Chapter 11 Spinal Cord Stimulation for Refractory Angina and Peripheral Vascular Disease
 Stable angina typically arises during physical or emotional stress secondary to severe stenotic lesions affecting more than 70% of the affected coronary artery lumen of one or more arteries that causes myocardial ischemia. Unstable angina involves acute formation of a thrombosis within an already stenotic coronary vessel, which may or may not immediately lead to a myocardial infarction. Refractory angina (RA) is marked by severe chronic chest pain for more than three months secondary to coronary insufficiency; it occurs in patients who have failed to obtain or undergo appropriate control via other modalities including medical therapy, percutaneous revascularization, and CABG, yet who continue to have a reversible ischemia.
Stable angina typically arises during physical or emotional stress secondary to severe stenotic lesions affecting more than 70% of the affected coronary artery lumen of one or more arteries that causes myocardial ischemia. Unstable angina involves acute formation of a thrombosis within an already stenotic coronary vessel, which may or may not immediately lead to a myocardial infarction. Refractory angina (RA) is marked by severe chronic chest pain for more than three months secondary to coronary insufficiency; it occurs in patients who have failed to obtain or undergo appropriate control via other modalities including medical therapy, percutaneous revascularization, and CABG, yet who continue to have a reversible ischemia. Patients who are unlikely candidates for SCS include patients with RA, who typically have failed either percutaneous coronary interventions (PCIs), CABG, or who have not been candidates for either procedure because of poor coronary anatomy, prior surgical repairs not amenable to further manipulation, impaired left ventricular function, co-morbid noncardiac disease compounding their cardiovascular status, or advanced age.
Patients who are unlikely candidates for SCS include patients with RA, who typically have failed either percutaneous coronary interventions (PCIs), CABG, or who have not been candidates for either procedure because of poor coronary anatomy, prior surgical repairs not amenable to further manipulation, impaired left ventricular function, co-morbid noncardiac disease compounding their cardiovascular status, or advanced age. Before choosing SCS as a treatment modality, the patient must be properly assessed according to various diagnostic algorithms, ideally ones that have been standardized. The evaluation team must include at a minimum the following specialists: a pain physician and/or a neurosurgeon, an anesthesiologist, a cardiologist, and a psychologist.
Before choosing SCS as a treatment modality, the patient must be properly assessed according to various diagnostic algorithms, ideally ones that have been standardized. The evaluation team must include at a minimum the following specialists: a pain physician and/or a neurosurgeon, an anesthesiologist, a cardiologist, and a psychologist. Although pain relief and limb salvage are the primary goals of SCS in PVD, careful patient selection must be undertaken to maximize the likelihood of success, while minimizing inappropriate use of the device and health care resources.
Although pain relief and limb salvage are the primary goals of SCS in PVD, careful patient selection must be undertaken to maximize the likelihood of success, while minimizing inappropriate use of the device and health care resources. Patients who develop unstable angina after SCS implantation will manifest angina that breaks through the SCS-imposed pain relief. Increased mortality with SCS in patients with RA has not been observed in several studies, thus indicating that the use of this device is safe in this patient population.
Patients who develop unstable angina after SCS implantation will manifest angina that breaks through the SCS-imposed pain relief. Increased mortality with SCS in patients with RA has not been observed in several studies, thus indicating that the use of this device is safe in this patient population. SCS use has not demonstrated any arrhythmogenic effects leading to adverse coronary events. Secondarily, the observed perceived decrease in arrhythmogenic events may be related to decreased ischemia with the use of SCS.
SCS use has not demonstrated any arrhythmogenic effects leading to adverse coronary events. Secondarily, the observed perceived decrease in arrhythmogenic events may be related to decreased ischemia with the use of SCS. Prior to implanting a SCS it is absolutely imperative that all causes of angina, both intrinsic and extrinsic, be ruled out prior to labeling a patient with RA.
Prior to implanting a SCS it is absolutely imperative that all causes of angina, both intrinsic and extrinsic, be ruled out prior to labeling a patient with RA. Be aware that RA may be not only secondary to a lack of medical, procedural, or surgical options, but also secondary to a patient that is in a location where such options are not available or the patient chooses not to partake in them.
Be aware that RA may be not only secondary to a lack of medical, procedural, or surgical options, but also secondary to a patient that is in a location where such options are not available or the patient chooses not to partake in them. The key to successful employment of a SCS relies on a multidisciplinary approach to these complex patients, whether they have RA or PVD with CLI. For those with RA it is critical that the cardiologist and pain physician maintain excellent communication first to determine feasibility and then to optimize the complex cardiac patient prior to implantation of the SCS.
The key to successful employment of a SCS relies on a multidisciplinary approach to these complex patients, whether they have RA or PVD with CLI. For those with RA it is critical that the cardiologist and pain physician maintain excellent communication first to determine feasibility and then to optimize the complex cardiac patient prior to implantation of the SCS. It has been suggested that ideal lead placement for coverage of RA is between T1 and T2 with the right side lead in the midline and the left lead slightly to the left of midline. For PVD with CLI, lead placement is based upon the side and the level to which the CLI reaches up the patient’s lower extremities.
It has been suggested that ideal lead placement for coverage of RA is between T1 and T2 with the right side lead in the midline and the left lead slightly to the left of midline. For PVD with CLI, lead placement is based upon the side and the level to which the CLI reaches up the patient’s lower extremities. When placing pocket, consult with patient as to where to place it. The person’s occupation and lifestyle may dictate where its location should be placed. If placing on anterior chest, consider the right side, as pacemakers are typically placed on left side.
When placing pocket, consult with patient as to where to place it. The person’s occupation and lifestyle may dictate where its location should be placed. If placing on anterior chest, consider the right side, as pacemakers are typically placed on left side. Patients with CLI should be carefully assessed for feasibility of revascularization, because if revascularization is possible, then the risk of amputation decreases, thus decreasing their overall morbidity and mortality risks associated with PVD.
Patients with CLI should be carefully assessed for feasibility of revascularization, because if revascularization is possible, then the risk of amputation decreases, thus decreasing their overall morbidity and mortality risks associated with PVD. One of key benefits associated with SCS for PVD is that it not only helps with pain, but also likely helps in the treatment of PVD and CLI through improved vascularization resulting in limb salvage.
One of key benefits associated with SCS for PVD is that it not only helps with pain, but also likely helps in the treatment of PVD and CLI through improved vascularization resulting in limb salvage. When trialing SCS for PVD, consider a two-week trial with an examination of transcutaneous partial pressure of oxygen. A significant increase during the trial period correlates with increased limb salvage.
When trialing SCS for PVD, consider a two-week trial with an examination of transcutaneous partial pressure of oxygen. A significant increase during the trial period correlates with increased limb salvage. Be aware that RA may be not only secondary to a lack of medical, procedural, or surgical options, but also secondary to a patient that is in a location where such options are not available or the patient chooses not to partake in them.
Be aware that RA may be not only secondary to a lack of medical, procedural, or surgical options, but also secondary to a patient that is in a location where such options are not available or the patient chooses not to partake in them. Without involvement of a cardiologist to optimize the patient on blood thinners, risk of developing an adverse bleeding incident, including a epidural hematoma, leading to significant morbidity or mortality exists.
Without involvement of a cardiologist to optimize the patient on blood thinners, risk of developing an adverse bleeding incident, including a epidural hematoma, leading to significant morbidity or mortality exists. If a patient is on warfarin, consider placing the trial leads as if they are to be the permanent lead, thus avoiding the risk of potentially developing an epidural hematoma twice.
