CHAPTER 107 Spinal Cord Stimulation in Chronic Pain
INTRODUCTION
Since the first published paper on spinal cord stimulation (SCS) by Shealy in 1967 there have been a total of over 2000 articles, presentations, symposia, and abstracts on the topic of neuroaugmentation.88,92 The long-term results of SCS published in the 1970s were disappointing yet promising.28,30,32,93 Most of the studies published in the 1970s and early 1980s demonstrated success rates of approximately 40%.12 As with many new devices, problems included poorly designed hardware and software, inadequate patient selection criteria, and suboptimal surgical technique. Early on, the hardware typically consisted of a single or dual electrode system that was implanted epidurally. They provided a small electrical field and thus were unable to consistently stimulate the spinal cord. In addition, these systems were implanted via laminectomy or laminotomy with the patient under general anesthesia, thus eliminating the possibility of surgeon–patient interaction. The electrodes were commonly implanted in the high thoracic or lower cervical region for lumbar pain syndromes and patients were not consistently screened for psychological dysfunction, drug habituation, secondary gain issues, pain topography, and quality of pain. All of these factors have considerable impact on the overall efficacy of SCS.
Significant advances in SCS have been made in recent years. The hardware is more durable and the electrode size and interelectrode distance have been improved. Improved software allows for more complex programming and multiple, simultaneously operating programs which generally allows for improved coverage and pain relief of difficult pain patterns. The devices may be implanted percutaneously under fluoroscopic guidance, which allows operator–patient interaction and may lead to more accurate positioning of SCS leads. Paddle-style leads implanted via laminectomy may have certain advantages relating to energy consumption and long-term maintenance of back pain. Three decades of experience have provided improved patient selection criteria. The net result is an improved capacity to control chronic pain.12 This chapter will discuss the clinical indications, outcomes, complications, and other methods of implantation which may positively impact future outcomes and areas of study.
PAIN ANATOMY AND PHYSIOLOGY
Pain is an uncomfortable sensation associated with an emotional response.35,106 The International Association for the Study of Pain (IASP) defined pain as ‘an unpleasant sensory and emotional experience associated with actual and potential tissue damage, or described in terms of such damage.’35 It may originate from stimulation of chemical, mechanical, or thermal receptors found in free nerve endings within injured tissue. This is known as afferent pain, and can occur in ligamentous or muscular injuries of the spine.11,47,54,90 Pain can also occur from direct injury to the peripheral nerve, which results in burning or shooting pain in the distribution of the affected nerve. This is called peripheral deafferentation (neuropathic) pain and is demonstrated in conditions such as complex regional pain syndrome, peripheral neuropathy, or radiculopathy.30,64,65 Central deafferent pain appears after injury to certain structures within the central nervous system, such as the thalamus, that are responsible for the transmission of pain.
Peripheral pain signals are transmitted by either thinly myelinated A-delta or unmyelinated C fibers. The A-delta fibers convey discrete, sharp, fast pain at approximately 15 m/sec, whereas the C fibers transmit vague, chronic, burning, slow pain at less than 1 m/sec.25,108
Pain fibers typically enter the spinal cord through the dorsal root and then ascend or descend two to six segments within the dorsolateral fasciculus (Lissauer’s tract)25,108 The A-delta fibers synapse with the dorsal gray horn neurons located in laminae 1, 2, 5, and 10, whereas the C fibers synapse with dorsal gray horn neurons located in laminae 1, 2, and 5. The majority of fibers then cross to the opposite ventrolateral portion of the spinal cord before ascending in the spinothalamic, spinoreticulothalamic, and spinomesencephalic tracts. The lateral spinothalamic fibers terminate in the thalamic ventralis posterolaterales and posteromedialis nuclei, from which fibers are projected into other areas of the thalamus and to the somatic sensory cortex. The medial spinothalamic, spinoreticulothalamic, and spinomesencephalic tracts end in the reticular activating system within the medulla, pons, midbrain, periaqueductal gray, hypothalamus, and thalamic medial and intralaminar nuclei (Fig. 107.1).
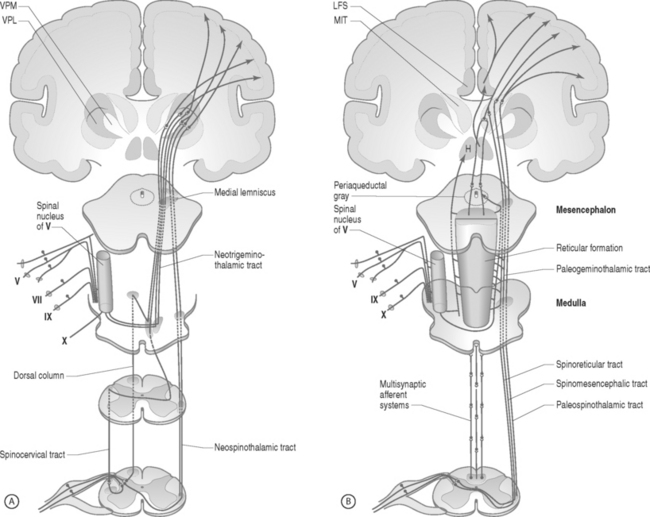
Fig. 107.1 Neuroanatomic pathway for nociceptive pain transmission. (A) Lateral tracts and (B) medial tracts.
In: Bonica JJ, ed. The Management of Pain. 2nd edn. Philadelphia: Lea & Febiger; 1990:89.
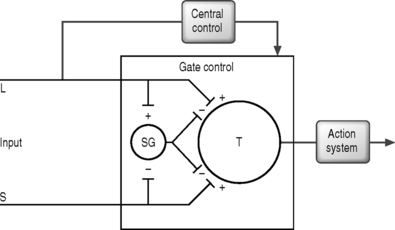
In 1965, Melzack and Wall published their ‘gate control’ theory in which they hypothesized that a ‘gate’ system existed for pain modulation located in the dorsal gray horn within the substantia gelatinosa (laminae 2 and 3).60 They proposed that excess tactile signals traveling along the large myelinated A-delta fibers closed the gate, which then inhibited the propagation of pain impulses along the unmyelinated C fibers (Fig. 107.2).
Although the pain pathway is still not completely understood, researchers have uncovered important parts of the neuronal system. This includes descending inhibitory influences from the brain, which have been shown to suppress transmission of pain.10,80,86 There is also evidence of an endogenous system of opioids that modulate sensory input.41,89,95
Today, there is a better awareness that the pain experience is not just physiologic but is also influenced by culture, religion, and psychological makeup.29,37,61,62 In order to provide appropriate treatment, all of these factors must be taken into consideration when evaluating patients.
