CHAPTER 90 Spinal Intradural Infections
Bacterial Pathogens
Spinal Subdural Empyema
Background
The current understanding of the pathophysiology, diagnosis, treatment, and prognosis of spinal subdural empyemas (SSEs) is based on anecdotal evidence (case reports and case series). It is the rarity of the condition that has necessitated this anecdotal approach. Since the initial description by Sittig in 1927,1 a total of 50 cases have been published.1–39 The exact incidence, therefore, is unknown.
The demographics of the reported cases indicate a slight female predominance, with 27 females and 19 males.36–39 The age at presentation ranges from 9 months to 77 years.35 Approximately 50% of the patients, however, fall into the fifth to seventh decades of life.35 The predominant location of these abscesses tends to be the thoracolumbar spine, with only 10 of the 50 cases being isolated to the cervical spine.35–39
Pathophysiology
Several theories have been proposed to account for the relative infrequency of SSEs when compared with either cranial subdural empyemas or spinal epidural abscesses (SEAs). The absence of air sinuses in the spine may be one of the main factors. Another is the presence of a true epidural space rather than a potential space (cranial) that acts as a filter protecting the subdural space. This accounts for the greater incidence of SEAs as compared with SSEs.40,41 The final theory is the pattern of blood flow of the spine as compared with the brain. The blood is directed centripetally in the spine, whereas it is directed centrifugally in the brain. This again increases the incidence of SEAs when compared with SSEs.20,23,35
The pathogenesis of these infections can be categorized into one of four major categories. The first and most common mechanism for the development of an SSE is hematogenous spread from a distant source.* Although the primary site of infection may be anywhere, Bartels and colleagues35 found the most common site to be peripheral infections such as furuncles and cellulitis. Other primary infections include respiratory tract infections, endocarditis, urinary tract infections, and septic abortions.35,36 The next most common source is iatrogenic. This includes lumbar punctures, local anesthetic injections, and discography.* A third category comprises all infections arising from direct extension into the subdural space. These infections may be due to dermal sinus tracts associated with spinal dysraphism,4,21,23,39 spinal infections,35 or trauma to the spine.11 The fourth major category is an unknown primary source.11,13,20 Bartels and colleagues35 found that the patients in 10 of 45 cases reviewed had no known primary source for their infections.
The organisms responsible for SSEs reflect the primary sites of infection. It is not surprising, therefore, that the most frequently cultured organism is Staphylococcus aureus. Of the total of 50 reported cases, 24 have been due to S. aureus.† The remainder of cases can be attributed to a variety of organisms including other Staphylococcus species,13,25,30 Streptococcus,8 Escherichia coli,35,39 Pseudomonas aeruginosa,16 Streptococcus pneumoniae,14 and Peptococcus magnus (Box 90–1).21
Clinical Presentation
†References 1–3, 5, 7–9, 11, 12, 15, 18, 19, 22, 23, 28, 29, 36–38.
Fraser and colleagues15 described the classical presentation of an SSE in 1973. The triad includes fever and neck/back pain followed by symptoms of spinal cord/cauda equina compression. Levy and colleagues36 found the triad was present in 18 of 47 cases they reviewed. In their review, Bartels and colleagues35 confirmed the progression of symptoms as described by Fraser and associates. They found that at the time of initial onset of symptoms, 84.4% had spinal/limb pain and 55.6% had fever.35 By the time the patients had reached medical attention, 86.7% had fever, 84.4% had spinal/limb pain, and 82.2% had a motor deficit.35 A key feature of SSE is the absence of spinal tenderness, which helps distinguish this process from the more common SEA.15,16 However, the presence of spinal tenderness does not preclude the diagnosis of an SSE. Levy and colleagues36 found 14 of the 47 patients they reviewed did have spinal tenderness.
On the basis of their findings, Bartels and colleagues35 proposed a sequence of three stages in the progression of these infections. Stage 1 included fever with or without spinal pain. Stage 2 adds motor, sensory, and/or sphincter disturbances to the picture. Stage 3 is defined as complete motor and sensory loss below the lesion.35 The rate of progression from one stage to the next, however, is variable and unpredictable.
Laboratory Evaluation
The complete laboratory evaluation of patients who present with a clinical picture suggestive of an SSE is essential, although often nonspecific. Serum leukocytosis may be mild to moderate.16,35–39 The erythrocyte sedimentation rate (ESR) may be prolonged,35,36 and the C-reactive protein level may be elevated.39
Another essential part of the workup is obtaining cultures from all sources. This does not include the routine use of a lumbar puncture for obtaining cerebrospinal fluid (CSF) because a lumbar puncture risks contaminating deeper meningeal layers.36 If CSF is obtained, it will often show characteristics of a parameningeal process (not meningitis). This includes moderate pleocytosis, moderately elevated protein content, and low to normal glucose levels. The CSF cultures are generally negative.15,16,19,36,38
Imaging Studies
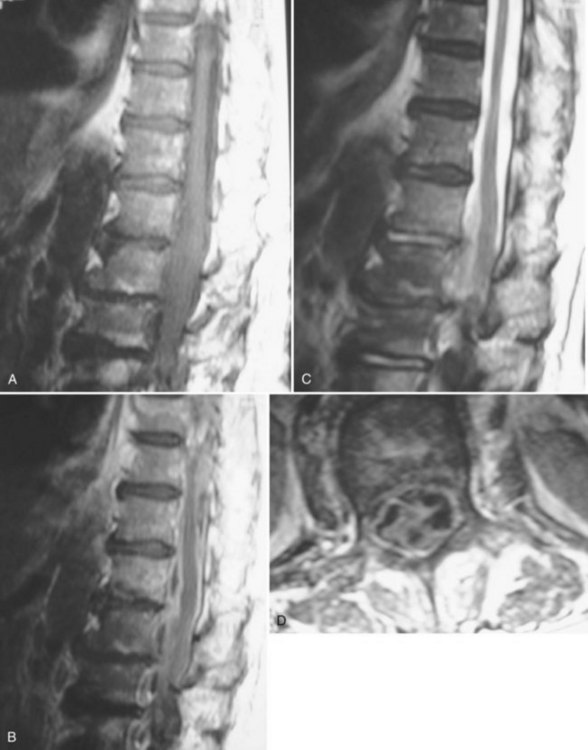
Much of the literature regarding SSEs was published before the routine use of magnetic resonance imaging (MRI) in the evaluation of patients with a clinical presentation suggestive of an SSE. The diagnostic study of choice at that time was a myelogram with or without a postmyelographic computed tomography (CT).1–29,31–35 The current definitive diagnostic study of choice is a contrast medium-enhanced MRI because it is noninvasive and allows better visualization of the spinal cord, vertebrae, disc spaces, extent of lesion, and extent of compression. Because there are only a total of six published cases of the imaging characteristics of SSEs on MRI, the exact findings are not well described.30,36–39 The basic finding, however, is an intraspinal, space-occupying, variably enhancing mass (Fig. 90–1).36–39 The major limitation of MRI, however, remains its inability to distinguish whether the lesion is intradural or extradural.38,42 The differentiation of epidural versus subdural may be aided by an evaluation (using plain radiographs and MRI) for the presence of discitis/osteomyelitis, which accompanies two thirds of SEAs.43
Treatment and Prognosis
The mainstay of treatment for these infections is surgical decompression (laminectomy) with irrigation and drainage of the subdural space followed by appropriate antibiotic therapy.35,36,44 The exposure should encompass the extent of the abscess.44 After copious irrigation, most authors advocate the primary closure of the dura. The arachnoid should be preserved if possible.36,44 Although some have advocated using postoperative irrigation via external drains, this has not been adopted as part of the conventional treatment algorithm.18 A significant indication for surgery is obtaining a definitive organism to treat; therefore cultures should be obtained before using antibiotic irrigation.
The use of postoperative antibiotics should be based on the given organism found during surgery. Empiric antibiotic coverage for these infections must cover gram-positive cocci.36 Some advocate the additional use of corticosteroids (dexamethasone) during the perioperative period as a prophylaxis against the development of thrombophlebitis.15,36 This is another technique that is not universally applied.35,37–39,44
The prognosis for patients with SSE is directly related to the treatment methodology.35,36 Although some have had success with conservative treatment,37 the overall prognosis is much better if patients undergo aggressive irrigation and drainage.35 In a review of 45 patients by Bartels and colleagues,35 the outcomes correlated with the treatment approaches. Among the surgically treated group, 82.1% made a complete recovery or improved, whereas 17.9% died. In the conservatively treated group, 80% died (four of five patients) and only 20% (one of five patients) improved.35 On the basis of these numbers, the current recommendations are for aggressive surgical treatment followed by antibiotic therapy.43,44
Spinal Intramedullary Abscess
Background and Demographics
Intramedullary spinal cord abscesses (ISCAs) are also an uncommon condition. Since the initial case description was published by Hart in 1830, 96 total cases have been published45–91 Although the exact incidence is unknown, an autopsy study conducted by Courville in 1950 found only one spinal cord abscess among 40,000 postmortem examinations.92 As with SSE, much of the current understanding regarding intramedullary spinal cord abscesses is based on case reports and several retrospective reviews of these published cases. Arzt46 reviewed all 42 published cases reported in the preantibiotic era between 1830 and 1944. In 1977 Menezes and colleagues and DiTullio47,48 independently reviewed all published cases of ISCA between 1944 and 1977. Most recently, Bartels and colleagues,72 as well as Chan and Gold,78 have published reviews encompassing the cases described since 1977.