If a patient is on warfarin, consider placing the trial leads as if they are to be the permanent lead, thus avoiding the risk of potentially developing an epidural hematoma twice.Introduction
As technologies and research into the treatment of cardiovascular disease and peripheral vascular disease (PVD) advance, applications of treatment regimens not only reduce morbidity and mortality, but they also can lead to undesirable, unintentional consequences—the development of a population of patients who no longer respond to the therapies that initially prolonged their lives, increased their functional capacity, or increased their quality of life. Neuromodulation via spinal cord stimulation (SCS) may be a partial answer to the challenge being faced for patients with refractory angina (RA) or irreparable PVD. It has not been Food and Drug Administration (FDA)–approved for use in either RA or PVD as of yet in the United States. Originally used to treat a patient with cancer pain by Shealy, Mortimer, and Reswick in 1967,1 SCS therapy has been extended to many other areas with varying amounts of success. Not everyone is a candidate for this technique. But appropriately screened patients who meet appropriate diagnostic and psychological criteria may benefit from SCS, which may alleviate chronic pain while adding an anti-ischemic benefit so patients’ quality of life and functional capacity improve.
Epidemiology: Coronary Artery Disease and Peripheral Vascular Disease
Coronary artery disease (CAD) remains the number one cause of morbidity and mortality in the United States, yet the prevalence of RA in the population has not been well-defined in the literature. Several estimates have been proposed. According to the American Heart Association’s Heart Disease and Stroke Statistics—2010 Update, approximately 10.2 million people suffer from angina pectoris. Currently the annual incidence is estimated to be approximately 500,000.2 In 1999 Mukherjee and associates3 attempted to estimate the incidence of RA. Their approximation of 12% of the total population of those with angina is based on the percentage of people undergoing angiography at tertiary referral centers who ultimately are not eligible for percutaneous or coronary artery bypass graft (CABG) revascularization procedures.3 This percentage is also endorsed by Mannheimer and associates,4 who remarked on the paucity of data but noted that one study they reviewed had a prevalence of 5% to 15% of the population as having RA. In 2002 Holmes5 noted that approximately 2.4 million people in the United States suffer from CAD untreatable by either percutaneous revascularization or CABG. With a large and ever-expanding population, the demand for novel treatments will certainly climb. This demand will undoubtedly be mirrored in the population that suffers from PVD.
PVD likely affects more than 10 million people in the United States according to Vallejo et al estimates in 2006, and it affects 12% to 20% of the population aged 65 and older.6 Of the people who develop PVD with signs of intermittent claudication, approximately 20% are believed to develop chronic critical limb ischemia (CLI); 25% of CLI sufferers require an amputation.7 The mortality for this population also is quite high, ranging from 25% to 30% at 2 years and increasing to 50% to 75% at 5 years after the onset of CLI. Although the amputation rate and mortality are significant in this subpopulation, the implementation of SCS shows promise in alleviating some of the suffering and morbidity and mortality of this disease.
Establishing Diagnosis: Angina and Role of Spinal Cord Stimulation
As defined by the American Heart Association in 1999,8 angina comprises a clinical syndrome of pain and discomfort in the chest, jaw, shoulder, back, or arm that may be exacerbated by physical exertion or emotional stress. Although angina typically is associated with CAD involving the epicardial vessels and subsequent ischemia, a more robust differential diagnosis of angina must be considered from both a cardiac and noncardiac standpoint. Cardiovascular origins of angina may also include valvular heart disease, severe hypertension, hypertrophic cardiomyopathies, acute aortic dissection, acute pericarditis, severe aortic stenosis, coronary vasospasm, and cardiac syndrome X (CSX).8–10 CSX patients present similarly to those with CAD-related angina. Although patients with CSX tend to feel pain with exertion and their electrocardiograms (ECGs) may show ST-segment depression during exercise stress tests that induce angina, these patients do not have signs of obvious CAD on angiography; in fact, in some patients, evidence suggests that ischemia may not be a causative factor.11 Proposed mechanisms for CSX include estrogen deficiency, abnormal function and distribution of adenosine receptors, and coronary microvascular dysfunction.9,11,12 Noncardiac origins of chest pain include trauma and esophageal conditions, including reflux and motility disorders, biliary colic, costochondritis, and pulmonary embolism or pulmonary hypertension.
In addition, angina may be classified as stable or unstable. Stable angina typically arises during physical or emotional stress secondary to severe stenotic lesions affecting more than 70% of the affected coronary artery lumen of one or more arteries that causes myocardial ischemia.13 Conversely, unstable angina involves acute formation of a thrombosis within an already stenotic coronary vessel, which may or may not immediately lead to a myocardial infarction (MI). Unstable angina is of greatest concern since it predicts an elevated short-term risk of a cardiac event8 and necessitates immediate coronary revascularization to alleviate. Unstable angina is beyond the scope of this chapter; however, episodes of unstable angina pain manifest even in patients with spinal cord stimulators for RA, as discussed in the following paragraphs.
To improve clinical classification of angina, the Canadian Cardiovascular Society (CCS)8,14 modified the New York Heart Association’s (NYHA) Functional Classification (Box 11-1). Campeau and Letter14 developed four classes—I through IV. In class I angina patients perceive angina only with strenuous activity. Class II patients experience only slight limitation of activity secondary to angina. Class III patients experience marked limitation of normal activity, and in class IV they experience angina with any activity, including being at rest. MI falls within classes III and IV.
Box 11-1
Grading of Angina of Effort by the Canadian Cardiovascular Society
From Campeau L: Letter: Grading of angina pectoris, Circulation 54(3):13, 1976.
RA is marked by severe chronic chest pain for more than 3 months secondary to coronary insufficiency; it occurs in patients who have failed to obtain or undergo appropriate control via other modalities, including medical therapy, percutaneous revascularization, and CABG, yet who continue to have a reversible ischemia.9,15,16 RA occurs when all reversible causes for ischemia have been ruled out. SCS has been used successfully to target RA—decreasing the frequency and severity of the episodes and improving patient functionality and quality of life.
Anatomy: Pain Pathway for Angina
Signals for angina pain are initiated at both the chemosensitive and mechanoreceptive nociceptors in the adventitia of the coronary arteries and myocardium.16 Because the majority of these nerves are slow-conducting C fibers, the predominant pain experienced is of a dull, aching, heavy, and squeezing type.8 Aδ fibers that carry stabbing and sharp pain are typically not involved in angina. On their activation these nociceptors release a variety of chemical mediators, including adenosine, bradykinin, prostaglandins, and others, which initiate signals in the sympathetic and parasympathetic (vagal) afferent pathways to dorsal spinal cord and parasympathetic ganglia located from C7 to T5.17,18 The pain experienced by the patient during an episode of angina is related to the convergence of common pathways at the dorsal spinal cord between C7 and T5, where afferent myocardial inputs and cutaneous nociceptors converge on the same interneurons at the same level within the spinal cord.16 Thus the pain perceived by the patient is distributed within the dermatome from where the cutaneous afferents converge on the same spinal segment as from the heart.
Indications: Spinal Cord Stimulation and the Alternative Therapies Available for Refractory Angina
As medical, interventional, and operative treatment modalities for angina and occlusive vascular disease have developed along with an aging population, so has the portion of the population with angina that lacks viable options for further improvement, despite optimization from one of the novel or improved therapies. This subgroup contains patients with RA who typically have failed either percutaneous coronary interventions (PCIs) or CABG, or who have not been candidates for either procedure because of poor coronary anatomy, prior surgical repairs not amenable to further manipulation, impaired left ventricular function, co-morbid noncardiac disease compounding their cardiovascular status, or advanced age.19,20 Jolicoeur and associates19 also mentioned that a person with RA may fall into the category of “nonrevascularization” secondary to either their geographical location where practitioners may not have the expertise or the patients lack of interest in pursuing a particular therapeutic path.
