CHAPTER 83 Spinal Orthoses for Traumatic and Degenerative Disease
Orthoses are externally applied devices that serve to restrict motion, correct deformity, or improve function in a particular body segment.1 Spinal orthoses for treatment of traumatic or degenerative disease primarily function to restrict motion through externally applied force. They have played an integral role in the management of spinal pathologic processes for thousands of years. Smith, in his 1908 article “The Most Ancient Splints,” described brace use in ancient Egypt more than 2500 years ago.2 Much of the early literature focused on treatment of spinal deformities including Galen’s chest-strapping method and Paré’s leather-lined metal jacket, popular in the late 16th century.3 Nicholas Andry developed the “iron cross,” consisting of a straight metal upright with a cross and metal ring attached to the top for support of the head, in the early 18th century for cervical immobilization.4 Today, spinal bracing continues to be a mainstay of treating deformity (covered elsewhere in this text) and the management of traumatic and degenerative spinal disorders.
Spinal orthoses can be broadly categorized on the basis of the body segment they are employed to immobilize: cervical (CO), cervicothoracic (CTO), thoracolumbosacral (TLSO), lumbosacral (LSO), and sacroiliac (SIO). This chapter focuses on the biomechanics, laboratory studies, and the classic and recent clinical results of commonly used, commercially available spinal orthoses. The U.S. Food and Drug Administration has classified spinal orthoses as class I devices. As a result, they have not been subject to the strict regulations and scrutiny applied to implantable devices. The currently available knowledge on these devices is the product of observations of varying degrees of scientific merit. Additionally, many commercially available products do not have peer-reviewed data to support their purported effectiveness. The prosthetics and orthotics community has produced numerous publications in print and online trade journals regarding their products and design developments, but comparatively little is published in medical journals familiar to practicing spine specialists. We recently conducted a Medline search covering the past 10 years using the key words “spinal orthoses.” This search yielded 579 unique results. A similar search restricted to new listings over the past year using the online search engine “Google” yielded 607,000 “hits.” The paucity of readily available peer-reviewed literature has presumably contributed to the variability of prescribing patterns among spine specialists. Bible and colleagues5 recently illustrated this point using a questionnaire study of the postoperative bracing preferences among spine surgeons. Most of the respondents braced their patients postoperatively, but there was no consensus on the type, duration, or indications for bracing. In this chapter, therefore, we have attempted to discuss the available data and review design features of various orthoses rather than make specific recommendations on the superiority of one commercial product versus another.
Biomechanics and Biomaterials
An improved understanding of spinal biomechanics has led to advances in spinal bracing. Conceptually, the spine can be thought of as a series of semirigid bodies interconnected by viscoelastic linkages. Spinal kinematics involves motion in six degrees of freedom, with rotation about three axes and translation along the three coordinates.6 For clinical considerations, testing (particularly involving human subjects) has generally been confined to three planes of motion: flexion-extension, axial rotation, and lateral bending.
The efficacy of a particular orthosis to limit spinal motion has been tested using various methods including radiography and goniometry. Standard radiography, typically using flexion-extension views, has been commonly employed. Cineradiography evaluates motion through the use of fluoroscopy. Goniometry uses external devices attached to the subject to measure spinal motion. It has been demonstrated to correlate fairly well with radiographic techniques7 and avoids exposing subjects to radiation. However, there is some decreased accuracy and lack of information on motion at any particular segment.
Because bracing attempts to control the position of the spine through the application of external forces, orthotic design must account for regional variations of the surrounding anatomy and accommodate vulnerable structures. This includes the vital soft tissue about the anterior neck, the rigid thoracic rib cage, the compressible abdomen, and the bony pelvis at the base of the lumbar spine. The surrounding soft tissue envelope has a substantial effect on the ability of an externally applied force to control spinal movement. Pressure measurements on the soft tissues may be an objective way to assess the fit of a spinal orthosis. The role of soft tissue pressure measurement as an index of applied corrective or stabilizing force, however, remains unclear.8,9 The intervening soft tissue envelope is also an area of potential complications with problems including breakdown, local pain, decreased vital capacity, and increased lower extremity venous pressure.10
Along with our improved understanding of the biomechanics of spinal bracing, improvements in the materials available for brace manufacture have led to dramatic advancements in their design. During the 18th century, braces were generally constructed of leather, iron, and wood. German developments in the 19th and early 20th centuries led to many new brace designs with paper cellulose and glue being added to wooden or iron frames.4 Newer composite materials, polymer resins, and thermoplastics have led to a proliferation of commercially available orthoses that are lightweight and comfortable without sacrificing the stability afforded by the heavier, more cumbersome designs of the past. Commonly used materials in the fabrication of orthoses are listed in Box 83–1.
Beyond the advancements in our understanding of biomechanics and biomaterials, advances in computer engineering have led to innovative methods for customized production of orthoses with decreased time requirements. At the primary authors’ institutions, efforts to limit the length of inpatient admissions have necessitated a close working relationship between the treating physicians and the orthotists. A computerized database of key measurements that have been recorded over the years has allowed our orthotists to develop digitized models of various sizes and body types using computer-aided design/computer-aided manufacturing technology (CAD/CAM). Using this approach, simple measurement at the sternal notch, xyphoid, and waist along with linear measurements can be performed and integrated with CAD-CAM technology to rapidly design a precision fit orthosis in a fraction of the time needed for standard mold-based orthoses. Recent studies have evaluated this approach for bracing patients with idiopathic scoliosis. Braces comparable with those made using traditional mold techniques (in comfort and curve correction) could be produced in roughly one third of the time previously required.11 Continuing advancements in this area have included the development of handheld three-dimensional laser imagers that use reflectors placed on the patient to capture shape information. This technology is already used in limb applications with efforts under way to apply this technology for spinal applications.
Cervical Orthoses
The cervical region provides a challenge for externally applied spine immobilization due to the soft tissue structures about the neck and high level of mobility of the spine segments. Cervical orthoses can be divided into two broad categories: soft and rigid. Soft collars provide comfort and proprioception, but little immobilization, decreasing flexion and extension by 5% to 15%, lateral bending by 5% to 10%, and axial rotation by 10% and 17%.12 They are often used in the treatment of whiplash-type injuries or muscular strains about the neck, where proprioceptive feedback helps “remind” a patient to voluntarily restrict motion. The use of soft cervical collars in the management of cervical myelopathy is controversial.
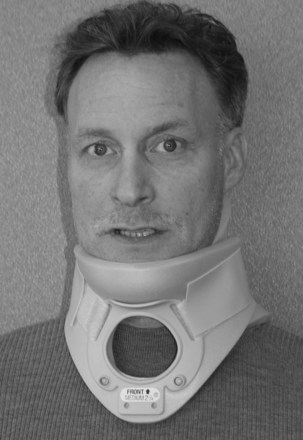
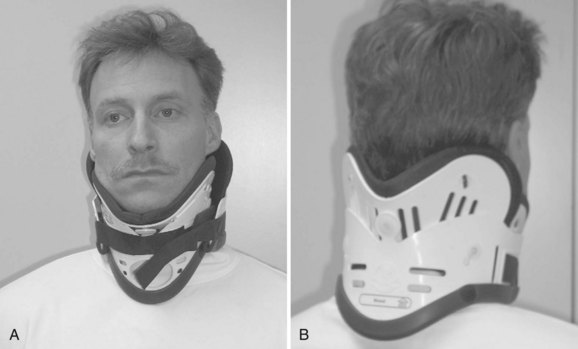
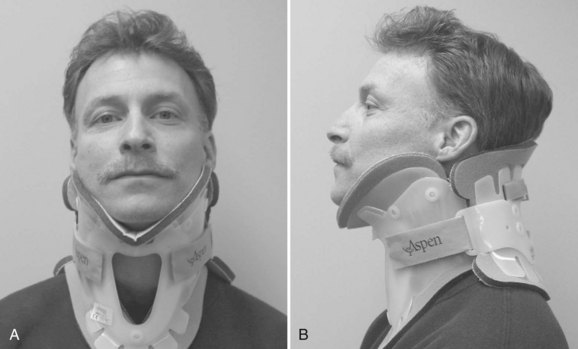
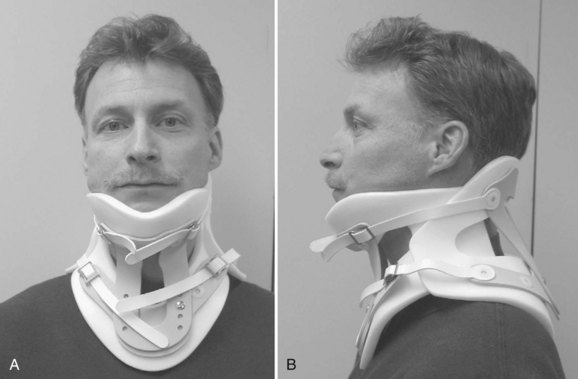
Rigid cervical orthoses are available in several forms, but all forms must be able to accommodate the vital soft tissue structures in the neck while simultaneously providing rigid immobilization of the mobile cervical spine. This is generally accomplished by firm seatings about the base of the skull and upper thorax connected by a rigid column. Most include an anterior opening to accommodate a tracheostomy tube. Rigid cervical orthoses are generally used to provide stabilization to the midcervical spine. Examples of cervical orthoses include the Philadelphia collar (Fig. 83–1), the Miami J collar (Fig. 83–2), the Aspen cervical orthosis (Fig. 83–3), and the Malibu collar (Fig. 83–4).
