Chapter 12 Spinal Cord Stimulation for Visceral Abdominal Pain
 Appropriately long trial of SCS is required to objectively assess patient’s improvements in pain and function.
Appropriately long trial of SCS is required to objectively assess patient’s improvements in pain and function. Psychological evaluation and interdisciplinary committee for implantable devices assessment are now frequently required before conducting an SCS trial and are an essential part of the patient selection process.
Psychological evaluation and interdisciplinary committee for implantable devices assessment are now frequently required before conducting an SCS trial and are an essential part of the patient selection process. Collaboration and consultation with the referring physician (frequently gastroenterologist) and continued exchange of information on implantation techniques should advance our understanding of mechanisms and refine this novel technique of SCS for abdominal visceral pain.
Collaboration and consultation with the referring physician (frequently gastroenterologist) and continued exchange of information on implantation techniques should advance our understanding of mechanisms and refine this novel technique of SCS for abdominal visceral pain. Midline position of the leads is most frequently used, and the number of leads used for trialing is not related to the success of the trial.
Midline position of the leads is most frequently used, and the number of leads used for trialing is not related to the success of the trial. Retrograde differential epidural block can assist in proper selection of patients by identifying those who have predominantly visceral abdominal chronic pain.
Retrograde differential epidural block can assist in proper selection of patients by identifying those who have predominantly visceral abdominal chronic pain. On the basis of the currently available data, SCS for abdominal pain may be an exciting therapeutic option for patients with severe visceral pain. Food and Drug Administration (FDA) approval of the new indication for SCS is still required; that process should be initiated as soon as possible in the form of a randomized, prospective clinical trial.
On the basis of the currently available data, SCS for abdominal pain may be an exciting therapeutic option for patients with severe visceral pain. Food and Drug Administration (FDA) approval of the new indication for SCS is still required; that process should be initiated as soon as possible in the form of a randomized, prospective clinical trial.Introduction
Abdominal pain is one of the most frequent complaints to a primary care physician, accounting for nearly 2.5 million office visits a year.1 In many patients who present with abdominal pain, a definitive diagnosis can be made, but in 35% to 51% of patients, no identifiable cause is found.2 There are approximately 2 million patients in the United States who currently experience severe abdominal pain. Most of them undergo a multitude of imaging studies and consultations with gastroenterology and surgery before being referred to a chronic pain specialist.3,4 Despite adequate evaluations by multiple physicians, the etiology of some abdominal pain remains unknown.
Characteristics of visceral pain have been described extensively in literature as poorly localized, referred to somatic structures, and not evoked by all viscera.5 Visceral nociceptors are capable of responding to a variety of noxious stimuli such as mechanical and chemical stimuli. However, pain is not necessarily evoked from all of the viscera or from visceral injury. Some internal organs lack nociceptors and can be damaged without the individual perceiving pain. Spinal and vagal afferent fibers convey sensory information from the upper gastrointestinal tract to the central nervous system. Vagal afferents transmit predominantly physiological information, whereas spinal afferents transmit noxious information.6 The dorsal root ganglia of the spinal nerves contain cell bodies of both vagal and spinal afferents. Vagal afferents enter the brainstem, whereas spinal afferents enter the spinal cord, making synaptic connections with second-order neurons, thereby conveying visceral information to the central nervous system. The ascending spinal pathways project to the thalamic nuclei. Afferent fibers with visceral and somatic information converge onto spinothalamic and spinoreticular pathways.
The role of the dorsal column pathway in transmission/amplification of visceral pain has been described more recently by Palacek and Willis7 and Palacek,8 and its role as participating in the neuromodulation of visceral painful information by spinal cord stimulation (SCS) has been hypothesized.9 Still the spinothalamic tracts are considered to be the major pathways for visceral nociception.10
Spinal sensitization process following tissue injury is characterized as a leftward shift in pain sensation/behavior (hyperalgesia) and enlargement of receptive fields within cutaneous areas able to activate the dorsal horn neuron (allodynia). This pattern is analogous to the change in functional bowel disorders, where a leftward shift in the stimulation-pain curve and enlargement of the somatic referral area occur.11 The expansion of convergent cutaneous fields after repetitive distension of a viscus indicates the presence of a central mechanism contributing to the alteration of excitability of central neurons.12 Brain-gut axis abnormalities can manifest as gastrointestinal motility and functional disorders. Because the neural activity plays a major role in these disorders, interventions aiming to modulate these processes can possibly improve the symptoms.
