Spinal Injuries
Perspective
Epidemiology
Statistics from the National Spinal Cord Injury Database show that motor vehicle collisions (MVCs) account for roughly 40% of all spinal injuries.1 Speeding, alcohol intoxication, and failure to use restraints are major risk factors. The next most common cause of spinal cord injury (SCI) is falls, followed by acts of violence (primarily gunshot wounds) and sporting activities. There are currently more than 260,000 spinal injury victims living in the United States, and 12,000 new cases occur each year. Approximately 80% of victims are male, and the average age at injury is 40 years. The lifetime cost to care for SCI victims ranges from $500,000 for people older than 50 years with incomplete motor function to over $3 million for people younger than 25 years with complete paraplegia. The total cost to society from lifelong medical expenses and lost productivity for all age groups and types of spinal injuries is estimated to be more than $5 billion.2 The devastating emotional and psychological impact on the victims and their families is incalculable.
Principles of Disease
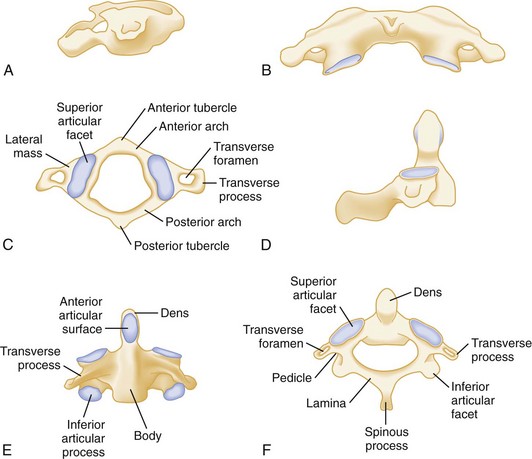
The human spine consists of 33 bony vertebrae: 7 cervical, 12 thoracic, 5 lumbar, 5 sacral (fused into one), and 4 coccygeal (usually fused into one) (Fig. 43-1).3 These 26 individual units are separated from one another by flexible intervertebral disks and connected to form a single functioning unit by a complex network of ligaments. In addition to providing basic structural support, the vertebral column protects the spinal cord, which extends from the midbrain to the level of the second lumbar vertebra. Nerves that receive and transmit sensory, motor, and autonomic impulses pass to and from the spinal cord through intervertebral foramina.
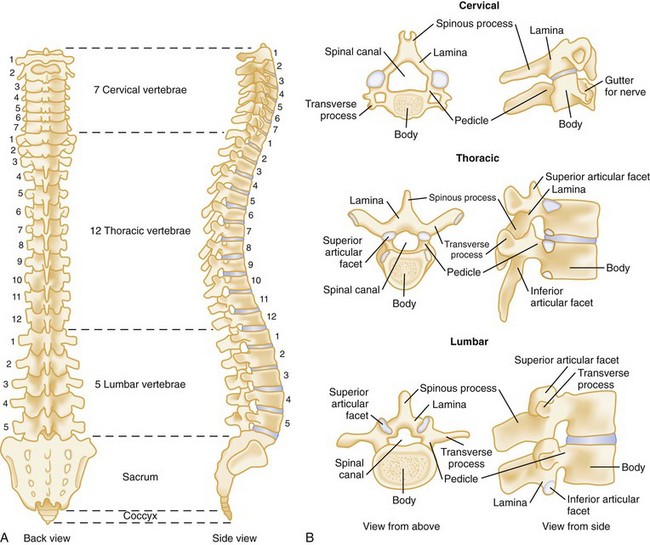
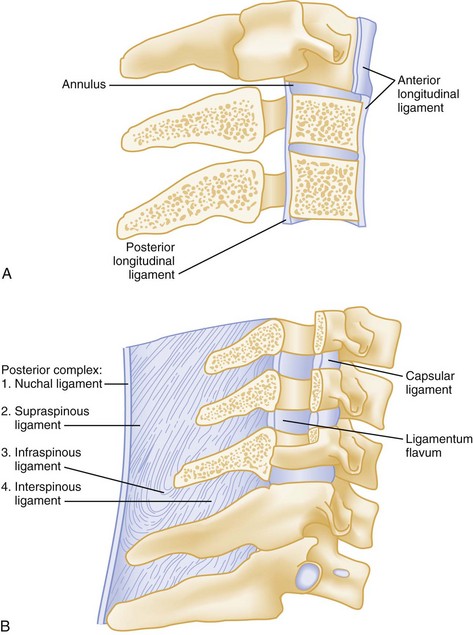
Figure 43-1 A, Vertebral column. B, Typical vertebrae.
Spinal injuries involve fractures in 85% of cases. Ten percent are purely ligamentous injuries, and 5% are SCI without radiographic abnormality (SCIWORA) in which the spinal cord is injured directly without any radiographic evidence of bony or ligamentous injury.4 Stability of a spinal injury refers to the resistance to displacement of fracture fragments or, in the case of ligamentous injury, the entire vertebral unit. Displacement may occur at the time of injury or progressively over hours to weeks and can cause or worsen damage to the spinal cord or nerve roots. There are several conceptual models for assessing the stability of subaxial spinal column injuries, but the most commonly used one is the three parallel vertical column model proposed by Denis (Fig. 43-2)5 The anterior column is formed by alternating vertebral bodies and intervertebral disks surrounded by the annulus fibrosus capsule and the anterior longitudinal ligament. The middle column consists of the posterior part of the annulus fibrosus and posterior vertebral wall, the posterior longitudinal ligament, the spinal cord, the paired laminae and pedicles, the articulating facets, the transverse processes, and the nerve roots and vertebral arteries and veins. The posterior column consists of the spinous processes, nuchal ligament, interspinous and supraspinous ligaments, and ligamentum flavum. Disruption of only a single column usually preserves a high degree of stability but does not preclude SCI from displaced fracture fragments. Disruption of two columns results in an injury that is stable in one direction but unstable in another (e.g., stable in flexion but unstable in extension). Disruption of all three columns produces a highly unstable injury. Until the full extent of the injury is clear, all spinal injuries should be treated as potentially unstable, and spinal immobilization should be maintained.
Pathophysiology
Classification of Spinal Column Injuries
Acute spinal injuries are classified according to the mechanism of trauma: flexion, flexion-rotation, extension, and vertical compression (Table 43-1).6,7
Table 43-1
Classification of Spinal Injuries
| MECHANISM OF SPINAL INJURY | STABILITY |
| Flexion | |
| Wedge fracture | Stable |
| Flexion teardrop fracture | Extremely unstable |
| Clay shoveler’s fracture | Stable |
| Subluxation | Potentially unstable |
| Bilateral facet dislocation | Always unstable |
| Atlanto-occipital dislocation | Unstable |
| Anterior atlantoaxial dislocation with or without fracture | Unstable |
| Odontoid fracture with lateral displacement fracture | Unstable |
| Fracture of transverse process | Stable |
| Flexion-Rotation | |
| Unilateral facet dislocation | Stable |
| Rotary atlantoaxial dislocation | Unstable |
| Extension | |
| Posterior neural arch fracture (C1) | Unstable |
| Hangman’s fracture (C2) | Unstable |
| Extension teardrop fracture | Usually stable in flexion; unstable in extension |
| Posterior atlantoaxial dislocation with or without fracture | Unstable |
| Vertical Compression | |
| Bursting fracture of vertebral body | Stable |
| Jefferson fracture (C1) | Extremely unstable |
| Isolated fractures of articular pillar and vertebral body | Stable |
Flexion.: Pure flexion injuries involving the C1-C2 complex can cause unstable atlanto-occipital or atlantoaxial joint dislocation, with or without an associated fracture of the odontoid (Fig. 43-3). These injuries are considered unstable because of their location and the relative lack of muscle and ligamentous support.
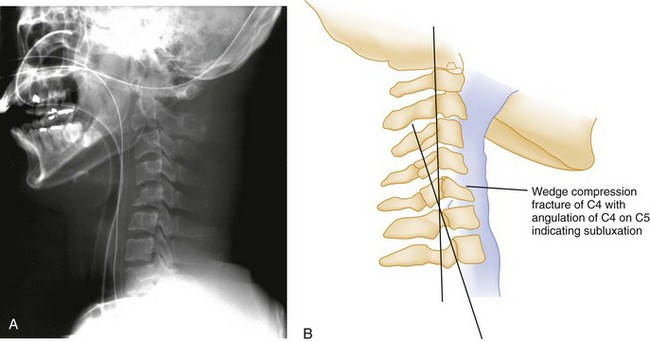
In pure flexion injuries below C2, a longitudinal pull is exerted on the strong nuchal ligament complex, which usually remains intact. Most of the force is expended on the vertebral body anteriorly, causing a simple wedge fracture. Radiographically, there is a diminished height and increased concavity of the anterior border of the vertebral body, an increased density of the vertebral body resulting from bony impaction, and prevertebral soft tissue swelling (Fig. 43-4). Because the posterior column remains intact, this injury is usually stable and rarely accompanied by nervous system damage. However, spinal instability may occur with severe wedge fractures (loss of more than half the vertebral height) or multiple adjacent wedge fractures, and such injuries are best treated as being potentially unstable.
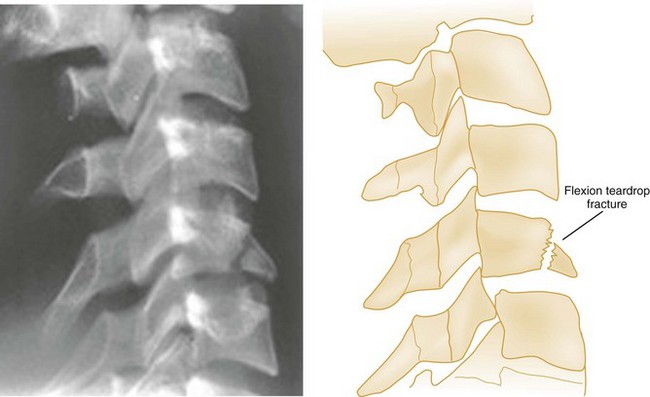
A flexion teardrop fracture results when severe flexion forces cause anterior displacement of a wedge-shaped fragment (resembling a teardrop) of the anteroinferior portion of the involved vertebral body (Fig. 43-5). Because this injury commonly involves anterior and posterior ligamentous disruption, it is often associated with neurologic injury and is highly unstable.
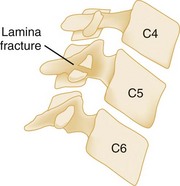
The clay shoveler‘s fracture is an oblique fracture of the base of the spinous process of one of the lower cervical segments (Fig. 43-6). The injury derives its name from its common occurrence in clay miners in Australia during the 1930s. When a miner lifted a heavy shovelful of clay, abrupt head flexion against the supraspinous ligament resulted in an avulsion fracture of the spinous process. Today, this fracture is more commonly seen after direct trauma to the spinous process and after sudden deceleration MVCs that result in forced neck flexion. Because this injury involves only the spinous process, it is stable and not associated with neurologic involvement.
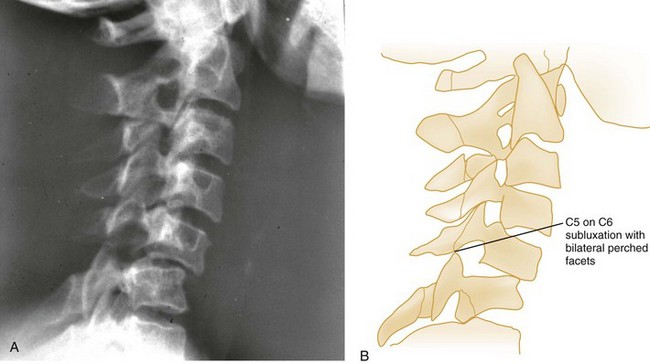
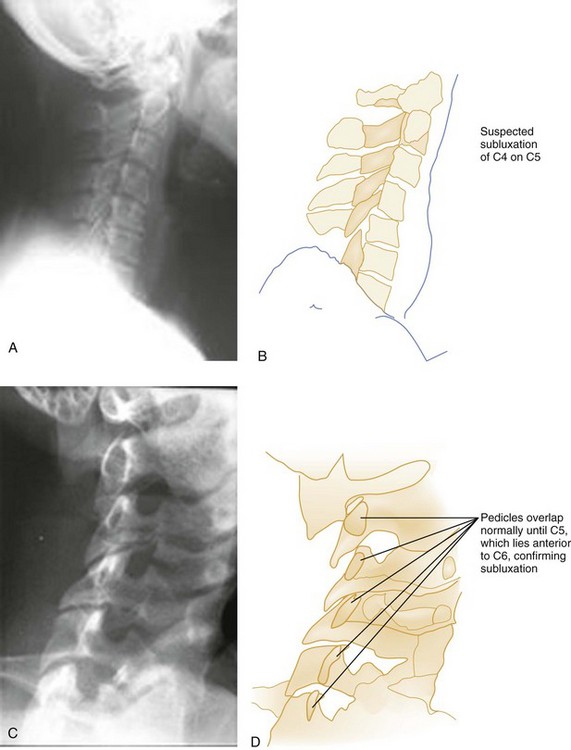
Pure spinal subluxation occurs when the ligamentous complexes rupture without an associated bony injury. This injury begins posteriorly in the nuchal ligament and proceeds anteriorly to involve other ligaments. The lateral radiograph with the neck in the neutral position may show a widening of both interspinous and intervertebral spaces posteriorly at the level of injury, and oblique views may demonstrate a widening or abnormal alignment of the facets (Fig. 43-7). These findings are often subtle and may be missed if flexion and extension views are not obtained. Although rarely associated with neurologic damage, this injury is potentially unstable.