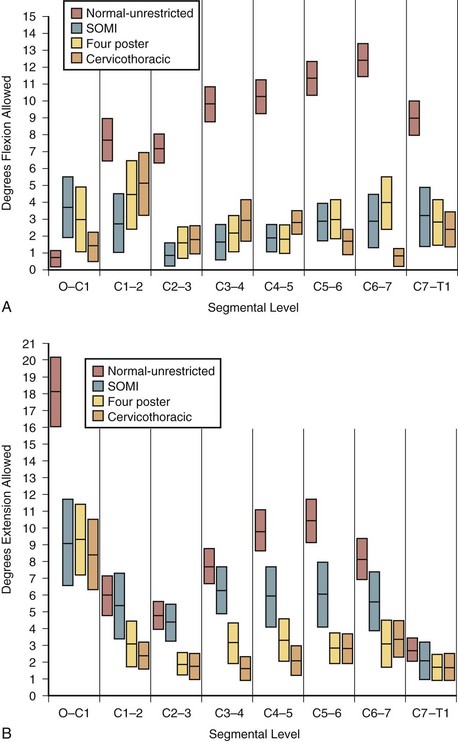
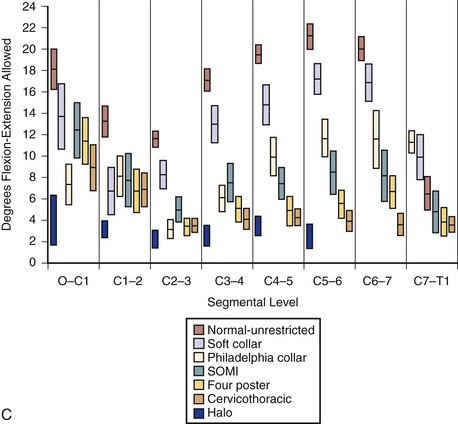
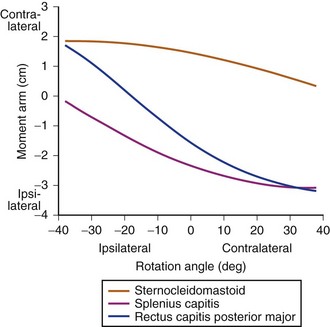
The classic study evaluating the effectiveness of various orthoses in immobilizing the cervical spine was performed by Johnson and colleagues.13 The methods of this study have often been emulated and its results frequently quoted since its publication in 1977. The authors evaluated the soft collar, Philadelphia collar, four-poster orthosis, sterno-occipito-mandibular immobilizer (SOMI), and a cervicothoracic orthosis. They used radiographs and overhead photographs taken at the extremes of motion in flexion-extension, rotation, and lateral bending. They quantified sagittal plane motion for each brace at every level of the cervical spine (Fig. 83–5). As others had demonstrated, they found that a soft collar offered no restriction of motion in any plane. They found that increasing the length of the orthosis (extending it onto the thorax) and increasing the rigidity of the connection improved the flexion control, but lateral bending and total flexion and extension were less controlled. They also demonstrated increased motion between the occiput and C1 in all the braces compared with the unbraced state. This “snaking,” or paradoxical motion, has subsequently been described throughout the cervical and thoracolumbar spine.14–16
Another study by Askins and colleagues17 compared five commonly used cervical orthoses in terms of their efficacy in restricting cervical motion. Radiographic and goniometric measurements found the NecLoc orthosis to be superior to the Miami J, Philadelphia, Aspen, and Stifneck orthoses in terms of flexion-extension, rotation, and lateral bending. The Miami J collar was also found to be significantly superior to the Philadelphia and Aspen orthoses in extension and combined flexion-extension.
A recent article by Bell and colleagues18 using electromagnetic sensors investigated the adverse effects of an ill-fitting Miami J cervical orthosis. The authors compared motion in healthy subjects using a Miami J of correct size to one too small and one too big, among other orthoses. They found that the ill-fitting cervical orthoses allowed significant increase in motion in multiple planes when compared with the correctly sized Miami J. The authors reiterated the importance of correct fitting of a cervical orthosis to avoid the potentially detrimental effects of excessive motion.
Known complications of cervical orthoses include skin breakdown over bony prominences such as the occiput, mandible, and sternum. Skin breakdown is especially prevalent in multitrauma patients with prolonged recumbency and in patients with altered sensorium. One study reported orthosis-related decubiti in 38% of patients with associated severe closed-head injuries.19 Molano-Alvarez and colleagues20 reported a 23.9% incidence of pressure sores in their series of patients with acute cervical spine injury placed in a cervical collar. The pressure sores were found on average at day 7 and were most frequently noted over the chin, occipital, and suprascapular areas. The authors found that pressure sores were associated with a higher injury severity score, longer time on mechanical ventilation, longer stays, and a greater percentage of patients with catheters for intracranial pressure monitoring. In addition, days spent in a cervical collar and presence of edema are thought to be significant predictors of skin breakdown.21 Plaisier and colleagues22 compared the skin pressure associated with the use of the Stifneck, Philadelphia, Miami J, and Aspen/Newport collars in supine patients. They found that the Miami J and Aspen collar produced the lowest chin and occiput pressures, both being below the mean capillary closing pressure.22 Tescher and colleagues23 assessed the effects of four commercially available cervical collars on mandibular and occipital tissue-interface pressure using custom-sized pads from a proprietary capacitive pressure-sensing transducer system. These authors found that the Miami J with and without Occian back had the lowest levels of mandibular and occipital pressure when compared with the Aspen and Philadelphia.23 Recently, increased intracranial pressure as a consequence of rigid cervical orthotic immobilization has been described. Hunt and colleagues24 studied the effects of rigid collar placement on intracranial pressure in head-injured patients. They found that rigid collars cause a small but significant increase in intracranial pressure, which may have deleterious effects in patients with severe head injuries and preexisting intracranial hypertension. They recommend early removal of rigid collars from head-injured patients once cervical spine injury has been excluded. Aspiration and dysphagia are at times anecdotally attributed to cervical collar use, but studies have been inconclusive. When using healthy volunteers performing swallowing studies, some mechanical changes in swallowing physiology were noted but no aspirations occurred.25 A causal link between cervical collar use and aspiration in injured or elderly patients has been suggested but not proven.