ISCA is a disease that commonly afflicts children. In reviewing patients presenting either before or after the discovery of antibiotics, there is a consistent plurality of patients (40%) presenting at younger than 20 years of age.47,48,72,78 In fact, 25% to 27% of patients are younger than the age of 10.47,48 The age range, however, is quite broad and extends from 7 months to 72 years.47,48,72,78 There is a male predilection, with 60% to 70% of cases occurring in men.48,72
ISCAs occur throughout the spine, but they are most frequently found in the thoracic region.47,48,72,78 Bartels and colleagues72 found in their review that 32% of lesions were isolated to the thoracic cord, as compared with 17% and 12% in the cervical and lumbar regions, respectively. Overall, 69% of all abscesses involved some portion of the thoracic cord.72 Six cases of holocord abscesses have also been published.81
Pathophysiology
The pathogenesis of ISCA can be divided into two broad categories: direct implantation and hematogenous spread. The more complex of the two is hematogenous spread via arterial supply, venous drainage, or lymphatics. The venous system that drains the spinal cord is a low-pressure system that communicates with the venous drainage of the chest and abdomen. Changes in the intrathoracic or abdominal pressures may generate backflow in these veins and allow seeding of the spinal cord with an infectious embolus from the thorax or abdomen.48,93
Arterial metastasis of an infection to the spinal cord is another route for developing ISCA. An understanding of the pathophysiology of this process can be derived from experimental work done by Hoche.94 He found that transient bacteremia generated by injecting various organisms into the arterial supply of the brain or spinal cord was not sufficient for the production of a parenchymal abscess. However, injection of an aseptic embolus followed by an injection of bacteria into the arterial supply of the brain or spinal cord did result in the formation of an abscess. In addition, injection of septic emboli also formed abscesses. The basic finding is that metastatic abscesses form in the central nervous system (CNS) as a result of septic emboli or bacterial infection of an area that was previously infarcted by an aseptic embolus.48,94
Menezes and colleagues48 describe a third route for the formation of metastatic abscess in the spinal cord. On the basis of experiments conducted by Galkin in the 1930s, the Virchow-Robin spaces of the spinal cord have connections with the lymphatics that drain the mediastinum, abdomen, and retroperitoneum.95 This connection is the lymphatic channels found along the spinal nerves. It is, therefore, theoretically possible for infections of the chest and abdomen to reach the spinal cord simply through their lymphatic drainage.48
The site of origin for cases of metastatic ISCA is variable. The most common primary site is pulmonary, owing to conditions such as pneumonia and bronchitis.47,48,66,72,78 Other primary infections include endocarditis,46–48 urinary tract infections,57 peritonitis,90 and peripheral skin infections.48,72,76
The other major route for the pathogenesis of ISCA is direct implantation. The most common source of direct implantation/contiguous spread is via a congenital midline neuroectodermal defect.52,55,59,60,69 Chan and Gold found that among the 25 cases they reviewed, 24% were a result of contiguous spread from a dermal sinus tract.78 The other sources include postoperative,64 post-traumatic (stab wound),48 and postprocedural infections.48
The most common mechanism for the development of ISCA, however, has become cryptogenic. In their review of 25 cases reported between 1977 and 1997, Chan and Gold78 found that 16 cases (64%) were cryptogenic. In Menezes’48 review, the cause of infection was unknown in 50% of cases reported after 1960. The case reports published since Chan and Gold’s series also support the observation that cryptogenic ISCAs are a highly frequent finding.77,81,85,87,88
Chan and Gold78 also compared their review of 25 patients treated in the antibiotic era (1977-1997) with the 42 patients reported in the preantibiotic era (1830-1944) and reviewed by Arzt. Several trends were elucidated by this comparison. One, highlighted earlier, is the increased frequency of cases with a cryptogenic mechanism. Another is the dramatic drop in the number of cases caused by hematogenous spread. In the preantibiotic era, cases of ISCA were far more frequently caused by hematogenous spread from an extraspinal source than during the antibiotic era (45% vs. 8% of cases).78 This trend may be attributed to the effectiveness of antibiotics in treating primary infections.
The organisms responsible for ISCA reflect the sources of the infection. In their review of 93 reported cases of ISCA, Bartels and colleagues72 found that a causative organism was reported in 56 cases. Among these, Staphylococcus accounted for 22 cases and Streptococcus was found in another 16 cases.72 The other significant organisms were Actinomyces, Proteus mirabilis, Pneumococcus, Listeria monocytogenes, Hemophilus, and Escherichia coli (Box 90–2).48,56,72,77,78
The organisms responsible for ISCAs can be further divided on the basis of the mechanism of formation for the given abscess. Cases of contiguous spread via a dermal sinus tract are most commonly due to †References 48, 55, 56, 63, 67, 73–75, 77, 78, 85.
Staphylococcus epidermidis, S. aureus, Enterobacteriaceae, anaerobes, and Proteus mirabilis.* Postsurgical (contiguous) cases are most often due to S. epidermidis, S. aureus, Enterobacteriaceae, and Pseudomonas aeruginosa.78 The cases that arise from hematogenous spread reflect the site of primary infection. The organisms found most often in cases that have a cryptogenic etiology include Listeria monocytogenes, Streptococcus viridans, Actinomyces meyeri, and Hemophilus species.† The high frequency of L. monocytogenes is based on the bacteria’s trophism for the CNS.78 The other major organisms are oral flora and may lead to ISCA after bacteremia from an odontogenic source.78
The histopathology of ISCA follows the same basic pattern as cerebral abscesses. The early stages of acute abscess formation appear microscopically as nodules composed of monocytes, lymphocytes, polymorphonuclear leukocytes, and endothelial cells. These lesions are often in proximity to vessels. There are organisms in both the abscess and the associated vessel. Areas of hemorrhage often surround these septic nodules. Veins in the area of the abscess are frequently thrombosed. As these lesions grow larger, they begin to develop purulent myelitis with areas of central necrosis. The chronic abscesses have a well-defined three-layer capsule surrounding a central area of pus and necrosis. The inner layer is composed of collagen fibers and polymorphonuclear leukocytes. The middle layer contains fibroblasts, capillaries, histiocytes, and plasma cells. The outer layer is essentially connective tissue.48,76,78 The growth of ISCAs begins in the gray matter of the spinal cord. It then extends rostrad and caudad along fiber tracts.76
Clinical Presentation
The presenting signs and symptoms in patients with ISCAs almost always involve motor deficits, which vary depending on the location of the abscess. At the time of diagnosis, 83% to 94% of patients will have some type of motor deficit.47,72,78 The extent of the deficits is variable from slight paresis to complete paralysis.72 Sensory disturbances are almost as frequent as motor findings, with 60% to 78% of patients having some degree of sensory loss before treatment.42,72,78 Although it does not appear as early as motor and sensory disturbances, 51% to 56% of patients will have loss of sphincter control at the time of diagnosis.72,78
The other two commonly encountered signs are spinal pain localized to the involved area and fever. The percentage of patients who are febrile at the time of their diagnosis ranges from 25% to 50%. The presence of spinal pain at some point before diagnosis occurs in 36% to 60% of patients.71,72,78 Less commonly found symptoms include Horner syndrome,56,76 brainstem findings,85 and Brown-Séquard syndrome.90
Menezes and colleagues48 divided the ISCAs into three clinical categories on the basis of symptomatology and chronicity. Acute infections are defined as infections with symptoms lasting less than 2 weeks. The symptoms in this group consist of complete or partial transverse myelitis commonly associated with fever and leukocytosis.48,76 The subacute lesions are defined as having a clinical history longer than 2 weeks but shorter than 6 weeks. The symptoms in this category consist of a stuttering onset of spinal cord dysfunction similar to the presentation of intramedullary tumors. Chronic abscesses are those with a clinical history longer than 6 weeks. These chronic ISCAs also present with symptoms that mimic the presentation of an intramedullary tumor. These patients rarely have fever or leukocytosis at presentation.48,76
Laboratory Evaluation
Laboratory studies are not consistently abnormal in patients with ISCAs. Leukocytosis, although more common in cases with acute presentation,76,81,85,87 may be present in chronic cases.48,76 It may also be absent in both acute56,72 and chronic cases.77,88,90 An abnormally prolonged ESR and elevated C-reactive protein levels are also inconsistent findings.76,77,81,88 The analysis of the CSF is not consistent, either. The one consistent finding is that CSF cultures are routinely negative.* If the CSF is abnormal, the profile reflects the presence of a parameningeal process with elevated white blood cells (WBCs), mildly elevated protein count, and normal glucose levels.48,56,71,72,76,90
Imaging Studies
Plain radiographs, myelograms, CT scans, and MRI have all been used to evaluate patients with ISCAs. Plain radiographs are almost always normal.53,71 The utility of plain radiographs is in evaluating for the presence of osteomyelitis, spinal deformity, spinal stenosis, and spinal dysraphism, which have all been shown to be predisposing factors for the development of ISCA.78 Myelography was the diagnostic study of choice before the routine availability of MRI. The findings on myelogram include symmetric or asymmetric widening of the spinal cord at a focal segment, partial or complete obstruction to flow, and low-lying conus/tethered spinal cord (spinal dysraphism).48,56,72,78 The use of CT of the spine was often in conjunction with a myelogram. Postmyelographic CT may show segmental widening of the spinal cord and/or partial or complete obstruction to CSF flow in approximately 60% of cases.78 CT done without intrathecal contrast medium enhancement may show widening of the cord, and some scans done with enhancement reveal an intramedullary process.72,76