Multiple pharmacological and nonpharmacological (mechanical intervention) therapies for RA are reviewed in the following paragraphs. The reviews are based primarily on Jolicoeur and colleagues’ report of the working group on clinical and research issues regarding chronic advanced CAD.19 Although their reviews are not comprehensive, they highlight the current management options for RA.
Pharmacological agents mitigate angina symptoms by a variety of mechanisms so the patient has reduced myocardial oxygen demand and increased supply via a reduction in heart rate (HR), decreased afterload, and decreased contractility. Agents with antianginal properties that are helpful in accomplishing these changes include β-blockers, nitrates, calcium channel blockers, and opioids. Each provides angina relief via different mechanisms, but each is not without side effects that lead to dose limitation or intolerance. Antithromboembolic agents such as aspirin and clopidogrel (Plavix) target the platelets; whereas antihyperlipidemics such as statins promote vessel patency, thus decreasing risk for myocardial ischemia and resultant angina. Ranolazine (Ranexa) is a relatively new drug with direct antianginal and anti-ischemic properties. A debate as to its mechanism of action exists; however, it permits an increase in exercise performance without changing HR or blood pressure.21 In addition, ranolazine improves angina and exercise threshold when used as a monotherapy and in combination with more traditional therapies listed previously.21,22
Mechanical interventions are also available for RA: percutaneous stent placement, transmyocardial laser revascularization, and CABG. Despite these interventions, people continue to have angina symptoms, and some people are not candidates for such therapies. Chronic total occlusion (CTO) recanalization via the percutaneous approach has been conducted after the development of chronic occlusions (greater than 3 months) with a substantial improvement in 10-year survival.19 The reported success of revascularization achieved by CTO recanalization remains a stable 71% despite attempts at canalizing longer lesions, whereas reported complications of the procedure range from 3.8% to 5.1%.23–25 Technical advances may eventually make this technique more effective and safe.19
Another mechanical technique sometimes used is enhanced external counterpulsation (EECP), approved by the FDA in 1995 for angina. In EECP three bilateral lower extremity cuffs are inflated sequentially during diastole to provide increased venous return and diastolic augmentation.26 Currently this technique requires 35 consecutive days of 1-hour sessions to show some lasting benefit of decreased angina symptoms and a longer time to greater-than–1-mm ST depression on an exercise stress test.26 EECP has not been studied in a large, randomized controlled setting; and it has side effects, including edema, bruising, and pain in the lower extremities. According to Soran and associates,27 patients undergoing EECP noted a substantial improvement in the quality of life; 72% improved from severe to mild or moderate angina, 52% stopped nitroglycerin use, and at 2 years 55% of the patients reported a maintained decrease in their angina.
Before choosing SCS as a treatment modality for RA, the patient must be properly assessed according to various diagnostic algorithms, ideally ones that have been standardized. One such algorithm for assessing a person’s appropriateness for SCS was proposed by the European Society of Cardiology.20 First a team of cardiologists and cardiac surgeons should determine if a patient’s angina is of ischemic origin and evaluate him or her for revascularization. A recent angiogram should be used to rule out newly treatable coronary pathology. In addition, all other forms of chest pain should be eliminated. For example, the differential diagnosis of chest pain should include noncardiac origins of chest pain, including esophageal pain, gastroesophageal reflux, musculoskeletal pain, costochondritis, anemia, uncontrolled hypertension, atrial fibrillation, and thyroid disorder.19,20 Once these criteria have been met, the patient’s medical therapy must be optimized. After an appropriate diagnosis has been established and subsequent medical and surgical optimization has occurred, the focus shifts to psychological evaluation to determine if the patient’s perception of pain has a significant co-morbidity of depression or anxiety that should be treated before SCS implantation or if the person should be evaluated on the basis of compliance with other treatments. Finally, risk factor management and cardiac rehabilitation must continue.
Basic Science: Mechanism of Action of SCS for Angina
SCS for angina enables both antianginal and anti-ischemic effects to be integrated to achieve pain relief. The severity of the angina decreases; and cardiac function improves, as evidenced by the following: decreased need for short-acting oral nitrates and 24-hour cardiac monitoring and markers of functional status and increased perceived quality of life.28 Multiple mechanisms have been proposed to explain these effects, a number of which are outlined here. note: What follows is not an exhaustive review of the literature on this topic.
Given the benefits of the antianginal and anti-ischemic effects of SCS, one should also consider its safety for patients with RA. An early concern was that SCS could mask angina pain associated with myocardial ischemia and eliminate a patient’s subjective sensation of RA. This concern has not been borne out in several studies.28 Instead, SCS merely raises the patient’s threshold for sensing angina, thus permitting the patient to increase exercise capacity. Investigators have not noted any resultant increase in morbidity and mortality with SCS.
The following mechanisms have been suggested.
Direct Suppression of Pain
The antianginal effect of the SCS may be caused by direct suppression of pain. According to the gate control theory proposed by Melzack and Wall in 1965,29 fast-conducting myelinated A fibers modulate slow-conducting unmyelinated C fibers via a negative feedback mechanism at the level of the dorsal horn of the spinal cord. Thus applying neuromodulation techniques to the dorsal column of the spinal cord at the level where input from nociceptors occurs inhibits signals ultimately responsible for achieving the decreased sensation of pain. The electricity of the SCS provides a continuous, selective, low-level activation of the sensitive afferent A fibers, which in turn inhibits the Aδ and C fibers of nociception presynaptically.9 Chandler and associates’30 support of this mechanism comes in their studies of SCS on anesthetized monkeys. They demonstrated that SCS of the dorsal column decreased the output of spinothalamic tract neurons that were triggered by electrical stimulation of cardiac sympathetic afferent fibers with sensory endings in the ventricles. These spinothalamic tract cells also received somatic input from the chest and upper extremities; this type of input is also blocked with SCS. Chandler and associates30 also demonstrated that intracardiac injection of bradykinin, which duplicates the effects of either cardiac sympathetic or somatic nociception, is blocked by the use of an SCS.30 What was not resolved was whether suppression of the spinothalamic tract is a direct effect or a decrease in information from nociceptive afferent.31
Molecular Mechanism
More recently a molecular mechanism leading to reduced angina has been investigated. Excitatory amino acids involved in transmitting signals within the dorsal horn include glutamate and aspartate. The release of these neurotransmitters has been shown to decrease in the presence of elevated gamma aminobutyric acid (GABA) that occurs during neuromodulation of the dorsal horn.32 Cui and associates32 further pointed out that this observed effect was transiently reversed with the addition of a GABA β-receptor antagonist placed at the dorsal horn. In addition, Oldroyd and colleagues33 found that β-endorphins are released from the pituitary in response to myocardial ischemia.33 The significance of these findings is that β-endorphins may participate in pain reduction through their action as endogenous opioids. In addition, β-endorphins may affect the regulation locally at the level of the myocardium by directly aiding in decreased oxygen consumption.34 According to Eliasson and associates,34 β-endorphin release at the myocardium increased with the use of SCS during conditions of rest and during pacing to angina in humans. They suggest that the data be interpreted cautiously since they conducted their study by applying an accepted method of evaluation of myocardial ischemia involving the myocardial lactate extraction ratio and using it to look at myocardial turnover of peptides. While conducting the study, they derived a wide range of individual values.34 Thus one current hypothesis suggests that SCS promotes release of biologically active molecules that may have both direct and indirect effects on angina pain and myocardial ischemia.
