonstration of nitric oxide in herniated lumbar discs: A clinical and animal model study. Spine. 1997;22:1080-1084.
43 Yabuki S, Kikuchi S, Olemarker K, et al. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine. 1998;23:2517-2523.
44 Singh K, Samartzis D, Vacarro AR, et al. Congenital lumbar spinal stenosis: A prospective, control-matched, cohort radiographic analysis. Spine J. 2005;5:615-622.
45 Hall S, Bartleson JD, Onofrio BM, et al. Lumbar spinal stenosis: Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103:271-275.
46 Jones RA, Thomson JL. The narrow lumbar canal: A clinical and radiological review. J Bone Joint Surg Br. 1968;50:595-605.
47 Tile M, McNeil SR, Zarins RK, et al. Spinal stenosis: Results of treatment. Clin Orthop. 1976;115:104-108.
48 Blau JN, Logue V. The natural history of intermittent claudication of the cauda equina: A long term follow-up study. Brain. 1978;101:211-222.
49 Rosomoff HL, Rosomoff RS. Nonsurgical aggressive treatment of lumbar spinal stenosis. Spine. 1987;1:383.
50 Postacchini F, Cinotti G, Gumina S, Perugia D. Long-term results of surgery in lumbar stenosis: 8-year review of 64 patients. Acta Orthop Scand Suppl. 1993;251:78-80.
51 Herno A, Airaksinen O, Saari T, Luukkonen M. Lumbar spinal stenosis: A matched-pair study of operated and non-operated patients. Br J Neurosurg. 1996;10:461-465.
52 Benoist M. The natural history of lumbar degenerative spinal stenosis. Joint Bone Spine. 2002;69:450-457.
53 Atlas SJ, Keller RB, Robson D, et al. Surgical and nonsurgical management of lumbar spinal stenosis. Spine. 2000;25:556-562.
54 Johnsson KE, Rosen I, Uden A. The natural course of lumbar spinal stenosis. Clin Orthop. 1992:82-86.
55 Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the Maine Lumbar Spine Study. Spine. 2005;30:936-943.
56 Miyamoto H, Sumi M, Uno K, et al. Clinical outcome of nonoperative treatment for lumbar spinal stenosis, and predictive factors relating to prognosis, in a 5-year minimum follow-up. J Spinal Disord Tech. 2008;21:563-568.
57 Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or non-operative treatment of lumbar spinal stenosis? Spine. 2007;32:1-8.
58 Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794-810.
59 Waters WC, Baisden J, Gilbert TJ, et al. Degenerative lumbar spinal stenosis: An evidence based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008;8:305-310.
60 Lin S-I, Lin R-M, Huang L-W. Disability in patients with degenerative lumbar spinal stenosis. Arch Phys Med Rehabil. 2006;87:1250-1256.
61 Hellstrom PA, Tammela TL, Niinimaki TJ. Voiding dysfunction and urodynamic findings in patients with lumbar spinal stenosis and the effect of decompressive laminectomy. Scand J Urol Nephrol. 1995;29:167-171.
62 Johnsson KE. Lumbar spinal stenosis: A retrospective study of 163 cases in southern Sweden. Acta Orthop Scand. 1995;66:403-405.
63 Kawaguchi Y, Kanamori M, Ishihara H, et al. Clinical symptoms and surgical outcome in lumbar spinal stenosis patients with neuropathic bladder. J Spinal Disord. 2001;14:404-410.
64 Aalto TJ, Malmivaara A, Kovacs F, et al. Preoperative predictors of postoperative clinical outcome in lumbar spinal stenosis. Spine. 2006;31:E648-63.
65 Ciric I, Mikhael M, Tarkington J, et al. The lateral recess syndrome. J Neurosurg. 1980;53:433-443.
66 Amundsen T, Weber H, Lilleas F, et al. Lumbar spinal stenosis: Clinical and radiologic features. Spine. 1995;20:1178-1186.
67 Frymoyer JW, Newberg A, Pope MH, et al. Spine radiographs in patients with low-back pain: An epidemiological study in men. J Bone Joint Surg Am. 1984;66:1048-1055.
68 Kent DL, Haynor DR, Larson EB. Diagnosis of lumbar spinal stenosis in adults: A meta-analysis of the accuracy of CT, MR, and myelography. AJR Am J Roentgenol. 1992;158:1135-1144.
69 Herkowitz HN, Garfin SR, Bell GR, et al. The use of computed tomography in evaluating non-visualized vertebral levels caudad to a complete block on a lumbar myelogram: A review of 32 cases. J Bone Joint Surg Am. 1987;69:218-224.
70 Bell GR, Rothman RH, Booth RE, et al. A study of computer assisted tomography: II. Comparison of metrizamide myelography and computed tomography in the diagnosis of herniated lumbar disc and spinal stenosis. Spine. 1984;9:552-556.
71 Herno A, Airaksinen O, Saari T, et al. The predictive value of preoperative myelography in lumbar spinal stenosis. Spine. 1994;19:1335-1338.
72 Modic MT, Masaryk T, Boumphrey F, et al. Lumbar herniated disk disease and canal stenosis: Prospective evaluation by surface coil MR, CT, and myelography. AJR Am J Roentgenol. 1986;147:757-765.
73 Schnebel B, Kingston S, Watkins R, et al. Comparison of MRI to contrast CT in diagnosis of spinal stenosis. Spine. 1989;14:332-337.