The current diagnostic method of choice, however, is an enhanced MRI. The imaging of ISCA follows essentially the same progression that has been documented in the development of brain abscesses. The two main stages are early and late myelitis.96 Early myelitis shows up as hyperintense signal on T2-weighted and proton density-weighted sequences. The T1-weighted sequences show isointense to hypointense signal changes with a widened spinal cord. There is poor contrast medium enhancement on T1-weighted sequences during the early myelitis phase. The T2-weighted hyperintense signal changes are more diffuse and extensive than the signal changes on the T1-weighted sequences. The late myelitis stage corresponds to the pathologic stage of capsular formation. At this point there is more clearly defined marginal enhancement on contrast medium-enhanced T1-weighted images. Generally, the well-defined enhancement classically described in abscess formation is not seen until 7 days after initial presentation. The T2-weighted hyperintense signal changes become less diffuse during the late myelitis phase.71,72,76,78,81,85,88
The resolution of abnormalities on MRI is variable. With treatment, the T2-weighted hyperintense signal changes resolve over several weeks. The resolution of the T1-weighted contrast medium enhancement takes several months.96
Treatment and Prognosis
Several factors have been found to be prognostically significant. Menezes and colleagues,48 in their review of the 55 cases described between 1830 and 1977, found that prognosis was linked to the clinical presentation. Patients presenting with acute onset of symptoms had a 90% mortality rate as compared with 66% for subacute presentations and 53% for chronic presentations.48 The confounding factor within this group, however, is the use of antibiotics. Among the 55 cases reviewed, 17 were treated during the antibiotic era (after 1944). The mortality rate within this group was 23%.48 Chan and Gold corroborated this finding in their review. They found the mortality rate among patients treated without antibiotics (1830-1944) was 90% as compared with 8% among those treated with antibiotics (1977-1997).78 Aggressive treatment with antibiotics requires empirical therapy until an organism has been isolated. The choice of antibiotics for empirical therapy should be based on the suspected source of infection and then adjusted on the basis of the operative culture results.48,71,72,78 The exact duration of antibiotic therapy has not been well defined. The current recommendation is a minimum of 4 to 6 weeks of parenteral therapy.48,78,85
Despite the confounding effects of antibiotic therapy, the chronicity of the patient’s symptoms does appear to be a prognostic factor. Other studies indicate that patients presenting with acute symptoms have a worse prognosis in terms of neurologic recovery.71
Although case reports do exist of patients treated successfully without surgical decompression and drainage,90 the current recommendations are for immediate surgical treatment.47,48,71,72,78 The surgery should include laminectomies at the involved levels, intradural exploration, midline myelotomy, and irrigation and drainage of the abscess cavity. Prompt surgical drainage has a significant effect on neurologic recovery and mortality. In their review of 93 cases, Bartels and colleagues72 found that the mortality among patients treated nonoperatively was 100%. In contrast, surgically treated patients improved or had a complete recovery in 77.9% of cases. One case (1.7%) worsened after surgery, 4 (6.8%) were unchanged, and in 8 (13.6%) the patients died after surgery. The modern surgical outcomes are even better because 6 of the 8 patients who died did not receive antibiotics after surgery.72 Overall, the death of a patient diagnosed with an ISCA is most frequently due to the presence of multiple CNS abscesses and, specifically, to brain or brainstem abscesses.48,66,67,78
Mycobacterial Pathogens
Spinal Intradural Tuberculosis
Background
The initial description of spinal tuberculosis was written by Percival Pott in 1779.97 It included the surgical drainage of a spinal abscess for the treatment of paraplegia. Despite this initial surgical approach, the mainstay of treatment remained rest (in a sanatorium), nutrition, and fresh air until the discovery of antituberculosis drugs in 1944. Chemotherapeutic agents dramatically reduced the incidence and improved the prognosis of tuberculosis. The role of surgery, however, has always been a significant part of the management of spinal tuberculosis.
The term Pott disease refers to tuberculous spondylitis with any associated epidural extension but does not include intradural tuberculosis. There are four major classifications for intradural spinal tuberculosis. These include subdural tuberculomas, meningitis, arachnoiditis, and intramedullary tuberculomas. The most common type of neurotuberculosis, tuberculous meningitis, is not discussed in this chapter because it is a diffuse CNS pathologic process that presents mainly as brain and cranial nerve dysfunction.98 The initial description of a spinal intradural lesion caused by tuberculosis was published by Abercrombie in 1828.99 He specifically described the case of an intramedullary tuberculoma.
Epidemiology
In the latter half of the 20th century, the incidence of tuberculosis in developed countries steadily declined with the use of antituberculosis drugs. Beginning in 1985, however, the incidence began to rise secondary to the spread of human immunodeficiency virus/acute immunodeficiency syndrome (HIV/AIDS).100,101 The demographics of tuberculosis can still be divided between developing and developed countries.97,102–104 In developing countries with higher disease prevalence, the disease commonly affects children and young adults. It also tends to be more aggressive. In developed countries, the disease tends to afflict older individuals, as well as those with HIV/AIDS and recent immigrants.97,104,105
Pott’s disease occurs in less than 1% of patients with tuberculosis.106 It accounts for 50% of musculoskeletal tuberculosis.105 CNS involvement is even less common. Bucy and Oberhill107 found evidence of CNS tuberculomas in 1 of every 53 cases of tuberculosis. In their review of 38,510 patients treated for tuberculosis between 1935 and 1957, Arseni and Samitca found CNS tuberculomas in 210 cases. Only five cases were intramedullary tuberculomas, and another three were intradural extramedullary tuberculomas.108
The rarity of intradural infections is also exemplified by the fact that since the initial description by Abercrombie in 1828 approximately 178 cases of intramedullary tuberculomas of the spine have been reported. These have been compiled in three major reviews. Lin published a review in 1960 of 105 cases reported between 1828 and 1960.109 MacDonell and colleagues104 reviewed another 42 cases reported between 1960 and 1990. In the most recent review, Ratliff and colleagues110 compiled 31 cases published between 1990 and 1999.
The majority of these cases come from developing countries. In MacDonell and coworkers’104 study only 4 of 43 cases come from the United States and Italy. The remaining 39 come from India (26 cases), Morocco, Sri Lanka, and other developing nations.104 This distribution is also echoed in the work of Ratliff and colleagues.110 The average age at presentation in MacDonell and colleagues’104 study was 28.6 years. The average age in those cases from developing countries is younger (24.9 years), which corresponds to the more aggressive nature of infection in these regions.104,111–115 The average age in cases from developed countries is older (47.8 years).104,110,116,117
Pathophysiology
Spinal intradural tuberculosis is a secondary site of infection for Mycobacterium tuberculosis. The primary site is either in the pulmonary or genitourinary systems.102 The spread to extrapulmonary sites (e.g., CNS, vertebrae, kidneys, intestines) is most often hematogenous. As with other secondary infections, the body has already established immunity to the tubercle bacilli and, therefore, is able to mount an immune response. The response is a delayed (type IV) hypersensitivity reaction. The process involves the migration of macrophages and lymphocytes to the area of infection and formation of an early granuloma. This early granuloma progresses to an epithelioid granuloma that contains Langhans-type giant cells, epithelioid macrophages, and lymphocytes surrounding a central area of caseous necrosis. The caseous granuloma has a surrounding layer of fibroblasts that produces a collagen wall.97,102,113,116,118
There are three sources for spinal intradural tuberculosis. The most common route of entry is hematogenous spread, with the spinal lesion being the first expression of tuberculosis in the CNS.114 The second is downward extension from intracranial disease. This commonly involves tuberculous meningitis but may also be secondary to intracranial tuberculomas.114,115 The final route is direct extension into the intradural space from vertebral body involvement.119
Intradural infections within the spinal canal can be delineated by location. Exclusive of tuberculous meningitis, the other three can be further categorized into two forms. The first is circumscribed tuberculous granulomas. This category encompasses both subdural and intramedullary tuberculomas.117 The second is a proliferative encasing granulomatous arachnoiditis that leads to cord compression and vasculitis.117 Tuberculous arachnoiditis has been postulated to arise from two sources. One is as a late complication of classic tuberculous meningitis. This mechanism is by far the most commonly reported etiology of tuberculous arachnoiditis.111,117,119,120 The other mechanism lacks the antecedent tuberculous meningitis. This form of arachnoiditis may arise from a local rupture of an intramedullary tuberculoma.107,117
These cases have a thick, fibrous exudate on the spinal cord connecting the leptomeninges to the inner surface of the dura. Microscopically, the leptomeninges are thickened and fibrotic with a granulomatous infiltration.117 This process leads to compression of the spinal cord and obstruction to CSF flow. In addition, there is a rim of necrosis affecting the spinal cord to a depth of 3 to 4 mm. The spinal vessels traversing the involved levels also show infiltration and thrombosis.117 All of this is similar to the basilar meningitis found intracranially in patients with tuberculous meningitis.