Methods for immobilizing the cervical spine of patients in the field have also been extensively studied. These include use of a cervical collar, a short board or sand-bag technique, or a combination of a collar and short board. Cline and colleagues26 compared the Hare extrication collar, the Philadelphia collar, and their immobilization protocol, which consists of a short board with forehead and chin straps. They concluded that the short board with straps provided the best immobilization and that the addition of a Philadelphia collar did not provide additional benefit. Podolsky and colleagues27 used goniometry to evaluate the immobilization provided by soft collar, hard collar, Philadelphia collar, Hare extrication device, and their sand-bag technique (which uses a board plus forehead tape). They found the sand-bag technique provided the most effective immobilization but that the addition of a Philadelphia collar provided additional benefit.27
The role of cervical collars following surgery for degenerative disease is an area of ongoing investigation and controversy. Bible and colleagues5 in their questionnaire of orthosis usage among spine surgeons found that orthoses were more often used after anterior cervical spine procedures as the complexity of the procedure increased. Campbell and colleagues28 evaluated the effect of a cervical collar after single-level anterior cervical fusion with plating on fusion rates and clinical outcomes in a U.S. Food and Drug Administration–regulated, multicenter controlled trial. They found similar clinical outcome parameters and a trend toward higher rates of fusion in the nonbraced group compared with those treated with a cervical collar. In addition, there was no difference between the proportion of patients returning to work.
Cervicothoracic Orthoses
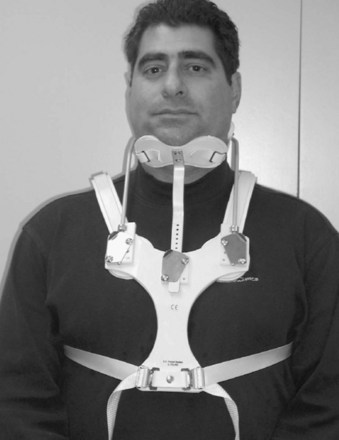
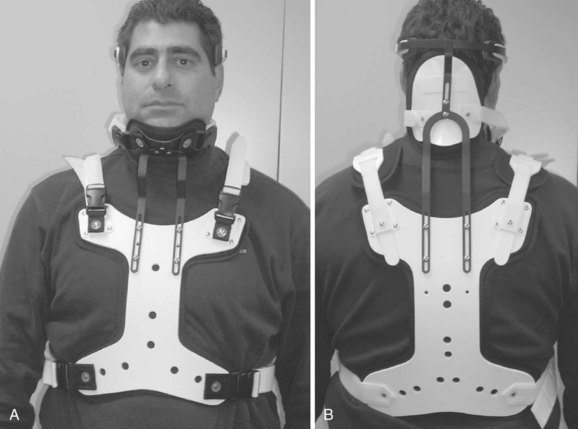
Cervicothoracic orthoses (CTOs) generally consist of occiput and chin supports attached to anterior and/or posterior thoracic plates. Examples include the sterno-occipito-mandibular immobilizer, or SOMI (Fig. 83–6), the Minerva brace (Fig. 83–7), and the Yale brace (Fig. 83–8). Compared with cervical orthoses, these devices improve control in all planes of motion and are particularly suited for immobilization of the middle to lower cervical spine and upper thoracic spine. This improved rigidity, however, comes at the expense of patient comfort. Some of the earlier authors distinguished between the two/four-poster designs and those with more extensive connections between the head and thoracic components.13 The more recent, standardized classification system, however, categorizes the poster braces as CTOs along with the other designs. The traditional four-poster brace was shown to limit 79% of overall cervical flexion-extension and limit midcervical flexion to a comparable degree as the more rigid CTOs.1,29 Because of their heavy design and high resting pressures on the chin and occiput,7 this brace is less commonly used today.
The SOMI (see Fig. 83–6) uses metal uprights to connect occipital and mandibular rests to a sternal plate that is secured to the thorax by padded metal “over-the-shoulder” straps and additional circumferential straps that cross in the back. Because there is no posterior thoracic plate, the occipital rests are supported by uprights from the sternal piece. This results in adequate control of flexion but deficient control of extension throughout the cervical spine.13,29 These braces are fairly comfortable to patients but are associated with high resting pressures at the chin and occiput.7
The original Minerva brace consisted of a heavy, custom, plaster jacket that created difficulties in maintaining patient hygiene and obtaining radiographs.30 As a result of the difficulties encountered in managing patients with this device, the halo came into popular use. Later, the thermoplastic Minerva body jacket (TMBJ) was developed. It preserved the noninvasive nature of the original concept, and its lightweight, bivalved, Polyform shell allowed improved patient comfort and hygiene and interfered less with follow-up radiographs.31 Donning this brace is somewhat complex, often requiring an orthotist for proper application. More recently a prefabricated version of the Minerva body jacket, the Minerva CTO (see Fig. 83–7), has been developed. Its design features a forehead band attached to a large occipital flare. Sharpe and colleagues32 reported that this orthosis limits overall sagittal plane motion by 79%, axial rotation by 88%, and lateral bending by 51%.
The Yale brace (see Fig. 83–8) was originally designed as a modified Philadelphia collar with custom-molded anterior and posterior polypropylene thoracic extensions.33 The modern version is prefabricated and usually made of Kydex. Although lighter and less cumbersome than most of the other CTOs, the Yale brace has similar efficacy in controlling motion. In Johnson’s study, the Yale brace restricted 87% of overall flexion-extension, 75% of axial rotation, and 61% of lateral bending.34 Whereas the CTOs have been shown to be fairly effective at limiting motion of the cervical spine, they should not be expected to rigidly immobilize below the C7-T1 level despite their thoracic components.
Halo Vest
It is generally agreed that the halo vest provides the most rigid immobilization of the cervical spine of all the currently used orthoses and is particularly effective in the upper cervical spine. Originally inspired by a device used by Bloom to treat facial fractures in pilots with overlying burns during World War II, modified versions were used by Perry and Nickel to immobilize patients with polio who had undergone posterior cervical fusion.35,36 The early halo devices consisted of a circumferential stainless steel ring with four pins for skull fixation. The ring was attached to a plaster jacket by upright posts.37 Numerous improvements have been made to the various components of the halo vest, but the overall design principles remain the same. A ring is fixed to the skull with multiple pins. The ring is then attached to a vest by four connecting rods. Newer rings are made of composite materials, which have the beneficial properties of light weight, radiolucency, and compatibility with magnetic resonance imaging. There does not appear to be a difference in fixation strength between newer radiolucent graphite rings and the earlier titanium ones.38 Rings that are open posteriorly, or have crown-type designs, have been developed and are frequently used. These designs allow for ease in placement because the head of the patient does not need to be passed through the ring. Additionally, because the patient is not lying on the back of the ring, there is less risk of cervical spine fracture displacement through ring manipulation.
The initial halo vest was fashioned from heavy plaster of Paris. With the development of plastic technology, lightweight, easily applied vests of various sizes based on chest circumference have been fabricated (Fig. 83–9). Adjustable straps and supports help customize the fit. The connecting rods have been anodized to prevent seizing of the metal during tightening. The connecting rods in many designs are made of carbon fiber for their radiographic lucency, as well as for compatibility with magnetic resonance imaging. Torque wrenches are included in the application sets to prevent overtightening of the bolts that connect the rods to the vest and the ring. Mirza and colleagues39 found that most commercially available vests provide comparable immobilization. Factors that they associated with decreased motion include increasing vest snugness, decreasing the deformability of the vest, and appropriate fit and application.39
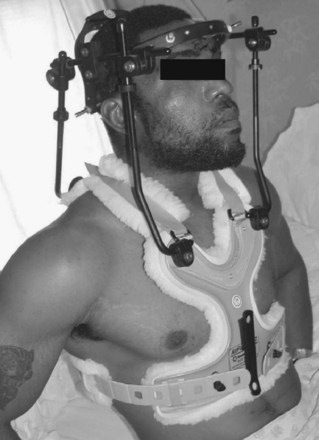
FIGURE 83–9 The halo vest (Bremer). This crown type design allows easier placement in the supine position.
Technologic advances have also been made in the field of halo pin materials and pin design. The popular pin composition at this time is stainless steel. Different pin-tip designs have been studied to determine which may provide the greatest resistance to shear frequently encountered at the pin-bone interface. Interest has arisen regarding a bullet-type tip that may be able to withstand higher shear forces.40,41 Some systems have torque wrenches that break off at a set torque.42 These wrenches are made to be low profile, allowing for ease of usage in cramped areas such as the posterior aspect of the skull.