Spinal Cord Stimulation in Visceral Pain: Possible Mechanisms of Pain Relief
Several possible mechanisms may be involved in the phenomena of chronic abdominal pain relief produced by SCS. Published studies used the model of visceral hyperalgesia by colorectal distention in rats. Suppression of lumbosacral spinal neuron responses to the noxious colorectal stimuli by SCS could be produced by placing the electrical lead either near the lumbar or cervical dorsal column.13,14 It was suggested that such suppression of the visceromotor reflex (VMR) by SCS may be the result of antidromic activation of primary efferent fibers within the dorsal column.13,14 Spinal gating mechanisms15 might also be operant as an explanation for the reduction in pain transmission of small-diameter visceral fibers by stimulating large afferents using relatively low-intensity electrical stimulation.15
Although interruption of the recently described midline dorsal column pathway relieves visceral pelvic pain in cancer patients,7,8,10,11 at this time it is not clear if this visceral pathway can be modulated, excited, or suppressed by SCS.
Suppression of the sympathetic nervous system could play a significant role in control of the abdominal visceral pain;16 chemical or surgical neurectomy/sympathectomy involving the superior hypogastric or celiac plexus can suppress chronic abdominal pain.16,17 Suppression of the sympathetic nervous system has been proposed as an important mechanism of pain control in intractable angina.18 It may be that such sympathectomy by SCS plays a role in suppression of chronic visceral abdominal pain.19
Clinical Experience in Using Spinal Cord Stimulation for Chronic Visceral Pain
Compelling data suggest that SCS may decrease pain and improve functional capacity in patients with various visceral chronic pain syndromes (Table 12-1). However, it may require years to accumulate sufficient clinical evidence.
Table 12-1: Reported Causes of Severe Chronic Abdominal Pain Treated with Spinal Cord Stimulation
| Causes of Abdominal Pain Treated With SCS | Study/Case Series/Case Report | Number of Patients Studied |
|---|---|---|
| Mesenteric ischemia | Ceballos et al, 200020 | 1 |
| Esophageal dysmotility | Jackson and Simpson, 200427 | 1 |
| Irritable bowel syndrome | Krames and Mousad, 200521 | 1 |
| Chronic pancreatitis | Khan et al, 200522 | 5 |
| Familial Mediterranean fever | Kapur, Mutagi, and Raphael, 200628 | 2 |
| Pelvic visceral pain | Kapural et al, 200619 | 6 |
| Gastroparesis | Tiede et al, 200626 | 2 |
| Chronic pancreatitis | Kapural and Rakic, 200823 | 1 |
| Chronic pancreatitis | Kim et al, 200924 | 1 |
| Chronic pancreatitis, abdominal adhesions, gastroparesis, mesenteric ischemia, postgastric bypass pain | Kapural et al, 201029 | 35 |
| Chronic pancreatitis, postsurgical intraabdominal adhesion, gastroparesis | Kapural et al, 201030 | 76 |
| Chronic pancreatitis | Kapural and Bensitel, 201025 | 30 |
SCS, Spinal cord stimulation.
The first case report on SCS for the treatment of abdominal pain described the case of a 78-year-old male with chronic, unrelieved severe postprandial pain caused by mesenteric ischemia. The patient experienced full pain relief after a SCS lead was placed epidurally at the T6 vertebral level.20 The only case report dealing with irritable bowel syndrome (IBS) described a female patient who suffered from 11 to 14 diarrheal episodes per day and extreme pain from IBS and who, after placement of a thoracic SCS system, became immediately diarrhea free. However, her initial reduction in pain relief was not sustained.21
Khan, Raza, and Khan22 described five successful cases of patients with nonalcoholic pancreatitis who received both single and dual leads placed at the T5-T7 vertebral height in the posterior epidural space. This report was followed by several others describing improvements in pain and function in patients with severe chronic pancreatitis23–25 (see Table 12-1).
Tiede and associates26 described improvements in pain scores of patients with gastroparesis, whereas Jackson and Simpson27 reported improved pain control and swallowing in a patient with a rather complicated history of esophageal problems. A most recently published report describes two cases of familial Mediterranean fever (FMF) in which painful abdominal intermittent attacks responded positively to SCS at T8-T9 and T7-T8, respectively.28
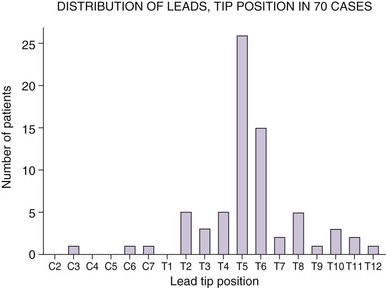
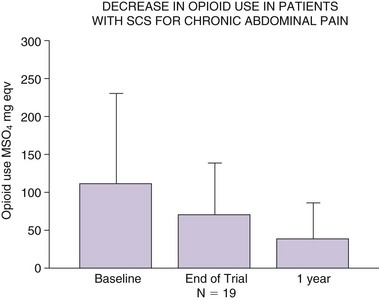
Recently much larger clinical experience using SCS for treatment of chronic abdominal pain has been published.29 In this study 35 patients were trialed with SCS over 4 to 14 days. Consistent with most previous reports (Figs. 12-1 and 12-2), SCS lead tips were positioned at T5 (n = 11) or T6 (n = 10) height within the epidural space. Thirty patients (86%) reported at least 50% pain relief on completion of the trial. Among 28 patients who received permanent implant, 19 were followed at least a year. Their visual analog scale (VAS) pain scores remained low (3.8 ± 1.9 cm; p < 0.001) at 1 year, as was opioid use (138.3 ± 134 to 38 ± 48 mg morphine equivalents [Fig. 12-3]). This report for the first time suggested that SCS may provide consistent long-term improvements as a very useful therapeutic option for patients with severe visceral pain.