The location of most spinal intradural infections is the thoracic spine. In MacDonell and colleagues’104 review, 18 of 42 patients had sufficient information in their case reports to be fully analyzed. Among these 18 patients, 65% had lesions in the thoracic spine, whereas 23% and 12% had lesions in the cervical and lumbar spine, respectively.104 This predilection for the thoracic region is also evident in other case reports.103,109,110,112,117,121,122
Although CNS tuberculomas have consistently been an uncommon extrapulmonary manifestation of tuberculosis, the discrepancy in relative prevalence between brain and spinal cord tuberculomas has been the source of much debate. Arseni and Samitca108 reviewed 38,510 patients with tuberculosis and reported 210 cases of CNS tuberculosis in 1960. They found 201 cases of intracranial tuberculomas as compared with 1 epidural, 3 subdural, and 5 intramedullary tuberculomas. The percentage of spinal intradural to intracranial tuberculomas is 3% to 95%. These percentages are similar to those derived by MacDonell and colleagues.104 In their 1990 review, they analyzed 947 patients with CNS tuberculomas. Intramedullary spinal tuberculomas account for 2.3% of cases, whereas intracranial tuberculomas constitute 97.7% of cases.104 The overall ratio, therefore, comes to 1 : 42 for spinal versus intracranial tuberculomas. This ratio is significant because it reflects the ratio of spinal cord to brain weight (27 g to 1275 g), which is 1 : 47.104 Although the exact pathophysiologic mechanism remains unknown, the distribution of neural tissue and its blood flow may explain the relative infrequency of spinal intradural tuberculomas.
Clinical Presentation
The most commonly described presentation of spinal intradural tuberculosis is one of subacute to chronic spinal cord compression.103,104,112,117 MacDonell and colleagues104 found the mean duration for symptoms of motor and sensory dysfunction to be 2.3 and 4.9 months, respectively. Other case reports also point to a gradual onset of symptoms.103,110,112,113,116,120–122 Those reports in which the onset of symptoms is acute are in children living in developing countries.114,115 They commonly present with back pain and motor dysfunction.
†References 103, 104, 110, 112, 114, 115, 120.
103,104,110,112–116,120,121 MacDonell and colleagues104 found paraparesis/paraplegia to be present in 94% of the cases they reviewed. Sensory changes affecting either the lateral or dorsal columns are also quite common.* The extent of sensory loss is variable, as are the sensory modalities affected. Bowel or bladder dysfunction is also quite common (66% of cases).104 Back pain, however, tends to be a less frequent symptom in these patients, occurring in approximately 33% of cases.† The constitutional symptoms that often accompany tuberculosis tend to be less commonly found in patients with intradural disease. They occur in approximately a third of cases.104 The most frequent symptom tends to be fever/chills.103,104,112–114,121 Night sweats and weight loss are far less common.
Intradural lesions are secondary lesions, with approximately 69% of patients having either a history of or active pulmonary tuberculosis.103,104 In addition to pulmonary tuberculosis, there are several reports of intradural tuberculosis secondary to genitourinary tuberculosis either with concurrent pulmonary disease116,122 or without it.121 Patients may also present without any extraneural disease. MacDonell and colleagues, Ratliffe and colleagues, and Vlcek and colleagues104,110,117 published case reports in which the patients had no systemic manifestations of tuberculosis and were purified protein derivative (PPD) negative.
This pattern of presentation is applicable to subdural and intramedullary tuberculomas, as well as arachnoiditis. However, the patients with arachnoiditis do have some differences in terms of their presentation. One is that they more commonly have a history of tuberculous meningitis than patients with tuberculomas.111,117,119,120 The other major difference is that patients with arachnoiditis tend to have a two-stage history. They initially have a chronic myelopathy that progresses over several months. The second stage, however, is a more rapid loss of motor and sensory function (usually less than 5 days’ duration).120
Laboratory Studies
In the setting of intradural tuberculosis, laboratory tests tend to aid in making the diagnosis but none is consistently abnormal. PPD tests are frequently negative even in immune-competent patients.104,110,120,122 In their review, MacDonell and colleagues104 found that only a third of cases had a positive PPD test. An elevated white blood cell count (WBC) count tends to be an infrequent finding as well. MacDonell and colleagues104 found only eight cases in which the WBC count was reported in their patients. Among these cases, only two patients had an elevated WBC count. Of several recent cases, only Rhoton and colleagues116 found an elevated WBC count. The other patients all had normal WBC counts.103,110,112–115,120–122 The ESR does appear to be the most consistently abnormal laboratory study among these patients. In almost all cases with a reported ESR, the rate is abnormal.104,112,114–116,120,121 It ranges from 18 to 123 mm/hr, with a mean of 68 mm/hr.104 There are, however, cases with normal sedimentation rates.122
The CSF was examined in the majority of reported cases. In the CSF profile, the two consistently abnormal values were the protein levels and the WBC count. Some cases have had normal protein levels.104,122 The majority of cases, however, have elevated levels. The mean is approximately 200 mg/dL.104 In several other published case reports, the protein levels range from 91 to 990 mg/dL.103,112–116,121 The cases of arachnoiditis, however, often have much higher protein levels (>1000 mg/dL).120 The WBC count in the CSF is also elevated in a significant majority of cases. Exclusive of two patients with normal counts,104,122 the range of WBCs in the CSF is from 20 to 570/mm3 (most mononuclear cells).103,112–116,120–122 The CSF cultures and the acid-fast stain are almost invariably negative.104,112,114–116,121,122 Nevertheless, it is important to perform an acid-fast stain and culture because in some cases they come back with diagnostic results.103
In addition to a complete blood cell count, complete metabolic panel, ESR, and full CSF analysis, a complete workup of a patient suspected of having an intradural tuberculous infection requires both sputum and urine cultures. These are the two primary sites of infection that can lead to CNS disease. Approximately 70% of cases have either pulmonary or genitourinary disease at the time of presentation.104 A positive sputum or urine culture makes diagnosis and treatment simpler. It also allows for an assessment of the contagiousness of the patient.
Imaging Studies
Imaging of patients should begin with a chest radiograph to evaluate the patient for pulmonary disease. Further evaluation should include radiographs of the spine at the levels indicated by the patient’s neurologic examination. Plain radiographs are useful to exclude the presence of Pott disease. Patients with intradural infections do not have abnormal plain radiographs.103,104,112,114,116,122 Although there are individual cases of intradural disease associated with tuberculous spondylitis,119 this is an extremely rare occurrence. Gupta and colleagues111 presented a series of 20 cases of intradural tuberculosis without any patient having Pott disease. Nussbaum and colleagues106 also reported that in their 20-year experience with spinal tuberculosis, only 4 of their 29 patients had intradural disease and none of the 4 had evidence of bone involvement.