Application Principles
Because many of the complications of halo fixation are related to inappropriate site selection or technique, a thorough understanding of pin insertion principles is essential. Many years of clinical data have fine-tuned the optimal location of halo pin placement. To minimize pin complications but maximize the rigidity of the halo vest frame, two anterior and two posterior pins are usually placed. The standard position of the two anterior pins is 1 cm superior to the orbital rim, over the lateral two thirds of the orbit, below the level of the greatest circumference of the skull (Fig. 83–10). This is considered the safe zone. In a cadaveric study, it was found that the skull thickness in this region averaged approximately 2 mm for the outer cortical table and 3 mm for the intercalvarial space or inner diploë. Pins placed too medial may damage the supraorbital or supratrochlear nerves. Also, the frontal sinus has a varied position in the midline. The outer table of the frontal sinus is often thin, which can lead to perforation with medial pin placement. Laterally placed anterior pins over the temporalis fossa have been suggested to avoid unsightly scarring over the anterior forehead. At this location, however, the zygomaticotemporal nerve may be injured, which provides sensation to the area over the temple. Also, by entering through the temporalis muscle, the pin often causes irritation during mandibular motion (e.g., eating). Additionally, in cadaveric studies, in this region the skull was found to have a thin outer and inner table with minimal cancellous diploë.43
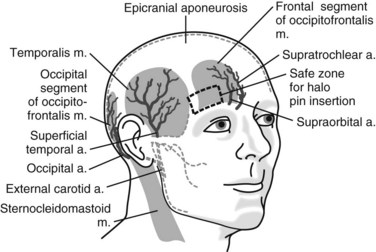
FIGURE 83–10 Diagram of the safe zone for placement of anterior halo fixator pins.
(From Ballock RT, Botte MJ, Garfin SR: Complications of halo immobilization. In Garfin SR [ed]: Complications of Spine Surgery. Baltimore, Williams & Wilkins, 1989, p 376.)
Because skull shapes differ, placing pins perpendicular to the tangent of the skull may be difficult. Because the halo is not a static unidirectional device, shear forces act at each of the pin sites. Triggs and colleagues,44 in a biomechanic study, evaluated the transverse shear forces to failure of pins placed in decremental angles from 90, 75, and 60 degrees. The load to deformity and failure were substantially higher for pins inserted perpendicular to the outer cortex of the skull as compared with 60 degrees.44 To minimize the incidence of pin loosening, it is important to try to place pins as perpendicular to the skull as possible to provide the most strength at the pin-bone interface.
The original pin-insertion torque recommendations of 6 in-lb were based on empiric observations. Cadaver studies, however, have shown that pins inserted at up to 10 in-lb of pressure barely penetrate the outer table.42 Biomechanical testing has demonstrated that 8 in-lb of insertional torque offers a more rigid pin-bone interface when compared with 6 in-lb.41 Clinical trials in adults have borne this out, with reduced pin site loosening and less infection at the pin sites.42
Application of the halo apparatus proceeds in a stepwise fashion (Box 83–2). After ring application, with the cervical spine protected by manual traction, the patient’s trunk can be elevated 30 degrees for vest placement. The posterior portion of the vest is applied and connected to the halo, followed by the anterior portion. Alternative methods include log rolling the patient, although it may be difficult to maintain cervical alignment. In rare instances of a stable fracture pattern, or after surgical internal fixation, the patient can be instructed to sit upright and the vest can be applied. All the vest locking bolts are then tightened to 28 in-lb of torque preset on the screwdriver. Once all the bolts are tightened, cervical spine alignment should be confirmed radiographically. The pins should be retightened 24 to 48 hours after placement. Baum and colleagues45 have documented an immediate drop of 2 to 4 in-lb in pin fixation purchase after vest placement. A commonly used pin care regimen consists of daily cleansing with dilute hydrogen peroxide (50/50 with water) on a cotton swab. Patients should follow up at predetermined intervals to confirm lack of halo vest complications.
Biomechanical Analysis
Whereas the halo is the most rigid external orthosis for immobilization of the cervical spine, some motion and force transmission to the cervical elements does occur. In early studies the halo device was found to permit only 4% of flexion-extension, 4% of lateral bending, and 1% of rotational motion of the normal native cervical spine.13 However, follow-up studies have shown that such significant immobilization is not routinely accomplished. One study has demonstrated up to 51 degrees of motion with halo immobilization.16 Segmentally, the greatest motion was observed at the occiput-C1 level (11.5 degrees). The least motion was observed at C2-3 (6.7 degrees). Interestingly, when flexion was observed at one segment of the spine, extension was observed at another level, the phenomenon known as “snaking.” Motion of the cervical spine was observed with patient position changes from supine to prone or from supine to sitting. However, there was no increased motion at the level of injury. In contrast, Anderson and colleagues46 reported in a clinical series greater than 3 degrees of angulation and 1 mm of translation at the fracture site in 77% of the patients evaluated.
Studies have also examined the directional force generated by the halo device. Maximal forces appear to be exerted in the mediolateral plane. However, in daily activities, anteroposterior and vertical forces are much larger. This was confirmed by Lind and Sihlbom,16 who observed no horizontal motion in the halo device. They reported significant differences in distractive forces between the supine and upright positions that can be attributed to the added weight of the head. Distractive forces were most increased with deep breathing, shoulder shrugging, and arm elevation, although no patient experienced any discomfort. These high distractive forces were most elevated in patients with tight-fitting vests. It was noted that the halo vest can be elevated by the sternum and the scapulae. They recommended that there be at least 30 mm of space between the sternum and the vest to prevent gross motion of the vest with daily activities. A vest called the “four pad” has been designed to minimize these forces by using four separate pads strategically placed on the thorax to provide snug fit without elevation from the scapulae.47 It has not, however, had significant clinical usage. Patients should be cautioned regarding exercises that involve twisting and bending because these tend to transmit undesirable forces to the cervical spine through the halo.
Complications
Although clinical studies have clearly demonstrated the efficacy of the halo device, complications with its use are not infrequent. Absolute contraindications to halo usage include cranial fracture, infection, and severe soft tissue injury at the proposed pin sites.48 Awareness of the most commonly seen complications can help minimize their severity and avoid catastrophic sequelae. Pin loosening has been identified as one of the most common problems with the halo device.49 In two large studies pin loosening was observed in 36% and 60% of patients.50,51 The hypothesized decrease in compressive pin forces with time was confirmed in a biomechanical study using instrumented halos in three patients. Pin force values at the time of halo vest application and at subsequent clinical visits were compared by Fleming and colleagues,52 who measured an 83% drop in torque pressure by the time of halo removal.52 They believed the mechanism of pin loosening was through bone resorption at the pin tip. To help decrease loss of the halo because of loosening, if there is no sign of infection, the pins may be retightened to 8 in-lb of torque as long as resistance is met on the first few turns of the pin. If no resistance is met, then a new pin should be placed in another position. The old pin should be kept in place until the new pin has been rigidly placed, to keep the correct orientation of the ring on the skull.
Halo pin infection rates have been documented to occur in approximately 20% of cases.50,51 If drainage and erythema continue at a pin site even with aggressive pin care, bacterial cultures should be obtained and appropriate oral antibiotics started. If cellulitis persists, or an abscess forms, the pin should be removed and new pins placed in another position. The patient may require incision and drainage of the abscess with parenteral antibiotics.50
Skull and dural penetration by a halo pin are rare complications. It is often related to patient falls. Clinically, the patient may present with a headache, malaise, or visual disturbances if symptomatic pin penetration has occurred. Radiographs taken tangential to the skull may demonstrate whether pin perforation of the inner table has occurred. Clear cerebrospinal fluid leakage from the pin site is a definitive sign that dural puncture has occurred. In these circumstances a new pin should be placed in another region and the old pin removed.51 Elevation of the head decreases intracerebral pressure and assists closure of the dural tear. These tears usually heal in 4 to 5 days. If the tear does not heal or an infection is suspected (subdural abscess), formal surgical intervention may be necessary.53
Patients may report difficulty with swallowing during halo immobilization. Deglutition dysfunction leading to aspiration has been reported.54 Many instances of swallowing difficulty are a result of the cervical spine being immobilized in the extended position. Efforts to flex the cervical spine, while maintaining cervical reduction, may assist in resolution of dysphagia.53
Pressure sores have been reported in 4% to 11% of patients during halo immobilization.50 These sores frequently develop underneath the vest, or cast vest, secondary to pressure against prominent bony surfaces or due to insufficient padding or incorrect sizing of the vest. Principles of pressure sore prevention include frequent turning, adequate vest padding, and routine skin inspections. Pressure sores are more prevalent in patient populations using a cast vest, rather than the padded prefabricated plastic vest. This is especially important in patients with neurologic deficits who may not have normal sensation over their trunk. Alternative strategies to halo immobilization, such as rigid internal fixation, should be considered in this patient population (spinal cord injury). Pressure sore treatment may require split-thickness skin grafting and/or employing rotational muscle flaps for coverage.51
A prospective study by van Middendorp and colleagues55 investigated the incidence and risk factors associated with complications during halo vest immobilization over a 5-year period in 239 patients. There was a 6% incidence of death, although only one was directly related to the immobilization. Although there was a 5% incidence of pneumonia, elderly patients did not have an increased risk of pneumonia. A 12% incidence of pin site infection, which was significantly related to pin penetration through the outer table of the skull, occurred. The authors reiterate that although there were relatively low rates of major complications, awareness of the substantial rates of minor complications is necessary to prevent further morbidity.