Central Nervous System Mechanism
A central mechanism of pain control may be triggered by the use of neuromodulation. According to Eckert and Horstkotte,16 functional neuroimaging has been used to examine areas of cerebral blood flow in patients with known CAD. In such patients angina and ECG changes are elicited by dobutamine infusion. According to Hautvast and colleagues,35 dynamic positron emission tomography (PET) scans during such periods of chemically induced ischemia demonstrated areas of varying regional cerebral blood flow. When using SCS, Zonenshayn and associates36 noticed corresponding changes of increase and decrease in cerebral blood flow during periods with and without stimulation, respectively. Eckert and Horstkotte16 pointed out that, when they examined the two groups, they saw similarities in increased cerebral blood flow to the hypothalamus bilaterally and the periaqueductal grey area, and decreased cerebral blood flow in the posterior insular cortex that modulates sympathetic nervous system (SNS) activity. Thus SCS may be influencing pain perception and processing in the central nervous system. These findings suggest that the thalamus may be acting as a filter for afferent pain signals.
Anti-Ischemic Mechanisms
One hypothesis to explain this decreased ischemia suggests that coronary blood flow (CBF) is redistributed to areas of poor perfusion, likely secondary to collateral flow.28 It has been suggested that SCS improves myocardial perfusion via vasodilation of microvessels within the myocardium, alleviating angina pain in patients who continue to have a small amount of coronary reserve, despite the ischemia. SCS may eliminate this reserve. Many techniques (i.e., intracoronary pressure and flow measurements, stress echo, myocardial scintigraphy, and PET scanning) have been attempted to evaluate possible mechanisms to explain why the SCS has an anti-ischemic effect, but they have not been entirely successful in elucidating such mechanisms. These techniques may not be able to fully identify the changes in microcirculation that result in decreased angina symptoms.16
The ability of neuromodulation to promote blood flow change remains controversial since evidence is lacking and somewhat contradictory. Mobilia and associates37 used PET to evaluate CBF and suggested that the SCS promoted increased CBF and allowed for redistribution from areas of high and low flow.37 SCS promotion of increased CBF was contradicted by Norrsell and colleagues38 who demonstrated an anti-ischemic effect independent of CBF velocity. Studies on dogs with normal hearts did not show an increase in local flow or a redistribution.39 Wu, Linderoth, and Foreman31 also point out that long-term use of SCS has been shown to decrease myocardial ischemia, perhaps because of better coronary collateralization secondary to increased physical activity of the patients.31
Remodeled Neural Pathways
The myocardium contains intracardiac neurons (ICNs) that are the primary integrators of the nervous system within the heart.40 Neuromodulation with an SCS has been suggested to “remodel the neural pathways” by altering the firing rate of the intracardiac neurons and stabilizing their activity during ischemia.41 Hypothetically decreases in angina secondary to SCS allow patients to increase and prolong their exercise. Initially SCS was believed to modulate the sympathetic branch of the SNS with respect to the perception of pain; however, this is likely not the case because there is no change in HR variability or in epinephrine and norepinephrine metabolism.28 Instead it is hypothesized that SCS acts on the myocardium via the ICNs to permit a redistribution of blood flow from the areas of normal perfusion to areas of ischemia.42 Speculation exists as to how this blood flow redistribution occurs. Possibilities include angiogenesis, collaterals, and preconditioning.
Intrinsic Cardiac Nervous System
Another working hypothesis suggests that the SCS neuromodulates the intrinsic cardiac nervous system. Wu, Linderoth, and Foreman31 witnessed decreased magnitude of ST-changes in the ECG and decreased risk for development of arrhythmias secondary to ischemia in patients with functioning SCSs.31 The intrinsic cardiac nervous system is comprised of parasympathetic and sympathetic efferent nerves, sensory afferents, and interconnecting local neurons. It resides in the cardiac ganglion plexi of the pericardial fat pads near and within the myocardium.43 According to Armour,40 these neurons interact both locally and regionally, which allows “reflex coordination” of autonomic neuronal outflow to the heart. It is important to note that SCS effects are blocked by stellectomy.44 Wu, Linderoth, and Foreman31 point out that SCS is believed to stabilize the activity of the intrinsic cardiac nervous system typically activated by ischemia.
In addition, it has been shown that SCS has decreased pain and O2 consumption in similar HRs in atrial-paced studies.45 Those who develop myocardial ischemia will still experience angina with a quality and distribution similar to that of their original symptoms.9 There has been no demonstrated arrhythmogenic effect associated with SCS.46 Furthermore, the perceived decrease in arrhythmic events may be secondary to the decreased myocardial ischemia from the use of the SCS. This observation may also be associated with the hypothesis that the SCS stabilizes the intracardiac neurons so the chance for an arrhythmic event is lessened as noted previously.
Early work by Mannheimer and colleagues47 suggested that SCS does not manifest changes at rest; instead, under conditions of stress, its effects occur. It has been proposed that SCS does not decrease cardiac sympathetic activity, leading to its anti-ischemic effect. Instead, it has been suggested that SCS globally decreases SNS activity, which leads to a decreased oxygen demand.45 According to Mannheimer and colleagues47, the total body norepinephrine spillover, but not that of the myocardium, was reduced, suggesting that SCS affects global sympathetic activity. During stress the SCS can lower HR, which counters the increase in activity in the intrinsic nervous system that would otherwise lead to dysrhythmias.48 This activation of the intrinsic neurons leads to changes in the system that persist after cessation of the SCS, which suggests that a remodeling exists that may limit the excitatory input induced by ischemia.
Murray and colleagues49 point out that 53% of those with RA responding to SCS have a history of sustaining a non-Q wave MI, whereas only 20% to 30% of all MIs are characterized by this.49 These patients are unique because they survive despite severe CAD; consequently they have further problems such as RA. To account for the difference in non-Q wave MIs between the two groups, Ganz and Braunwald50 note that these patients typically have severe CAD yet extensive collateral flow. This difference may reflect an advantage to this population, because subendocardial ischemia is believed to be aggravated by adenosine-mediated myocardial steal. Neuromodulation may promote the redistribution of myocardial blood flow from nonischemic to ischemic areas by reducing the adenosine-mediated steal phenomena.49 Thus patients who respond well to SCS may be doing so via adenosine antagonism, whereby blood is drawn from the extensive collateral reservoir and shifted to areas of ischemia.
Guidelines: Patient Selection for Spinal Cord Stimulation in Refractory Angina
Careful consideration of a patient’s candidacy for implantation involves a multidisciplinary approach to assess the patient’s cardiovascular status and other issues typically faced with SCS implantation. The evaluation team must include at a minimum the following specialists: a pain physician and/or a neurosurgeon, an anesthesiologist, a cardiologist, and a psychologist.28,51 De Vries and associates28 suggest a series of inclusion and exclusion criteria to determine patients’ suitability for SCS in RA (Box 11-2).
Box 11-2 Inclusion and Exclusion Criteria
Spinal Cord Stimulation for Ischemic Heart Disease
From De Vries J et al: Spinal cord stimulation for ischemic heart disease and peripheral vascular disease, Adv Tech Stand Neurosurg 32:71, 2007.