Standard CT scans of the spine through the region of interest are not clinically useful. There are several cases, however, in which a plain axial CT scan showed evidence of an enlarged spinal cord.104,116 Myelography and CT myelography were the principal imaging modalities in evaluating patients before MRI. Currently, they are of use in patients who cannot have an MRI. There are a variety of findings described in the setting of intraspinal tuberculomas. Although normal studies are reported, the majority of patients do show abnormalities.122 The range of findings includes widening of the cord, overt intramedullary mass, partial spinal block, complete spinal block, and extramedullary compressive lesion.104,113 The findings in the setting of tuberculous arachnoiditis also include partial or complete blockage of CSF flow.122 In addition, the images in the region of the block show irregular flow of contrast agent. Clumping of nerve roots is common in the lumbar region.111,122
The imaging modality of choice in the setting of a compressive spinal cord lesion is contrast medium-enhanced MRI. The scan should be focused on the region of interest on the basis of the patient’s clinical findings, but if suspicion of tuberculosis is high, the imaging studies should include the entire neuraxis to exclude other (clinically silent) lesions.115,116 Rhoton and colleagues116 reported the initial description of the MRI characteristics of an intramedullary tuberculoma in 1988. There is no consensus regarding the MRI characteristics of spinal tuberculomas. This may be because tuberculomas are evolving granulomas that have variable signal characteristics at different stages in their evolution.113 The most common description is of an isointense to hypointense lesion on both T1- and T2-weighted imaging that with gadolinium has either homogeneous or ring enhancement.103,111,113,114,116 The description by Rhoton and colleagues involves two scans performed on the same patient over a 6-week period. The initial scan shows cord enlargement, but the lesion was isointense to the cord. The follow-up scan done 6 weeks later shows a hypointense nodular lesion on both T1- and T2-weighted imaging.116 Gupta and colleagues111 reviewed the magnetic resonance images of eight patients with tuberculomas. The signal intensities on T1-weighted imaging were isointense for three and hypointense for five. The T2-weighted images were isointense for three and hyperintense for three. The enhancement patterns varied between nodular and ring enhancement.111 Enhancement patterns may reflect the evolution of the tuberculoma. Early granulomas with active inflammation but without caseating necrosis will homogeneously enhance (nodular), whereas late granulomas with caseating necrosis have ring enhancement.113 The other consistent finding in the cases is cord swelling.103,104,113,114,116,121,122
The MRI findings in cases of tuberculous arachnoiditis differ considerably from those seen with subdural and intramedullary tuberculomas. Significant findings include meningeal enhancement (most common), obliteration of subarachnoid space, CSF loculation, loss of spinal cord outline, clumping of nerve roots (lumbar spine), and irregular spinal cord surface.111 These patients may also have associated cord infarctions due to vasculitis. They may also have cord edema and formation of syringomyelia cavities.111
Treatment and Prognosis
Medical management of intradural spinal tuberculosis is generally advocated as the first line of treatment.103,104,112–114 This involves antituberculous drug therapy for an extended course with three to four medications.104,110,114,115,120,122 The exact duration of treatment is not delineated, but most patients are treated for a minimum of 1 year.103,104,113,114,116,122 The role of corticosteroids is also not fully defined.104 Some groups strongly advocate their use,103,113–115 whereas others use corticosteroids in the setting of acute deterioration112 and some do not use them at all.104,110,116,122
Although medical therapy is used as the first line of treatment, the majority of reported cases include surgical intervention. MacDonell and colleagues104 found that among 18 cases reviewed, 16 patients underwent surgery. There are two basic indications for surgery. The first is to obtain a diagnosis in the setting of a patient presenting with subacute to chronic cord compression with an enhancing intradural lesion on MRI but no extraneural or CSF evidence of tuberculosis. The need to obtain a diagnosis and rule out other pathologic processes (e.g., tumor, bacterial infection) is one reason to proceed with surgery.104,110,116,120 The other reason for surgical intervention is the failure of medical management. There are multiple reports of patients diagnosed as having subdural or intramedullary tuberculomas who neurologically deteriorated during the course of their medical treatment.103,112,113,121 The most common surgical approach is an excision of the tuberculoma.103,104,110,112,113,116 However, simple drainage of the tuberculoma has also been performed with good results.121 In cases of arachnoiditis, the surgical approach is slightly different. These patients can be treated with decompressive laminectomies if there is a need to treat them for neurologic deterioration. If obtaining a diagnosis is the goal of the surgery, then a laminectomy and biopsy of the leptomeninges would be sufficient.120
The overall prognosis for patients with isolated spinal intradural tuberculosis is good. The majority of patients (65%) improves neurologically.104 MacDonell and colleagues104 found that among 17 patients reviewed, 11 regained partial or complete neurologic function, 2 patients were unchanged, and 4 died. The deaths were due to disseminated disease (pulmonary) or lack of antituberculous drug therapy after surgery.104 Case reports published since the 1990 review by MacDonell and colleagues show an even better prognosis. In 10 cases reviewed, 9 patients either had a complete recovery114,115,122 or were ambulating independently.103,110,112,113,116,121 There was one death in a patient with arachnoiditis and concomitant tuberculous meningitis. She died as a result of the meningitis.120 There is no clear difference in outcome for patients who undergo surgery versus those who are treated medically.104 In the cases reviewed by MacDonell and colleagues, both medically and surgically managed patients had a good outcome. Among the 15 patients who had surgery, 9 had good outcomes, 2 experienced no change, and 4 died.104 Among the cases reported since 1990, 7 patients were treated medically and 3 had surgery as the first line of therapy. Four of the seven medically managed patients failed therapy and required surgery.103,112,113,121 The other three successfully completed their medical therapy and are all neurologically normal.114,115,122 The four who failed medical therapy and underwent surgery had excellent outcomes and are walking independently. Among the three patients who had surgery before medical treatment, two regained the ability to walk independently.110,116
Fungal Pathogens
Spinal Intradural Fungal Infections
Background
Fungi are ubiquitous organisms that, in general, have low virulence. They can be divided into two groups: true pathogens and opportunistic pathogens. The true pathogens cause infection in humans with normal immune defenses. These organisms include Coccidioides immitis, Cryptococcus neoformans, Blastomyces dermatitidis, and Histoplasma capsulatum.123 The other group of organisms is opportunistic pathogens that cause infections in immune-compromised hosts. These fungi include Candida albicans, Aspergillus species, Nocardia asteroides, and those causing mucormycoses (Zygomycetes class).123 Immune suppression can be the result of immune suppressive drugs for transplantation,124 chemotherapy/malignancy,125–127 chronic corticosteroid use,128 intravenous drug abuse,129 HIV/AIDS,130 diabetes mellitus,131,132 alcoholism,129 and congenital diseases of cellular immunity.133
Despite the omnipresent nature of fungi in the environment and the high prevalence of a compromised immune state in the modern population, spinal intradural fungal infections remain a rare event. Several large studies reviewing fungal CNS infections have confirmed this fact.123,134 Young and colleagues123 found that among 78 cases of fungal CNS infections, only 2 cases were spinal infections and neither was intradural. Fungal infections of the spine are most often extradural infections involving the disc space and vertebral bodies.135 Overall, the literature on intradural fungal infections is composed of single case reports and small case series.124–132,136–138
Pathophysiology
Intradural infections can be classified into two major categories: extramedullary and intramedullary. Extramedullary infections most often present as focal areas of inflammation affecting the leptomeninges (i.e., arachnoiditis).124,129–132,137 A second manifestation of extramedullary disease is granulomas involving the leptomeninges.126 Lastly, extramedullary infections can involve fungal thromboemboli in the arterial supply of the spinal cord.125,127,130,136 In contrast to the variety of pathologic manifestations of extramedullary disease, intramedullary fungal infections are granulomas of the spinal cord.138
The histopathology of arachnoiditis reveals fibrosis and a chronic granulomatous process containing giant cells.124,129,131,137 Some cases may also involve more of an acute inflammatory process with cell debris and a fibrinous exudate.128,132 Fungal elements may be seen on either routine microscopic preparations124,130 or, more specifically, on Gomori methenamine silver stain.128,131,137
Kingsley and colleagues126 published a case report of an extramedullary granuloma. These were distinct circular white masses apparently adherent to spinal cord or nerve roots.126 A conspicuous lack of meningeal irritation was found.126 The histopathology of these lesions was not described except to note that fungal elements were present within them.126
One type of intradural extramedullary infection is unique to fungi: septic thromboemboli to the arterial supply of the spinal cord. These cases may involve minimal to no evidence of arachnoiditis.125,127,136 In other cases, however, the septic arterial thrombus may be secondary to direct vessel invasion from acute purulent arachnoiditis.130,132 On microscopic examination, the arterial supply to the spinal cord is occluded by fibrinoid thromboemboli that contain fungal elements. This affects vessels ranging from the anterior spinal artery127,136 to small arterioles and capillaries.125 The associated spinal cord infarct may show evidence of fungal invasion.125,127
The intramedullary fungal infections are granulomas of the spinal cord. These lesions, similar to other fungal infections of the CNS, are necrotic, noncaseating, highly cellular lesions with Langhans multinucleated giant cells.128,138
Aspergillus species are the most commonly cited organisms both among immune competent135,136 and immune-suppressed patients.124,126,127,129–131 Other (Box 90–3) organisms found in the literature include Candida albicans,125 Histoplasma capsulatum,138 Rhizopus species,132 and Cryptococcus neoformans.121
Although the source of these infections is often unknown,124,129,131,136 the most commonly identified primary site of infection is pulmonary.125–128,130,132 It is therefore important to examine patients for signs and symptoms of pulmonary disease. The other commonly found source of intradural infection is direct extension from either vertebral osteomyelitis or epidural abscess.124,128,130
Presentation
The most consistent facet of the clinical presentation of intradural fungal infections is a focal neurologic deficit secondary to spinal cord compression,* infarction,125,127,136 or both.130,132 The neurologic deficit is often preceded by new onset of localizable neck/back pain.124,126,128–130,132,137,138 The rapidity of onset of these symptoms is variable, ranging from a few days127–130136 to more than 3 months.129,138 Most commonly, however, these patients present with complaints of weakness starting 2 to 8 weeks before presentation.124–126,129,131,132,137
Fever, although an inconsistent finding among these patients, is present in the initial symptomatology of a majority of cases.†References 124, 126, 128–130, 132, 136, 137.