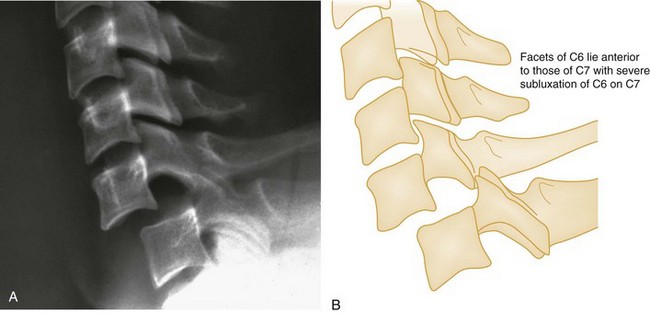
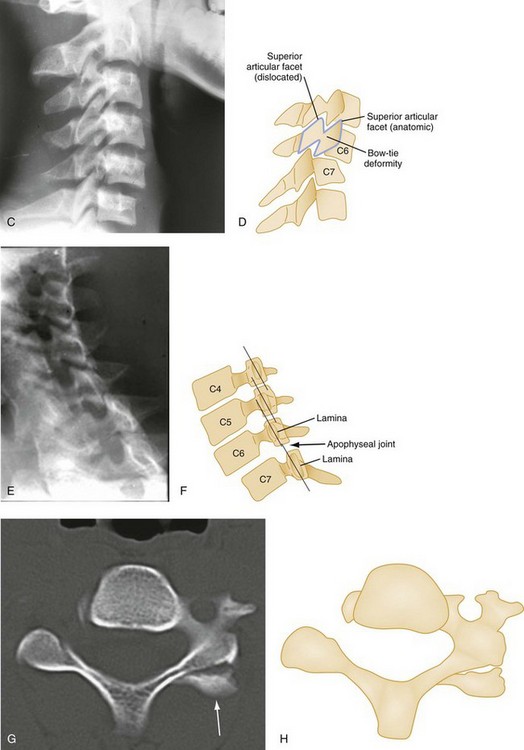
Bilateral facet dislocations occur when a greater force of flexion causes soft tissue disruption to continue anteriorly to the annulus fibrosis of the intervertebral disk and the anterior longitudinal ligament, resulting in an extremely unstable condition. The forward movement of the spine causes the inferior articulating facets of the upper vertebra to pass upward and over the superior facets of the lower vertebra, resulting in anterior displacement of the spine above the level of injury. Radiographically, the anterior displacement will appear to be greater than one half of the anteroposterior (AP) diameter of the lower vertebral body with the superior facets anterior to the inferior facets (Fig. 43-8).
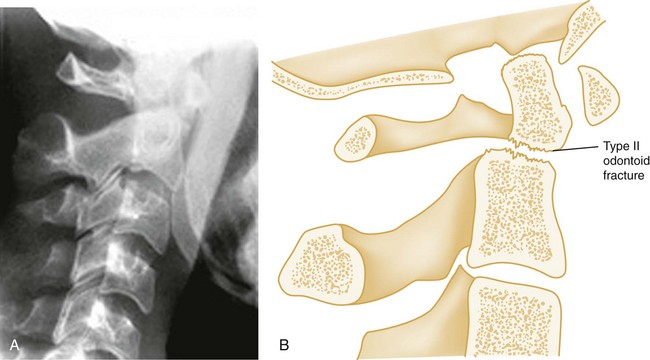
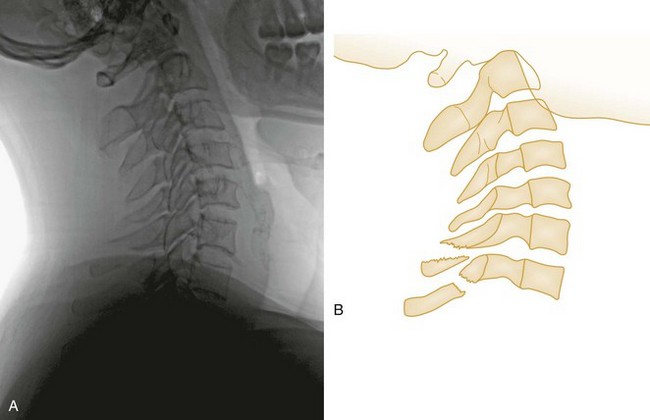
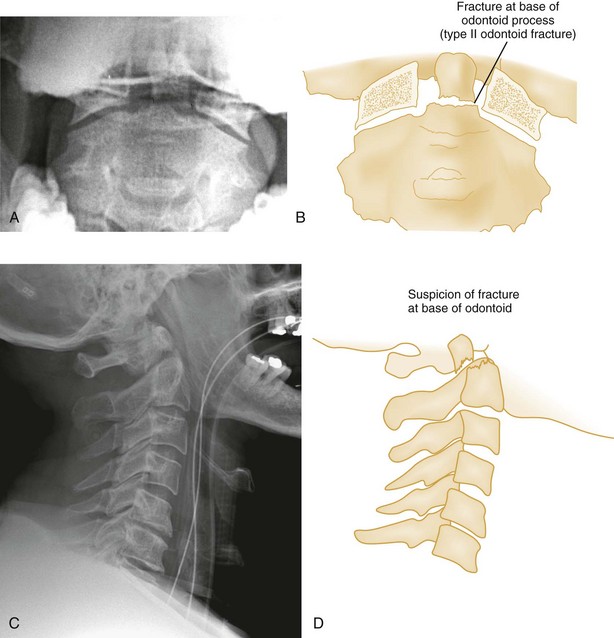
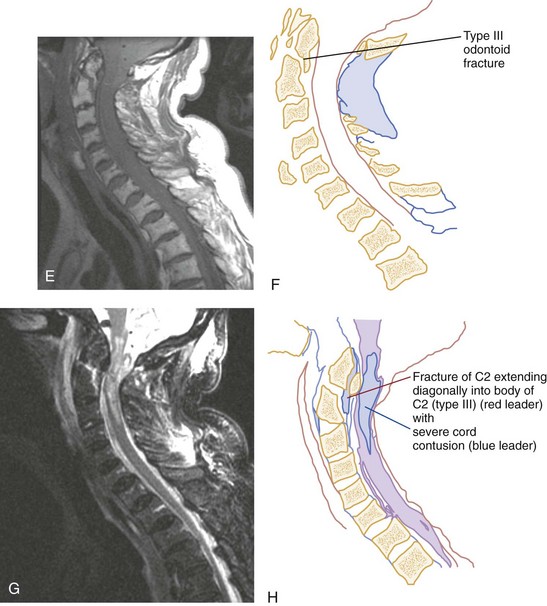
Shear Injury.: Trauma to the head directed in an AP direction may result in fracture of the odontoid process above the transverse ligaments (type I) or, more commonly, at the base of the odontoid process where it attaches to C2 (type II) (Fig. 43-9). Slight angulation of the force may result in extension of the fracture into the body of C2 (type III). Type I odontoid fractures are usually stable because they are an avulsion injury to the odontoid tip. However, if traction forces injure the apical and alar ligaments, then the fracture may become unstable. Type II odontoid fractures are unstable and often complicated by nonunion. SCI is uncommon but can occur. Type III odontoid fractures are also mechanically unstable as they can extend laterally into the superior articular facet of the atlas.
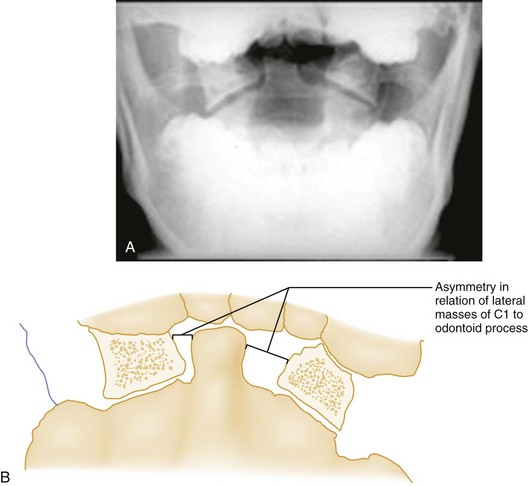
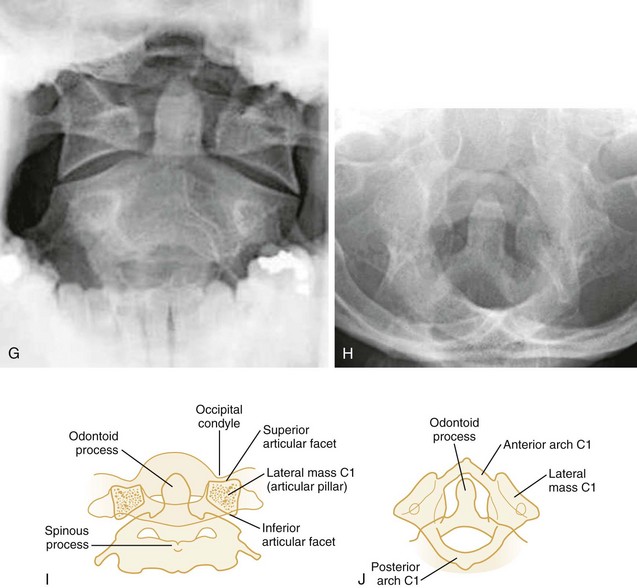
Flexion-Rotation.: Rotary atlantoaxial dislocation is an unstable injury visualized best on open-mouth odontoid radiographs (Fig. 43-10). If the skull is shown obliquely, there may be a false-positive asymmetry between the odontoid process and the lateral masses of C1. However, when the x-ray film reveals symmetrical basilar skull structures, a unilaterally magnified lateral mass confirms a C1-C2 dislocation.
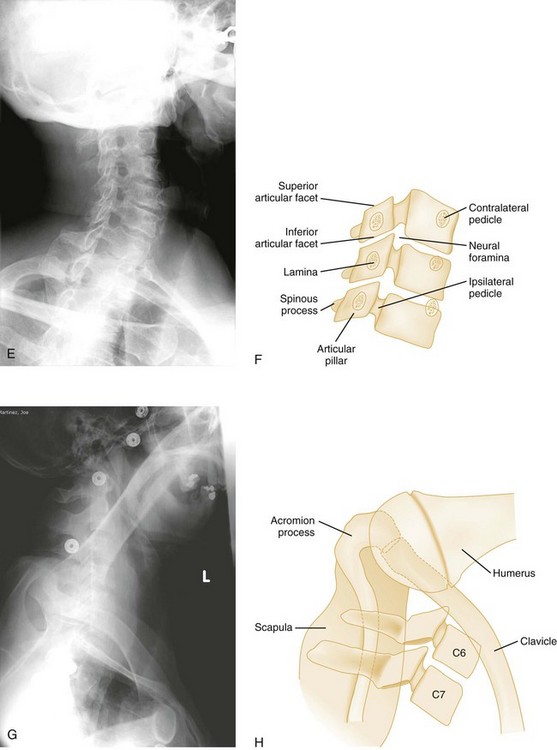
A unilateral facet dislocation involves both flexion and rotation. The rotational component of this injury occurs around one of the facet joints, which acts as a fulcrum. Simultaneous flexion and rotation cause the contralateral facet joint to dislocate, with the superior facet riding forward and over the tip of the inferior facet and coming to rest within the intervertebral foramen. In this position, the dislocated articular mass is mechanically locked in place, making this a stable injury, although the posterior ligament complex is disrupted. The frontal radiograph shows the spinous processes above the level of dislocation displaced from the midline in the direction of the rotation (Fig. 43-11A and B). The lateral radiograph shows a forward displacement of the dislocated segment on the vertebra below (less than one half the AP diameter of this vertebral body) and a rotation of the dislocated vertebra and those above it (Fig. 43-11C and D).
Any cervical fracture or dislocation may cause torticollis, but torticollis may also be caused by a benign process such as a muscle spasm. It may be difficult to differentiate torticollis caused by cervical fracture or dislocation from torticollis caused by severe muscle spasm, however, and oblique projections may be necessary to demonstrate the dislocated facet joint (Fig. 43-11E and F).
Owing to the varying shapes of the articular processes, particularly between the cervical and lumbar regions, different types of flexion-rotation injuries result. In the cervical region, where articular processes are small, flat, and almost horizontal, unilateral facet dislocations occur as described previously. In the lumbar region, however, where articular processes are large, curved, and nearly vertical, unilateral facet dislocation is rare. Instead, one or both articular processes fracture, and the upper vertebra swings forward. Commonly seen in the thoracolumbar and lumbar region, this rotation fracture-dislocation is unstable (Fig. 43-12).
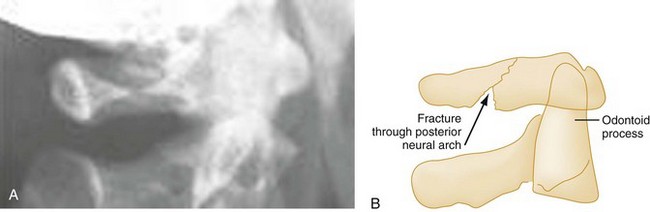
Extension.: The posterior neural arch fracture of the atlas (C1) results from the compression of the posterior elements between the occiput and the spinous process of the axis (C2) during forced neck extension (Fig. 43-13). Although the anterior arch and the transverse ligament remain intact, this fracture is potentially unstable because of its location.
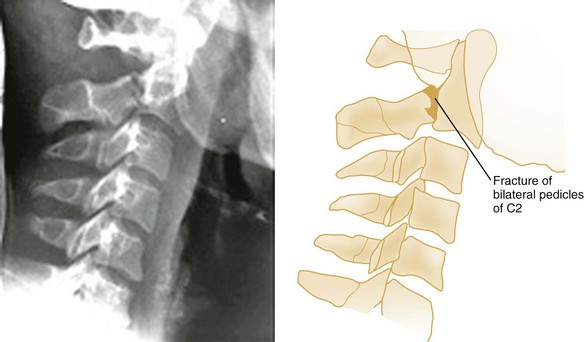
The hangman‘s fracture, or traumatic spondylolysis of C2, occurs when the cervicocranium (the skull, atlas, and axis functioning as a unit) is thrown into extreme hyperextension as a result of abrupt deceleration. Bilateral fractures of the pedicles of the axis occur with or without dislocation (Fig. 43-14). Although this lesion is unstable, cord damage is often minimal because the AP diameter of the neural canal is greatest at the C2 level, and the bilateral pedicular fractures permit the spinal canal to decompress itself. Originally described in victims of hanging injury, today it is most often the result of head-on MVCs.