The cardiologist’s evaluation is the most critical one, because the pain physician should operate under the assumption that the angina is not refractory until proven otherwise. The cardiologist evaluates the patient’s cardiac status to determine whether the patient suffers from RA or a nonoptimized cardiac condition. If the cardiologist finds the patient to be an SCS candidate, he or she can also comment on the patient’s cardiac stability in terms of blood pressure, ejection fraction, and other parameters that aid the anesthesiologist in determining the risk and tolerability for the patient to undergo anesthesia. The cardiologist’s other significant contribution is to optimize the patient’s drugs that impact bleeding, including aspirin, warfarin (Coumadin), and medications that alter platelet function51 (Table 11-1). Use of blood thinners must be stopped secondary to the risk of surgical bleeding and, more important, the risk of epidural hematoma that may lead to paralysis. The cardiologist must determine the feasibility of stopping these medications for the period of device implantation. The patient may remain on baby aspirin but should be off warfarin for a minimum of 3 days with an international normalized ratio (INR) check before the time of trial or implantation. Clopidogrel and associated drugs should be stopped for 10 days. If there is a question about continued alteration of platelet function caused by clopidogrel, a platelet function assay should be considered. Because placement of an SCS device is an elective procedure, the injunction, “Do no harm to the patient,” takes precedence. If taking a patient off these medications causes excessive risk, the procedure should not be attempted. If it is a concern that the patient is off medications, he or she may be a candidate for hospital admission for initiation of heparin therapy, which may be stopped 6 hours before surgery and restarted 6 hours afterward. Low–molecular weight heparin should be avoided secondary to the increased risk of epidural bleeding after SCS lead placement for patients on this therapy.
| Drug | Recommendation |
|---|---|
| Warfarin | Off 3 days before implant with normal INR |
| Clopidogrel and similar drugs | 10 days and until the trial lead is removed |
| Baby aspirin | Most physicians do not recommend stopping baby aspirin |
| Conventional aspirin and NSAIDs | Physician discretion |
INR, International normalized ratio; NSAID, nonsteroidal antiinflammatory drug.
From Deer, T.R (ed): Spinal cord stimulation for the treatment of angina and peripheral vascular disease. Curr Pain Headache Rep 2009, pp. 20.
Equipment and Technique for Spinal Cord Stimulation in Refractory Angina
With implantation of SCS in any patient, the most critical step is placement of the SCS leads at the proper level and location to achieve paresthesias over the area the patient experiences the angina. Typically the tip of the stimulator lead should be placed, under fluoroscopic guidance, at the C7 level in the dorsal epidural space since the afferent fibers are located from approximately T1 to T4. Most successful placement involves stimulation between T1 and T2.46,48,52 For percutaneous placement the 14-gauge Tuohy needle usually used for insertion should be introduced at approximately the T4-T5 to T6-T7 levels, depending on the patient’s anatomy. The further down the vertebral column a needle is introduced, the more difficult it may become to advance the percutaneous stimulator lead. Because the most common cause of failure is improper position of the stimulator electrode,9,51 care should be taken to direct one stimulator lead slightly to the left of midline and one at midline to capture most of the angina pain.
Other considerations exist. One is whether the patient has a pacemaker; an automatic implantable cardioverter-defibrillator (AICD) is a contraindication to SCS. SCS compatibility with the pacemaker should be confirmed before implantation, and the pacemaker should be interrogated before and after implantation of the final device. Because of the acuity of this patient population, another consideration is how to trial the patient. If the patient requires titration on and off of blood thinners and/or has difficult lead placement, he or she may be a candidate for implanting trial leads so the leads don’t have to be reintroduced if the trial is successful; instead the patient may go directly to generator placement. Deer51 points out that no outcome studies have been published to elucidate a better trial technique.
Outcomes for Spinal Cord Stimulation and Refractory Angina
Since SCS was introduced in 1987 as a treatment modality for RA, no large or long-term randomized controlled clinical trials have been conducted to examine its efficacy, likely because of the cost. Instead, several observational, retrospective, and small randomized controlled trials (RCTs) have been done, each measuring different primary and secondary endpoints, excluding any common standardized outcome measures. In addition, as Mannheimer and associates53 pointed out in their study, patients enrolled for SCS caused by RA must be considered in relation to the medical knowledge and technology at that time. Studies conducted over too long a period potentially lead to differences between early and late enrollees secondary to advancing surgical, interventional, and anesthetic techniques.53
Most of the outcomes literature comes from European studies, which focused on the following measures: exercise capacity, ischemic burden, nitrate drug consumption, functional class of angina, health-related quality of life, adverse events and SCS-related complications, cost, reduced frequency and severity of angina, change in pain scores, sleep, and morbidity and mortality. To put many of these outcome measures into perspective, Taylor and associates54 prepared a systematic literature review and meta-analysis of SCS use for RA as of February 2008. Following the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions, the team ultimately examined 11 papers involving seven RCTs with similar inclusion criteria: all patients developed RA despite medical optimization, they were not candidates for revascularization, and they were included in NYHA angina classes III and IV. All seven trials were short term, except for the electrical stimulation versus coronary artery bypass surgery study (ESBY) in severe angina pectoris conducted by Mannheimer and associates53 and the open label, single-centre, randomized trial of spinal cord stimulation versus percutaneous myocardial laser revascularization in patients with refractory angina pectoris (SPiRiT trial) conducted by McNab and associates55 that followed up beyond 1 year.55
Taylor and colleagues54 examined seven specific outcome categories across the seven RCTs: exercise capacity, ischemic burden, nitrate drug consumption, functional class of angina, health-related quality of life, adverse events and SCS-related complications, and cost. The RCTs reviewed included one that examined SCS implantation vs. no implantation conducted by de Jongste et al in 1994.56 Four of the RCTs involved an SCS on state vs. an SCS off state with all patients in the study being implanted.52,57–59 The ESBY study compared CABG to SCS.53 The SPiRiT trial examined percutaneous myocardial laser revascularization vs. SCS.55 Much of the following discussion is based on Taylor and associates’ findings with respect to these investigations. Adverse events and SCS-related complications are addressed in a different section, and cost is examined last within this section from the perspective of investigators other than Taylor and colleagues.
According to Taylor and associates, investigators in six RCTs studied exercise capacity. However, investigators in only three RCTs found statistical significance between baselines and follow-up with the SCS out of four studies that looked at change in exercise capacity within the SCS group. The authors pointed out that in the fourth, the ESBY study, the investigators turned off the stimulator while the patients were undergoing evaluation for exercise capacity; thus the patients did not demonstrate any significant change in work capacity. They stated that a pooled analysis demonstrated an improvement in exercise capacity in the SCS-on vs. SCS-off state (p = 0.03) but noted no difference when compared to CABG or percutaneous myocardial laser revascularization (PMR) to SCS and exercise capacity.54
Next the authors evaluated ischemic burden in four of the studies they examined. This burden was measured in these studies by evaluating the patient’s 24- or 48-hour ECG monitoring for the frequency and magnitude of ST depression.54 No statistical difference with regard to ischemic burden was noted between SCS and CABG (p = 0.44), and only a trend favored SCS when compared to no SCS or nonoperating SCS (p = 0.12).
Only two RCTs evaluated a change functional class of angina by using the CCS angina classification (similar to NYHA). At 3 and 12 months, McNab and associates55 reported an improvement in CCS class (lower) of p = 0.049 and p = 0.093, respectively, when compared to patients receiving PMR. But a decrease in CCS class was only statistically significant at 3 months.55 The other study by Eddicks and associates58 was statistically significant with p = 0.002, with a mean decrease in CCS functional class of 1.6.
Taylor and colleagues concluded that SCS is a viable option for patients with RA citing the significant improvement in both exercise capacity and health related quality of life in most of the RCTs they evaluated.54 Although they noted that none of the parameters differed tremendously from CABG or PMR, it must be pointed out that these techniques have not been very beneficial for patients with RA. Not one of the randomized controlled trials in the literature use change in pain (any scale) as a primary outcome measure. Instead, all primary outcomes are linked to either exercise capacity in five studies, and ischemic burden in one study. The focus on these outcome measures points to the importance of SCS use for RA to improve primarily function and quality of life as opposed only to pain.