† These patients lack symptoms of acute meningitis (e.g., nuchal rigidity, altered mental status, headaches). Other reported systemic manifestations include malaise, anorexia, and weight loss.124,126,129
The site of infection is predominantly within the thoracic spine.124,126–132 The second most common site is the cervical spine.125,129,136,138 There are also cases involving diffuse lesions of the entire spinal canal,137 as well as isolated lesions of the lumbar spine.129
Laboratory Evaluation
The WBC count may be elevated.128,132,136 Often, however, it is not reported in the case reports.125,126,130,131,137,138 Given the compromised immune status of many of these patients, they may also present with leukopenia.124,127 In only two cases was either the ESR or C-reactive protein (CRP) level reported, and they were elevated in both.136,137
The most important laboratory evaluation in these patients is the CSF analysis. The primary process is not meningitis but a parameningeal inflammatory process. The CSF profile, therefore, shows elevated protein levels associated with leukocytosis.128,130–132,137 The glucose levels are usually normal in such cases. There are other profiles reported, however, in these cases. One is isolated leukocytosis with normal protein levels.126,129 The other is an elevated protein level with normal cell counts.124,129 One key feature found in all case reports is the lack of growth on CSF cultures. This is even true when cultures are specifically aimed at fungal organisms.128–132137 In cases in which the infection is isolated to the blood vessels of the spinal cord, the CSF may be entirely normal.136
Imaging
It is important to begin the evaluation of these patients with plain radiographs of the spine to rule out bony pathologic processes such as discitis/osteomyelitis because direct extension is a source of intradural infection.124,128,130 However, when case reports present the results of plain radiographs, they are universally normal.124,126,129,131,136,137
Before the MRI era, myelography was the gold standard technique for the diagnosis. In cases of arachnoiditis the most consistent finding is a block in the myelogram.129,131,137 This block may be partial or complete. If it is a complete block, a cisternal puncture may be necessary to inject contrast agent into the cervical spine to find other lesions above the level of the initial block.137 Several cases of a complete block affecting the lumbar region resulted in no egress of CSF from the lumbar puncture.129
The cases involving spinal cord necrosis have had variable findings on the myelogram. One report found dilatation of the spinal cord consistent with swelling of the cord.125 Another report found an entirely normal myelogram in the setting of spinal cord necrosis due to septic thromboemboli.136
Enlargement of the spinal cord is also a myelographic finding reported in the case of a fungal intramedullary granuloma.138 Intradural extramedullary granulomas have been reported to result in myelographic defects surrounding the spinal cord.126
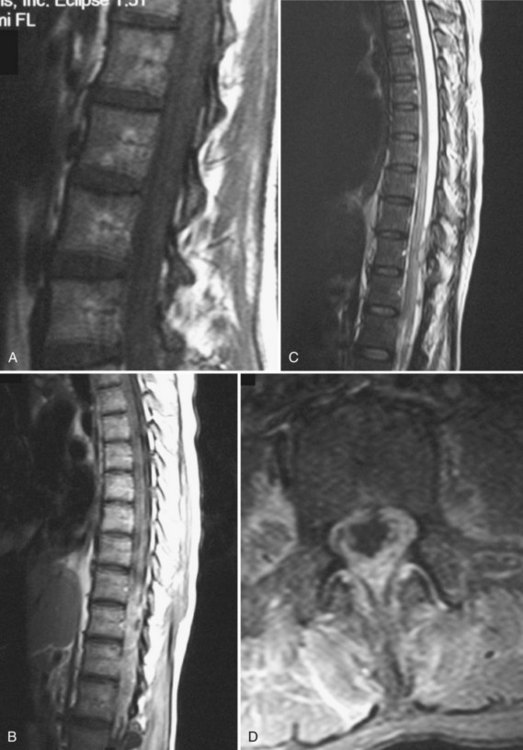
The current gold standard in evaluating patients suspected to have intradural fungal infections is an MRI with and without gadolinium (Fig. 90–2). Because of the small number of cases with any description of the MRI findings, no definitive MRI characteristics exist for these infections. In the case of arachnoiditis, Cravens and colleagues131 found a cyst surrounding the spinal cord that was hypointense on T1-weighted imaging with associated thickening of the meninges around the cord. Two other case reports, however, could not find any evidence of arachnoiditis on MRI.130,132 The cases of intramedullary lesions have been reported in two other reports. Pfausler and colleagues136 described the findings in the case of septic thromboemboli to the spinal cord with resultant cord infarction. They found hyperintensity of the cervical spinal cord on T2-weighted and proton density sequences associated with an increase in the size of the cord. There was no enhancement of the cord on T1-weighted sequences.135 Voelker and colleagues138 described the findings in a case of an intramedullary granuloma in the cervical cord. They found the abnormality to be a hypointense ring surrounding an area of hyperintensity on T2-weighted images.138
Treatment
The most commonly cited approach to treating these cases is surgical decompression/débridement followed by medical therapy.124,126,129–131,132,137 Given the difficulty of diagnosing intradural fungal infections, one of the main reasons for operating on these patients is the need for a diagnosis by means of direct tissue culture and histopathology.124,128,129,131,137 The other major indication for operating is to obtain adequate decompression and débridement because these patients often present with spinal cord compression.124,126,129,131,132,138 In cases involving granulomas (either intramedullary or extramedullary) the surgery may involve a resection of a discrete lesion.126,138
As important as surgery is the long-term medical treatment of these patients. They require extended intravenous treatment with amphotericin B. The extent of treatment, however, remains uncertain, ranging from 3 to 9 months.124,126,128–132,137 Some groups also recommend intrathecal administration of amphotericin B.126,129,131 Adjuvant medical therapy with 5-fluorocytosine or rifampicin has also been recommended.124,126,129,137
Prognosis
The overall prognosis for these cases is dismal. Since 1978, 14 cases have been reported, with 10 patients eventually dying.124–132,136–138 All four survivors were aggressively treated with surgical decompression and antifungal medications.129,131,137,138 Three of the four survivors made complete recoveries.129,137,138 Among the 10 mortalities, in only 3 cases were the patients treated with both aggressive surgery and medical therapy.124,126,129
Pearls/Pitfalls
Key Points
1 Bartels RH, de Jong TR, Grotenhuis JA. Spinal subdural abscess: Case report. J Neurosurg. 1992;76:307-311.
2 DiTullio MVJr. Intramedullary spinal abscess: A case report with a review of 53 previously described cases. Surg Neurol. 1977;7:351-354.
3 MacDonell AH, Baird RW, Bronze MS. Intramedullary tuberculomas of the spinal cord: Case report and review. Rev Infect Dis. 1990;12:432-439.
4 Menezes AH, Graf CJ, Perret G. Spinal cord abscess: A review. Surg Neurol. 1977;8:461-467.
1 Sittig O. Metastatischer Rückenmarksabscess bei septichem Abortus. Z Neurol Psychiatr. 1927;107:146-151.
2 Bennett AE, Keegan JJ. Circumscribed suppurations of the spinal cord and meninges: Report of a case of subdural abscess with functional recovery following operation. Arch Neurol Psychiatry. 1928;19:329-333.
3 Arnett JH. Meningococcic (later also staphylococcic) meningitis, low spinal subarachnoid block, abscess, laminectomy, recovery. Med Clin North Am. 1930;13:1051-1062.
4 Walker AE, Bucy PC. Congenital dermal sinuses: A source of spinal meningeal infection and subdural abscesses. Brain. 1934;57:401-421.
5 Chi CY. Intraspinal subdural abscess. Chin Med J. 1936;50:921-926.
6 Abott KH. Acute subdural spinal abscess: Report of case. Bull Los Angeles Neurol Soc. 1940;5:227-231.
7 Freedman H, Alpers BJ. Spinal subdural abscess. Arch Neurol Psychiatry. 1948;60:49-60.
8 Negrin JJr, Clark RAJr. Pyogenic subdural abscess of the spinal meninges: Report of two cases. J Neurosurg. 1952;9:95-100.
9 Dús V. Spinal peripachymeningitis (epidural abscess): Report of 8 cases. J Neurosurg. 1960;17:972-983.
10 Schiller F, Shadle OW. Extrathecal and intrathecal suppuration: Report of two cases and discussion of the spinal subdural space. Arch Neurol. 1962;7:33-36.
11 Hirson C. Spinal subdural abscess. Lancet. 1965;2:1215-1217.
12 Hesketh KT. Subdural abscess of the lumbar cord: Report of a patient with recurring paraplegia. Paraplegia. 1965;3:161-164.
13 Raskind R, Weiss SR. Subdural and extradural abscess with unusual complication and complete recovery. Int Surg. 1969;52:440-443.
14 Reddy DR, Rao GN, Krishnamurthy D. Pneumococcal spinal subdural abscess (a case report). J Postgrad Med. 1973;19:190-192.
15 Fraser RAR, Ratzan K, Wolpert SM, et al. Spinal subdural empyema. Arch Neurol. 1973;28:235-238.
16 Dacey RG, Winn HR, Jane JA, et al. Spinal subdural empyema: Report of two cases. Neurosurgery. 1978;3:400-403.
17 Kumar S, Gulati DR. Spinal abscesses: A report on 22 cases. Neurol India. 1978;26:193-195.
18 Patronas NJ, Marx WJ, Duda EE. Radiographic presentation of spinal abscess in the subdural space. AJR Am J Roentgenol. 1979;132:138-139.
19 Heindel CC, Ferguson JP, Kumarasamy T. Spinal subdural empyema complicating pregnancy: Case report. J Neurosurg. 1974;40:654-656.
20 Grubel G. Bericht über ein spinales subdurales Empyem. Acta Neurochir. 1970;22:213-216.
21 Schnegg JF, Glauser M, de Tribolet N. Infection of a lumbar dermoid cyst by an anaerobic peptococcus. Acta Neurochir. 1981;58:127-129.
22 Penkert G, Fleidner E. Spinales subdurales Empyema nach Stromverletzung. Unfallheilkunde. 1982;85:473-477.
23 Probst C, Wicki G. Spinale subdurale Empyeme und Abszesse. Schweiz Arch Neurol Neurochir Psychiatr. 1984;134:53-70.
24 Scully RE, Mark EJ, McNeely BU. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises: Case 47-1984. N Engl J Med. 1984;311:1365-1370.
25 Theodotou B, Woosley RE, Whaley RA. Spinal subdural empyema: Diagnosis by spinal computed tomography. Surg Neurol. 1984;21:610-612.
26 Lomholdt Knudsen L, Voldby B, Stagaard M. Computed tomographic myelography in spinal subdural empyema. Neuroradiology. 1987;29:99.
27 Abramovitz JN, Batson RA, Yablon JS. Vertebral osteomyelitis: The surgical management of neurologic complications. Spine. 1986;11:418-420.