The extension teardrop fracture occurs when abrupt extension of the neck causes the anterior longitudinal ligament to pull the anteroinferior corner of a vertebral body away from the remainder of the vertebra, producing a triangular fracture that is radiographically similar to the flexion teardrop fracture. Often occurring in lower cervical vertebrae (C5-C7) from diving accidents, this injury may be associated with a central cord syndrome, which is discussed later, and is caused by the ligamentum flavum buckling into the spinal cord.8 Because the posterior elements remain intact, this injury is stable in flexion but potentially unstable in extension.
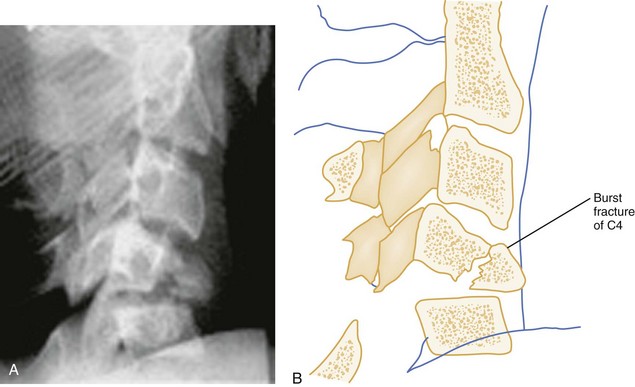
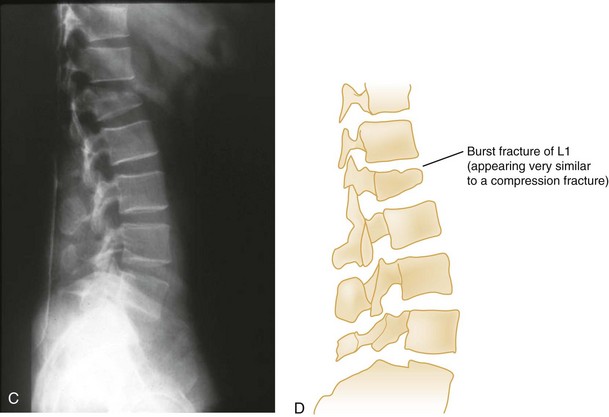
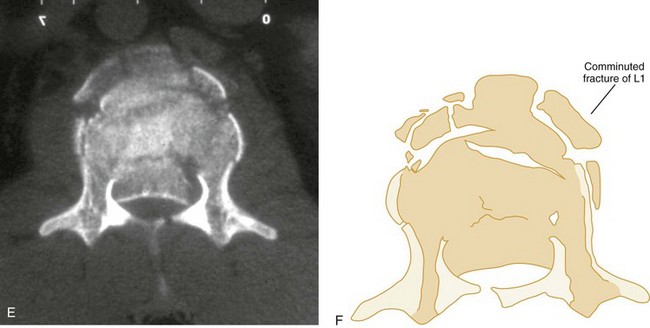
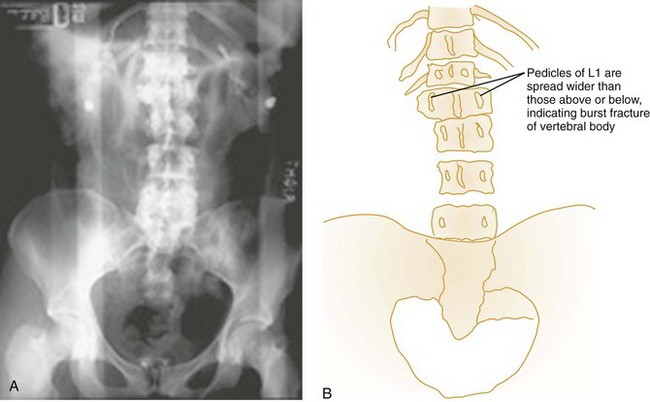
Vertical Compression.: Vertical compression injuries occur in the cervical and lumbar regions, which are capable of straightening at the time of impact. When forces are applied from either above (skull) or below (pelvis or feet), one or more vertebral body endplates may fracture. The nucleus pulposus of the intervertebral disk is forced into the vertebral body, which is shattered outward, resulting in a burst fracture (Fig. 43-15). The lateral radiograph shows a comminuted vertebral body, and there will typically be greater than 40% compression of the anterior vertebral body, which helps differentiate it from the simple wedge fracture. The frontal radiograph demonstrates a characteristic vertical fracture of the vertebral body, which will also help differentiate it from the simple wedge fracture and the flexion teardrop fracture. This is a stable fracture because all the ligaments remain intact. However, fracture fragments may impinge on or penetrate the ventral surface of the spinal cord and cause an anterior cord syndrome (see Fig. 43-15).
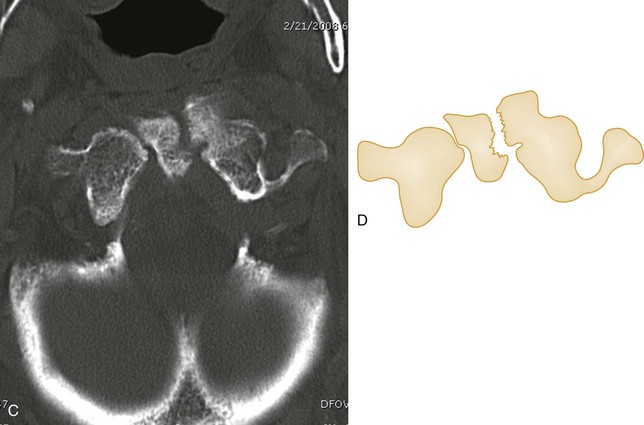
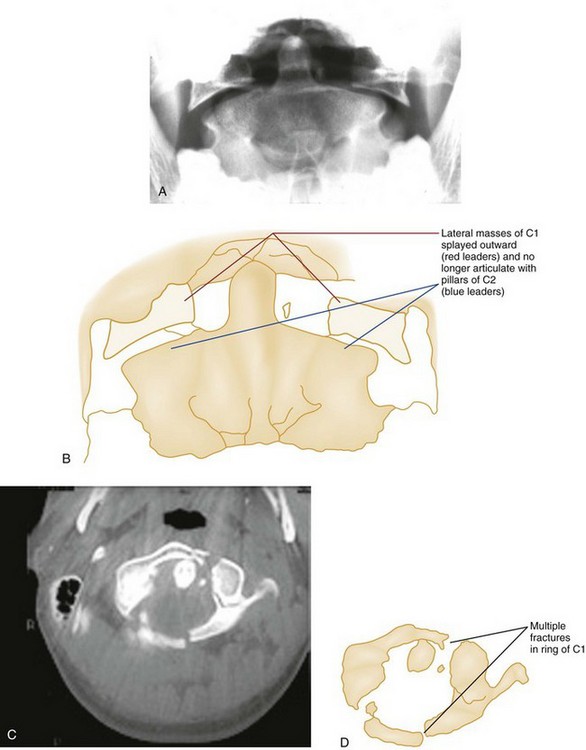
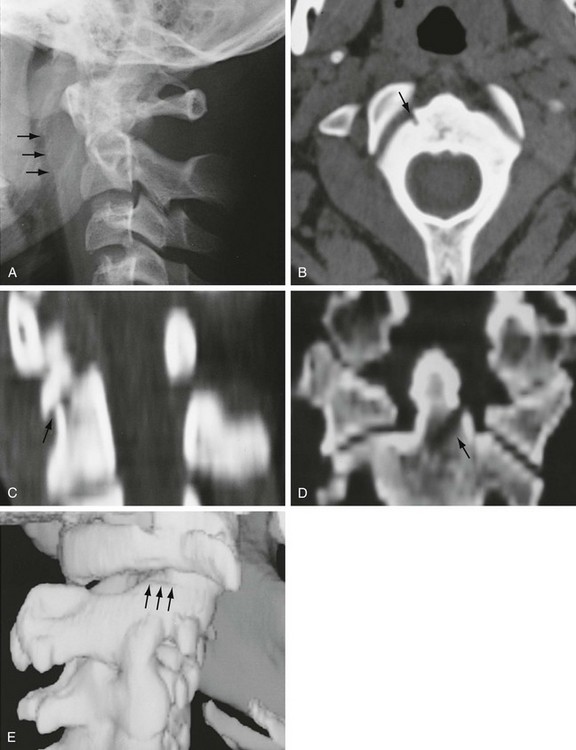
The Jefferson fracture of C1 is an extremely unstable injury that occurs when a vertical compression force is transmitted through the occipital condyles to the superior articular surfaces of the lateral masses of the atlas. This force drives the lateral masses outward, resulting in fractures of the anterior and posterior arches of the atlas and a disruption of the transverse ligament. Because this injury is often associated with prevertebral hemorrhage and retropharyngeal swelling, the lateral film may demonstrate a widening of the predental space between the anterior arch of C1 and the odontoid, or dens. The open-mouth view will demonstrate a bilateral offset of both right and left lateral masses of C1 relative to the lateral masses of C2. A fracture should be diagnosed when the sum of the offset distances from the right and left sides exceeds 7 mm (Fig. 43-16). However, when the fragments are minimally displaced, the Jefferson fracture is difficult to recognize, and computed tomography (CT) may be necessary.
Classification of Spinal Cord Injuries
Primary Spinal Cord Injury.: The spinal cord may be injured in a number of ways.8 First, penetrating trauma or massive blunt trauma with disruption of the vertebral column may cause the transection of neural elements. Because neurons within the central nervous system do not regenerate, such injuries are irreversible. Less severe blunt trauma may have similar effects resulting from a displaced bony fragment or a herniated disk.
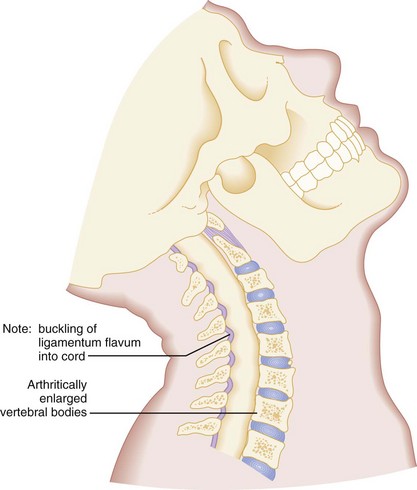
Second, when elderly patients with cervical osteoarthritis and spondylosis are subjected to forcible cervical spine extension, the spinal cord may be compressed between an arthritically enlarged anterior vertebral ridge and a posteriorly located hypertrophic ligamentum flavum (Fig. 43-17). This injury frequently results in a central cord syndrome.
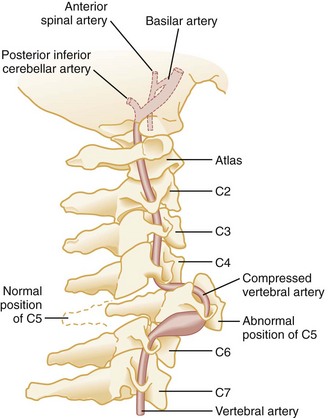
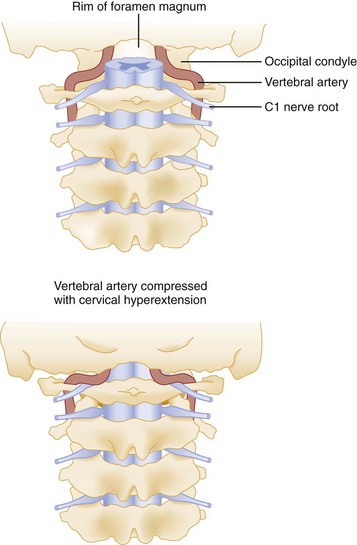
Primary vascular damage to the spinal cord, a third mechanism of injury, may occur in several ways. The spinal cord may be compressed by an extradural hematoma, particularly in patients who are on anticoagulants or have bleeding disorders. Vascular injuries should also be suspected when there is a discrepancy between the clinically apparent neurologic deficit and the known level of spinal injury. For example, a lower cervical dislocation may compress the vertebral arteries as they travel within the spinal foramina of the vertebrae. This compression may result in thrombosis and decreased blood flow through the anterior spinal artery that originates from both vertebral arteries at the level of C1 (Fig. 43-18). On physical examination, such an injury may erroneously appear to be localized to the level of C1 or C2. Also, the great radicular artery of Adamkiewicz, originating from the aorta and entering the spinal canal at the level of L1, sends branches as cephalad as T4. Therefore a lumbar fracture or dislocation can produce a neurologic deficit as high as T4.
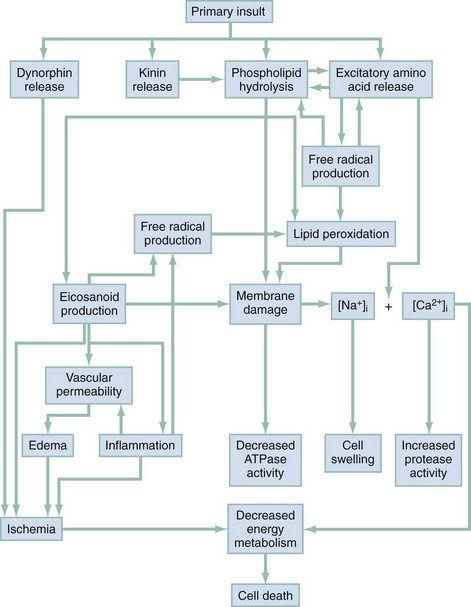
Secondary Spinal Cord Injury.: The maximum neurologic deficit after blunt spinal cord trauma is often not seen immediately and may instead progress over many hours. The histopathology of the so-called “secondary SCI” has been studied extensively in experimental animal models.9–11 It is now thought that primary SCI initiates a complex cascade of biochemical events that result in progressive ischemia of gray and white matter during the postinjury period (Fig. 43-19). Other factors, such as hypoxia, hypotension, hyperthermia, hypoglycemia, and mishandling by medical personnel, also affect the ultimate extent of SCI.
Clinical Features
The initial neurologic evaluation of a patient with a suspected spinal injury should begin with simple observation. Careful inspection, beginning with the head and proceeding downward, may reveal signs of possible spinal involvement. Significant head and facial trauma have a 5 to 10% incidence of associated cervical spine injuries.12,13 Scapular contusions suggest a rotation or flexion-rotation injury of the thoracic spine. Chest and neck abrasions from automobile shoulder belts and lower abdominal markings from lap belts indicate possible blunt carotid and vertebral injuries, as well as spinal, intrathoracic, and intra-abdominal injuries. As occurs with falls from considerable heights, injuries to the gluteal region, calcaneal fractures, and severe ankle fractures suggest a compression type of spinal injury.