Before the review by Taylor and associates,54 the American College of Cardiology and American Heart Association 2002 Guideline Update on Chronic Stable Angina classified SCS as class IIb (usefulness/efficacy is less well established by evidence/opinion).60 Up to the time the guidelines were written, only two small RCTs had been conducted. Since that time, no multiple large-scale RCTs have been conducted demonstrating intermediate and long-term benefit that would elevate the use of SCS to level IA data.
A recent review by Simpson and associates61 out of the United Kingdom evaluated clinical and cost-effectiveness of SCS for both neuropathic and ischemic pain. Their review uses four of the same studies analyzed by Taylor and associates. From a clinical standpoint Simpson and associates come to a similar conclusion. In addition, they went on to assess the cost-effectiveness of SCS as a treatment. Because the paucity of data available to determine comparative efficacy to other treatments, including medical management, CABG, or PCI, Simpson and associates61 conducted a threshold analysis to determine what the cost benefit would be with the choice of SCS instead of one of the other treatments. Each analysis suggested that each comparison examined would require the patient receiving SCS to live longer to achieve benefit for a life year group. Besides a lack of evidence for comparison, the comparison does not take into account the fact that the suggested use of SCS is for patients who would not be candidates for one of the other interventions on account of the fact that their RA is not amenable to other such treatments.
In addition, several studies by investigators other than Taylor and colleagues have demonstrated cost-effectiveness with the use of SCS. In a study by Rasmussen and associates,62 use of the device was shown to save approximately 30% on medical costs because of decreased invasive testing alone. In addition, in a retrospective study by Yu and associates63 the cost of the total SCS procedure was recuperated within 16 months, which they point out was less than 40% of the life span of the device as of 2004. Murray and associates49 noted that rehospitalization and length of stay after SCS when compared to revascularization was significantly lower (p = 0.002). All of these studies have been observational or retrospective; none have been prospective RCTs.
After approximately a decade of use, a study by TenVaarwerk and colleagues64 demonstrated in a retrospective clinical outcome study that the use of SCS in RA did not lead to any increase in the rate of adverse events when compared to populations that did not undergo SCS implantation. This retrospective study evaluated 517 patients by questionnaire at 14 centers in Europe. They concluded that the patients died from either events unrelated to heart disease or coronary events linked to lower ejection fraction and the severity of CAD, not the presence of the SCS. In addition, when compared to patients receiving medical management, there was no change in mortality.
Complications for Spinal Cord Stimulation and Refractory Angina
Whenever SCS is implanted, several complications are possible—from the most common, lead migration, to a variety of others, including loss of paresthesia, electrode failure, premature battery exhaustion, and infection. Buchser and Durrer65 stated that the combined rate of complication is 6.8%. Simpson and associates61 reported that four studies totaling 403 patients showed a 1% incidence for device removal secondary to infection and a 5%- to 38%-range for device complications in studies with follow-ups greater than 2 months. They suggested such a range for device complications because of the variable sizes of studies, different follow-up periods, or clinical circumstances.
Establishing Diagnosis: Peripheral Vascular Disease and Role of Spinal Cord Stimulation
PVD most commonly develops secondary to advancing atherosclerosis; however, a variety of other conditions lead to the final common pathway, but for brevity’s sake they are not discussed here.66 If left unchecked, PVD progresses to CLI and eventual infarction secondary to a decrease in blood flow rate necessary to maintain tissue metabolic function. Patients suffering from PVD typically first notice symptoms of vascular claudication or intermittent pain with ambulation that resolves at rest. If left untreated, the disease progresses to infarction and gangrene, ultimately requiring amputation if other measures fail.
Establishing a CLI diagnosis is critical for selecting patients for various therapies. The second European consensus conference on chronic CLI recommended two ways to identify and define CLI. First, in patients with and without diabetes CLI may be defined by either of two criteria: (1) persistently recurring ischemic rest pain requiring regular adequate analgesia for more than 2 weeks, with an ankle systolic pressure ≤50 mm Hg and/or a toe systolic pressure of ≤30 mm Hg; or (2) ulceration or gangrene of the foot or toes, with an ankle systolic pressure ≤50 mm Hg and/or a toe systolic pressure of ≤30 mm Hg.7 Second, the conference recommended a more precise way to identify and define CLI (i.e., use angiography to identify large-vessel disease, take toe arterial pressure, and use methods such as transcutaneous partial pressure of oxygen (TcpO2) to delineate local microcirculation.7 It is important to note that not every patient with CLI falls within these definitions, despite having severe disease. Best clinical judgment must be used to evaluate all patients for extent of disease.
For those patients with concomitant diabetes, a few important factors must be considered during patient evaluation. First, because patients with diabetes may also suffer from painful diabetic neuropathy, their neuropathic pain should be distinguished from their CLI pain at rest. In addition, as the consensus conference points out, because patients with diabetes may have falsely high ankle systolic pressures, pressure readings with a toe cuff should be taken.7 Patients with diabetes may also lack palpable pulses or be so weak that observer variability may affect diagnosis. Thus the consensus conference suggested evaluating absolute pressure at the ankle or toe as a more appropriate method of determining extent of disease than the standard ankle/arm pressure index.
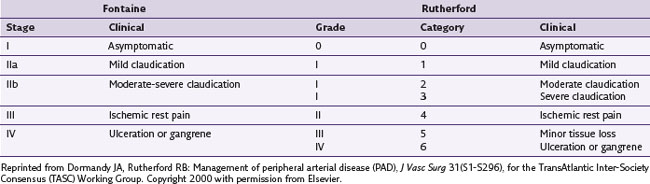
Two classification systems, Fontaine stages and Rutherford categories, have been developed to help determine the extent of CLI in PVD (Table 11-2). The Fontaine classification helps assess limb ischemia and the severity of PVD. In stage I the patient is asymptomatic; in stage II the patient demonstrates intermittent claudication; in stage III the patient develops pain at rest; and in stage IV the patient has evidence of tissue loss, including ulcers and gangrene, and pain at rest.10,67 Fontaine stages III and IV (Rutherford categories 4, 5, and 6), along with the associated blood pressure criteria noted later in the paragraph, define CLI.28,66 Although these definitions help categorize the extent of CLI, they do not aid in prognosis for the affected limb. According to De Vries and associates,28 no consensus currently exists as to how to most accurately determine prognosis. Patients with Fontaine stages III or IV disease have affected macrocirculation and microcirculation, each of which is evaluated differently. According to Jacobs and Jorning, 68 macrocirculation is best evaluated by systolic ankle/arm pressure measurements at rest and after treadmill exercise. Microcirculatory (cutaneous) blood flow may be evaluated by tissue oxygen pressure measurement, laser Doppler flowmetry, and radioisotope clearance.28,68 Ubbink and associates69 evaluated prognostic capabilities of these methods. They determined that microcirculatory classification predicted the need for eventual amputation; but Fontaine stage, ankle blood pressure, or diabetes did not. The combination of toe blood pressure of 38 mm Hg and TcpO2 of 35 mm Hg supine was of good prognostic value.28,70
Indications: Spinal Cord Stimulation and the Alternative Therapies Available for Peripheral Vascular Disease
Conservative therapies include a variety of pharmacological agents, but options are sparser than for the treatment of angina. As of 2005 several drugs were under investigation to treat CLI. Pentoxifylline (Trental), a xanthine derivative with vasodilator and hemorrheologic properties, did not demonstrate any significant benefit for patients with CLI. Cilostazol, approved for intermittent claudication patients, as of 2005 had not been demonstrated as effective in patients with CLI.66 According to Hirsch and associates,66 three prostaglandins (PGs)—PGE-1, iloprost, and ciprostene—with vasodilatory properties have undergone multiple trials in patients with inoperable CLI without success. More traditional conservative therapies, including analgesics, vasodilators, or anticoagulants, either slightly limit the progression or aid in alleviating the symptoms of the disease.71
Many patients with CLI are candidates for revascularization procedures before being considered for SCS. Approximately 5% of the patients with intermittent claudication develop CLI, and approximately half of those require revascularization.66 Revascularization may be conducted by either endovascular stenting or surgical arterial vascular reconstruction. According to Hirsh and associates,66 the mortality associated with surgical revascularization ranges from 0% to 6%, whereas the mortality for amputation is 4% to 30% during the first 30 days. Furthermore, if patients do undergo amputation, they are at greater risk for other complications, especially with decreased mobility in the elderly population. Thus, if at all possible, revascularization by one technique or another should be considered as the primary mode of treatment for patients with CLI. Not all of them are candidates for revascularization for one reason or another, but they may benefit from SCS implantation.