MECHANISM OF ACTION
Although the exact mechanism for pain control from SCS is not entirely understood, it is believed to result from direct or facilitated inhibition of pain transmission.28,30,32,40,60,92 There exist five mechanistic theories for SCS which should be noted: (1) gate control theory – segmental, antidromic activation of A-beta efferents; (2) SCS blocks transmission in the spinothalamic tract; (3) SCS produces supraspinal pain inhibition; (4) SCS produces activation of central inhibitory mechanisms influencing sympathetic efferent neurons; and (5) SCS activates putative neurotransmitters or neuromodulators.40
The gate control theory motivated Shealy et al. in 1967 to apply SCS as a means to antidromically activate the tactile A-beta fibers through dorsal column stimulation.92 Shealy et al. reasoned that sustained stimulation of the dorsal columns would keep the gate closed and provide continuous pain relief. While the theoretical model put forth by Melzack and Wall has been shown not to be precisely accurate, pain gating or pain control has been shown to exist.28,30,32,60
Others believe that pain relief from SCS results from direct inhibition of pain pathways in the spinothalamic tracts and not secondary to selective large fiber stimulation.21 This theory has been supported by Hoppenstein, who showed that the posterolateral stimulation of the spinal cord provided effective contralateral pain relief with substantially less current than posterior stimulation.34
Some investigators speculate that the changes in blood flow and skin temperature from spinal cord stimulation may affect nociception at the peripheral level.11,17,27,54,55 This postulate is further supported in part by data from Marchand et al. who investigated the effects of SCS on chronic pain using noxious thermal stimuli.22,31,36,58,70,87 Since it was discovered that SCS causes vasodilation in animal studies, clinicians have used this modality for the treatment of chronic pain due to peripheral vascular disease and this is one of the leading indications for SCS in Europe today.31,36,40,87,100 The precise methods of action of pain modulation by SCS are still being elucidated. A better understanding of the pain system may lead to continued improvement in SCS hardware and software, implantation techniques, and even improved clinical outcomes.
SPINAL CORD STIMULATION LEADS
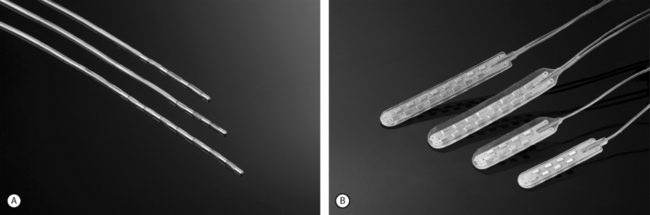
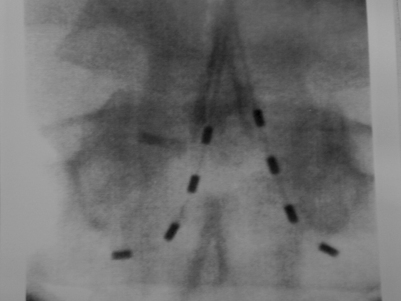
Advanced Neuromodulation Systems (ANS) produces several leads for percutaneous placement with four or eight electrodes (Fig. 107.3A). The four-electrode lead has four 3 mm electrodes separated by an interelectrode distance of either 4 mm or 6 mm and spans a distance of either 24 mm or 30 mm. The eight-electrode lead has eight 3 mm electrodes with an interelectrode distance of 4 mm. The notion of spanning a larger distance is to help thwart the effects of possible migratory pain patterns which is thought to be a quality of complex regional pain syndrome type 1 or to provide programming redundancy in case of lead migration. The four-electrode lead spans one average thoracic vertebral body while the eight-electrode lead spans two average vertebral bodies or three average cervical vertebral bodies.
ANS also produces several different paddle electrodes for surgical implantation via laminotomy or laminectomy. They have a four-electrode Lamitrode Four, an eight-electrode Lamitrode 44 which has four electrodes side by side, and eight-electrode Lamitrode 8, and a 16-electrode Lamitrode 88 with eight electrodes side by side. ANS recently released curved contour laminotomy leads in dual quad, and dual Octrode configurations. The new curved contour laminotomy leads conform more accurately to the shape of the epidual space in the dorsal spinal canal and potentially provide more consistent service than previous laminotomy leads (Fig. 107.3B).
Medtronic also produces five electrodes for implantation via laminotomy: the Symmix, the dual-paddle Symmix, the Resume TL, the Resume, and the Specify. The Symmix, Resume TL, and the Resume leads have four electrodes each, the Specify has eight, and the dual-paddle Symmix has two paddles with two electrodes each. The Symmix has four electrodes arranged in a diamond pattern and the Specify has a total of eight electrodes with four electrodes arranged side by side. Both of these electrode arrangements facilitate bilateral extremity stimulation. The dual-paddle Symmix has two separate paddles designed to allow implantation over two contact sites on the spinal cord to cover a more complex pain pattern. The Resume lead is the most commonly implanted paddle electrode.
Both Medtronics and ANS have internally implanted batteries with pulse generators (internal pulse generator or IPG) and an externally powered radiofrequency (RF) controlled implanted receiver. Both IPG powered systems have the advantage of having the device completely internalized, which makes the system more flexible in that the patient can swim with it on if desired. However, the IPG powered system is limited in that at this time it can only power a maximum of eight electrodes at a limited frequency and will require surgical replacement at some point in time. The RF receiver systems have the advantage of being externally powered, thus obviating the requirement for surgical replacement. The ANS receiver also has the capacity to run at a frequency of up to 1500 Hz, thus potentially allowing for a local anesthetic effect on the stimulated nerves.2,50 Both the ANS IPG and receiver have the capacity for complex programming and running several different programs simultaneously, which may have benefits when dealing with complex pain patterns, and has yet to be evaluated in a controlled study (Fig. 107.4).
PATIENT SELECTION CRITERIA – INDICATIONS
Proper patient selection is essential to the long-term success of a SCS system. Improper selection criteria were one of the main reasons for suboptimal results reported in the 1970s. During the 1970s and early 1980s, most studies evaluating the long-term efficacy of dorsal column stimulation quoted success rates of approximately 40%. Technical advances leading to improved hardware coupled with improved patient selection, have improved the rate of long-term efficacy to approximately 70%.12,92,93
An SCS system should be considered for patients who have failed all reasonable medical rehabilitation and inteventional spine techniques.12 An ideal patient should be motivated, compliant, and free of drug dependence.56 Psychological screening is recommended but not mandatory to exclude conditions that predispose to failure of the procedure. It is extremely important that the patient and the physician have a discussion regarding probable outcome prior to implantation and that the patient has realistic expectations. If a patient has unrealistic expectations of the intervention, the treating physician should not consider that patient for implantation.