Halo vest immobilization in the elderly is an area of increasing concern due to reports of significant morbidity and mortality. Tashjian and colleagues56 investigated the morbidity and mortality of halo usage in patients older than the age of 65 in treating odontoid fractures, compared with a rigid cervical orthosis or operative fixation. Of the patients treated with a halo vest, 42% died, compared with 20% in the nonhalo group. This difference was statistically significant. There was also a significantly increased rate of major complications in the halo vest group. The authors concluded that treatment of odontoid fractures in the elderly with a halo is associated with significant morbidity and mortality. Other authors have evaluated the treatment of elderly patients with type II odontoid fracture with cervical collars and found that fracture stability by either osseus or fibrous union could be achieved with acceptable clinical results.57
Pediatric Considerations
Halo immobilization has been successful in the treatment of children and infants after both unstable cervical injuries and congenital abnormalities.58,59 The recommended pin torque pressure in children is 2 in-lb to 5 in-lb owing to the skull being thinner and more pliable than adults.58 In infants younger than 3 years old a multiple, low-torque pin insertion technique is recommended to achieve maximal stability.60
In young children up to 12 pins can be inserted at 2 in-lb of torque pressure under general anesthesia. Halo pins should be placed under the largest diameter of the skull, with care to avoid the frontal sinus and temporal regions. Some authors recommend a computed tomogram of the head to identify the location of suture lines and bone fragments (in congenital cases) before placement of the halo.48 Furthermore, in the presence of open suture lines and fontanelles, vigilant care must be taken to make sure that equal pressure is placed symmetrically on the skull through the halo pins to prevent skull deformity.
Owing to sizing issues in this age group, a custom-made ring and vest are often required. Once the halo is placed, the vest is applied in normal fashion and connected to the halo. Children require the same pin care as adults. Baum and colleagues45 observed that children have a higher rate of pin loosening than adults. Children with halo fixators should have close parental and medical supervision.
Noninvasive Halo
A noninvasive halo (NIH) has been investigated as an alternative that avoids invasive pin placement while potentially providing more immobilization than a CTO. This orthosis consists of a halo mask that encircles the patient’s head over the forehead and the chin and attaches to a rigid anterior vest. DiPaola and colleagues61 performed a biomechanical evaluation of conventional and noninvasive halos in cadaveric C1-2 and C5-6 instability models using various maneuevers. The authors found less cervical spine motion during application of the NIH compared with the conventional halo. The conventional halo, however, did provide superior immobilization during the logroll maneuver, although the halos were similar during the sit-up maneuver. Skaggs and colleagues62 treated 30 children with stable spines in an NIH postoperatively and found only one complication. Sawers and colleagues63 retrospectively reviewed the use of an NIH to treat 19 patients for cervical trauma. All fractures healed in acceptable alignment without neurologic deterioration. There was one case of occipital ulceration and two cases of noncompliance. Although NIH is a tempting option to avoid the potential complications associated with traditional halo usage, there are insufficient data to recommend its usage as an equivalent alternative.
Thoracolumbar and Sacral Orthoses
Flexible TLSOs and LSOs
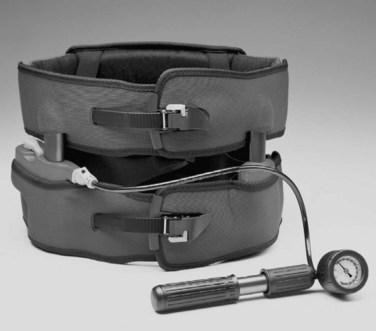
Flexible LSOs and TLSOs are prescribed by some for the treatment of low back pain. These corset-style devices are adjustable by means of laces, hooks, or Velcro straps. Some authors have reported that these types of orthoses decrease the myoelectric activity of the paraspinal muscles and increase intra-abdominal pressure, possibly resulting in decreased loads on the intervertebral discs.64,65 Others have reported increased myoelectric activity, as measured through surface electrodes on the paraspinal muscles, when certain tasks are performed in braced subjects.66 Less controversy surrounds the effect on the abdominal muscles, with several authors reporting decreased measured myoelectric activity with brace wear.65,67 Design modifications to improve brace effectiveness have included the use of pneumatic lifters designed to transfer some of the patient’s upper body weight from the lumbar spine to the iliac crests (Fig. 83–11). In a survey of 44 patients who completed an 8-week regimen using the Orthotrac Pneumatic Vest, 59% reported moderate to significant improvement of mechanical back pain symptoms.68 The role of lumbar supports for prevention and treatment of low back pain continues to be an area of controversy because clinical studies on their efficacy are conflicting. Jellema and colleagues69 performed a systematic review of the literature to assess this issue. They searched the Medline, Cinahl, and Current Contents databases; the Cochrane Controlled Trials Register; and the Embase database. Two reviewers independently assessed methodologic quality and performed data review. In performing a quantitative analysis, they classified the strength of evidence as strong, moderate, limiting or conflicting, or no evidence. Only 4 of the 13 studies identified were believed to be high quality. They determined that on the basis of the literature available, there is no strong evidence to support the use of lumbar supports for prevention or treatment of low back pain.69
Rigid TLSOs and LSOs
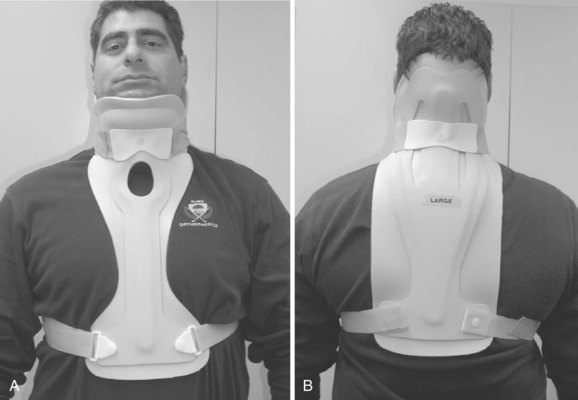
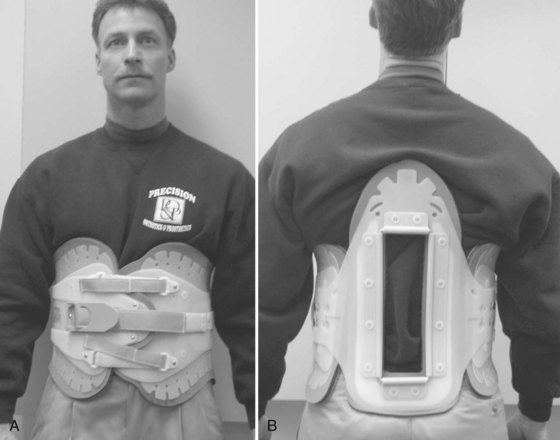
Most conventional TLSOs are more effective in limiting motion in the sagittal plane than in controlling rotation or lateral bending. The Jewett hyperextension brace is an example of a non-custom-molded TLSO brace (Fig. 83–12). It applies three-point fixation to the torso through anterior pads on the symphysis pubis and sternum and a posterior pad midway between the anterior pads. This arrangement of forces places the spine in slight extension. Similar to cervical collars, this brace is best in controlling motion in the flexion-extension plane and less effective in controlling lateral bending and rotation. The Knight-Taylor brace (Fig. 83–13) is another commonly prescribed TLSO and can be prefabricated or custom molded. It has a corset-style front for abdominal compression and lateral and posterior uprights attached to over-the- shoulder straps for thoracic control. Prefabricated TLSOs often consist of clamshell-style braces that can be ordered to measurements and usually are fabricated of  – to
– to 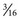 -inch low-density polyethylene with a full
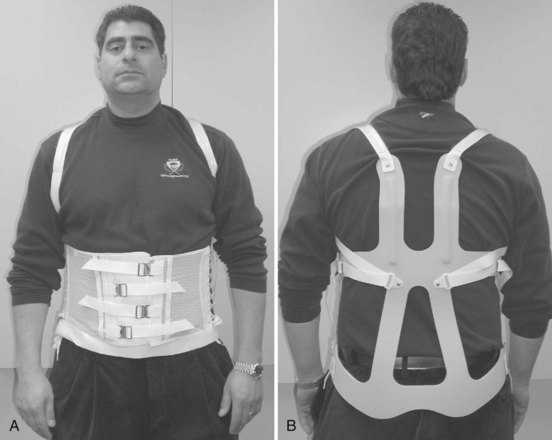
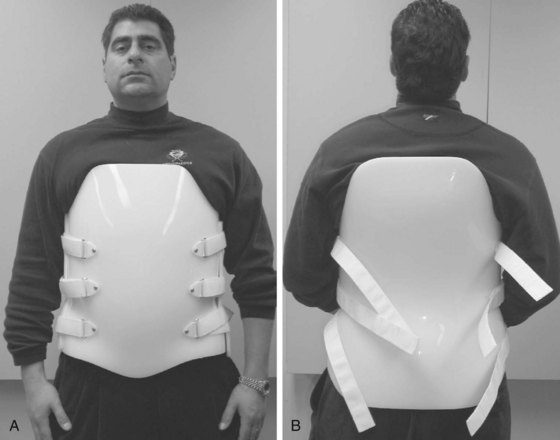