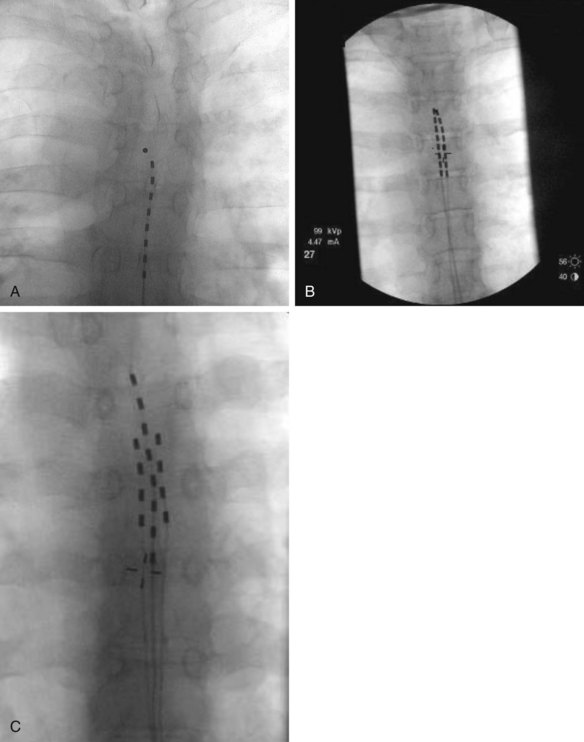
Fig. 12-1 Fluoroscopic anterior-posterior (AP) radiographs of thoracic spine with appropriately positioned leads in midline and with the lead tips positioned anywhere from T4 (A) to T6 (C). Used were either 1, 2, or 3 leads (A,B,C, respectively) without significant difference in pain score improvements if one, two, or three leads were trialed.29
(From Kapural L, et al: Spinal cord stimulation for visceral abdominal pain. Pain Med 11(3):347–355, 2010.)
To initiate the formation of consensus between pain physicians on patient selection and technical aspects of SCS for abdominal visceral pain, a national survey directed to physicians who implant SCS for such pain was conducted recently and collected 76 case reports.30 Considering so few cases reported by the physicians responding to this survey, SCS for abdominal visceral pain is still rarely used, despite possibly high therapeutic success rate. Causes for this include very few studies describing basic mechanisms of neuromodulation for long-standing visceral pain, comfort levels by the physicians, and issues with coverage of such treatment by the payers.
The technical aspects of SCS for the treatment of abdominal visceral pain seem to be uniform among physicians who use such technology across the United States and are also consistent with our larger retrospective study described previously.29,30 In most patients, the SCS leads were positioned with their tips at the level of the T5 (26 patients) or T6 vertebral body (15 patients) (see Fig. 12-2). Pain relief exceeded 50% in 66 of 70 patients reported. VAS pain scores before an implant were 8 ± 1.9 cm, whereas after the implant they were 2.49 ± 1.9 cm. The opioid use before an implant was 158 ± 160 mg; at the last office visit after the implant, it was 36 ± 49 mg. The weakness of this survey was that the subgroup of responding physicians may not adequately represent the population of all of the physicians who trialed SCS for chronic visceral abdominal pain but rather those who had largely positive results; therefore results may be biased. However, the aim was to examine technical aspects of SCS for chronic abdominal pain, and this was not an efficacy study.30
Spinal Cord Stimulation in the Algorithm for the Treatment of Chronic Abdominal Pain
The definite place for SCS within a pain treatment continuum for chronic visceral abdominal pain is still unclear. Following a multidisciplinary evaluation, possibly within a comprehensive abdominal pain center, an interdisciplinary treatment plan should be established. Such treatments include cognitive and behavioral therapies; pharmacological pain management; adjuvant therapies for co-morbidities, including the management of sleep disturbances; and interventional diagnostic and therapeutic nerve blocks—including retrograde epidural differential block; splanchnic, celiac plexus and hypogastric blocks; and radiofrequency ablation. Based on the limited human experience reported in the literature, SCS might be indicated when conservative therapies fail to improve analgesia and function. Psychological evaluation for implantable devices and case discussion within the interdisciplinary medical team should precede SCS trialing. Only after trial is completed can a decision be made if SCS is an appropriate next step in the therapeutic continuum. Following such a rigorous algorithm would likely produce a smaller group of candidates for an implant. In our published study, only 28 patients received SCS implant from 237 initial candidates.29 The data in support of SCS for visceral pain presently are encouraging. However, randomized controlled trials need to be initiated to support the role of SCS for long-term treatment of visceral pain.