The patient should be observed for the presence and symmetry of both voluntary and involuntary movements. Because the diaphragm is innervated by the phrenic nerve, which originates at C3-C4, an abnormal, abdominal breathing pattern may provide an important clue to a cervical injury. The presence of Horner’s syndrome, characterized by unilateral ptosis, miosis, and anhidrosis, may result from disruption of the cervical sympathetic chain, usually between C7 and T2.5 Priapism may occur with severe SCI, and it is often associated with spinal shock, which is a transient reflex depression of the spinal cord below the level of the injury.
The motor activity of the body is complex. Because a single motion is often governed by muscles innervated by multiple spinal segments, localizing a spinal lesion based solely on an assessment of motor function is extremely difficult. Testing the presence and strength of those motions outlined in Table 43-2, however, provides a rapid baseline assessment. When a deficit is noted, the motor and neurologic examination should be repeated at frequent intervals because progression of dysfunction may occur. If there is apparent total loss of function, every effort should be made to elicit the most minimal of motor responses because any response markedly improves the prognosis. A slight toe flicker in an otherwise paralyzed individual indicates that the patient may again eventually walk unassisted.
Table 43-2
| LEVEL OF LESION | RESULTING LOSS OF FUNCTION |
| C4 | Spontaneous breathing |
| C5 | Shrugging of shoulders |
| C6 | Flexion at elbow |
| C7 | Extension at elbow |
| C8-T1 | Flexion of fingers |
| T1-T12 | Intercostal and abdominal muscles* |
| L1-L2 | Flexion at hip |
| L3 | Adduction at hip |
| L4 | Abduction at hip |
| L5 | Dorsiflexion of foot |
| S1-S2 | Plantar flexion of foot |
| S2-S4 | Rectal sphincter tone |
*Localization of lesions in this area is best accomplished with the sensory examination.
The presence of cord-mediated deep tendon reflexes can be helpful as a localizing, diagnostic aid (Table 43-3). Classically, muscle paralysis associated with intact deep tendon reflexes indicates an upper motor neuron (spinal cord) lesion, whereas paralysis associated with absent deep tendon reflexes indicates a lower motor neuron (nerve root or cauda equina) lesion. This differentiation is important because the latter condition is often caused by a surgically correctable lesion. After the initial period of areflexia, reflexes gradually return after 1 to 3 days, and after 1 to 4 weeks, patients with SCI will manifest characteristic hyper-reflexia and spasticity.14 Because reflexes are typically absent during the initial phase of spinal shock, the examination of reflexes is less useful in the emergency department.
Table 43-3
| LEVEL OF LESION (AT OR ABOVE) | RESULTING LOSS OF REFLEX |
| C6 | Biceps |
| C7 | Triceps |
| L4 | Patellar |
| S1 | Achilles |
Sensory function can be quickly evaluated through use of a structured approach (Table 43-4) or a graphic sensory dermatome chart (Fig. 43-20). After locating an area of hypesthesia, one should move the sensory stimulus from areas of decreased sensation outward, rather than the reverse, because patients are more sensitive to the appearance of sensation than to its disappearance. This test should be performed first with a cotton wisp to assess sensitivity to light touch, a posterior column function. A pin should be used to assess pain sensation, which is an anterior spinothalamic tract function. The presence of islands of preserved sensation within an affected dermatome or below the level of apparent total dysfunction, even in the presence of complete motor paralysis, indicates potential for functional motor recovery.15 An accurate baseline sensory examination is imperative because a cephalad progression of hypesthesia is the most sensitive indicator of deterioration. When this is observed in the cervical region, one should anticipate impending respiratory failure and stabilize the airway.
Table 43-4
| LEVEL OF LESION | RESULTING LEVEL OF LOSS OF SENSATION |
| C2 | Occiput |
| C3 | Thyroid cartilage |
| C4 | Suprasternal notch |
| C5 | Below clavicle |
| C6 | Thumb |
| C7 | Index finger |
| C8 | Small finger |
| T4 | Nipple line |
| T10 | Umbilicus |
| L1 | Femoral pulse |
| L2-L3 | Medial aspect of thigh |
| L4 | Knee |
| L5 | Lateral aspect of calf |
| S1 | Lateral aspect of foot |
| S2-S4 | Perianal region |
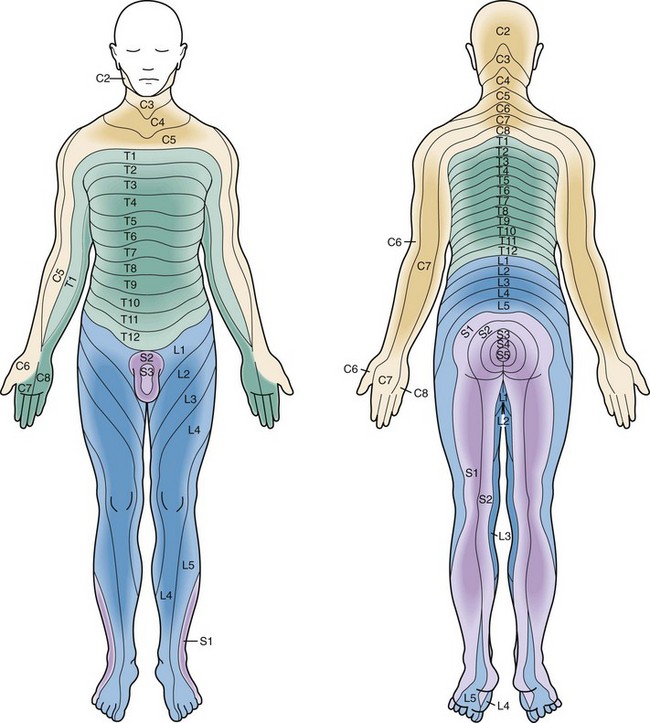
Figure 43-20 Sensory dermatomes.
Complete Spinal Cord Lesions
A complete spinal cord lesion is defined as total loss of motor power and sensation distal to the site of an SCI. Functional motor recovery is rare in a patient with a complete cord syndrome that persists for longer than 24 hours after the injury.7 Before diagnosis of a complete cord syndrome, however, two points should be considered. First, any evidence of minimal cord function, such as sacral sparing, excludes the patient from this group. Signs of sacral sparing include perianal sensation, normal rectal sphincter tone, or flexor toe movement. The presence of any of these signs indicates a partial lesion, usually a central cord syndrome, and the patient may have marked functional recovery, including bowel and bladder control and eventual ambulation.
Second, it is important to note that a complete spinal cord lesion may be mimicked by a condition known as spinal shock, which may persist from a few days to a few weeks. Spinal shock results from a concussive injury to the spinal cord that causes total neurologic dysfunction distal to the site of injury.16 The end of spinal shock is heralded by the return of the bulbocavernosus reflex, which is a normal cord-mediated reflex elicited by placing a gloved finger in the patient’s rectum and then squeezing the glans penis or clitoris or by tugging gently on the Foley catheter. An intact reflex results in rectal sphincter contraction. Absence of this reflex indicates the presence of spinal shock, during which time the patient’s prognosis cannot be accurately assessed. A complete spinal cord lesion will remain unchanged after the cessation of spinal shock.
Incomplete Spinal Cord Lesions
Approximately 90% of incomplete spinal injuries can be classified as one of three clinical syndromes: the central cord syndrome, the Brown-Séquard syndrome, and the anterior cord syndrome (Fig. 43-21).7 The most common is the central cord syndrome, often seen in patients with degenerative arthritis of the cervical vertebrae when their necks are hyperextended. The ligamentum flavum buckles into the cord, resulting in a concussion or contusion of the central gray matter in the most central portions of the pyramidal and spinothalamic tracts. Because fibers that innervate distal structures are located in the periphery of the spinal cord, the upper extremities are more severely affected than the lower extremities. With more severe injuries, patients may appear to be almost completely quadriplegic and have only sacral sparing. The prognosis is variable, but more than 50% of patients with central cord syndrome become ambulatory and regain bowel and bladder control, as well as some hand function.15
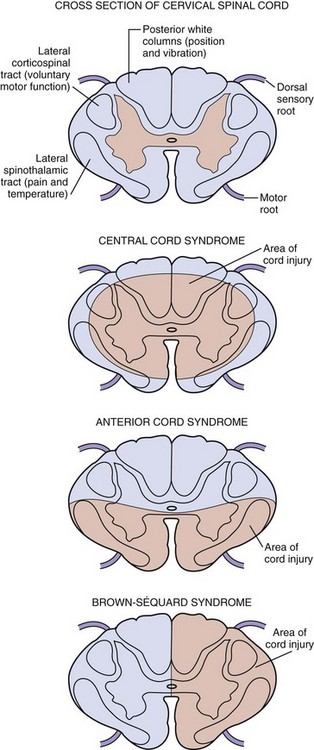
Figure 43-21 Incomplete spinal cord syndromes.
The Brown-Séquard syndrome, or hemisection of the spinal cord, usually results from penetrating trauma but may also be seen after lateral mass fractures of the cervical spine. Patients with this lesion have ipsilateral loss of position and vibration sense as well as motor paralysis but contralateral loss of pain and temperature sensation distal to the level of injury; however, either finding may predominate depending on the exact location and extent of injury. Moreover, because the fibers of the lateral spinal thalamic tract cross at a different level to the contralateral side, the pain and temperature loss may be found variably one or two segments above the lesion. Virtually all patients maintain bowel and bladder function and unilateral motor strength, and most become ambulatory.7
The anterior cord syndrome usually results from hyperflexion injuries causing cord contusion, by the protrusion of a bony fragment or herniated disk into the spinal canal, or by laceration or thrombosis of the anterior spinal artery. It can also occur after prolonged (longer than 30 minutes) cross-clamping of the aorta. This syndrome is characterized by paralysis and hypalgesia below the level of injury with preservation of posterior column functions, including position, touch, and vibratory sensations. Suspicion for an acute anterior cord syndrome warrants immediate neurosurgical consultation because it may be a result of a surgically correctable lesion. After surgical intervention, patients have variable degrees of recovery during the first 24 hours but little improvement thereafter.7
There are several less common spinal cord syndromes that may result from a direct injury to the cervicomedullary junction and upper cervical segments or from vertebral artery occlusion resulting from severe hyperextension (Fig. 43-22).7 The posteroinferior cerebellar artery syndrome may produce dysphagia, dysphonia, hiccups, nausea, vomiting, dizziness or vertigo, and cerebellar ataxia. The Dejeune onion skin pattern of analgesia of the face is caused by damage to the spinal trigeminal tract. Horner‘s syndrome results from damage to the cervical sympathetic chain and is characterized by ipsilateral ptosis, miosis, and anhidrosis. Injuries below the L2 level can result in an acute cauda equina syndrome, characterized by perineal or bilateral leg pain, bowel or bladder dysfunction, perianal anesthesia, diminished rectal sphincter tone, and lower extremity weakness.
The syndrome of SCIWORA is seen primarily in younger children but may occur in any age group.17–19 The mechanism is unclear but has been ascribed to the increased ligamentous elasticity seen in the young, leading to transient spinal column subluxation, stretching of the spinal cord, and vascular compromise. Patients often experience a brief episode of upper extremity weakness or paresthesias followed by neurologic deficits that appear hours to days later. The prognosis for patients with SCIWORA is variable, depending on the degree of neurologic impairment and the rate of resolution.
Diagnostic Strategies
Indications
Clinicians have historically taken a liberal approach to imaging the cervical spine in the setting of trauma because failure to recognize an SCI may result in devastating neurologic consequences. According to the National Hospital Ambulatory Medical Care Survey, only 4% of all cervical spine radiographs demonstrate a fracture.20 However, there is controversy as to what is the most efficient and accurate method of imaging, leading to great practice variation among emergency physicians, with a sixfold range in radiography ordering rates.21
In an effort to standardize clinical practice and guide physicians to be more selective in their use of radiography without jeopardizing patient care, two clinical decision rules have been developed. Use of selective but safe imaging may decrease overall health care costs, reduce radiation exposure, and avoid the discomfort and complications (e.g., aspiration and decubitus ulcerations) associated with patients lying flat on backboards with rigid collars while waiting for spine imaging. The first rule to be developed, the National Emergency X-Radiography Utilization Study (NEXUS) Low-Risk Criteria (NLC), was based on a multicenter prospective observational study involving 34,069 trauma patients seen at 21 U.S. emergency departments. The decision instrument required patients to meet five criteria to be classified as having a low probability of injury: (1) no midline cervical tenderness; (2) no focal neurologic deficit; (3) normal alertness; (4) no intoxication; and (5) no painful, distracting injury. The decision rule identified all but eight of the 818 patients who had spinal injuries. Only two of these patients had a clinically significant injury, only one of whom required surgical stabilization, and neither sustained a permanent neurologic injury.22 Sensitivity, specificity, and negative predictive value of the NLC were calculated and were found to be 99.6, 12.9, and 99.8%, respectively.22
Owing to concerns about the low specificity of the NLC, Stiell and colleagues developed the Canadian C-Spine Rule (CCR) based on 25 selected clinical predictor variables associated with spine injury. In 2003 the CCR was prospectively studied and compared with the NLC in the nine Canadian tertiary care hospitals. Of 8283 patients, 162 were found to have “clinically significant” injuries, and the sensitivity, specificity, and negative predictive values of the CCR were, respectively, 99.4, 45.1, and 100%.23 The CCR is composed of the following three questions:
1. Are there any high-risk factors that mandate radiography?
2. Are there any low-risk factors that allow safe assessment of range of motion?
3. Is the patient able to actively rotate his or her neck 45 degrees to the left and right?
There is some controversy as to which of the two rules to implement. There are methodological differences in the respective study designs, such as different inclusion and exclusion criteria.23 Note that both studies excluded patients with penetrating trauma and those who had sustained direct blows to the neck. Still, both rules have been well validated and are sensitive, and the use of either rule has been shown to significantly limit the number of unnecessary radiographs while missing only rare patients with clinically significant injuries.