-inch low-density polyethylene with a full 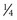 -inch foam liner. The anterior and posterior shells are fastened in place with three Velcro closures per side. Some manufacturers include pneumatic bladders in the abdominal and/or lumbar areas to help customize the fit and accommodate volume changes. Prefabricated “customizable” TLSOs are also commercially available with apron-style fronts that can be adjusted with Velcro straps and telescoping sternal pads (Fig. 83–14). These braces provide good control in all three planes, but once again the major restriction is in the flexion-extension plane. For optimal control in all three planes between T5 and L4, a fully custom-molded TLSO (Fig. 83–15) should be used. These are often formed from high-temperature thermoplastic that is custom fitted from a plaster cast formed from the patient. When immobilization proximal to T5 is required, a cervical extension should be included.70 If immobilization distal to L4 is necessary, a thigh cuff should be added to the orthosis to control pelvic motion.71,72
-inch foam liner. The anterior and posterior shells are fastened in place with three Velcro closures per side. Some manufacturers include pneumatic bladders in the abdominal and/or lumbar areas to help customize the fit and accommodate volume changes. Prefabricated “customizable” TLSOs are also commercially available with apron-style fronts that can be adjusted with Velcro straps and telescoping sternal pads (Fig. 83–14). These braces provide good control in all three planes, but once again the major restriction is in the flexion-extension plane. For optimal control in all three planes between T5 and L4, a fully custom-molded TLSO (Fig. 83–15) should be used. These are often formed from high-temperature thermoplastic that is custom fitted from a plaster cast formed from the patient. When immobilization proximal to T5 is required, a cervical extension should be included.70 If immobilization distal to L4 is necessary, a thigh cuff should be added to the orthosis to control pelvic motion.71,72
Compared with cervical orthoses, few studies have been performed to scientifically evaluate the ability of external orthoses to immobilize the thoracolumbosacral spine. Brown and Norton reported the earliest data on motion restriction with lumbar external supports.71 They evaluated three rigid LSOs, one flexible LSO, and a TLSO. Kirschner wires were inserted into the spinous processes of volunteers. The angles between the wires were measured to determine the amount of motion. Radiographic evaluation was also performed. Of note, they reported increased motion across the lumbosacral junction in all of the braces. Increased motion at L4-5 was also noted while the subjects were sitting. Compared with the unbraced state, all of the braces resulted in some flexion at L4-5 and L5-S1 while standing. Lumsden and Morris reported similar findings when they studied lumbosacral rotational motion in subjects wearing either a chairback brace or an LSO corset. Volunteers had Steinmann pins placed in their posterior superior iliac spines. Motion was determined by radiographs and measurement of pin rotation. In each case, they found the braces increased motion at the lumbosacral level.73 Fidler and Plasmans72 used radiographs to compare the effect on lumbosacral motion of a corset, a brace, and a plaster jacket with and without a thigh cuff. They found the custom-molded plaster jacket to provide the best immobilization at the L1-3 level. To improve immobilization at the L4-S1 levels, they recommended adding a thigh cuff to the orthosis.
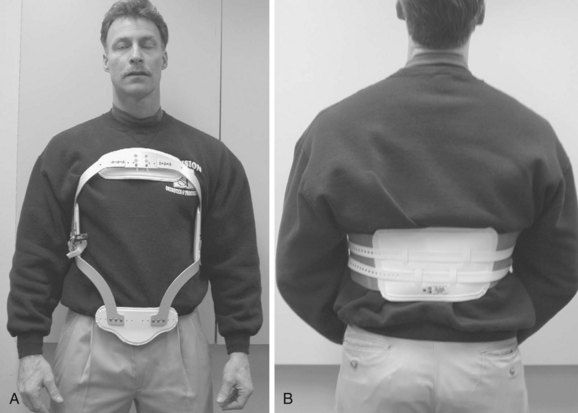
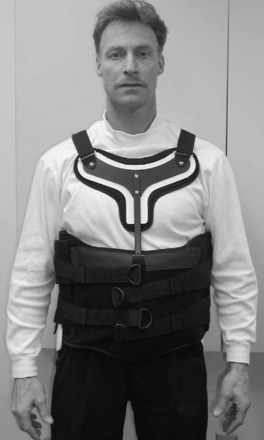
Indications for TLSOs are less well defined than for COs. Bracing for compression fractures of the thoracolumbar spine is not tolerated well by the elderly. Treatment in these patients usually consists of early mobilization and close follow-up. A soft binder or corset may provide support and symptomatic relief. The cruciform anterior spinal hypertension (CASH) brace (Fig. 83–16) is another lightweight alternative that may be reasonably tolerated. In younger patients, however, anterior column fractures are often the result of greater energy than their osteoporotic counterparts, and therefore a more cautious approach is often favored. These patients are commonly treated with a rigid Jewett or Knight-Taylor brace. However, the need for rigid bracing of these fractures is still a matter of debate. Ohana and colleagues74 retrospectively reviewed the outcome of 129 young patients with mild compression fractures who were treated with or without a Jewett hyperextension brace. They found that one-column fractures of the thoracolumbar spine with as much as 30% compression can be safely treated without bracing, instead prescribing early ambulation, hyperextension exercises, and close follow-up.74 In addition, there is no consensus on the use of TLSOs after lumbar spine surgery for degenerative disease. In one questionnaire study, 49% of spine surgeons stated they use braces after lumbar spine surgery, with no statistically different frequency of use between instrumented and noninstrumented fusions.5
Burst fractures comprise another entity in the spectrum of thoracolumbar injuries. Historically, neurologically intact patients with burst fractures were treated with bed rest for 4 to 12 weeks, followed by progressive mobilization. Today, controversy exists as to which injuries require surgical stabilization and which can be treated with bracing and early mobilization. Any neurologic deficit is often an indication for operative management. Other fairly well accepted criteria for surgery, rather than bracing, include canal compromise of 50% or more, kyphotic deformity of greater than 30 degrees, and posterior column involvement. Chow and colleagues75 retrospectively studied functional outcomes in 24 patients treated with hyperextension body casts and/or Jewett hyperextension braces for thoracolumbar burst fractures. None of these patients had posterior column fractures, significant kyphosis, or neurologic deficit. Patients were initially treated with bed rest and logroll precautions until the predictable ileus and abdominal distention resolved 2 to 3 days later. At that point patients were placed in a cast or brace and progressively mobilized. Patients were followed for a minimum of 1 year. They concluded that hyperextension casting or bracing with early mobilization reduced hospital time, avoided costs and risks of surgery, and allowed patients a relatively early return to work. Additionally, the authors mentioned that patients treated nonoperatively tended to experience moderate back pain up to a year after the injury and that this pain eventually diminished over time.75
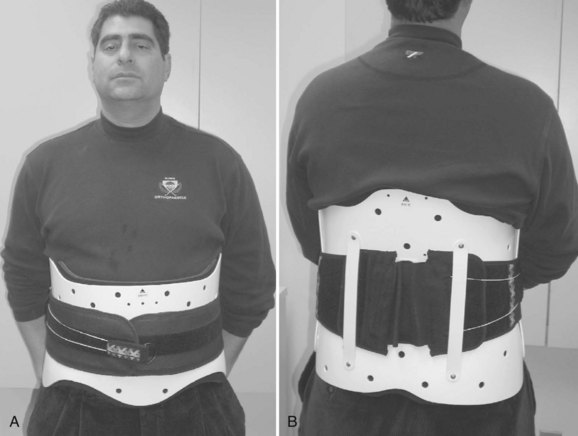
LSOs (Figs. 83-17 and 83-18) are often prescribed for treatment after arthrodesis for degenerative conditions. As discussed earlier, several studies demonstrate little or no immobilizing effect from wearing LSOs and possibly an increase in L4-5, L5-S1 motion after application of these orthoses. The point continues to be debated. Postlumbar fusion bracing is believed by some to help relieve pain and decrease the risk of pseudarthrosis and fixation failure and is, therefore, prescribed by some surgeons for up to 12 weeks postoperatively. Others believe that lumbosacral orthoses do little to immobilize the lumbar spine beyond the stabilization provided by rigid operative fixation.76 Rohlmann and colleagues77 evaluated the effect of a brace on loads on internal spinal fixation devices. The implant loads were measured using telemeterized fixators in six patients for several positions and activities including sitting, standing, walking, bending forward, and lifting an extended leg in a supine position. They found that none of the braces tested was able to markedly reduce the loads on the fixators. They observed that higher fixator loads were often observed while wearing a brace.