74 Postacchini F, Amatruda A, Morace GB, et al. Magnetic resonance imaging in the diagnosis of lumbar spinal canal stenosis. Ital J Orthop Trauma. 1991;17:327-337.
75 Bischoff RJ, Rodriguez RP, Gupta K, et al. A comparison of computed tomography-myelography, magnetic resonance imaging, and myelography in the diagnosis of herniated nucleus pulposus and spinal stenosis. J Spinal Disord. 1993;6:289-295.
76 Hamanishi C, Matukura N, Fujita M, et al. Cross-sectional area of the stenotic lumbar dural tube measured from the transverse views of magnetic resonance imaging. J Spinal Disord. 1994;7:388-393.
77 Speciale AC, Pietrobon R, Urban CW, et al. Observer variability in assessing lumbar spinal stenosis severity on magnetic resonance imaging and its relation to cross-sectional spinal canal area. Spine. 2002;27:1082-1086.
78 Weiner BK, Patel NM, Walker MA. Outcomes of decompression for lumbar spinal canal stenosis based upon pre-operative radiographic severity. J Orthop Surg. 2007;2:3.
79 Song K, Jang E, Jung H, et al. Observer variability in the evaluation of multiple lumbar stenosis by routing MR-myelography and MRI. J Spinal Disord Tech. 2008;21:569-574.
80 Lurie JD, Tosteson AN, Tosteson TD, et al. Reliability of readings of magnetic resonance imaging features of lumbar spinal stenosis. Spine. 2008;33:1605-1610.
81 Uden A, Johnsson KE, Johnsson K, et al. Myelography in the elderly and the diagnosis of spinal stenosis. Spine. 1985;10:171-174.
82 Ogikubo O, Forsberg L, Hansson T. The relationship between the cross-sectional area of the cauda equine and the pre-operative symptoms in central lumbar spinal stenosis. Spine. 2007;13:1423-1428.
83 Geisser ME, Haig AJ, Tong HC, et al. Spinal canal size and clinical symptoms among persons diagnosed with lumbar spinal stenosis. Clin J Pain. 2007;23:780-785.
84 Haig AJ, Geisser ME, Tong HC, et al. Electromyography and magnetic resonance imaging to predict lumbar stenosis, low back pain, and no back symptoms. J Bone Joint Surg. 2007;89:358-366.
85 Schmid MR, Strucki G, Duewell S, et al. Changes in cross-sectional measurements of the spinal canal and intervertebral foramina as a function of body position: in vivo studies on an open-configuration MR system. Am J Radiol. 1999;172:1095.
86 Zamani AA, Moriarty T, Hsu L, et al. Functional MRI of the lumbar spine in erect position in a superconducting open-configuration MR system: Preliminary results. J Magn Reson Imaging. 1998;8:1329-1333.
87 Vitzthum HE, Konig A, Seifert V. Dynamic examination of the lumbar spine by using vertical, open magnetic resonance imaging. J Neurosurg. 2000;93:58-64.
88 Weishaupt D, Schmid MR, Zanetti M, et al. Positional MR imaging of the lumbar spine: Does it demonstrate nerve root compromise not visible at conventional MR imaging? Radiology. 2000;215:247-253.
89 Willen J, Wessberg PJ, Danielsson B. Surgical results in hidden lumbar spinal stenosis detected by axial loaded computed tomography and magnetic resonance imaging. Spine. 2008;33:E109-115.
90 Madsen R, Jensen TS, Ope M, et al. The effect of body position and axial load on spinal canal morphology. Spine. 2008;33:61-67.
91 Jacobsen RE. Lumbar stenosis: An electromyographic evaluation. Clin Orthop. 1976;115:68-72.
92 Spengler DM. Degenerative stenosis of the lumbar spine. J Bone Joint Surg Am. 1987;69:305-308.
93 Johnsson KE, Rosen I, Uden A. Neurophysiologic investigation of patients with spinal stenosis. Spine. 1987;12:483-487.
94 Chiodod A, Haig AJ, Yamakawa KSJ, et al. Magnetic resonance imaging vs electrodiagnostic root compromise in lumbar spinal stenosis. Am J Phys Med Rehabil. 2008;87:789-797.
95 Dvonch V, Scoff T, Bunch WH, et al. Dermatomal somatosensory evoked potentials: Their use in lumbar radiculopathy. Spine. 1984;9:291-293.
96 Keim HA, Hajdu M, Gonzales EG, et al. Somatosensory evoked potentials as an aid in diangnosis and intraoperative management of spinal stenosis. Spine. 1985;10:338-388.
97 Kondo M, Matsuda H, Kureya S, et al. Electrophysiological studies of intermittent claudication in lumbar stenosis. Spine. 1989;14:862-866.
98 Tenhula J, Lenke LJ, Bridwell KH, et al. Prospective functional evaluation of the surgical treatment of neurogenic claudication in patients with lumbar spinal stenosis. J Spinal Disord. 2000;13:276-282.
99 Graff ID, Park A, Bierma-Zeinstra S, et al. Diagnosis of lumbar spinal stenosis: A systematic review of the accuracy of the diagnostic tests. Spine. 2006;31:1168-1176.
100 Konno S, Hayashino Y, Fukuhara S, et al. Development of a clinical diagnosis support tool to identify patients with lumbar spinal stenosis. Eur Spine J. 2007;16:1951-1957.