Cervical Plain Radiographs
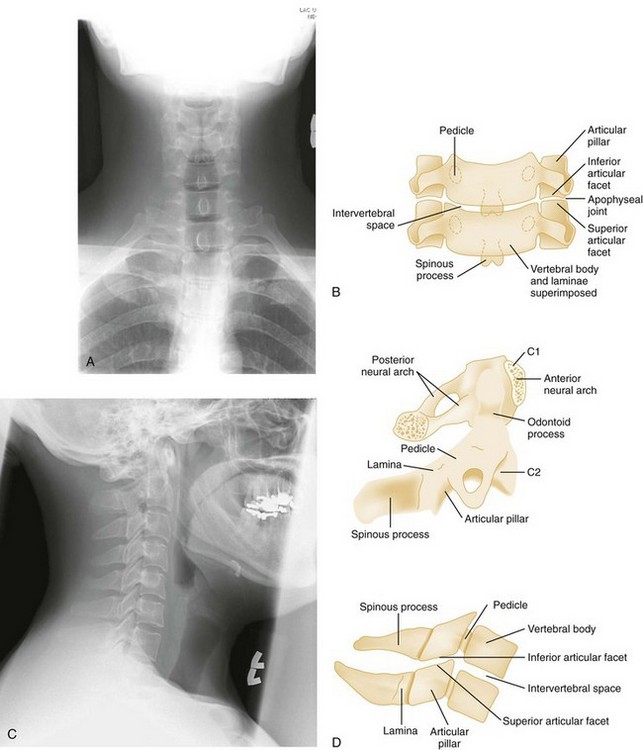
Normal cervical spine radiographs in AP, lateral, swimmer’s, oblique, and odontoid views are presented in Figures 43-23 and 43-24. All vertebrae suspected of being injured must be visualized. The C7-T1 vertebra may be obscured in muscular or obese patients, as well as in patients with spinal lesions that result in paralysis of the muscles that act to depress the shoulders. The paralysis leaves the trapezius muscles, which elevate the shoulders, unopposed. Such lesions are located in the lower cervical region. The shoulders can usually be depressed by pulling the patient’s hands toward the feet, using slow, steady traction, but caution is exercised to avoid causing or worsening a distraction injury. Movement should occur only at the shoulder girdle while the head and neck remain immobilized. Note that the head and neck should not be pulled in opposition to the person pulling down the shoulders because this may induce a distraction injury. If this maneuver is unsuccessful or difficult to perform because of upper extremity injuries, a transaxillary or swimmer’s view of the lower cervical vertebrae may be helpful. The upper three or four thoracic vertebrae are also difficult to visualize on routine lateral views of either the cervical or thoracic spine, and a swimmer’s view, an oblique radiograph, or CT is often needed for adequate evaluation of the thoracic spine.
Although the cross-table lateral view of the cervical spine is the most helpful x-ray study in diagnosing spinal injures, its inadequacy as the sole view is well documented.24 Because the diagnostic yield is significantly increased when the AP and odontoid views are included, all three views of the cervical spine should be evaluated before discontinuation of cervical spine immobilization.25 The NLC shows that a technically adequate three-view trauma series will fail to diagnose spinal injury in only 0.07% of patients with injuries and in only 0.008% of patients with unstable injuries.26
Cross-Table Lateral View.: The inspection of the lateral cervical spine film should be methodical and complete. For this to be accomplished, it is helpful to remember the “ABCs” of interpreting the lateral film, where A stands for alignment, B for bony abnormalities, C for cartilage-space assessment, and s for soft tissues.
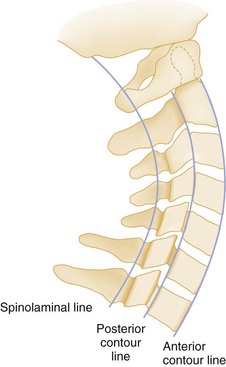
For the alignment to be checked, two imaginary lines are drawn that separately connect the anterior and posterior margins of the vertebral bodies, the anterior and posterior contour lines. A third line, the spinolaminal line, connects the bases of the spinous processes extending to the posterior aspect of the foramen magnum (Fig. 43-25). All three lines should form a smooth, continuous lordotic curve, and any disruption of these lines suggests a bony or ligamentous injury. An exception to this rule is the pseudosubluxation of C2 and C3, which is commonly seen in infants and children. This phenomenon is attributed to their immature muscular development and a hypermobile spine. Thus if a high cervical injury is suspected in a child, the posterior cervical line, which connects the points bisecting the bases of the spinous processes of C1 and C3, should be used (Fig. 43-26). If the base of C2 lies more than 2 mm anterior or posterior to the posterior cervical line, an injury at that level should be suspected.27 On the lateral view, the predental space, which is the distance between the anterior aspect of the odontoid process and the posterior aspect of the anterior ring of C1, should not exceed 3 mm in an adult or 5 mm in a child (see Fig. 43-26). A widening of this space may indicate a Jefferson fracture of C1.
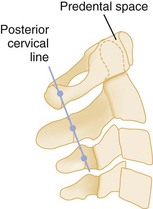
Figure 43-26 Posterior cervical line of a normal lateral spine.
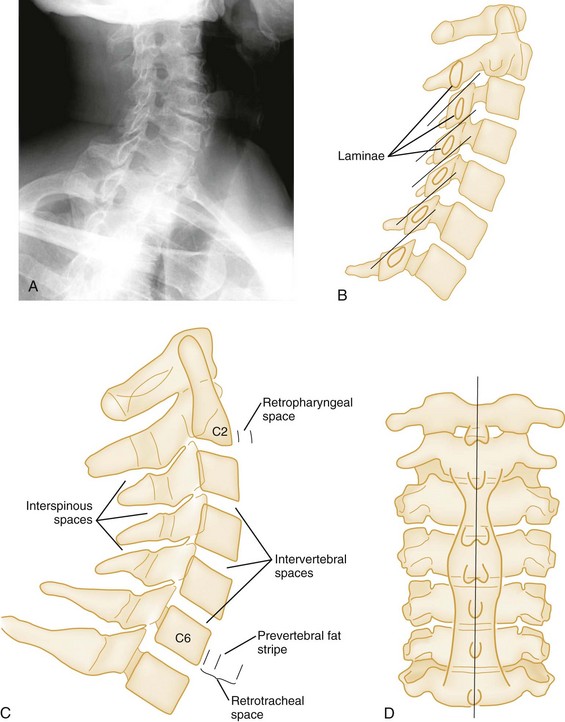
Finally, the soft tissues of the retropharyngeal space should be assessed for the presence of prevertebral swelling and hemorrhage, often the only radiographic sign of spinal injury. The retropharyngeal space, measured from the anterior border of the body of C2 to the posterior wall of the pharynx, should not exceed 6 mm in children or adults (Fig. 43-27A and C). At the level of C3 and C4, this measurement should not exceed 5 mm or should be less than one half the width of the vertebral body at that level. Below the level of C4, the prevertebral soft tissue space is widened by the esophagus and the cricopharyngeal muscle. Here, the retrotracheal space, measured from the anterior border of the body of C6 to the posterior wall of the trachea, should not exceed 22 mm in adults or 14 mm in children younger than age 15 years. In children younger than age 2 years, the retropharyngeal space may normally appear widened during expiration; therefore inspiratory films should be obtained.28 Air in the prevertebral space may indicate rupture of the esophagus or some portion of the respiratory tree, and anterior bulging of the prevertebral fat stripe is an excellent sign of an underlying bony or soft tissue injury.
Odontoid View.: The second film obtained in the emergency department is the open-mouth or closed-mouth view of the atlas and axis (see Fig. 43-24G-J). Nonfusion of the odontoid in children and congenital anomalies of the odontoid in adults may mimic fractures.
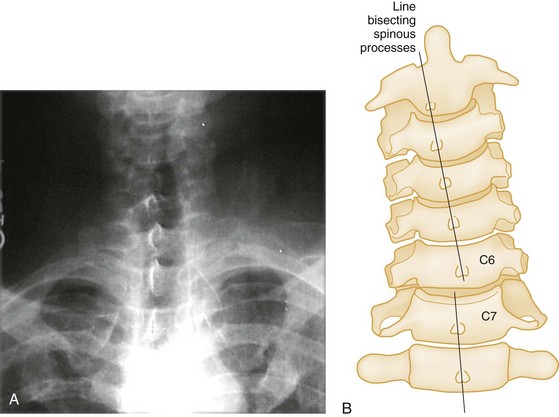
Anteroposterior View.: The AP spinal film completes the spinal series. The spinous processes should form a straight line, and the laryngeal and tracheal air shadows should be midline (see Figs. 43-23B and 43-27D). The regular outline of the lateral masses should be verified, and the pedicles viewed end on can be checked for fracture. Widening of the interpedicular distance compared with adjacent vertebrae suggests a burst fracture (Fig. 43-28).
Oblique Views
Oblique views of the cervical spine may be helpful to confirm a suspected posterior laminar fracture, a unilateral facet dislocation, or a real subluxation.29 However, with the advent of high-resolution CT, suspicion of these injuries on plain films ordinarily leads to CT rather than the obtaining of oblique views. The normal lamina appears as an intact ellipse (see Fig. 43-23E and F), and a posterior laminar fracture disrupts the appearance of this ellipse (Fig. 43-29). Normal overlapping laminae have the appearance of shingles on a roof (see Fig. 43-27A and B). Integrity of these shingles excludes the presence of a unilateral facet dislocation, whereas disruption confirms it (see Fig. 43-11E and F). The normal interlaminar distance, measured from the center points of successive laminar ellipses on an oblique view, should be equidistant. An increased interlaminar distance, caused by lack of capsular ligamentous integrity, indicates true subluxation (Fig. 43-30).
Flexion and Extension Views
There is controversy as to the role of flexion and extension (F/E) views in blunt cervical trauma, but they are often obtained when there is concern for ligamentous injury, despite negative standard radiographs when magnetic resonance imaging (MRI) is not available. F/E views should be obtained only in patients who are alert and able to articulate the presence of pain, numbness, or paresthesias, because such symptomatology may indicate instability. Other potential indicators of instability of the cervical spine include any of the following: more than 3.5 mm of horizontal displacement between the disks, displaced apophyseal joints, widened disk spaces, loss of greater than 30% of the disk height, or the presence of a prevertebral hematoma.30 The NEXUS investigators demonstrated that 86 of the 818 (10.5%) patients ultimately found to have cervical injury underwent F/E testing.31 Although two patients had bony injuries and four patients had subluxations demonstrated only on F/E views, all 6 patients had other injuries apparent on routine radiographs. F/E views are also deemed inadequate for interpretation in nearly one third of studies.32 In addition, in the acute setting, F/E radiographs have been reported to have unacceptably high false-positive and false-negative rates because minor subluxations may be masked by concomitant muscle spasm.33 Thus delayed F/E views obtained a week or two after injury may be more helpful. Moreover, other studies, such as CT and MRI, appear to be superior imaging modalities in patients with severe, localized symptoms who have normal radiographs.34
Computed Tomography
Conventional radiography is limited as a result of the nature of equipment, difficulties in positioning, lack of patient cooperation, and the often critical status of trauma patients. The incidence of an inadequate lateral cervical view, specifically visualization of C7 to T1, is as high as 25%.29 The CT scan effectively deals with these limitations and in many cases is the technique of choice for the definitive evaluation of acute cervical spine trauma.35–38 CT also permits examination without moving the patient from the supine position and is thus preferable in terms of fracture stabilization, airway control, and other life-support measures. In fact, practice guidelines from the Eastern Association for the Surgery of Trauma (EAST) recommend that CT from the occiput to T1 be used as the primary screening method in blunt cervical trauma patients.39
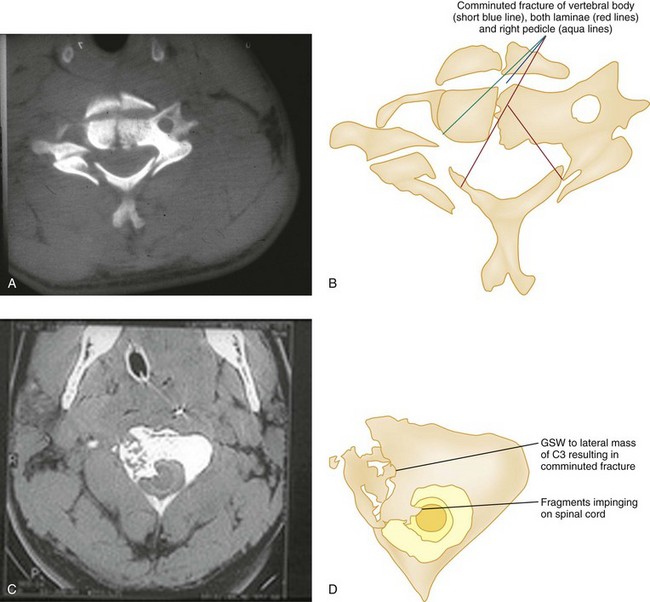
The superiority of CT in fracture detection is a critical advantage (Figs. 43-31, 43-32, and 43-33); therefore unclear fractures or displacements on standard radiographs should be further evaluated by a CT scan. Because fractures in contiguous and noncontiguous vertebrae are fairly common, CT scan should be obtained to visualize the entire cervical spine. CT can also identify bony fragments, acute disk herniation, a foreign body, paraspinal hematoma, or an extramedullary hematoma.
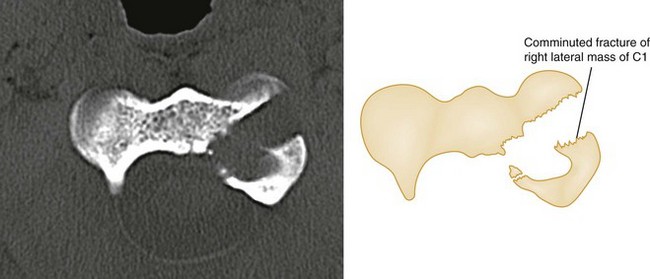
Figure 43-32 C1 fracture through the left lateral mass.