Acknowledgments
Pearls
Pitfalls
Key Points
1 Botte MJ, Garfin SR, Byrne TP, et al. The halo skeletal fixator: Principles of application and maintenance. Clin Orthop. 1989;239:12-18.
2 Garfin SR, Botte MJ, Waters RL, Nickel VL. Complications in the use of halo fixation device. J Bone Joint Surg Am. 1986;68:320-325.
The authors report the complications observed with their extensive use of the halo device.
3 Jellema P, van Tulder MW, van Poppel MNM. Lumbar supports for prevention and treatment of low back pain: A systematic review within the framework of the Cochrane Back Review Group. Spine. 2001;26:377-386.
4 Johnson RM, Hart DL, Simmons EF, et al. Cervical orthoses: A study comparing their effectiveness in restricting cervical motion in normal subjects. J Bone Joint Surg Am. 1977;59:332-339.
5 Norton PL, Brown T. The immobilizing efficacy of back braces. J Bone Joint Surg Am. 1957;39:111-138.
1 American Academy of Orthopaedic Surgeons. Atlas of Orthotics. St. Louis: CV Mosby; 1975.
2 Smith G. The most ancient splints. BMJ. 1908;1:732-734.
3 Peltier L. Orthopedics, a History and Iconography. San Francisco: Norman Publishing; 1993.
4 Edwards J. Orthopaedic Appliance Atlas. St. Louis: American Academy of Orthopaedic Surgeons; 1952.
5 Bible JE, Biswas D, Whang PG, et al. Postoperative Bracing after Spine Surgery for Degenerative Conditions-A Questionnaire Study. Spine J. 2009;9:309-316.
6 White A, Panjabi M. Physical properties and functional biomechanics of the spine. In White A, Panjabi M, editors: Clinical Biomechanics of the Spine, 2nd ed, Philadelphia: JB Lippincott, 1990.
7 Fisher SV, Bowar JF, Awad EA, et al. Cervical orthoses effect on cervical spine motion: roentgenographic and goniometric method of study. Arch Phys Med Rehabil. 1977;58:109-115.
8 Chase AP, Bader DL, Houghton GR. The biomechanical effectiveness of the Boston brace in the management of adolescent idiopathic scoliosis. Spine. 1989;14:636-642.
9 Wilner F. The effect of the Boston brace on the frontal sagittal curves of the spine. Acta Orthop Scand. 1984;55:457-460.
10 Sypert GW. External spinal orthotics. Neurosurgery. 1987;20:642-649.
11 Wong MS, Cheng JC, Lo KH. A comparison of treatment effectiveness between the CAD/CAM method and the manual method for managing adolescent idiopathic scoliosis. Prosthet Orthot Int. 2005;29:105-111.
12 Hsu JD, Michael JW, Fisk JR. AAOS Atlas of Orthoses and Assistive Devices, 4th ed. Philadelphia: Elsevier Health Sciences; 2008.
13 Johnson RM, Hart DL, Simmons EF, et al. Cervical orthoses. A study comparing their effectiveness in restricting cervical motion in normal subjects. J Bone Joint Surg Am. 1977;59:332-339.
14 Colachis SCJr., Strohm BR. Radiographic studies of cervical spine motion in normal subjects: flexion and hyperextension. Arch Phys Med Rehabil. 1965;46:753-760.
15 Hartman JT, Palumbo F, Hill BJ. Cineradiography of the braced normal cervical spine. A comparative study of five commonly used cervical orthoses. Clin Orthop Relat Res. 1975:97-102.
16 Lind B, Sihlbom H, Nordwall A. Forces and motions across the neck in patients treated with halo-vest. Spine. 1988;13:162-167.
17 Askins V, Eismont FJ. Efficacy of five cervical orthoses in restricting cervical motion. A comparison study. Spine. 1997;22:1193-1198.
18 Bell KM, Frazier EC, Shively CM, et al. Assessing range of motion to evaluate the adverse effects of ill-fitting cervical orthoses. Spine J. 2009;9:225-231.
19 Chendrasekhar A, Moorman DW, Timberlake GA. An evaluation of the effects of semirigid cervical collars in patients with severe closed head injury. Am Surg. 1998;64:604-606.
20 Molano Alvarez E, Murillo Perez Mdel A, Salobral Villegas MT, et al. [Pressure sores secondary to immobilization with cervical collar: a complication of acute cervical injury]. Enferm Intensiva. 2004;15:112-122.
21 Powers J, Daniels D, McGuire C, et al. The incidence of skin breakdown associated with use of cervical collars. J Trauma Nurs. 2006;13:198-200.
22 Plaisier B, Gabram SG, Schwartz RJ, et al. Prospective evaluation of craniofacial pressure in four different cervical orthoses. J Trauma. 1994;37:714-720.
23 Tescher AN, Rindflesch AB, Youdas JW, et al. Range-of-motion restriction and craniofacial tissue-interface pressure from four cervical collars. J Trauma. 2007;63:1120-1126.
24 Hunt K, Hallworth S, Smith M. The effects of rigid collar placement on intracranial and cerebral perfusion pressures. Anaesthesia. 2001;56:511-513.
25 Stambolis V, Brady S, Klos D, et al. The effects of cervical bracing upon swallowing in young, normal, healthy volunteers. Dysphagia. 2003;18:39-45.
26 Cline JR, Scheidel E, Bigsby EF. A comparison of methods of cervical immobilization used in patient extrication and transport. J Trauma. 1985;25:649-653.
27 Podolsky S, Baraff LJ, Simon RR, et al. Efficacy of cervical spine immobilization methods. J Trauma. 1983;23:461-465.
28 Campbell MJ, Carreon LY, Traynelis V, et al. Use of cervical collar after single-level anterior cervical fusion with plate: is it necessary? Spine. 2009;34:43-48.
29 Johnson RM, Owen JR, Hart DL, et al. Cervical orthoses: a guide to their selection and use. Clin Orthop Relat Res. 1981:34-45.