28 Harris LF, Haws FP, Triplett JNJr, et al. Subdural empyema and epidural abscess: Recent experience in a community hospital. South Med J. 1987;80:1254-1258.
29 Butler EG, Dohrmann PJ, Stark RJ. Spinal subdural abscess. Clin Exp Neurol. 1988;25:67-70.
30 Kurokawa Y, Hashi K, Fujishige M, et al. [Spinal subdural empyema diagnosed by MRI and recovery by conservative treatment.]. No To Shinkei. 1989;41:513-517. (In Japanese)
31 Lownie SP, Ferguson GG. Spinal subdural empyema complicating cervical discography. Spine. 1989;14:1415-1417.
32 Takenaka K, Kobayashi H, Niikawa S, et al. [Spinal subdural abscess-report of a case and a review of the literature of 43 cases.]. No To Shinkei. 1989;41:331-336. (In Japanese)
33 Harries-Jones R, Hernandez-Bronchud M, Anslow P, et al. Meningitis and spinal subdural empyema as a complication of sinusitis [letter]. J Neurol Neurosurg Psychiatry. 1990;53:441.
34 Hershkowitz S, Link R, Ravden M, et al. Spinal empyema in Crohn’s disease. J Clin Gastroenterol. 1990;12:67-69.
35 Bartels RH, de Jong TR, Grotenhuis JA. Spinal subdural abscess: Case report. J Neurosurg. 1992;76:307-311.
36 Levy ML, Weider BH, Schneider J, et al. Subdural empyema of the cervical spine: Clinicopathological correlates and magnetic resonance imaging: Report of three cases. J Neurosurg. 1993;79:929-935.
37 Sathi S, Schwartz M, Cortez S, et al. Spinal subdural abscess: Successful treatment with limited drainage and antibiotics in a patient with AIDS. Surg Neurol. 1994;24:424-427.
38 Schneider P, Givens TG. Spinal subdural abscess in a pediatric patient: A case report and review of the literature. Pediatr Emerg Care. 1998;14:22-23.
39 Chen CY, Lin KL, Wang HS, et al. Dermoid cyst with dermal sinus tract complicated with spinal subdural abscess. Pediatr Neurol. 1999;20:157-160.
40 Baker AS, Ojemam RG, Swartz MN, et al. Spinal epidural abscesses. N Engl J Med. 1975;293:463-468.
41 Hlavin ML, Kminski HJ, Ross JS, et al. Spinal epidural abscess: A ten-year perspective. Neurosurgery. 1990;27:177-184.
42 Osborne AG. Nonneoplastic Disorders of the Spine and Spinal Cord. In: Osborne AG, editor. Diagnostic Neuroradiology. St. Louis: Mosby; 1994:826.
43 Krauss WE, McCormick PC. Infections of the dural spaces. Neurosurg Clin North Am. 1992;3:421-433.
44 Martin RJ, Yuan HA. Neurosurgical care of spinal epidural, subdural, and intramedullary abscesses and arachnoiditis. Orthop Clin North Am. 1996;27:125-136.
45 Hart J. Case of encysted abscess in the center of the spinal cord, 5. Dublin Hospital Rep. 1830.
46 Arzt PK. Abscess within the spinal cord: Review of the literature and report of three cases. Arch Neurol Psychiatry. 1944;51:533-543.
47 DiTullio MVJr. Intramedullary spinal abscess: A case report with a review of 53 previously described cases. Surg Neurol. 1977;7:351-354.
48 Menezes AH, Graf CJ, Perret G. Spinal cord abscess: A review. Surg Neurol. 1977;8:461-467.
49 Manfredi M, Bozzao L, Frasconi F. Chronic intramedullary abscess of the spinal cord: Case report. J Neurosurg. 1970;33:352-355.
50 Kendall MJ, Clark SW, Smith WT. Spinal abscess due to Listeria monocytogenes in a patient with hepatic cirrhosis. J Pathol. 1972;107:9-11.
51 Rengachary SS, Kugler K, Watanabe I. Intramedullary abscess following transpharyngeal stab injury. Int Surg. 1975;60:298-300.
52 Beau JR, Walsh JW, Blacker HM. Cervical dermal sinus and intramedullary spinal cord abscess: Case report. Neurosurgery. 1979;5:60-62.
53 Fortuna A, Contratti F, DiLorenzo N. Cervical intramedullary abscess: Expiration by means of microsurgical techniques. J Neurosurg Sci. 1979;23:159-162.
54 Morrison CR, Brown J, Gooding RS. Spinal cord abscess caused by Listeria monocytogenes. Arch Neurol. 1980;37:242-244.
55 Maurice-Williams RS, Pamphilon D, Coakham HB. Intramedullary abscess-a rare complication of spinal dysraphism. J Neurol Neurosurg Psychiatry. 1980;43:1045-1048.
56 Blacklock JB, Hood TW, Maxwell RE. Intramedullary cervical spinal cord abscess: Case report. J Neurosurg. 1982;57:270-273.
57 Marwah RK, Khosla VK, Agarwal KC, et al. Intramedullary spinal cord abscess. Indian Pediatr. 1982;22:71-74.
58 Koppel BS, Daras M, Duffy KR. Intramedullary spinal cord abscess. Neurosurgery. 1990;26:145-146.
59 Maheswaran J, Rathinam PV, Lakshimi S, et al. Intramedullary spinal abscess complicating a dermal sinus. J Indian Med Assoc. 1991;89:227.
60 Carus MEM, Anciones B, Castro A, et al. Intramedullary spinal cord abscess. J Neurol Neurosurg Psychiatry. 1992;55:225-226.
61 Benzil DL, Epstein MH, Knuckey NW. Intramedullary epidermoid associated with an intramedullary spinal abscess secondary to a dermal sinus. Neurosurgery. 1992;30:118-120.
62 Rogg JM, Benzil DL, Haas RL, et al. Intramedullary abscess: An unusual manifestation of a dermal sinus. Am J Neuroradiol. 1993;14:1393-1395.
63 Babu R, Jafa JJ, Huang PP, et al. Intramedullary abscess associated with a spinal cord ependymoma: Case report. Neurosurgery. 1992;30:121-124.
64 Amacher AL. Intramedullary epidermoid associated with an intramedullary spinal cord abscess secondary to a dermal sinus [letter]. Neurosurgery. 1992;31:979.
65 Tewari MK, Devi BI, Thakur RC, et al. Intramedullary spinal cord abscess: A case report. Childs Nerv Syst. 1992;38:287-290.
66 Erlich JH, Rosenfeld JV, Fuller A, et al. Acute intramedullary spinal cord abscess: Case report. Surg Neurol. 1992;38:287-290.
67 King SJ, Jeffree MA. MRI of an abscess of the cervical spinal cord in a case of Listeria monocytogenes meningoencephalomyelitis. Neuroradiology. 1993;35:495-496.
68 Hardwidge C, Palsingh J, Williams B. Pyomelia: An intramedullary spinal abscess complicating lumbar lipoma with spina bifida. Br J Neurosurg. 1993;7:419-422.
69 Gurbani SG, Cho CT, Lee KR. Staphylococcus epidermidis meningitis and an intraspinal abscess associated with a midthoracic dermal sinus tract. Clin Infect Dis. 1994;19:1138-1140.
70 Cokea F, Meco O, Arasil E, et al. An intramedullary dermoid cyst abscess due to Brucella abortus biotype 3 at T11-L2 spinal levels. Infection. 1994;22:359-360.
71 Byrne RW, Von Roenn KA, Whisler WW. Intramedullary abscess: A report of two cases and a review of the literature. Neurosurgery. 1994;35:321-326.
72 Bartels RHMA, Gonera EG, van der Spek JAN, et al. Intramedullary spinal cord abscess: A case report. Spine. 1995;242:153-156.
73 Pfadenhauer K, Rossmanith T. Spinal manifestation of neurolisteriosis. J Neurol. 1995;242:153-156.
74 Chu JY, Montaner W, Willinksy RA. Listeria spinal cord abscess-clinical and MRI findings. Can J Neurol Sci. 1996;23:220-223.
75 Brasme CDL, Peruzzi P, Bertault R, et al. Intramedullary abscess of the spinal cord in a patient with a right-to-left shunt: Case report. Clin Infect Dis. 1997;24:89-90.
76 Sverzut JM, Laval C, Smadja P, et al. Spinal cord abscess in a heroin addict: Case report. Neuroradiology. 1998;40:455-458.
77 Ushikoshi S, Koyanagi I, Hilda K, et al. Spinal intrathecal actinomycosis: A case report. Surg Neurol. 1998;50:221-225.
78 Chan CT, Gold WL. Intramedullary abscess of the spinal cord in the antibiotic era: Clinical features, microbial etiologies, trends in pathogenesis, and outcomes. Clin Infect Dis. 1998;27:619-626.
79 Derkinderen P, Duval X, Bruneel F, et al. Intramedullary spinal cord abscess associated with cervical spondylodiskitis and epidural abscess. Scand J Infect Dis. 1998;30:618-619.
80 Morandi X, Mercier P, Fournier HD, et al. Dermal sinus and intramedullary spinal cord abscess: Report of two cases and review of the literature. Childs Nerv Syst. 1999;15:202-208.
81 Desai KI, Muzumdar DP, Goel A. Holocord intramedullary abscess: An unusual case with review of literature. Spinal Cord. 1999;37:866-870.