Diagnoses that are typical indications for this procedure in the United States include chronic radiculopathy, FBSS, neuropathic pain, and complex regional pain syndrome.8,16,25,43,59 In Europe, SCS is also used for peripheral vascular disease and angina that are not amenable to medical therapy with reportedly excellent results.6,31,36,87,97,100 Other indications for SCS include transformed migraines, perineal pain, and interstitial cystitis.4,83
When considering pain topography, extremity pain responds better than axial pain, and the more distal the extremity pain the greater the clinical response.73,103 Middle and upper lumbar pain as well as thoracic, cervical, and chest wall pain are difficult to adequately control and maintain long term. Pain, due to severe nerve damage superimposed upon cutaneous numbness (i.e. anesthesia dolorosa) is also difficult to treat with SCS. Central pain syndromes do not respond to SCS and are best treated by other modalities.
The use of 3–7 day outpatient trials with an SCS system has proved helpful in determining which patients will respond well enough to warrant a permanent implantation.18,19,73,103 Absolute criteria that must be present for a patient to have a positive trial include tolerance of paresthesia, greater than 50% pain relief, and overall patient satisfaction. Relative requirements for a positive trial include improved functional level and reduced usage of pain medication. Long-term goals include reduced use of the medical system.
CLINICAL OUTCOMES
Original long-term results of pain control from spinal cord stimulation in the late 1960s and 1970s were disappointing.28,30,32,93,96 This led to widespread disenchantment with SCS. Poor patient selection, inadequate equipment, and failure to perform implantations with the patient awake accounted for the disappointing results. The advent of new technology, careful patient selection, trial implantation, percutaneous placement or strict attention to positioning a paddle electrode in the same place as a percutaneously placed lead during a trial, and active physician–patient interaction during the procedure have all contributed to the success of spinal cord stimulation over the past 15 years.
In 1998, Kumar et al. published a case series of 235 patients who had undergone an SCS implantation.44 The follow-up ranged from 6 months to 15 years after implantation with a mean of 5.6 years. One hundred and eighty-nine patients (80%) experienced satisfactory initial pain relief during a trial period of SCS and underwent a permanent SCS implant. The study population included 150 men and 85 women with a mean age of 51.4 years. Indications for SCS included FBSS (114 patients), peripheral vascular disease (39 patients), peripheral neuropathy (30 patients), MS (13 patients), RSD (13 patients) and other chronic pain etiology (26 patients). One hundred and eleven patients (59%) received satisfactory pain relief and 47 were gainfully employed (as compared with only 22 prior to implantation). Improvements in daily living as well as a decrease in analgesic usage were reported in successful outcomes. The shorter the duration of time to implantation after FBSS, the greater the rate of success. In the group with FBSS there were 101 successful stimulation trials and 13 unsuccessful stimulation trials. In this group, fifty-two patients received long-term pain relief while 49 patients who underwent permanent implantation were classified as failure. Out of the 235 patients who underwent permanent implantation, 189 had a successful trial SCS and 111 (58.7%) were successful long term.
In 2000, Villavicencio et al. published a retrospective nonrandomized case series comparing the long-term effectiveness of SCS using laminectomy-style electrodes versus percutaneously implanted electrodes.105 The etiology of pain was FBSS (n=24), CRPS (n=7), neuropathic pain (n=4) and other (n=6). Mean follow-up after implantation of SCS was 8.6 months for the laminectomy-type electrode and 10.3 months for the percutaneously placed electrodes. Twenty-seven of 41 patients (66%) participating in the trial SCS implantation went on to receive a permanent SCS implantation. The laminectomy-style electrodes were placed under general anesthesia, while the percutaneous ones were placed under local anesthesia. Outcome was measured per VAS scale via phone interview. Visual analogue scale scores decreased by an average of 4.6 points after laminectomy-style electrode stimulation and by 3.1 after percutaneously implanted electrode stimulation. Electrodes placed through laminectomy provided significantly greater long-term pain relief than percutaneously placed ones (p=0.02). Overall, 89% of patients had greater than 50% pain relief. Five of 12 patients in the laminectomy group and seven of 15 patients in the percutaneous group underwent revision due to loss of pain relief. All 3 patients under the age of 65 in the laminectomy group were able to go back to work. Of the four patients working in the percutaneous group, two were able to go back to work, one retired, and one remained disabled due to pain.
In 2001, Leveque at al. published the results of a retrospective study involving 30 patients with FBSS who underwent permanent SCS.53 Each patient had had multiple spinal surgeries and was deemed to no longer be a candidate for traditional spine surgery. Each patient had back and leg pain and all patients underwent a screening trial of SCS prior to permanent implantation. Sixteen patients had greater than 50% pain relief during the trial SCS and underwent permanent implantation. Of the 16 patients who underwent permanent implantation, 9 were implanted with percutaneously implanted catheter-style leads and the remaining 7 patients underwent permanent implantation with a paddle-style lead via a laminectomy. Mean follow-up was 34 months with a range of 6–66 months. At last follow-up, 12 of the 16 patients continued to have at least 50% pain relief. All 6 patients who underwent implantation via laminectomy continued to have greater than 50% pain relief with or without narcotics while only 6 out of the 9 percutaneously implanted patients had greater than 50% pain relief with or without narcotics. The authors concluded that SCS is an effective means of treating intractable back pain in the FBSS population. They also suggested that paddle-style SCS leads placed via laminectomy may be superior for long-term efficacy to catheter-style leads placed percutaneously.
In 2001, Ohnmeiss and Rashbaum published a retrospective study which evaluated patient satisfaction of SCS for predominant axial low back pain.79 The literature prior to this study does not support the use of SCS for chronic spinal pain that is primarily limited to the low back. The patient sample included 41 patients who underwent SCS for axial low back pain of an average duration of 83 months. Inclusion criteria included failure of aggressive nonoperative care and patients were not considered surgical candidates. Thirty-eight out of forty-one patients had FBSS with back pain greater than leg pain. In all 41 patients, a SCS trial was performed for an average of 5.9 days. Five patients did not receive significant benefit from SCS trial and were not implanted. The remaining 36 patients were implanted with a Medtronics Mattrix system. Of the 36 patients, 4 later had the system removed due to lack of efficacy. The study included a mean follow-up of 10.5 months.
In 2001, Barolat et al. published a retrospective review of 44 patients who had undergone SCS implantation with a paddle-style electrode and a radiofrequency receiver via a laminectomy for the treatment of intractable low back pain.9 Only patients in whom further lumbar surgery was not indicated or those in whom medical conditions contraindicated surgery underwent implantation. All patients had leg pain in addition to their back pain. The study was a multicenter, prospective study with follow-up at 6, 12, and 24 months. Follow-up data were available in 41 patients.