Basic Science: Mechanism of Action for Peripheral Vascular Disease
PVD develops through a process of slowly narrowing blood vessels, especially in the lower extremities. Eventually decreased vascular patency progresses to distal tissue ischemia and resultant limb pain. This limb pain is likely a combination of nociceptive pain from the ischemic process and neuropathic pain. Neuromodulation has been used successfully to relieve pain from tissue ischemia; but, more important, it promotes enhanced blood flow via vasodilation to the extremities.72,73 Promotion of blood flow was first demonstrated by Cook and associates72 in 1976, who observed ulcer healing in patients with lower extremity PVD and attributed the improvement to increased vascular flow.
As is the case of using neuromodulation to treat patients with RA, the mechanism of action for treating ischemic limb pain has not been fully elucidated. Despite this, two significant plausible mechanisms have been proposed.31 First, stimulation activates sensory fibers via an antidromic mechanism, resulting in a release of vasodilators. Second, neuromodulation decreases sympathetic outflow, resulting in vasodilation of distal arterial vessels.
The Antidromic Mechanism
Bayliss74 initially proposed the antidromic mechanism in 1901 when he observed that high-intensity, dorsal root stimulation caused peripheral vasodilation mediated by thin fibers. His proposal was confirmed by others who have significantly clarified the antidromic mechanism over the past decade.31 Tanaka and colleagues75 investigated antidromic activation of Aδ fibers or C fibers of primary afferent nerves by SCS.75 They used capsaicin to determine if C fibers were responsible for peripheral blood flow changes by SCS. Their results demonstrated that after capsaicin application, before SCS, vasodilation was decreased at higher levels of stimulation (percent of motor threshold [MT]), whereas vasodilation at lower levels (30% and 60% MT) was not affected. This result suggested that capsaicin blocked the unmyelinated fibers and thus prompted a decrease in the amount of calcitonin gene-related peptide (CGRP) to be released. CGRP may be antidromically released by small myelinated and unmyelinated fibers, and it is a potent vasodilator.76,77 The fact that vasodilation still occurred at 30 and 60% MT suggested that small myelinated fibers are also involved in vasodilation.31
Wu and colleagues78 further investigated the antidromic mechanism and demonstrated that transient receptor potential vanilloid-1 (TRPV-1)–containing sensory fibers mediate vasodilation when stimulated via SCS.78 In another study they determined that the SCS antidromically activated TRPV-1–containing sensory fibers, which in turn promoted the release of CGRP, the peptide involved in vasodilation.79 A review by Wu and colleagues31 discusses a possible mechanism for the vasodilation that is initiated by the SCS.CGRP is one of the most potent vasodilators; it targets the CGRP-1 receptor on vascular smooth muscle. In addition, it is proposed that the CGRP also activates nitric oxide (NO) release from endothelial cells, prompting further vasodilation.
The Sympathetic Mechanism
The second proposed mechanism is that SCS induces peripheral vasodilation via stimulation, which inhibits the efferent SNS. The evidence for this mechanism stems from the fact that sympathectomies or sympathetic blocks help with pain relief and vasodilation.28,31,80 On the basis of their understanding that vascular tone is maintained by the SNS via autonomic ganglia, Linderoth, Herregodts, and Meyerson81 evaluated this mechanism by applying various pharmacological agents that affect the SNS in the setting of SCS. Acetylcholine targets postsynaptic nicotinic receptors, which eventually trigger the peripheral adrenergic receptors that lead to vasoconstriction. They demonstrated that hexamethonium, a nonspecific ganglionic-blocking agent, and chlorisondamine, a neuronal nicotinic ganglionic blocker, both eliminated the vasodilation created by SCS. In addition, they pointed out that complete sympathectomy prevents the actions of SCS. However, most sympathectomies performed in humans are subtotal; thus SCS may still be efficacious.31,81
To reconcile these two seemingly independent mechanisms, Tanaka and associates82 examined whether temperature differentials affected the two mechanisms because they noted that Linderoth, Herregodts, and Meyerson81 rats were in a cooler climate, suggesting elevated peripheral sympathetic tone compared to their rats. When Linderoth, Herregodts, and Meyerson81 and Tanaka and colleagues82 conducted a collaborative study in which they examined SCS-caused vasodilation on cooled extremities in the presence of hexamethonium and CGRP (8-37), a CGRP-1 receptor antagonist, the results demonstrated that both the antidromic and sympathetic mechanisms likely are involved and that the antidromic mechanism likely occurs at moderate temperatures (25° to 28° Celsius).82 They further pointed out that the threshold for the sympathetically mediated mechanism is likely higher. According to Wu and associates,31 the mechanism that predominates likely relates to level of sympathetic activity, level of SCS, and the individual’s genetic variability.
In their review Wu and colleagues31 pointed out that other mechanisms have also been proposed (i.e., vasodilator improvement of endothelial function, stimulation of angiogenesis from released substances that would lead to long-term improvement in ischemic tissue, improved blood flow through collaterals to further tissue healing, and potential release of endogenous opioids with SCS).
Guidelines: Patient Selection for Spinal Cord Stimulation in Peripheral Vascular Disease
Although pain relief and limb salvage are the primary goals of SCS in PVD, patients must be selected carefully to maximize the likelihood of success and minimize inappropriate use of the device and health care resources. Patients deemed candidates for this modality should meet several criteria: have a CLI diagnosis (Fontaine stage III or IV) that is currently not evolving rapidly over a period of days or weeks,28 have failed conservative measures, have been considered unsuitable for endovascular or surgical revascularization, or have already failed such interventions. Furthermore, De Vries and associates28 suggest that ulcers or other skin lesions should not exceed 3 cm2 since lesions beyond this size suggest a more advanced stage of disease that typically cannot be reversed with SCS. Patients should also meet macrovascular and microvascular criteria, especially the latter. If patients have a Doppler ankle systolic pressure ≤50 mm Hg or an ankle/brachial index ≤35 mm Hg and TcpO2 between 10 and 35 mm Hg,9 they can be included. note: Patients with TcpO2 ≤10 mm Hg are at greater risk for impending amputation because of significantly decreased microvascular reserve, whereas patients with TcpO2 ≥30 mm Hg are likely to improve without the SCS.83
Exclusion criteria should be considered in the decision as well. Patients not suitable for implantation include those without at-rest pain, gangrene, or ulceration; and those with infection, cancer, a vascular disease other than atherosclerosis, and a psychological or social incompetence, as outlined in Box 11-3.