A study comparing CT with plain radiography demonstrated the sensitivity in the detection of spine fractures to be 70% for plain radiography compared with 100% for CT, and there was a significant reduction in mean time in the radiology department with CT (1.0 vs. 1.9 hours, P < .001).40 In another study, CT identified 99.3% of all fractures of the cervical, thoracic, and lumbar spine after high-energy trauma, and those missed by CT required minimal or no treatment.41 Finally, a systematic review of studies comparing plain radiography and CT also demonstrated CT to be superior with a pooled sensitivity of 52% (95% confidence interval [CI] 47-56) for plain radiography versus 98% (95% CI 96-99) for CT.42
Owing to the advantages of CT, some authorities argue that routine plain radiographs of the spine are unnecessary when CT is readily available. Investigators have assessed whether vertebral images reconstructed from CT scans of the abdomen and pelvis obtained for the evaluation of chest and abdominal injuries provide sufficient data to screen for spinal fractures, thereby decreasing the time and cost of spine injury evaluation. CT is also thought to be adequate to clear cervical spines even in the obtunded blunt trauma patient.43
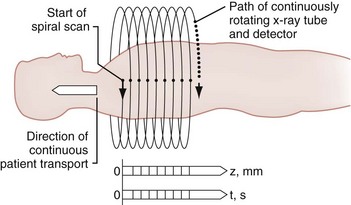
The spiral (helical) CT scan provides continuous acquisition of data (volume scan) via a rotating x-ray tube and simultaneous patient movement through the CT gantry (Fig. 43-34). The ability to reconstruct axial CT data in two-dimensional and three-dimensional formats is helpful to nonradiologists, who are less accustomed to performing the mental integration of multiplanar images (Fig. 43-35). The spiral CT scan can demonstrate cervical spine injuries not apparent on plain film or axial CT images (Fig. 43-36).
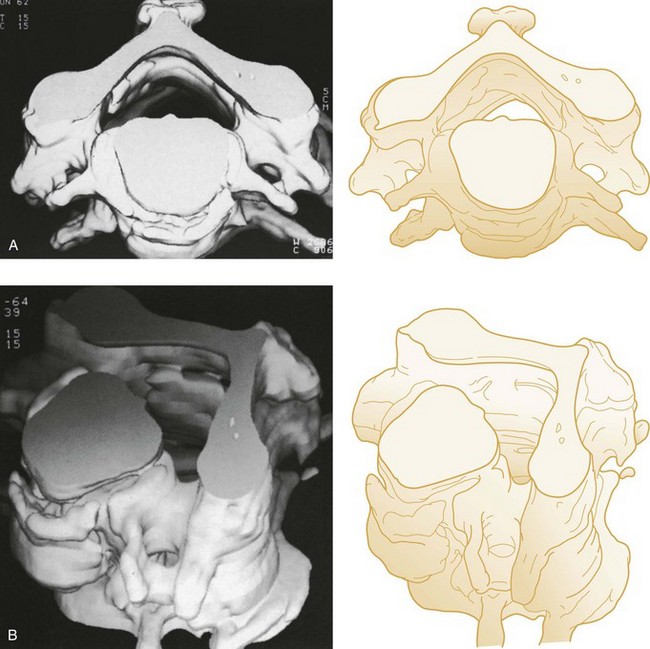
Figure 43-35 Three-dimensional computed tomography images of the cervical vertebrae. A, Superior view. B, Oblique view.
Potential disadvantages of CT include cost and radiation exposure. However, in one study, although CT was associated with higher mean overall spinal imaging charges than plain radiography ($4386 vs. $513, P < .001), there was no statistically significant difference when comparing spinal imaging cost per patient ($172 vs. $164, P > .05).40 In the same study, radiation exposure for CT was higher than for plain radiography for cervical spine imaging (26 vs. 4 mSv), but CT had lower levels of radiation exposure for thoracolumbar imaging (13 vs. 26 mSv).40
Magnetic Resonance Imaging
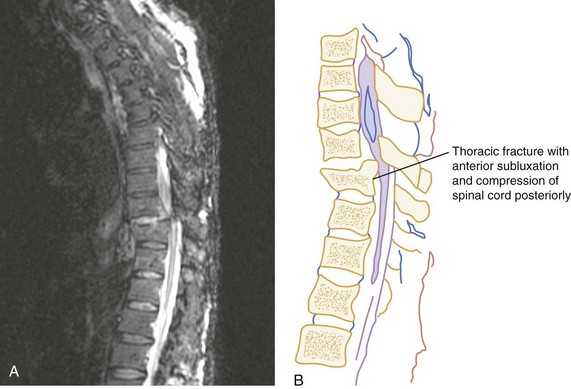
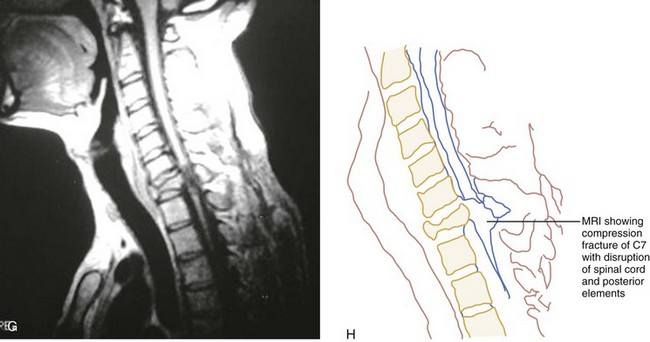
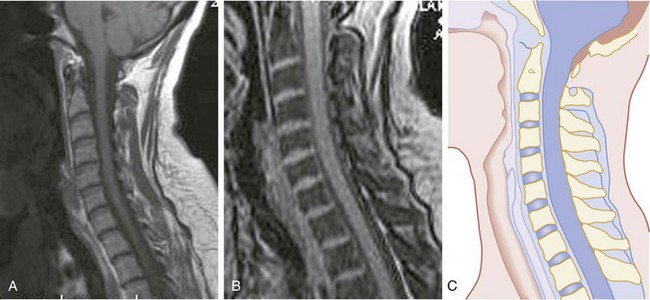
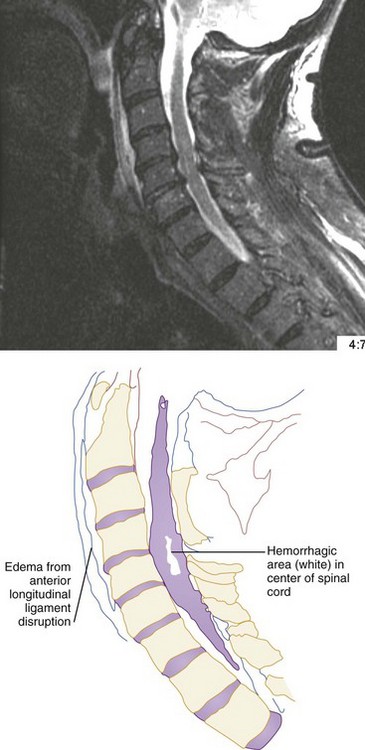
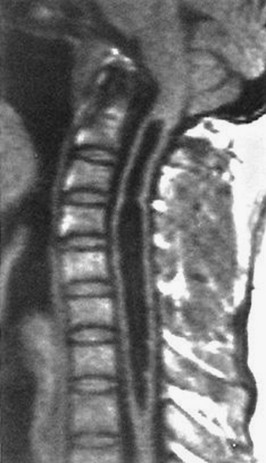
CT has a higher sensitivity than MRI to detect fractures and dislocations at the craniocervical junction, as well as fractures of the posterior elements of the spine.45 However, MRI, with its superior resolution and lack of ionizing radiation, also has the primary advantage of the ability to directly image nonosseous structures, including intramedullary and extramedullary spinal abnormalities that potentially cause neurologic deficit (Fig. 43-37). Its major impact has therefore been in demonstrating potentially surgically correctable lesions, including acute disk herniation, ligamentous injury, bony compression, epidural and subdural hemorrhage, and vertebral artery occlusion (Fig. 43-38). MRI can identify three separate patterns of SCI, including acute cord hemorrhage, cord edema or contusion, and mixed cord injury. Patients with cord edema or contusion show significant neurologic improvement, whereas those with cord hemorrhage (Fig. 43-39) fare far worse. MRI therefore has diagnostic and prognostic capabilities in the evaluation of cervical spine injury. MRI is also viewed as the best diagnostic imaging modality for SCIWORA, in which MRI may demonstrate central disk herniation, spinal stenosis, cord edema, and contusion.
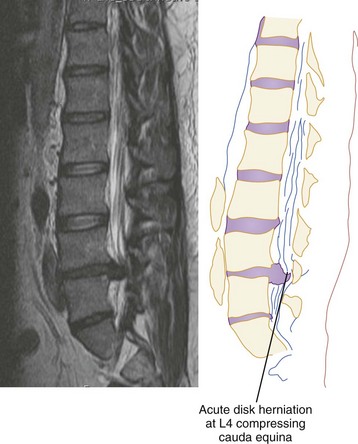
Figure 43-38 Magnetic resonance image showing acute L4 disk herniation with compression of the cauda equina.
Contraindications to MRI include the presence of a pacemaker, cerebral aneurysm clips (MRI-compatible clips are now available), and metallic (ferromagnetic) foreign bodies. There are risks to performing an MRI, as well, such as aspiration, secondary brain injury, and the difficulty of monitoring and resuscitating a patient in the MRI suite.46 In addition, MRI cannot be used when MRI-incompatible life-support, monitoring systems, and cervical traction devices are employed (although MRI-compatible support systems exist). Plain films and CT are still superior to MRI in evaluating osseous anatomy and fractures, particularly posterior-element fractures.
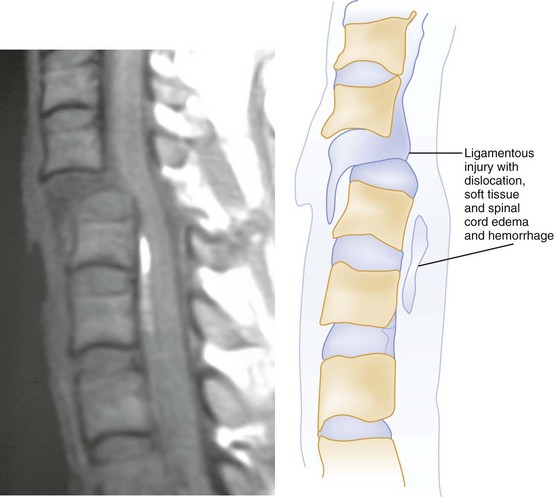
The role of MRI in the evaluation of acute cervical spine trauma continues to evolve, and some advocate an immediate MRI in patients with clinical signs or plain film evidence suggesting ligamentous injury, particularly when prevertebral swelling is noted (Fig. 43-40). Most recommend MRI for patients with a strong clinical suggestion of an occult spine injury who have normal plain radiographs, including those with persistent neck pain or neurologic deficit. However, when CT findings are normal, some studies have demonstrated that MRI may not be necessary to exclude unstable injuries, even in the obtunded or “unreliable” patient.47,48 A recent prospective study of the use of cervical spine CT in 402 obtunded patients reported a sensitivity of greater than 99%.49 MRI, however, is useful in the evaluation of the previously traumatized spinal cord.50 Progressive neurologic dysfunction in a previously stable cord-injury patient may indicate undiagnosed disk or bone impingement on the spinal cord, myelomalacia, a developing intramedullary (post-traumatic) syrinx, or subarachnoid cystic changes (Fig. 43-41), which can all be diagnosed by MRI. For fractures involving the transverse foramina or C1-C3, which are associated with vertebral artery dissection or thrombosis in up 22% of cases, as well as basilar artery stroke, magnetic resonance angiography (MRA), CT angiography (CTA), or four-vessel angiogram should be considered.51
Management
A spinal injury should be suspected in all trauma victims with an unknown or suggestive mechanism of injury associated with complaints of neck or back pain, evidence of significant head or facial trauma, spinal tenderness, signs of focal neurologic deficit, impaired consciousness, potentially distracting injuries, or unexplained hypotension (see Fig. 43-42 for management algorithm).
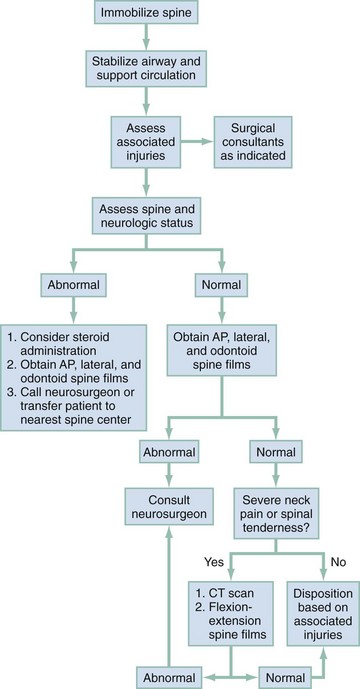
Figure 43-42 Approach to a patient with suspected cervical spine injury. AP, anteroposterior; CT, computed tomography.
Spinal Column Stabilization
Spinal immobilization should be initiated at the scene and maintained until a spinal injury is excluded in the emergency department. A variety of effective spinal immobilization orthoses are commercially available. The most widely used, and perhaps most effective, approach uses the combination of a spinal backboard, a rigid cervical collar, and supportive blocks placed on both sides of the head, which is held in place by straps or tape stretched across the forehead.52–56 The heads of children younger than age 8 are somewhat large in proportion to their bodies, resulting in mild neck flexion when they are placed on a flat backboard. This can be avoided by use of a special backboard with an occipital recess or by placing a rolled sheet under the shoulders.55 Further out-of-hospital care should be directed at evaluation and stabilization of associated injuries or problems requiring immediate intervention.