In 2001, Dario et al. published a five year retrospective analysis (1992–1997) of 49 patients treated for FBSS at the authors’ center.23 Twenty-one patients had predominantly leg pain, 22 had leg pain only, and six had back pain only. Outcome measures include change in VAS, Oswestry Scale, and Pain Disability Index as measured at entry and every three months for a period of 24–84 months with a mean duration of 42 months. The medical therapy was structured and all patients received the same protocol. After 6 months of medical therapy without significant benefit, patients underwent implantation of a Medtronic Itrel 2 or 3 SCS systems. Twenty-two patients had percutaneous leads implanted and 2 patients had laminectomy paddle-type leads implanted. The following measures were evaluated for SCS implanted patients: efficacy of therapy for leg and back pain, return to work status if previously employed, ADL activity if retired, and the concomitant use of medications and dosages after implant. The results revealed medical therapy improved both back and leg pain with mean VAS decreasing from 76 to 25. Oswestry scale results dropped from 23 to 6, and PDI scores went from 42 to 4. Of the 22 patients who underwent SCS implant, the VAS results were broken down into leg pain only and back pain only. In the leg pain only group, VAS went from 85 to 22, whereas in the back pain only group the VAS went from 45 to 40. The overall SCS groups mean PDI went from 51 to 7 and the Oswestry scoring went from 12 to 9. The authors concluded that neurostimulation only partially resolved the need for chronic medical therapy.
In 2002, North and Wetzel selected 26 patients with FBSS to undergo trial SCS.76 Selection criteria included FBSS with leg pain greater than back pain and one or more of the following: recent abnormal diagnostic imaging results, neurological deficit consistent with the patient’s complaints and history, and/or a well-documented history of surgery for appropriate indications. All patients underwent a percutaneous trial with a four-electrode SCS lead. Of the 26 patients trialed, 24 were chosen to undergo a permanent implantation at the same spinal level as the trial lead was placed. Twelve patients were implanted with a percutaneous four-electrode lead similar to the one temporarily implanted for the trial stimulation and the other twelve patients were implanted with an insulated four-electrode lead via laminectomy. The study demonstrated that the laminectomy-placed leads outperformed percutaneously placed leads with better patient-reported pain coverage and lower amplitude requirements which should, according to calculations, yield a doubling of battery life.
In 2002, Kumar et al. evaluated the overall cost-effectiveness of SCS therapy versus conventional pain therapy for a consecutive series of 104 patients with FBSS.45 Sixty patients with FBSS were treated with SCS versus 44 patients with conventional pain therapies (CPT). Outcome measures utilized included the Oswestry disability questionnaire at enrollment, yearly, and at 5-year follow-up. Cost of treatment was determined based on year 2000 Canadian dollars and expenditures were regulated by the Canadian Healthcare System. Treatment costs included primary care physician and specialist evaluations, imaging studies, surgery and devices for the interventional arm of the study and medication, physiotherapy, chiropractic, massage, and acupuncture for the control arm. The cumulative totals for the SCS arm were $29 123/patient compared to the control arm figure of $38 029/patient. The cost savings of SCS over the CPT group were realized at an average of 2.5 years due to the high initial cost of surgery and the device. After 2.5 years the savings of SCS versus CPT were realized through reduced drug use, reduced use of physical therapy services, and reduced use of the healthcare system in general. It is possible that in the US healthcare system the cost savings would be even greater. A prior study by Bell et al. puts the breakpoint at 2 years based on projected dollar values.13
In 2004, Turner et al. published a systematic review of the literature including the MEDLINE, EMBASE, Scientific Citation Index, Cochrane Controlled Trials Register, and Current Contents bibliographic databases back to their starting dates for articles published on the effectiveness of spinal cord stimulation (SCS) in treating failed back surgery syndrome (FBSS) and complex regional pain syndrome (CRPS) up to May 16, 2003.104 Seven of 583 articles met the inclusion criteria for review of SCS effectiveness (two CRPS, three FBSS, two mixed diagnoses) and 15 others met the criteria for the review of SCS complications. The average number of previous spine surgeries (in the three studies that reported it) ranged from 2 to 3.3. Mean follow-up time was 33.6 months (range 6–60 months) and an average of 72% (range 58–96%) of patients received the permanent implantation after trial implantation. The studies included different SCS implantation methods (percutaneous trial followed by percutaneous implantation, percutaneous trial followed by implantation by laminectomy, and direct implantation by laminectomy without trial). Both FBSS studies (Dario23 and Ohnmeiss and Rashbaum79) that reported leg and back pain separately found greater improvement in the leg pain than the back pain with SCS. Due to the study design of the seven publications, no conclusions could be drawn regarding effectiveness of SCS in returning patients to work. There was a suggestion that pain relief after SCS implantation decreases over time; however, no prospective study with a control group was available. Among 40 FBSS patients in an Ohnmeiss et al. study done in 1996, benefit from SCS decreased from 83% after 12 month to 70% after 24 months.78 One large prospective multicenter study published in 1996 by Burchiel et al. with 182 FBSS patients who received a permanent SCS was excluded due to the fact that only 70 completed the follow-up.19 In this study, a one-year follow-up, showed statistically significant pain decrease and improvement in quality of life measures, but not in medication use or work status. However, due to the large number of patients lost in the follow-up, it is unclear if the patients at follow-up were representative of the original sample.
Also, in 2004, Mailis-Gagnon et al. performed a systematic review of the medical literature from 1980 to September 2003 regarding the efficacy of SCS in relieving certain types of chronic pain, its complications, and its adverse effects.57 Studies that were only descriptive or observational were excluded. Primary outcome measure was pain relief. Secondary outcomes were indirect statements about pain relief, e.g. well-being, happiness, functional status, etc. A total of 1629 titles and abstracts were screened, 18 papers were retrieved, 16 of those were excluded, and two were included: one by Kemler et al.38 and one by North et al.74 Both studies were randomized, controlled trials but the Kemler et al. study was a truly prospective study while the North et al. study used a cross-over design. The Kemler et al. study evaluated CRPS type I and had 12-month follow-up while the North et al. study evaluated FBSS and had a 6-month cross-over between SCS and operation. Kemler et al. concluded that SCS is more effective and less expensive than standard treatments protocols for chronic CRPS and North et al. concluded that SCS should be considered prior to operation of the spine on a repeat disc herniation at a previously operated segment. The conclusion of Mailis-Gagnon et al. was that there is limited evidence in favor of SCS for FBSS and CRPS type I. Further, they felt that more trials were needed to confirm whether SCS is an effective treatment for certain types of chronic pain.
The most common SCS application in North America today is in the treatment of chronic low back and lower extremity pain due to chronic radiculopathy despite surgery.19,46,78,91,95,103 The largest SCS study incorporates 320 consecutive patients who underwent either temporary or permanent implants at the Johns Hopkins Hospital between 1971 and 1990.40 This series included follow-up on 205 patients, the majority of whom had the diagnosis of failed back surgery syndrome. Permanent SCS implants were placed in 171 of these patients. At follow-up (mean interval 7.1±4.5 yrs), 52% of patients had at least 50% continued pain relief, and 58% had a reduction or elimination of analgesic intake. About 54% of patients younger than 65 were working at the time of follow-up; 41% had been working preoperatively.