30 Benzel EC, Larson SJ, Kerk JJ, et al. The thermoplastic Minerva body jacket: a clinical comparison with other cervical spine splinting techniques. J Spinal Disord. 1992;5:311-319.
31 Millington PJ, Ellingsen JM, Hauswirth BE, et al. Thermoplastic Minerva body jacket–a practical alternative to current methods of cervical spine stabilization. A clinical report. Phys Ther. 1987;67:223-225.
32 Sharpe KP, Rao S, Ziogas A. Evaluation of the effectiveness of the Minerva cervicothoracic orthosis. Spine. 1995;20:1475-1479.
33 Zelenik R, Chapin W, Hart D, et al. Yale cervical orthosis. Phys Ther. 1978;58:861-864.
34 Johnson RM, Hart DL, Owen JR, et al. The Yale cervical orthosis: an evaluation of its effectiveness in restricting cervical motion in normal subjects and a comparison with other cervical orthoses. Phys Ther. 1978;58:865-871.
35 Perry J, Nickel V. Total cervical spine fusion for neck paralysis. J Bone Joint Surg Am. 1959;41:37-60.
36 Botte M, Garfin S, Byrne T, et al. The halo skeletal fixator: Principles of application and maintenance. Clin Orthop. 1989;239:12-18.
37 Botte MJ, Byrne TP, Abrams RA, et al. Halo Skeletal Fixation: Techniques of Application and Prevention of Complications. J Am Acad Orthop Surg. 1996;4:44-53.
38 Lerman JA, Haynes RJ. Open versus closed halo rings: comparison of fixation strengths. Spine. 2001;26:2102-2104.
39 Mirza SK, Moquin RR, Anderson PA, et al. Stabilizing properties of the halo apparatus. Spine. 1997;22:727-733.
40 Voor MJ, Khalily C. Halo pin loosening: a biomechanical comparison of experimental and conventional designs. J Biomech. 1998;31:397-400.
41 Garfin SR, Lee TQ, Roux RD, et al. Structural behavior of the halo orthosis pin-bone interface: biomechanical evaluation of standard and newly designed stainless steel halo fixation pins. Spine. 1986;11:977-981.
42 Botte MJ, Byrne TP, Garfin SR. Application of the halo device for immobilization of the cervical spine utilizing an increased torque pressure. J Bone Joint Surg Am. 1987;69:750-752.
43 Garfin SR, Botte MJ, Centeno RS, et al. Osteology of the skull as it affects halo pin placement. Spine. 1985;10:696-698.
44 Triggs KJ, Ballock RT, Lee TQ, et al. The effect of angled insertion on halo pin fixation. Spine. 1989;14:781-783.
45 Baum JA, Hanley ENJr., Pullekines J. Comparison of halo complications in adults and children. Spine. 1989;14:251-252.
46 Anderson PA, Budorick TE, Easton KB, et al. Failure of halo vest to prevent in vivo motion in patients with injured cervical spines. Spine. 1991;16:S501-S5015.
47 Fukui Y, Krag M, Huston D, et al. Halovest dynamic loads: full crossover comparison of three vest types. Spine. 2002;27:241-249.
48 Bono CM. The halo fixator. J Am Acad Orthop Surg. 2007;15:728-737.
49 Glaser JA, Whitehill R, Stamp WG, et al. Complications associated with the halo-vest. A review of 245 cases. J Neurosurg. 1986;65:762-769.
50 Lind B, Sihlbom H, Nordwall A. Halo-vest treatment of unstable traumatic cervical spine injuries. Spine. 1988;13:425-432.
51 Garfin SR, Botte MJ, Waters RL, et al. Complications in the use of the halo fixation device. J Bone Joint Surg Am. 1986;68:320-325.
52 Fleming BC, Krag MH, Huston DR, et al. Pin loosening in a halo-vest orthosis: a biomechanical study. Spine. 2000;25:1325-1331.
53 Garfin SR, Botte MJ, Triggs KJ, et al. Subdural abscess associated with halo-pin traction. J Bone Joint Surg Am. 1988;70:1338-1340.
54 Kelly P, Beregin D, Cunningham U, et al. Deglutition dysfunction in cervical orthosis. J Bone Joint Surg Br. 2002;84-B:2-3.
55 van Middendorp JJ, Slooff WB, Nellestein WR, et al. Incidence of and risk factors for complications associated with halo-vest immobilization: a prospective, descriptive cohort study of 239 patients. J Bone Joint Surg Am. 2009;91:71-79.
56 Tashjian RZ, Majercik S, Biffl WL, et al. Halo-vest immobilization increases early morbidity and mortality in elderly odontoid fractures. J Trauma. 2006;60:199-203.
57 Koech F, Ackland HM, Varma DK, et al. Nonoperative management of Type II odontoid fractures in the elderly. Spine. 2008;33:2881-2886.
58 Kopits SE, Steingass MH. Experience with the “halo-cast” in small children. Surg Clin North Am. 1970;50:935-943.
59 Osenbach RK, Menezes AH. Pediatric spinal cord and vertebral column injury. Neurosurgery. 1992;30:385-390.
60 Mubarak SJ, Camp JF, Vuletich W, et al. Halo application in the infant. J Pediatr Orthop. 1989;9:612-614.
61 DiPaola CP, Sawers A, Conrad BP, et al. Comparing cervical spine motion with different halo devices in a cadaveric cervical instability model. Spine. 2009;34:149-155.
62 Skaggs DL, Lerman LD, Albrektson J, et al. Use of a noninvasive halo in children. Spine. 2008;33:1650-1654.
63 Sawers A, DiPaola CP, Rechtine GR2nd. Suitability of the noninvasive halo for cervical spine injuries: a retrospective analysis of outcomes. Spine J. 2009;9:216-220.
64 Nachemson A, Schultz A, Andersson G. Mechanical effectiveness studies of lumbar spine orthoses. Scand J Rehabil Med Suppl. 1983;9:139-149.
65 Waters RL, Morris JM. Effect of spinal supports on the electrical activity of muscles of the trunk. J Bone Joint Surg Am. 1970;52:51-60.
66 Lantz SA, Schultz AB. Lumbar spine orthosis wearing. II. Effect on trunk muscle myoelectric activity. Spine. 1986;11:838-842.
67 Morris JM, Lucas DB. Biomechanics of Spinal Bracing. Ariz Med. 1964;21:170-176.
68 Loguidice V, Polaski E. Patient satisfaction with the Orthotrac Pneumatic Vest for low back pain. Care Manage. 2002;8:4.
69 Jellema P, van Tulder MW, van Poppel MN, et al. Lumbar supports for prevention and treatment of low back pain: a systematic review within the framework of the Cochrane Back Review Group. Spine. 2001;26:377-386.
70 White A, Panjabi M. Clinical Biomechanics of the Spine. Toronto: JB Lippincott; 1978.
71 Brown T, Norton PL. The immobilizing efficiency of back braces; their effect on the posture and motion of the lumbosacral spine. J Bone Joint Surg Am. 1957;39-A:111-139. passim
72 Fidler MW, Plasmans CM. The effect of four types of support on the segmental mobility of the lumbosacral spine. J Bone Joint Surg Am. 1983;65:943-947.
73 Lumsden RM2nd, Morris JM. An in vivo study of axial rotation and immoblization at the lumbosacral joint. J Bone Joint Surg Am. 1968;50:1591-1602.
74 Ohana N, Sheinis D, Rath E, et al. Is there a need for lumbar orthosis in mild compression fractures of the thoracolumbar spine? A retrospective study comparing the radiographic results between early ambulation with and without lumbar orthosis. J Spinal Disord. 2000;13:305-308.
75 Chow GH, Nelson BJ, Gebhard JS, et al. Functional outcome of thoracolumbar burst fractures managed with hyperextension casting or bracing and early mobilization. Spine. 1996;21:2170-2175.
76 Connolly PJ, Grob D. Bracing of patients after fusion for degenerative problems of the lumbar spine—yes or no? Spine. 1998;23:1426-1428.
77 Rohlmann A, Bergmann G, Graichen F, et al. Braces do not reduce loads on internal spinal fixation devices. Clin Biomech (Bristol, Avon). 1999;14:97-102.