82 Mukunda BN, Shekar R, Bass S. Solitary spinal intramedullary abscess caused by Nocardia asteroides. South Med J. 1999;92:1223-1224.
83 Bingol A, Yuceman N, Meco O. Medically treated intraspinal “Brucella” granuloma. Surg Neurol. 1999;52:570-576.
84 Bavdekar SB, Rao N, Kamat JR. Intramedullary spinal cord abscess. Indian J Pediatr. 1997;64:428-431.
85 Weng TI, Shih FY, Chen WJ, et al. Intramedullary abscess of the spinal cord. Am J Emerg Med. 2001;19:177-178.
86 Chidambaram B, Balasubramaniam V. Intramedullary abscess of the spinal cord. Pediatr Neurosurg. 2001;34:43-44.
87 Durmaz R, Atasoy MA, Durmaz G, et al. Multiple nocardial abscesses of cerebrum, cerebellum and spinal cord, causing quadriplegia. Clin Neurol Neurosurg. 2001;103:59-62.
88 Thome C, Krauss JK, Zevgaridis D, et al. Pyogenic abscess of the filum terminale: Case report. J Neurosurg Sci. 2001;95(1 Suppl):100-104.
89 Elmac I, Kurtkaya O, Peker S, et al. Cervical spinal cord intramedullary abscess: Case report. J Neurosurg Sci. 2001;45:213-215.
90 Lascaux AS, Chevalier X, Brugieres P, et al. Painful neck stiffness secondary to an intramedullary abscess of the spinal cord in a HIV infected patient: A case report. J Neurol. 2002;249:229-230.
91 Helvaci M, KasIrga E, Cetin N, et al. Intramedullary spinal cord abscess suspected of Brucella infection. Pediatr Int. 2002;44:446-448.
92 Courville CB. Pathology of the Central Nervous System Mountainview, CA. 3rd ed. 1950:pp 191-192.
93 Batson OV. The functions of vertebral veins and their role in the spread of metastasis. Ann Surg. 1941;112:138-149.
94 Hoche A. Experimentelle Beitrage zur Pathologie des Rückenmarkes. Arch Psych Nervenkr. 1899;209:975-1108.
95 Galkin M. Zur Methodik der Injektion des Lymphysystems beim Subarachnoidalraum aus. Ztschr D Ges Exper Med. 1930;74:482-489.
96 Murphy KJ, Brunberg JA, Quint DJ, et al. Spinal cord infection: Myelitis and abscess formation. Am J Neuroradiol. 1998;19:341-348.
97 Boachie-Adjei O, Squillante RG. Tuberculosis of the spine. Spinal Infect. 1996;27:95-103.
98 Dehoux E. Urinary retention revealing a tuberculoma of conus medullaris in a patient with intracranial tuberculosis: Case report. Spinal Cord. 1996;34:630-632.
99 Abercrombie J. Pathological and Practical Researches on Diseases of the Brain and Spinal Cord. Edinburgh: Waugh and Innes; 1828.
100 Barnes PF, Bloch AB, Davidson PT, et al. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644-1650.
101 Cantwell MF, Snider DEJr, Cauthen GM, et al. Epidemiology of tuberculosis in the United States, 1985 through 1992. JAMA. 1994;272:535-539.
102 Moorthy S, Prabhu NK. Spectrum of MR imaging findings in spinal tuberculosis. AJR Am J Roentgenol. 2002;179:979-983.
103 Citow JS, Ammirati M. Intramedullary tuberculoma of the spinal cord: Case report. Neurosurgery. 1994;35:327-330.
104 MacDonell AH, Baird RW, Bronze MS. Intramedullary tuberculomas of the spinal cord: Case report and review. Rev Infect Dis. 1990;12:432-439.
105 Moore SL, Rafii M. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North Am. 2001;39:343-355.
106 Nussbaum ES, Rockswold GL, Bergman TA, et al. Spinal tuberculosis: A diagnostic and management challenge. J Neurosurg. 1995;83:243-247.
107 Bucy PC, Oberhill HR. Intradural spinal granulomas. J Neurosurg. 1950;7:1-12.
108 Arseni C, Samitca DC-T. Intraspinal tuberculous granuloma. Brain. 1960;83:285-292.
109 Lin TH. Intramedullary tuberculoma of the spinal cord. J Neurosurg. 1960;17:497-499.
110 Ratliff JK, Connolly ES. Intramedullary tuberculoma of the spinal cord. J Neurosurg (Spine 1). 1999;90:125-128.
111 Gupta RK, Gupta S, Kumar S, et al. MRI in intraspinal tuberculosis. Neuroradiology. 1994;36:39-43.
112 Kemaloglu S, Gür A, Nas K, et al. Intramedullary tuberculoma of the conus medullaris: Case report and review of the literature. Spinal Cord. 2001;39:498-501.
113 Lin S-K, Wu T, Wai Y-Y. Intramedullary spinal tuberculomas during treatment of tuberculous meningitis. Clin Neurol Neurosurg. 1994;96:71-78.
114 Bansal D, Singhi PD, Ray M, et al. Cervical intramedullary tuberculoma: Acute presentation and rapid response to medical therapy. J Trop Pediatr. 2002;48:55-57.
115 Kalita J, Mistra UK. Intramedullary cervical tuberculoma. Spinal Cord. 1999;37:297-298.
116 Rhoton EL, Ballinger WE, Quisling R, et al. Intramedullary spinal tuberculoma. Neurosurgery. 1988;22:733-736.
117 Vlcek B, Burchiel KJ, Gordon T. Tuberculous meningitis presenting as an obstructive myelopathy. J Neurosurg. 1984;60:196-199.
118 Chandrasoma P, Taylor CR. Concise Pathology. Norwalk, CT: Appleton & Lange; 1995:pp 71-73 and pp 512-514.
119 Chang KH, Han MH, Choi YW, et al. Tuberculous arachnoiditis of the spine: Findings on myelography, CT and MR imaging. AJNR Am J Neuroradiol. 1989;10:1255-1262.
120 Brooks WDW, Fletcher AP, Wilson RR. Spinal cord complications of tuberculous meningitis: A clinical and pathological study. Q J Med. 1954;23:275-290.
121 Tacconi L, Arulampalam T, Johnston FG, et al. Intramedullary spinal cord abscess: Case report. Neurosurgery. 1995;37:817-819.
122 Sánchez Pernaute R, Berciano J, Rebollo M, et al. Intramedullary tuberculoma of the spinal cord with syringomyelia. Neuroradiology. 1996;38:S105-S106.
123 Young RF, Gade G, Grinnell V. Surgical treatment for fungal infections in the central nervous system. J Neurosurg. 1985;63:371-391.
124 Ingwer I, McLeish KR, Tight RR, et al. Aspergillus fumigatus epidural abscess in a renal transplant recipient. Arch Intern Med. 1978;138:153-154.
125 Ho K, Williams A, Gronseth G, et al. Spinal cord swelling and candidiasis: A case report. Neuroradiology. 1982;24:117-118.
126 Kingsley DPE, White E, Marks A, et al. Intradural extramedullary aspergilloma complicating chronic lymphatic leukaemia. Br J Radiol. 1979;52:916-917.
127 Nakazato I, Kamada Y, Taira T, et al. Massive spinal cord necrosis associated with adult T-cell leukaemia caused by Aspergillus. Virchows Arch [Pathol Anat]. 1993;423:397-400.
128 Sheth NK, Varkey B, Wagner DK. Spinal cord Aspergillus invasion—complication of an aspergilloma. Am J Clin Pathol. 1985;84:763-769.
129 Stein SC, Corrado ML, Friedlander M, et al. Chronic mycotic meningitis with spinal involvement (arachnoiditis): A report of five cases. Ann Neurol. 1982;11:519-524.
130 Woods GL, Goldsmith JC. Aspergillus infection of the central nervous system in patients with acquired immunodeficiency syndrome. Arch Neurol. 1990;47:181-184.
131 Cravens G, Robertson H, Banta C, et al. Spinal cord compression due to intradural extramedullary aspergilloma and cyst: A case report. Surg Neurol. 1989;31:315-318.
132 von Pohle WR. Disseminated mucormycosis presenting with lower extremity weakness. Eur Respir J. 1996;9:1751-1753.
133 White CJ, Kwon-Chung KJ, Gallin JI. Chronic granulomatous disease of childhood: An unusual case of infection with Aspergillus nidulans var. echinulatus. Am J Clin Pathol. 1988;90:312-316.
134 Salaki JS, Louria DB, Chemel H. Fungal and yeast infections of the central nervous system. Medicine. 1984;63:108-132.
135 Frazier DD, Campbell DR, Garvey TA, et al. Fungal infections of the spine: Report of eleven patients with long-term follow-up. J Bone Joint Surg Am. 2001;83:560-570.
136 Pfausler B, Kampfl A, Berek K, et al. Syndrome of the anterior spinal artery as the primary manifestation of aspergillosis. Infection. 1995;23:240-242.
137 Van de Wyngaert FA, Sindic CJM, Rousseau JJ, et al. Spinal arachnoiditis due to Aspergillus meningitis in a previously healthy patient. J Neurol. 1986;233:41-43.
138 Voelker JL, Muller J, Worth RM. Intramedullary Spinal Histoplasma Granuloma. J Neurosurg. 1989;70:959-961.