Emergency Department
Trauma victims who arrive at the emergency department with spinal immobilization should be quickly assessed by a physician. If the probability of spinal injury is moderate to high, it is advisable to remove the patient’s clothes, evaluate any associated injuries, and perform resuscitative maneuvers without removing the immobilization apparatus. If the patient’s spine can be clinically cleared by use of either the NEXUS criteria or the CCR, as described earlier, the immobilization device may be carefully removed. In addition, if the trauma victim was wearing a helmet and the helmet was not removed in the field, the face mask and/or the helmet may be carefully removed while immobilization is maintained.56a Ideally, at least two or three providers should be present to perform the task of helmet and face mask removal. The first provider should position himself or herself at the head of the bed and immobilize the cervical spine by placing both hands on the sides of the helmet while securing the patient’s mandibles bilaterally. The chin strap should then be cut while a second provider takes over the job of in-line stabilization by placing one hand on the patient’s occiput and another hand on the patient’s chin. The first provider may then use a screwdriver to remove the four screws from the face mask and remove the face mask, which opens access to the airway. Note that face mask removal is all that is necessary to intubate the patient. The helmet and the shoulder pads should be removed simultaneously; one provider pulls the sides of the helmet away from the patient’s ears while the second provider prevents hyperextension of the neck or overextension of the head onto the bed.56b Once the helmet and shoulder pads have been removed, a rigid collar should be placed if the patient’s cervical spine cannot be cleared by use of the NEXUS criteria or the CCR.
Airway Management
According to the American College of Surgeons (ACS) Advanced Trauma Life Support guidelines, the preferred method of airway management for patients with traumatic cardiopulmonary arrest, even with evidence of spinal injury, is rapid sequence intubation (RSI) orotracheally with in-line spinal stabilization.57 This is also the recommended approach for patients who are breathing but unconscious and in need of airway control or ventilatory support.58–67 Hypoxia should be prevented, as it may contribute to apoptosis and further cord damage.68
The value of in-line spinal stabilization has been questioned in a review of the topic that notes that the data supporting its use come from cadaver studies, observations on uninjured volunteers, and several case series; that there is little evidence to support the contention that orotracheal intubation without in-line stabilization, which causes less spinal motion than the chin thrust maneuver, is unsafe in spinal-injured patients; that the incidence of spinal injury in trauma patients requiring intubation, who often have intracranial injuries, is quite low; and that there is evidence that in-line stabilization diminishes the laryngoscopic view, possibly leading to failed intubation and severe hypoxemia, which has been shown to predict poor outcomes in central nervous system injury.69 The authors note that despite this information, clinicians will be hesitant to forgo in-line stabilization because it is recommended by the ACS and suggest that further studies in trauma airway management be performed with use of intubating supraglottic airways, optical stylets, and videolaryngoscopes. Cricothyrotomy or percutaneous jet ventilation should be considered for patients with severe maxillofacial injuries and when RSI is contraindicated or if attempts at orotracheal intubation fail (see Chapter 1).
Spinal Shock
Spinal shock is a clinical syndrome characterized by the temporary loss of neurologic function and autonomic tone below the level of an acute spinal cord lesion.16 Patients usually exhibit flaccid paralysis with loss of sensation, deep tendon reflexes, and urinary retention, along with bradycardia, hypotension, hypothermia, and intestinal ileus. The cessation of spinal shock, which may last from less than 24 hours to more than 2 weeks,70 is heralded by the return of the bulbocavernosus reflex.
Although there is no consensus in the literature regarding the most appropriate treatment of neurogenic hypotension, it is prudent to begin the resuscitation of all newly arrived, hypotensive trauma victims with crystalloid fluid infusion. Most cases of pure neurogenic hypotension are mild (e.g., systolic blood pressure >90) and will initially respond to fluids. Severe (e.g., systolic blood pressure <70) neurogenic hypotension, seen in 20 to 30% of all patients with spinal injuries, usually occurs with high cervical injuries associated with total or near-total loss of neurologic function.16 Hypotension can lead to hypoperfusion and secondary ischemic injury of the spinal cord, which can adversely affect prognosis. Therefore prolonged, severe hypotension must be prevented and treated.71 Fluid resuscitation is often ineffective in such patients and may result in fluid overload if aggressively pursued. As a result, symptomatic neurogenic hypotension should be managed with fluids, Trendelenburg positioning (unless contraindicated because of concomitant head injury), vasopressors, or cardiac pacing—based on hemodynamic monitoring—once the diagnosis is established and other causes of traumatic shock have been excluded.
Pharmacologics for Incomplete Cord Injury
Experimental animal models of SCI support the concept that delayed biochemical damage, occurring hours to days after an initial traumatic insult, contributes to ongoing tissue loss and worsening neurologic function. As a result, numerous neuroprotective and neuroregenerative treatment strategies, including induction of hypothermia, have been investigated in both laboratory animal studies and human clinical trials (Box 43-1).72–89
Methylprednisolone is currently the only agent used for human victims of SCI outside of the research setting, and its use is highly controversial. The National Acute Spinal Cord Injury Study (NASCIS) investigators published several flawed studies showing that early administration of high-dose methylprednisolone improves the neurologic outcome of SCI victims.77–80 Methylprednisolone is administered as an initial 30 mg/kg intravenous bolus followed by an infusion of 5.4 mg/kg/hr. The infusion is maintained for 24 hours in patients who are treated within 3 hours after injury, and it is maintained for 48 hours in patients who are treated within 3 to 8 hours after injury. The administration of steroids resulted in a worse outcome when started after 8 hours.
The NASCIS studies have been criticized for methodological flaws and for failing to assess whether the mild neurologic improvement seen in patients treated with methylprednisolone actually results in improved day-to-day functioning.90,91 Moreover, other studies have reported that patients treated with methylprednisolone have a higher rate of infectious, gastrointestinal, and metabolic complications compared with placebo.92 The most recent guidelines published by the Consortium for Spinal Cord Medicine (which includes the American Association of Neurological Surgeons, the American College of Emergency Physicians, the Congress of Neurological Surgeons, the American Association of Orthopaedic Surgeons, and the International Spinal Cord Society) in 2008 state that “no clinical evidence exists to definitively recommend the use of any neuroprotective pharmacologic agent, including steroids, in the treatment of acute SCI to improve functional recovery.”93 Despite this, the Cochrane Database review maintains that “high-dose methylprednisolone steroid therapy is the only pharmacologic therapy shown to have efficacy in a Phase Three randomized trial when it can be administered within 8 hours of injury…and…there may be additional benefit by extending the maintenance dose from 24 to 48 hours if treatment is not started until between 3 and 8 hours after injury.”94 Surveys performed in the United States, the United Kingdom, and Canada between 2006 and 2009 reveal the number of spine surgeons using steroids to treat patients with blunt SCI diminishing to a minority in recent times, with many of those still using steroids listing “fear of litigation” as the reason.93–96 Some authors continue to support the use of steroids until more effective treatments are discovered, feeling that the effectiveness of steroids in this setting has not been definitely disproved and that the majority of complications associated with steroid use in otherwise healthy young adults are most often minor and manageable.97–101 There is currently no substantive scientific evidence to support the use of prednisolone for acute blunt SCI, and because of the possibility of severe complications and side effects92,102,103 its use can be considered only, at best, a treatment option rather than the treatment standard. A risk-benefit assessment should be performed on a case-by-case basis.
Associated Injuries
Although deterioration in a trauma victim’s cardiopulmonary status is usually the result of hemorrhagic shock or direct injury to the heart or lungs, it may reflect the development of pulmonary edema that occasionally occurs in response to brain injury and SCI.104 Spinal cord trauma may stimulate an intense sympathetic discharge with two subsequent effects. First, pulmonary capillary endothelial cells are disrupted, leading to the pulmonary capillary leak syndrome, in which pulmonary edema occurs in the presence of normal pulmonary artery pressures (<18 mm Hg). Second, marked increases in afterload may cause left ventricular dysfunction, leading to pulmonary edema associated with a high pulmonary artery pressure (>18 mm Hg). Aggressive fluid resuscitation can also contribute to pulmonary edema. As a result, the management of such patients is often complex and requires carefully balancing fluid requirements, afterload-reducing agents, and artificial ventilation with positive end-expiratory pressure.97 In addition, because of the autonomic dysfunction that often occurs in response to SCI, patients may demonstrate extremely volatile fluctuations in blood pressure, sometimes resulting in a life-threatening hypertensive emergency.
Gastrointestinal and Genitourinary
If SCI renders the abdominal examination unreliable, an abdominal CT scan, ultrasound, diagnostic peritoneal lavage, or some combination is often necessary.105 In the acute stages of SCI, both the gastrointestinal tract and the bladder become atonic. Thus, once a patient has been stabilized, a nasogastric tube should be placed to prevent gastric distention, and a Foley catheter inserted to prevent bladder distention and to monitor fluid output. Because gastrointestinal bleeding from stress ulcers occurs in 2 to 20% of spinal trauma patients,106 ulcer prophylaxis with histamine H2 receptor antagonists or proton pump inhibitors should be initiated.93
Definitive Treatment and Prognosis
The role of immediate surgical intervention in the management of spinal injuries is currently limited to relieving impingement on the spinal cord caused by foreign bodies, herniated disks, bony fracture fragments, or an epidural hematoma.101,107 Surgery may be necessary later to stabilize severe bony injuries or to reduce spinal dislocations. The timing of surgical intervention is controversial, and there are no well-designed studies that have determined whether early (<12 hours) versus late decompression is beneficial.93
Once almost uniformly fatal, major spinal injury caused death as a result of pulmonary complications or sepsis resulting from skin necrosis or urinary infection. The advent of antibiotic therapy made long-term survival not only possible but also expected. Today, patients with SCIs are best managed by early referral to a regional spine injury center, where a team of neurosurgeons, orthopedic surgeons, psychologists, and physical therapists can initiate rehabilitation. Specialized SCI treatment centers offer patients a chance to return to a productive life within the limits of their disability. With the exception of patients with high cervical lesions (above C5), most patients attain sufficient independence to live outside of a high-level care environment.109
Disposition
Patients with musculoskeletal injuries of the spine who have only mild to moderate discomfort without neurologic impairment or abnormal radiographic findings are best managed as outpatients. Treatment should include analgesics and referral for follow-up evaluation. Up to 27% of patients experiencing neck pain in the emergency department after trauma will continue to have symptoms at 1 year.110,111
Minor Fractures
Most patients with spinal fractures require hospitalization. Patients with isolated cervical vertebral body compression fractures or spinous process fractures may be managed as outpatients if the mechanism of injury is not significant, there is no evidence of neurologic impairment or associated ligamentous instability, and the degree of patient distress is not severe. Appropriate follow-up should be arranged in all instances because even minor spinal injuries may be associated with prolonged disability resulting from chronic pain.110–114
For patients with minor wedge fractures (<10% wedge fractures) who do not have an associated ileus or neurologic deficit, outpatient management may also be possible. However, most wedge fractures of the thoracic and lumbar spine are usually best managed in the hospital for several reasons.115,116 First, patients with these injuries usually have marked discomfort, often requiring parenteral narcotics. Second, significant force is generally required to fracture thoracic or lumbar vertebrae, and associated intrathoracic or abdominal injuries are common. Elderly patients who have vertebral fractures and only minor associated injuries may require admission to facilitate assessment of fall risks and to expedite rehabilitation. Finally, lower thoracic and lumbar fractures are associated with prolonged and occasionally delayed gastrointestinal ileus, requiring continuous nasogastric suction.
References
1. Spinal Cord Information Network. Spinal Cord Injury: Facts and Figures at a Glance. https://www.nscisc.uab.edu.
2. Berkowitz, M. Assessing the socioeconomic impact of improved treatment of head and spinal cord injuries. J Emerg Med. 1993;11:63.
3. Snell RS, Smith MS, eds. Clinical Anatomy for Emergency Medicine. St Louis: Mosby, 1993.
4. Demetriades, D, Newton, E. Atlas of Emergency Trauma, 2nd ed. Cambridge UK: Cambridge University Press; 2011.
5. Looby, S, Flaanders, A. Spine trauma. Radiol Clin North Am. 2011;49:129–163.
6. Harris JH, Harris WH, eds. The Radiology of Emergency Medicine, 4th ed, Philadelphia: Lippincott: Williams & Wilkins, 2000.
7. Maroon, JC, Abla, AA. Classification of acute spinal cord injury, neurological evaluation and neurosurgical considerations. Crit Care Med. 1987;3:655.
8. Guthkelch, AN, Fleischer, AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147:428.
9. Ankeny, DP, Popovich, PG. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience. 2009;158:1112.
10. Matis, GK, Birbilis, TA. Erythropoietin in spinal cord injury. Eur Spine. 2009;18:314.
11. Darian-Smith, C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15:149.
12. Sinclair, D, Schwartz, M, Gruss, J, McLellan, B. A retrospective review of the relationship between facial fractures, head injuries and cervical spine injuries. J Emerg Med. 1988;6:109.
13. Tator, CH. Management of associated spine injures in head injured patients. In: Narayan RK, Wilberger JE, Poulishock JT, eds. Neurotrauma. New York: McGraw-Hill, 1996.
14. Ditunno, JF, Little, JW, Tessler, A, Burns, AS. Spinal shock revisited: A four phase model. Spinal Cord. 2004;42:383.
15. Merriam, WF, Taylor, TK, Ruff, SJ, McPhail, MJ. A reappraisal of acute traumatic central cord syndrome. J Bone Joint Surg Br. 1986;68:708.
16. Atkinson, PP, Atkinson, LD. Spinal shock. Mayo Clin Proc. 1996;71:384.
17. Pang, D, Pollack, IF. Spinal cord injury without radiographic abnormality in children—the SCIWORA syndrome. Trauma. 1989;29:658.
18. Kokoska, ER, Keller, MS, Rallo, MC, Weber, TR. Characteristics of pediatric cervical spine injures. J Pediatr Surg. 2001;36:10.
19. Apuzzo, ML. Spinal cord injury without radiographic abnormality. Neurosurgery. 2002;50:S100.
20. McCaig, LF, Burt, CW. National Hospital Ambulatory Medical Care Survey: 2003 emergency department summary. Adv Data. 2006;358:1.
21. Stiell, IG, et al. Variation in emergency department use of cervical spine radiography for alert, stable trauma patients. CMAJ. 1997;156:1537.
22. Hoffman, JR, Mower, WR, Wolfson, AB, Todd, KH, Zucker, MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med. 2000;343:94.