The percentage of patients having long-term pain relief is similar in the majority of large published SCS series of implants for FBSS. The success rate in most of these studies, which is generally reported as 50% or more pain relief, is approximately 50–60%.9,20,23,43,52,53,68,79,81,85 Some studies report success rates as high as 88% and others as low as 37%.39,94 Although these latter studies differ in implantation technique and screening protocols, the success rate for pain reduction generally remains the same.
More recently published reviews have specifically looked at the efficacy of SCS in FBSS for pain control, reduction in narcotic consumption, function, and work status.24,5069,70,84,105 According to these studies, long-term pain reduction (at least 2 years after implantation) can be expected to range 50–70% in approximately 60% of SCS patients. In 50–90% of individuals, there will be an elimination or reduction in the use of opioids. The return to full employment rate after SCS reported by two studies is 25–59%, which is significant when comparing it to the usual return to work rate in this population of 1–5%.50,70 Reasons for the disparity between pain reduction and return-to-work rates appear to reflect the high percentage of unskilled laborers among this population, the prolonged periods of disability and the attendant socio-behavioral changes tend to take place in chronic pain patients. Despite this disparity, there is a general increase in function and activities of daily living.
COMPLICATIONS
There are rarely any serious complications from of SCS implantation.7 In one study, one nonfatal pulmonary embolism and one case of paraplegia lasting 3 months occurred.49 The latter resulted during the laminectomy placement of a paddle-style lead. Other rarely reported complications include sphincter disturbance and gait abnormality.82
Most complications from SCS devices include reduced or altered paresthesia, lead migration, lead fracture, pain at the pocket site or connection site, infection, nerve injury, and epidural hematoma.6,7,25,26,42,66,70,99,109 In a comprehensive summary of different publications, lead migration or displacement varied from 3.7% to 69% although most studies reported migration between 16% and 25%.7 Rates of lead fractures were reported in various series from less than 1% to more than 20% and superficial infections occurred in 2–12% of cases. Serious surgical infections were rare as were clinically apparent epidural hematomas. Cerebrospinal fluid leakage was found in one series in 2% of patients.
In a more recent study, Kumar et al. published a case series of 235 patients who had undergone a SCS implantation.45 Complications included hardware malfunction (n=8), electrode displacement (n=65, of which 35 were repositioned and 30 were replaced), infection (4% of implanted devices; n=11 of which 8 required removal), CSF leak (n=2, resolved spontaneously), 1 SQ hematoma at the site of the pulse generator, 3 electrical leaks, and 8 electrode fractures. Development of tolerance was thought to be responsible for most of the 79 long-term failures.
In 2000, Heideche et al. published a retrospective review of 42 patients with FBSS who had undergone a permanent SCS implantation for the purposes of identifying the types and frequency of hardware failures.33 The patients were followed for 6–74 months. Thirty-five patients had undergone implantation with a single quadripolar SCS lead percutaneously placed and attached to a Medtronic X-trel receiver; three patients were implanted with dual leads attached to a Mattrix receiver; and the other four patients were implanted with a Medtronics Resume lead via laminectomy. In this study, lead breakage or insulation disruption (n=8) was the most frequently encountered hardware failure with receiver leakage being second most common (n=4).
In 2004, Turner et al. performed a systematic review of the literature involving SCS and found 583 articles.104 After screening for pertinent articles, they reviewed 21 studies. An average of 34% (range 0–81%) of patients had one or more complications after the implantation of the permanent SCS during the study follow-up period. Complications included superficial infection (mean 4.5%, range 0–12%), deep infection (one case), pain in the region of the stimulator components (mean 5.8%, range 0–40%), and equipment failure (mean 10.2%, range 0–40%). The median rate for equipment failure of 6.5% may be more accurate, since one study in 2002 by Alo et al. had higher than usual equipment failure rates, which may have skewed the rate upwards.5 Other complications include a need for stimulator revision, mostly due to dislodged or displaced electrodes and a need for stimulator removal.5,104
THE FUTURE
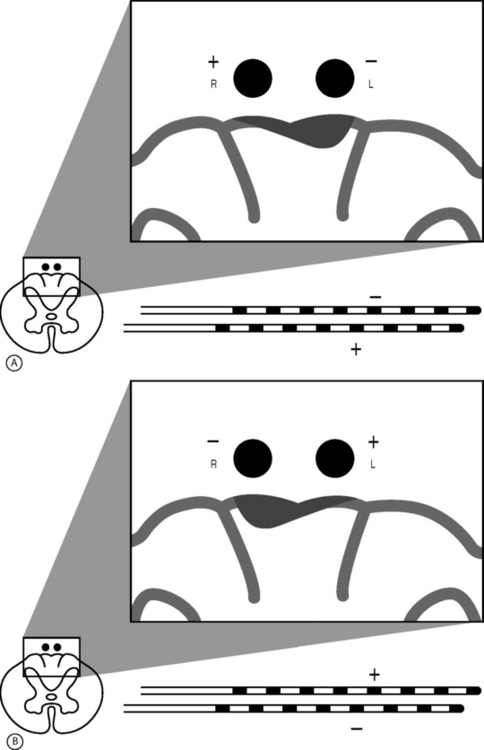
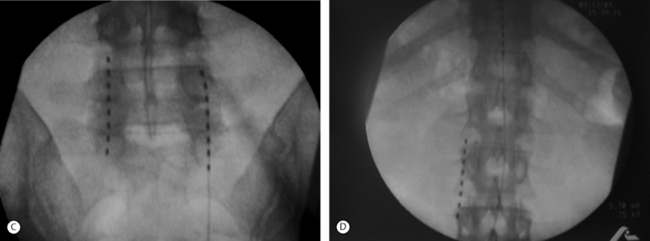
The retrograde placement of percutaneously placed leads has proven effective in the treatment of low back and leg pain (Fig. 107.5).2 This technique typically involves the cephalocaudal advancement of a lead into the lower lumbar or sacral spine via an epidural needle placed at the L2–3 or L3–4 level. Using this technique, the lead may be placed over one or two adjacent nerve roots or actually fed into a particular foramen. This technique allows for a very stable, focused paresthesia along one or a few nerve roots at very low amplitudes. When the lead is entered into a foramen it tends to be very stable and allows for very little positional or activity-related change in paresthesia. Thus, if a patient has a poor result from a SCS trial with a traditionally placed SCS lead, then the placement of a SCS lead via a retrograde approach may be indicated. In selected cases, it is the authors’ experience that by applying this method in lieu of or in addition to a traditionally placed lead, clinical outcomes will improve.