1 Everhart J. Overview. vol. NIH publication no. 94-1447. Everhart J, editor. Digestive diseases in the United States: epidemiology and impact. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 1994:3-53.
2 Klinkman MS. Episodes of care for abdominal pain in a primary care practice. Arch Fam Med. 1996;5:279-285.
3 Russo MW, et al. Digestive and liver diseases statistics. Gastroenterology. 2004;126:1448-1453.
4 Derbyshire SW. Imaging visceral pain. Curr Pain Headache Rep. 2007;11(3):178-182.
5 Giamberardino MA, Vecchiet L. Pathophysiology of visceral pain. Curr Pain Headache Rep. 1997;1:23-33.
6 Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(suppl I):2-5.
7 Palecek J, Willis D. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rat. Pain. 2003;104(3):501-507.
8 Palecek J. The role of dorsal columns pathway in visceral pain. Physiol Res. 2004;53(suppl. 1):S125-S130.
9 Krames ES, Foreman R. Spinal cord stimulation modulates visceral nociception and hyperalgesia via the spinothalamic tracts and the postsynaptic dorsal column pathways: a literature review and hypothesis. Neuromodulation. 2007;10(3):224-237.
10 Palecek J, Paleckova V, Willis WD. Fos expression in spinothalamic and postsynaptic dorsal column neurons following noxious visceral and cutaneous stimuli. Pain. 2003;104(1-2):249-257.
11 Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990;43:377-386.
12 Cervero F, Laird JMA, Pozo MA. Selective changes of receptive field properties of spinal nociceptive neurons induced by noxious visceral stimulation in the cat. Pain. 1992;51:335-342.
13 Qin C, et al. Spinal cord stimulation modulates intraspinal colorectal visceroreceptive transmission in rats. Neurosci Res. 2007;58:58-66.
14 Greenwood-Van Meerveld B, et al. Attenuation by spinal cord stimulation of a nociceptive reflex generated by colorectal distention in a rat model. Auton Neurosc-Basic Clin. 2003;104:17-24.
15 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
16 Steege JF. Superior hypogastric block during microlaparoscopic pain mapping. J Am Assoc Gynecol Laparosc. 1998;5:265-267.
17 Rauck RL. Sympathetic nerve blocks. In: Raj PP, editor. Practical management of pain. ed 2. St Louis: Mosby; 1992:778-812.
18 Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med. 2006;7(S1):S14-S26.
19 Kapural L, et al. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7(5):440-444.
20 Ceballos A, et al. Spinal cord stimulation: a possible therapeutic alternative for chronic mesenteric ischaemia pain. Pain. 2000;87(1):99-101.
21 Krames E, Mousad DG. Spinal cord stimulation reverses pain and diarrheal episodes of irritable bowel syndrome: a case report. Neuromodulation. 2005;8:82-88.
22 Khan Y, Raza S, Khan E. Application of spinal cord stimulation for the treatment of abdominal visceral pain syndromes: case reports. Neuromodulation. 2005;8:14-27.
23 Kapural L, Rakic M. Spinal cord stimulation for chronic visceral pain secondary to chronic non-alcoholic pancreatitis: a case report. Clin Gastroenterol Hepatol. 2008;42(6):750-751.
24 Kim JK, et al. Spinal cord stimulation for intractable visceral pain due to chronic pancreatitis. J Korean Neurosurg Soc. 2009;46(2):165-167.
25 Kapural L, Bensitel T: Spinal cord stimulation for pain management in chronic pancreatitis patients, 2010 American Academy of Pain Medicine Annual Meeting Abstracts, A256.
26 Tiede JM, et al. The use of spinal cord stimulation in refractory abdominal visceral pain: case reports and literature review. Pain Pract. 2006;6(3):197-202.
27 Jackson M, Simpson KH. Spinal cord stimulation in a patient with persistent oesophageal pain. Pain. 2004;112:406-408.
28 Kapur S, Mutagi H, Raphael J. Spinal cord stimulation for relief of abdominal pain in two patients with familial Mediterranean fever. Br J Anaesth. 2006;97:866-868.
29 Kapural L, et al. Spinal cord stimulation for visceral abdominal pain. Pain Med. 2010;11(3):347-355.
30 Kapural L, et al. Spinal cord stimulation for visceral abdominal pain: results of the national survey. Pain Med. 2010;11(5):685-691.