23. Stiell, IG, et al. The Canadian C-spine Rule for radiography in alert and stable trauma patients. JAMA. 2011;286:1841.
24. West, OC, Anbari, MM, Pilgram, TK, Wilson, AJ. Acute cervical spine trauma: Diagnostic performance of single-view versus three-view radiographic screening. Radiology. 1997;204:819.
25. Petri, R, Gimbel, R. Evaluation of the patient with spinal trauma and back pain: An evidence based approach. Emerg Med Clin North Am. 1999;17:25.
26. Mower, WR, et al. Use of plain radiography to screen for cervical spine injuries. Ann Emerg Med. 2001;38:1.
27. Cadoux, CG, White, JD, Hedberg, MC. High-yield roentgenographic criteria for cervical spine injuries. Ann Emerg Med. 1987;16:738.
28. Fesmire, FM, Luten, RC. The pediatric cervical spine: Developmental anatomy and clinical aspects. J Emerg Med. 1989;7:133.
29. Mann, FA, et al. Supine oblique views of the cervical spine: A poor proxy for the lateral view. Emerg Radiol. 1995;2:214.
30. Bagley, LJ. Imaging of spinal trauma. Radiol Clin North Am. 2006;44:1.
31. Pollack, C, et al. Use of flexion-extension radiographs of the cervical spine in blunt trauma. Ann Emerg Med. 2001;38:8.
32. Khan, SN, Erickson, G, Sena, MJ, Gupta, MC. Use of flexion and extension radiographs of the cervical spine to rule out acute instability in patients with negative computed tomography scans. J Orthop Trauma. 2011;25:132.
33. Panacek, EA, Mower, WR, Holmes, JF, Hoffman, JR, NEXUS Group. Test performance of the individual NEXUS low-risk clinical screening criteria for cervical spine injury. Ann Emerg Med. 2001;38:22.
34. Pitt, E, Thakore, S. Role of flexion/extension radiography in neck injuries in adults. Emerg Med J. 2004;21:587.
35. Murphey, MD, Batnitzky, S, Bramble, JM. Diagnostic imaging of spinal trauma. Radiol Clin North Am. 1989;27:855.
36. Domenicucci, M, et al. Three-dimensional computed tomographic imaging in the diagnosis of vertebral column trauma: Experience based on 21 patients and review of the literature. J Trauma. 1997;42:254.
37. Acheson, MB, Livingston, RR, Richardson, ML, Stimac, GK. High-resolution CT scanning in the evaluation of cervical spine fractures: Comparison with plain film examinations. Am J Radiol. 1987;148:1179.
38. Schleehauf, K, Ross, SE, Civil, ID, Schwab, CW. Computed tomography in the initial evaluation of the cervical spine. Ann Emerg Med. 1989;18:815.
39. Como, JJ, et al. Practice management guidelines for identification of cervical spine injuries following trauma: Update from the Eastern Association for the Surgery of Trauma practice management guidelines committee. J Trauma. 2009;67:651.
40. Antevil, JL, et al. Spiral computed tomography for the initial evaluation of spine trauma: A new standard of care? J Trauma. 2006;61:382.
41. McCulloch, PT, et al. Helical computed tomography alone compared with plain radiographs with adjunct computed tomography to evaluate the cervical spine after high-energy trauma. J Bone Joint Surg Am. 2005;87:2388.
42. Holmes, JF, Akkinepalli, R. Computed tomography versus plain radiography to screen for cervical spin injury: A meta-analysis. J Trauma. 2005;58:902.
43. Widder, S, et al. Prospective evaluation of computed tomographic scanning for the spinal clearance of obtunded trauma patients: Preliminary results. J Trauma. 2004;56:1179.
44. Reference deleted in proofs.
45. Holmes, JF, et al. Variability in computed tomography and magnetic resonance imaging in patients with cervical spine injuries. J Trauma. 2002;53:524.
46. Dunham, CM, Brocker, BP, Collier, BD, Gemmel, DJ. Risks associated with magnetic resonance imaging and cervical collar in comatose, blunt trauma patients with negative comprehensive cervical spine computed tomography and no apparent spinal deficit. Crit Care. 2008;12:R89.
47. Schuster, R, et al. Magnetic resonance imaging is not needed to clear cervical spines in blunt trauma patients with normal computed tomographic results and no motor deficits. Arch Surg. 2005;140:762.
48. Hogan, GJ, Mirvis, SE, Shanmuganathan, K, Scalea, TM. Exclusion of unstable cervical spine injury in obtunded patients with blunt trauma: Is MR imaging needed when multi-detector row CT findings are normal? Radiology. 2005;237:106.
49. Hennessy, D, et al. Cervical spine clearance in obtunded trauma patients: A prospective study. J Trauma. 2010;68:576.
50. Pimentel, L, Diegelmann, L. Evaluation and management of acute cervical spine trauma. Emerg Med Clin North Am. 2010;28:719–738.
51. Cothren, C, et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55:811–813.
52. Solot, JA, Winzelberg, GG. Clinical and radiographic evaluation of vertebrae extrication collars. J Emerg Med. 1990;8:79.
53. Chandler, DR, Nemejc, C, Adkins, RH, Waters, RL. Emergency cervical spine immobilization. Ann Emerg Med. 1992;21:1185.
54. Apuzzo, ML. Cervical spine immobilization before admission to the hospital. Neurosurgery. 2002;50:S7.
55. Apuzzo, ML. Management of pediatric cervical spine and spinal cord injuries. Neurosurgery. 2002;50:S85.
56. DeLorenzo, RA. A review of spinal immobilization techniques. J Emerg Med. 1996;14:603.
56a. Waninger, KN. Management of the helmeted athlete with suspected cervical spine injury. Am J Sports Med. 2004;32:1331–1350.
56b. Peris, MD, Donaldson, WF, 3rd., Towers, J, Blanc, R, Mussonigro, TS. Helmet and shoulder pad removal in suspected cervical spine injury: human control model. Spine (Phila Pa 1976). 2002;27:995–999.
57. American College of Surgeons. ATLS: Advanced Trauma Life Support Student Course Manual, 8th ed. Chicago: American College of Surgeons; 2008.
58. Ghafoor, AU, Martin, TW, Gopalakrishnan, S, Viswamitra, S. Caring for the patients with cervical spine injuries: What have we learned? J Clin Anesth. 2005;17:640.
59. Ollerton, JE, Parr, MJ, Harrison, K, Hanrahan, B, Sugrue, M. Potential cervical spine injury and difficult airway management for emergency intubation of trauma adults in the emergency department—a systematic review. Emerg Med J. 2006;23:3.
60. Crosby, ET. Airway management in adults after cervical spine trauma. Anesthesiology. 2006;104:1293.
61. Bivins, HG, Ford, S, Bezmalinovic, Z, Price, HM, Williams, JL. The effect of axial traction during orotracheal intubation of the trauma victim with an unstable cervical spine. Ann Emerg Med. 1988;17:25.
62. Rhee, KJ, O’Malley, RJ. Neuromuscular blockade-assisted oral intubation versus nasotracheal intubation in the prehospital care of injured patients. Ann Emerg Med. 1994;23:37.
63. Talucci, RC, Shaikh, KA, Schwab, CW. Rapid sequence induction with oral endotracheal intubation in the multiply injured patient. Am Surg. 1988;54:185.
64. Holley, J, Jordon, R. Airway management in patients with unstable cervical spine fractures. Ann Emerg Med. 1989;18:1237.
65. Rhee, KJ, Green, W, Holcroft, JW, Mangili, JA. Oral intubation in the multiply injured patient: The risk of exacerbating spinal cord damage. Ann Emerg Med. 1990;19:511.
66. Wright, SW, Robinson, GG, 2nd., Wright, MB. Cervical spine injuries in blunt trauma patients requiring emergent endotracheal intubation. J Emerg Med. 1992;10:104.
67. Scannell, G, Waxman, K, Tominaga, G, Barker, S, Annas, C. Orotracheal intubation in trauma patients with cervical fractures. Arch Surg. 1993;128:903.
68. Bilello, JF, et al. Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg. 2003;138:1127.
69. Manoach, S, Paladino, L. Manual in-line stabilization of suspected cervical spine injury: Historical review and current questions. Ann Emerg Med. 2007;50:236.
70. Srivastava, R. Spinal shock in spinal cord injury—is duration level dependent? J Bone Joint Surg. 2006;88:144.
71. Sekhan, L, Fehlings, MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2–S12.
72. Straley, KS, Foo, CW, Heilshorn, SC. Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma. 2009;27:1.
73. Bracken, MB. Pharmacological treatment of acute spinal cord injury: Current status and future projects. J Emerg Med. 1993;11:43.
74. Xu, XM, Onifer, SM. Transplantation-mediated strategies to promote axonal regeneration following spinal cord injury. Respir Physiol Neurobiol. 2009;169:171.
75. Cappuccino, A, et al. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine (Phila Pa 1976). 2010;35:E57.
76. Geisler, FH, Dorsey, FC, Coleman, WP. Past and current clinical studies with GM-1 ganglioside in acute spinal cord injury. Ann Emerg Med. 1993;22:1041.
77. Bracken, MB, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. N Engl J Med. 1990;322:1405.
78. Bracken, MB, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76:23.
79. Bracken, MB, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. JAMA. 1997;277:20.
80. Bracken, MB, et al. Methylprednisolone or tirilizad mesylate administration after acute spinal cord injury: 1-year follow-up. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. J Neurosurg. 1998;89:699.
81. Faden, AI, Stoica, B. Neuroprotection: Challenges and opportunities. Arch Neurol. 2007;64:794.
82. Qaio, F, et al. Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma. Am J Pathol. 2006;169:1039.
83. Brewer, KL, Hardin, JS. Neuroprotective effects of nicotinamide after experimental spinal cord injury. Acad Emerg Med. 2004;11:125.
84. Nikulina, E, Tidwell, JL, Dai, HN, Bregman, BS, Filbin, MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786.
85. Hata, K, et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47.
86. Nakayama, T, et al. Atrial natriuretic peptide reduces ischemia/reperfusion-induced spinal cord injury in rats by enhancing sensory neuron activation. J Pharmacol Exp Ther. 2007;322:582.
87. Cao, Q, et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947.
88. Fuller, DD, Johnson, SM, Olson, EB, Jr., Mitchell, GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993.
89. Donovan, WH. Spinal cord injury—past, present and future. J Spinal Cord Med. 2007;30:85.
90. Coleman, WP, et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13:185.
91. Nesathurai, S. Steroids and spinal cord injury: Revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45:1088.
92. Matusmoto, T, et al. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine (Phila Pa 1976). 2001;26:426.
93. Consortium for Spinal Cord Medicine. Early Acute Management in Adults with Spinal Cord Injury: A Clinical Practice Guideline for Healthcare Providers. Washington, DC: Consortium for Spinal Cord Medicine; 2008.
94. Bracken, MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2, 2002.
95. Eck, JC, Nachtigall, D, Humphreys, SC, Hodges, SD. Questionnaire survey of spine surgeons on the use of methylprednisolone for acute spinal cord injury. Spine (Phila Pa 1976). 2006;31:E250.
96. Frampton, AE, Eynon, CA. High dose methylprednisolone in the immediate management of acute, blunt spinal cord injury: What is the current practice in emergency departments, spinal units and neurosurgical units in the UK? Emerg Med J. 2006;23:550.
97. Hurlbert, RJ, Hamilton, MG. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci. 2008;35:41.
98. Nicholas, JS, Selassie, AW, Lineberry, LA, Pickelsimer, EE, Haines, SJ. Use and determinants of the methylprednisolone protocol for traumatic spinal cord injury in South Carolina acute care hospitals. J Trauma. 2009;66:1446.
99. Rozet, IR. Methylprednisolone in acute spinal cord injury: Is there any other ethical choice? J Neurosurg Anesthesiol. 2008;20:137.
100. Walsh, KA, Weant, KA, Cook, AM. Potential benefits of high-dose methylprednisolone in acute spinal cord injuries. Orthopedics. 2010;33:249.
101. Liu, JC, Patel, A, Vaccaro, AR, Lammertse, DP, Chen, D. Methylprednisolone after traumatic spinal cord injury: Yes or no? PM R. 2009;1:669.
102. Suberviola, B, González-Castro, A, Llorca, J, Ortiz-Melón, F, Miñambres, E. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury. 2008;39:748.
103. Ito, Y, Sugimoto, Y, Tomioka, M, Kai, N, Tanaka, M. Does high dose methylprednisolone sodium succinate really improve neurological status in patients with acute cervical cord injury? A prospective study about neurological recovery and early complications. Spine (Phila Pa 1976). 2009;34:2121.
104. Lemons, VR, Wagner, FC. Respiratory complications after cervical spinal cord injury. Spine (Phila Pa 1976). 1994;19:2315.
105. Soderstrom, CA, McArdle, DQ, Ducker, TB, Militello, PR. The diagnosis of intra-abdominal injury in patients with cervical cord trauma. J Trauma. 1983;23:1061.
106. Matsumura, JS, et al. Gastrointestinal tract complications after acute spine injury. Arch Surg. 1995;130:751.
107. Vaccaro, AR, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609.
108. Reference deleted in proofs.
109. Mizra, SK, et al. Early versus delayed surgery for acute cervical spinal cord injury. Clin Orthop Relat Res. 1999;359:104.
110. Dollfus, P. Rehabilitation following injury to the spinal cord. J Emerg Med. 1993;11:57.
111. McNamara, RM, O’Brien, MC, Davidheiser, S. Post-traumatic neck pain: A prospective and follow-up study. Ann Emerg Med. 1988;17:906.
112. Atherton, K, et al. Predictors of persistent neck pain after whiplash injury. Emerg Med J. 2006;23:195.
113. Ditunno, JF, Formal, CS. Chronic spinal cord injury. N Engl J Med. 1994;330:550.
114. Ross, PD. Clinical consequences of vertebral fractures. Am J Med. 1997;103:305.
115. Savitsky, E, Votey, S. Emergency department approach to acute thoracolumbar spine injury. J Emerg Med. 1996;15:49.
116. Vollmer, DE, Gegg, C. Classification and acute management of thoracolumbar fractures. Neurosurg Clin N Am. 1997;8:499.