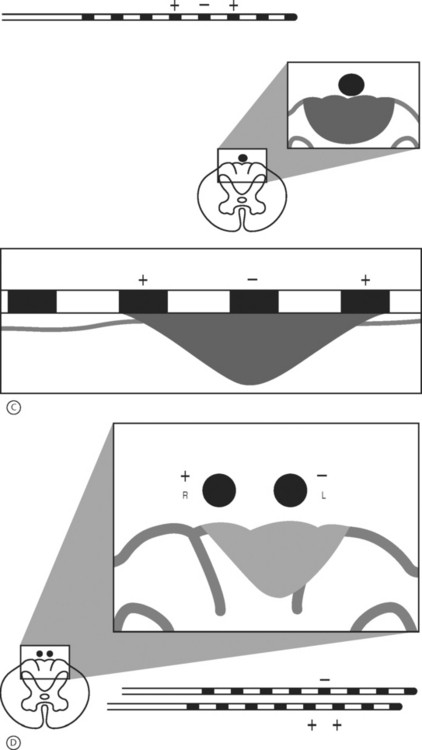
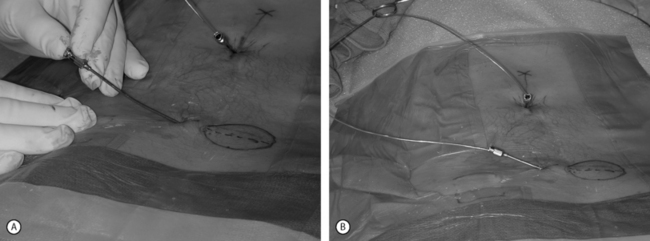
Peripheral nerve stimulation (PNS) is a technique that is considered to be very similar to SCS except that the neural elements being stimulated lie outside of the spinal column. This method has been successfully applied in the treatment of refractory transformed migraines.5 It has also been successfully applied in an anecdotal manner by the authors and others for the treatment of focal, intense low back pain. This technique involves the placement of an epidural needle immediately beneath the skin peripheral to the center of a focal region of pain (Fig. 107.6A). The needle is then directed into the long axis of the pain so that the distal 5 cm of the needle crosses through the middle of the most intense pain. An eight-channel SCS lead is then advanced into the needle and the needle is removed. The lead is then trialed for efficacy in a manner similar to an SCS trial. This method of PNS provides a very dense, focused paresthesia and is often successful at relieving intense low back pain when SCS alone fails. As a result, when a patient with an intense, focused region of low back pain has a poor result from a trial SCS, a PNS trial should be considered in lieu of or in addition to a traditional SCS trial (Fig. 107.6B-D). The authors have had several anecdotal cases in which a combination of PNS and SCS has succeeded in patients who have had a poor result from the antegrade placement of a percutaneously placed lead despite coverage of the patient’s pain pattern with paresthesia. Studies focused on the combination of PNS and SCS in selected cases may improve long-term success rates.
1 Abram SE. Pain pathways and mechanisms. Sem Anesth. 1985;4:267-274.
2 Alo K, Yland M, Radko R, et al. Lumbar and sacral nerve root stimulation (NRS) in the treatment of chronic pain: A novel anatomical approach and neurostimulation technique. Neuromodulation. 1999;2(1):23-31.
3 Alo K, Ziddan A. Selective nerve root stimulation in the treatment of end-stage, diabetic, peripheral neuropathy: A case report. Neuromodulation. 2000;3(4):201-208.
4 Alo K, McKay E. Selective nerve root stimulation (SNRS) for the treatment of intractable pelvic pain and motor dysfunction: a case report. Neuromodulation. 2001;4(1):19-24.
5 Alo K, Redko V, Charnov J. Four year follow up of dual electrode spinal cord stimulation for chronic pain. Neuromodulation. 2002;5(2):79-88.
6 Augustinsson LE, Carlsson CA, Holm J, et al. Epidural electrical stimulation in severe limb ischemia. Pain relief, increased blood flow, and a possible limb-saving effect. Ann Surg. 1985;202(1):104-110.
7 Augustinsson LE. Avoiding difficulties in spinal cord stimulation. In: Waldman SD, Winnie AP, editors. Interventional pain management. 1st edn. Philadelphia: WB Saunders; 1996:427-430.
8 Barolat G, Schwartzman R, Woo R. Epidural spinal cord stimulation in the management of reflex sympathetic dystrophy. Stereot Funct Neurosurg. 1989;53(1):29-39.
9 Barolat G, Oakley J, Law J, et al. Epidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable back pain. Neuromodulation. 2001;4(2):59-66.
10 Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci. 1984;7:309-338.
11 Bayliss WM. On the origin from the spinal cord of the vasodilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901;26(3):173-209.
12 Bedder MD. Spinal cord stimulation and intractable pain: patient selection. In: Waldman SD, Winnie AP, editors. Interventional pain management. 1st edn. Philadelphia: WB Saunders; 1996:412-418.
13 Bell GK, Kidd D, North RB. Cost-effectiveness analysis of spinal cord stimulation in treatment of failed back surgery syndrome. J Pain Symptom Manag. 1997;13(5):286-295.
14 Bonica JJ. Anatomic and physiologic basis of nociception and pain. Bonica J, editor. The management of pain, 2nd edn., Vol 1. Philadelphia: Lea & Febiger, 1990;28-94.
15 Bowsher D. Pain mechanisms in man. Res Staff Phys. 1983;29:26-34.
16 Broseta J, Roldan P, Gonzalez-Darder J, et al. Chronic epidural dorsal column stimulation in the treatment of causalgia pain. Appl Neurophysiol. 1982;45(1–2):190-194.
17 Broseta J, et al. Spinal cord stimulation in peripheral arterial disease. J Neurosurg. 1986;64:71-80.
18 Burchiel KJ, Anderson VC, Wilson BJ, et al. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery. 1995;36(6):1101-1110.
19 Burchiel KJ, Anderson VC, Brown FD, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21(23):2786-2794.
20 Burton CV. Session on spinal cord stimulation: Safety and clinical efficacy. Symposium on the safety and clinical efficacy of implanted neuroaugmentative devices. Neurosurgery. 1977;1:214-215.
21 Campbell JN. Examination of possible mechanisms by which stimulation of the spinal cord in man relieves pain. Appl Neurophysiol. 1981;44(4):181-186.
22 Crue BL. The neurophysiology and taxonomy of pain. In: Brena SF, Chapman SL, editors. Management of patients with chronic pain. Jamaica, NY: Spectrum; 1983:21-31.
23 Dario A, Fortini G, Bertollo D, et al. Treatment of failed back surgery syndrome. Neuromodulation. 2001;4(3):105-110.
24 De La Porte C, Van de Kelft E. Spinal cord stimulation in failed back surgery syndrome. Pain. 1993;52(1):55-61.
25 Devulder J, De Colvenaer L, Rolly G, et al. Spinal cord stimulation in chronic pain therapy. Clin J Pain. 1990;6(1):51-56.
26 Erickson DL, Long DM. Ten-year follow-up of dorsal column stimulation. Advin Pain Res Ther. 1983;6:583-589.
27 Fedorcsak I, et al. Peripheral vasodilation due to sympathetic inhibition induced by spinal cord stimulation. In: Proceedings of the IBRO World Congress of Neurosciences, Paris, France, 1991:126.
28 Feldman RA. Patterned response of lamina V cells: Cutaneous and dorsal funicular stimulation. Physiol Behav. 1975;15(1):79-84.
29 Fields H. Depression and pain: A neurobiological model. Neuropsychiatry, Neuropsychol Behav Neurol. 1991;4:83-92.
30 Foreman RD, Beall JE, Coulter JD, et al. Effects of dorsal column stimulation on primate spinothalamic tract neurons. J Neurophysiol. 1976;39(3):534-546.
31 Groth DE. Spinal cord stimulation for the treatment of peripheral vascular disease. Adv Pain Res Ther. 1985;9:861-870.
32 Handwerker HO, Iggo A, Zimmerman M. Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimuli. Pain. 1975;1(2):147-165.
33 Heideche V, Rainov N, Burket W. Hardware failures in spinal cord stimulation for failed back surgery syndrome. Neuromodulation. 2000;3(1):27-30.
34 Hoppenstein R. Percutaneous implantation of chronic spinal cord electrodes for control of intractable pain. Preliminary report. Surg Neurol. 1975;4(1):195-198.
35 International Association for the Study of Pain. Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms. Pain Suppl. 1986;3:S1-S225.
36 Jacobs MJ, Jorning PJ, Joshi SR, et al. Epidural spinal cord electrical stimulation improves microvascular blood flow in severe limb ischemia. Ann Surg. 1988;207(2):179-183.
37 Jensen MP, Turner JA, Romano JM, et al. Coping with chronic pain: A critical review of the literature. Pain. 1991;47(3):249-283.
38 Kemler M, Barendse G, van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):618-624.
39 Kolin MT, Winkelmuller W. Chronic pain after multiple lumbar discectomies – significance of intermittent spinal cord stimulation. Pain. 1990;40(5):S241.
40 Krames ES. Mechanisms of action of spinal cord stimulation. In: Waldman SD, Winnie A, editors. Interventional pain management. 1st edn. Philadelphia: WB Saunders; 1996:407-411.
41 Krieger DT, Martin JB. Brain peptides. N Eng J Med. 1981;304(15):876-885.
42 Kumar K, Wyant G, Ekong C, et al. Epidural spinal cord stimulation for relief of chronic pain. Pain Clin. 1986;1(2):91-99.
43 Kumar K, Nath RK, Wyant GM. Treatment of chronic pain by epidural spinal cord stimulation: 10-year experience. J Neurosurg. 1991;75(3):402-407.
44 Kumar K, Toth C, Nath RK, et al. Epidural spinal cord stimulation for treatment of chronic pain – some predictors of success: a 15-year experience. Surg Neurol. 1998;50(2):110-121.
45 Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurg. 2002;51(1):106-116.
46 Kupers RC, Van den Dever R, Van Houdenhove B, et al. Spinal cord stimulation in Belgium: a nation-wide survey on the incidence, indications and therapeutic efficacy by the health insurer. Pain. 1994;56(2):211-216.
47 Larson SJ, Sances A, Riegel DH, et al. Neurophysiological effects of dorsal column stimulation in man and monkey. J Neurosurg. 1974;41(2):217-223.
48 Law JD. Spinal stimulation: Statistical superiority of monophasic stimulation of narrowly separated, longitudinal bipoles having rostral cathodes. Appl Neurophysiol. 1983;46(1–4):129-137.
49 Law JD. Percutaneous spinal cord stimulation for the ‘failed back surgery syndrome.’. Pain Manage Update. 1990;1(l):12.
50 Law JD, Kirkpatrick AF. Update: Spinal cord stimulation. Am J Pain Manage. 1992;2(l):34-42.
51 LeDoux MS, Langford KH. Spinal cord stimulation for the failed back syndrome. Spine. 1993;18(2):191-194.
52 LeRoy PL. Stimulation of the spinal neuraxis by biocompatible electrical current in the human. Appl Neurophysiol. 1981;44(4):187-193.
53 Leveque J, Villavicencio A, Bulsara K, et al. Spinal cord stimulation for failed back surgery syndrome. Neuromodulation. 2001;4(1):1-9.
54 Linderoth B, Fedorcsak I, Meyerson BA. Is vasodilatation following dorsal column stimulation mediated by antidromic activation of small diameter afferents? Acta Neurochirurg Supple. 1989;46:99-101.
55 Linderoth B. Dorsal column stimulation and pain: Experimental studies of putative neurochemical and neurophysiological mechanisms (thesis). Stockholm: Karolinska Institute, 1992.
56 Loeser JD, Parker G. Assessment and investigation of the patient with chronic pain at the University of Washington Multidisciplinary Pain Center. In: Loeser JD, Egan KJ, editors. Pain management. New York: Raven Press; 1989:21-34.
57 Mailis-Gagnon A, Furlan AD, Sandoval JA, et al. Spinal cord stimulation for chronic pain (Cochrane Review). In: The Cochrane Library, Issue 3, 2004. Chichester, UK: John Wiley; 2004.
58 Marchand S, Bushnell MC, Molina-Negro P, et al. The effects of dorsal column stimulation on measures of clinical and experimental pain in man. Pain. 1991;45(3):249-257.
59 Meglio M, Cioni B, Rossi GF. Spinal cord stimulation in management of chronic pain: A 9-year experience. J Neurosurg. 1989;70(4):519-524.
60 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971-979.
61 Melzack R, Casey KL. Sensory, motivational, and central control determinates of pain. In: Kenshalo DR, editor. The skin senses. Springfield: Charles C Thomas; 1968:423-443.
62 Melzack R. Psychological aspects of pain: implications for neural blockade. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. 2nd ed. Philadelphia: JB Lippincott; 1989:845-860.
63 Melzack R. Anatomy and physiology of pain: clinical correlates. In: Waldman SD, Winnie AP, editors. Interventional pain management. 1st edn. Philadelphia: WB Saunders; 1996:1-9.
64 Meyerson BA. Electric stimulation of the spinal cord and brain. In: Bonica JJ, editor. The management of pain. 2nd edn. Philadelphia: Lea & Febiger; 1990:1862-1877.
65 Meyerson BA, Linderoth B, Lind G. Spinal cord stimulation in chronic neuropathic pain. Lakartidningen. 1991;88(9):727-732.
66 Mullet KR. Design and function of spinal cord stimulators – theoretical and developmental considerations. Pain Digest. 1992;1:281-287.
67 Nagase Y, Moritani M, Nakagawa S. Serotonergic axonal contacts on identified cat trigeminal motor neurons and their correlation with medullary raphe nucleus stimulation. J Compar Neurol. 1997;384(3):443-455.
68 Nielson KD, Adams JE, Hosobuchi Y. Experience with dorsal column stimulation for relief of chronic intractable pain. Surgi Neurol. 1975;4(1):148-152.
69 North RB, Ewend MG, Lawton MT, et al. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28(5):692-699.
70 North RB, Ewend MG, Lawton MT, et al. Spinal cord stimulation for chronic, intractable pain: superiority of ‘multi-channel’ devices. Pain. 1991;44(2):119-130.
71 North RB, Fowler K, Nigrin D, et al. Patient-interactive, computer-controlled neurological stimulation system: Clinical efficacy in spinal cord stimulator adjustment. J Neurosurg. 1992;76(6):967-972.
72 North RB, Kidd D, Zahurak M. Spinal cord stimulation for chronic intractable pain: Experience over two decades. Neurosurgery. 1993;32(3):384-394.
73 North RB, Kidd D, Lee MS, et al. A prospective, randomized study of spinal cord stimulation vs. reoperation for failed back surgery syndrome: initial results. Stereot Funct Neurosurg. 1994;62(1–4):267-272.
74 North RB, Kidd D, Piantadosi S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochirurg Suppl. 1995;62(1–4):273-278.
75 North B, Kidd D, Wimberly RL, et al. Prognostic value of psychological testing in patients undergoing spinal cord stimulation: A prospective study. Neurosurgery. 1996;39(2):301-311.
76 North RB, Wetzel FT. Spinal cord stimulation for chronic pain of spinal origin. Spine. 2002;27(22):2584-2591.
77 North RB, Kidd DH, Olin JC, et al. Spinal cord stimulation electrode design: prospective randomized, controlled trial comparing percutaneous and laminectomy electrodes. Part I: technical outcomes. Neurosurgery. 2002;51(2):381-390.
78 Ohnmeiss D, Rashbaum R, Bogdanffy GM. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine. 1996;21(11):1344-1350.
79 Ohnmeiss D, Rashbaum R. Patient satisfaction with spinal cord stimulation for predominant complaints of chronic, intractable low back pain. Spine J. 2001;1(5):358-363.
80 Oliveras JL, Redjemi G, Guilbaud G, et al. Analgesia induced by electrical stimulation of the inferior centralis of the raphe in the cat. Pain. 1975;1(2):139-145.
81 Pineda A. Dorsal column stimulation and its prospects. Surg Neurol. 1975;4(1):157-163.
82 Pineda A. Complications of dorsal column stimulation. J Neurosurg. 1978;48(1):64-68.
83 Popeney CA, Alo KM. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache. 2003;43(4):369-375.
84 Racz GB, McCarron R, Talboys P. Percutaneous dorsal column stimulator for chronic pain control. Spine. 1989;14(1):1-4.
85 Ray CD, Burton C, Lifson A. Neurostimulation as used in a large clinical practice. Appl Neurophysiol. 1982;45(1–2):160-166.
86 Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164(878):444-445.
87 Robaina FJ, Dominguez M, Diaz M, et al. Spinal cord stimulation for relief of chronic pain in vasospastic disorders of the upper limbs. Neurosurgery. 1989;24(1):63-67.
88 Robb LG, Spector G, Robb MP. Spinal cord stimulation: neuroaugmentation of the dorsal columns for pain relief. In: Weiner RS, editor. Pain management: a practical guide for clinicians. 5th edn. Boca Raton: St. Lucie Press; 1998:271-293.
89 Ruda MA. Opiates and pain pathways: Demonstration of enkephalin synapses on dorsal horn projection neurons. Science. 1982;215(4539):1523-1525.
90 Saade NE, Tabet MS, Soueidan SA, et al. Supraspinal modulation of nocioception in awake rats by stimulation of the dorsal column nuclei. Brain Res. 1986;369(1–2):307-310.
91 Segal R, Stacey BR, Rudy TE, et al. Spinal cord stimulation revisited. Neurolog Res. 1998;20(5):391-396.
92 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by dorsal column stimulation. Anesth Analg. 1967;46(4):489-491.
93 Shealy CN, Mortimer JT, Hagfors NR. Dorsal column electroanalgesia. J Neurosurg. 1970;32(5):560-564.
94 Siegfried J, Lazorthes Y. Long-term follow-up of dorsal column stimulation for chronic pain syndrome after multiple lumbar operations. Appl Neurophysiol. 1982;45(1–2):201-204.
95 Snyder SH. Opiate receptors in the brain. N Engl J Med. 1977;296(5):266-271.
96 Spiegelmann R, Friedman WA. Spinal cord stimulation: a contemporary series. Neurosurgery. 1991;28(1):65-71.
97 Steude U, Abendroth D, Sunder-Plassamann L. Epidural spinal electrical stimulation in the treatment of severe arterial occlusive disease. Acta Neurochirurg Suppl. 1991;52:118-120.
98 Stinson L, Roderer G, Cross N, et al. Peripheral subcutaneous electrostimulation for control of intractable post-operative inguinal pain: a case series. Neuromodulation. 2001;4(3):99-104.
99 Sweet WH, Wepsic JG. Stimulation of the posterior column of the spinal cord for pain control: indications, technique and results. Clin Neurosurg. 1974;21:278-310.
100 Tallis RC, Illis LS, Sedgwick EM, et al. Spinal cord stimulation in peripheral vascular disease. J Neurol Neurosurg Psychiatry. 1983;46(6):478-484.
101 Tasker RR. Deafferentation in central pain. In: Wall PD, Melzack R, editors. Textbook of pain. 2nd edn. Edinburgh: Churchill-Livingstone; 1989:154-180.
102 Thienhaus O, Cole BE. The classification of pain. In: Weiner RS, editor. Pain Management: a practical guide for clinicians. 5th edn. Boca Raton: St. Lucie Press; 1998:19-26.
103 Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery. 1995;37(6):1088-1096.
104 Turner JA, Loeser JD, Deyo RA, et al. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137-147.
105 Villavicencio AT, Leveque JC, Rubin L, et al. Laminectomy versus percutaneous electrode placement for spinal cord stimulation. Neurosurgery. 2000;46(2):399-405.
106 Wall PD, Melzack R. Introduction. In: Wall PD, Melzack R, editors. Textbook of Pain. 2nd edn. Edinburgh: Churchill-Livingstone; 1989:1-18.
107 Yaksh TL. Neurological mechanisms of pain. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. 2nd edn. Philadelphia: JB Lippincott; 1988:791-844.
108 Yaksh TL. Anatomy of the pain processing system. In: Waldman SD, Winnie AP, editors. Interventional pain management. 1st edn. Philadelphia: WB Saunders; 1996:10-18.
109 Young RF. Evaluation of dorsal column stimulation in the treatment of chronic pain. Neurosurgery. 1978;3(3):373-379.