Box 11-3
Inclusion and Exclusion Criteria Spinal Cord Stimulation for Peripheral Vascular Disease
Inclusion Criteria
From De Vries J et al: Spinal cord stimulation for ischemic heart disease and peripheral vascular disease, Adv Tech Stand Neurosurg 32:80, 2007.
Equipment and Technique for Spinal Cord Stimulation in Peripheral Vascular Disease
SCS for PVD requires epidural placement of the device leads at a level that will enable the patient to feel paresthesias over the same area as their pathology. Usually leads are introduced epidurally at a level two to three below the final resting place of the tip of the lead.28 For example, to cover lower-extremity PVD, placement typically is targeted at the T10 level initially and adjusted from there (see Table 11-3 for the complete listing suggested by Deer). Again, placement should be attempted at a 45-degree angle to minimize the risk of dural puncture with the Tuohy needle and to allow a smooth insertion of the stimulator lead, both for the trial and for the placement of the permanent lead. The patient should be sedated lightly enough to be aware of paresthesias as the lead is advanced. By confirming paresthesias of the affected areas, the patient can aid the physician in determining proper placement of the lead. Once paresthesias are confirmed, the lead may be tunneled laterally to minimize movement and decrease infection risk during the trial period.
| Site of Ischemia | Lead Placement |
|---|---|
| Chest, arm, jaw, shoulder | C7, T1, T2 |
| Upper extremity | C3 to C6 |
| Lower extremity, including foot | T10 to L3 |
| Failure to get foot stimulation with conventional placement | L5 or S1 foramen |
From Deer TR, editor: Spinal cord stimulation for the treatment of angina and peripheral vascular disease, Curr Pain Headache Rep 13(1):19, 2009.
To determine if the technique will be successful, Petrakis and Sciacca70 suggested that a trial time of up 2 weeks be conducted. Their suggestion is based on the finding that limb salvage was achieved in their patients who demonstrated a significant increase in their TcpO2 measured at the foot within 2 weeks of the trial and a 50% increase in TcpO2 after 2 months of implantation.
Outcomes for Spinal Cord Stimulation and Peripheral Vascular Disease
Recently Ubbink and Vermeulen65 reviewed the available literature on SCS for nonreconstructible chronic CLI for the Cochrane Collaboration. They conducted a meta-analysis based on 10 papers encompassing six randomized or controlled trials that met their selection criteria. The investigators picked studies that involved patients of advanced age and CLI secondary to arthrosclerosis that was considered nonreconstructible. The randomized trials picked by Ubbink and Vermeulen65 included those by Suy and associates, Jivegard and associates, Claeys and Horsch, Klomp and associates, and Spincemaille and associates; the controlled trial was by Amann and associates.84–93 Combined, these studies pooled 444 patients between 1994 and 2006. The primary outcome noted in all studies was limb salvage (defined as no major amputation of foot or higher after 12 months).94 Limb amputation leads to significant further morbidity caused by decreased mobility in the elderly population, which translates to an overall increased mortality. Secondary end points reviewed included pain relief, clinical improvement, change in macrocirculation and microcirculation, quality of life, SCS complications (see next section), and costs.
Ubbink and Vermeulen83 focused on limb salvage, the primary end point of the reviewed studies first. They noted that the overall amputation prevalence was 50% no matter what treatment was provided. However, they did note that all the studies that reviewed the SCS groups demonstrated a tendency toward improved limb salvage, especially if the patients were selected based on initial TcpO2. In addition, normotensive patients with SCS implanted had a lower amputation rate.86 They also proposed that, on the basis of their meta-analysis of pooling the results after 12 months, the number needed to treat to prevent one major amputation was nine.83
In terms of the secondary outcome of pain relief, Ubbink and Vermeulen83 noted that data from the selected studies could not be pooled secondary to lack of standard deviations.83 They did point out that with two studies pain was significantly better at 3 and 12 months with the SCS groups than with the medically treated groups by Jivegard and associates86 and Spincemaille and colleagues,91 respectively. In addition, a study by Spincemaille and colleagues90 noted that patients with SCS implantation for CLI used significantly less opioid and nonopioid analgesics, suggesting lower pain levels.
The next outcome examined was clinical improvement. The studies by Suy and associates93 and Claeys and Horsch85 showed clinical stage (measured by use of the Fontaine stages) improvement from CLI to intermittent claudication (p = 0.0014) when compared to those receiving medical treatment alone.65 When Ubbink and Vermeulen65 pooled the data, they showed that the number of patients who needed treatment to convert from rest pain to intermittent claudication was three. Two of the reviewed studies evaluated wound healing; but, when the data were pooled, no significant differences between the groups were determined. In addition, no significant differences were noted between patients with and without diabetes.85
To determine the effects of SCS on circulation, investigators have looked for improved macrocirculation (measured by the ankle/brachial pressure index [ABPI]) and microcirculation. In the Cochrane review SCS-treated patients in Claeys’ study demonstrated improved ABPI of 10% with SCS and a decrease of 17% in the medically treated group (p < 0.02), whereas Jivegard and associates did not demonstrate a difference.85,86 Ubbink and Vermeulen94 reported that they were not able to pool the data for these studies. When evaluating the studies for an improvement in microcirculation (change in TcpO2), no determination of significant improvement in TcpO2 values over medical management could be made. However, when looking at individual studies, significant increases in microcirculation were observed (Claeys: p < 0.001; Ubbink and associates: p < 0.05).85,95
Investigators in each of the studies evaluated quality of life in only a few of the studies. Unfortunately their different methods of evaluation of outcomes prevented overall compilation and analysis of data. When looking at the reviewed studies individually, the study by Amann and colleagues84 demonstrated no overall decrease in quality of life. Spincemaille and colleagues90 demonstrated quality of life significantly improved in patients treated with SCS (p < 0.01). In addition, some of the study groups evaluated only the SCS arm and not the conservatively treated one. Spincemaille and colleagues92 used the Nottingham Health Profile, which demonstrated improved quality of life in both the conservative and SCS groups, but the mobility score was significantly improved in the SCS group (p < 0.01).
Only one study reviewed,90 done in Europe, compared costs between the two groups at 2 years. The SCS group cost substantially more than the conservative group (p < 0.009), even when adjusted for mortality (p < 0.002).
Complications for Spinal Cord Stimulation and Peripheral Vascular Disease
In Ubbink and Vermeulen’s review,94 they noted no differences between the mortality of medically managed and SCS groups. SCS implantation complications in patients with PVD included implantation difficulties (inability to place lead) approximately 8% of the time, increasing to 12% in multicenter trials. This level of difficulty suggests the need for better-trained specialists in this technique. Other complications included battery failure, infection of lead or generator pocket, and lead fracture—all of which may potentially occur with any patient undergoing an SCS implantation.
1 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46(4):489-491.
2 Lloyd-Jones D, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46-e215.
3 Mukherjee D, et al. Direct myocardial revascularization and angiogenesis—how many patients might be eligible? Am J Cardiol. 1999;84(5):598-600.
4 Mannheimer C, Camici P, Chester MR, et al. The problem of chronic refractory angina: report from the ESC joint study group on the treatment of refractory angina. European Heart Journal. 2002;23:355-370.
5 Holmes DRJr. Treatment options for angina pectoris and the future role of enhanced external counterpulsation. Clin Cardiol. 2002;25(12sSuppl 2):II22-25.
6 Vallejo R, et al. Spinal neuromodulation: a novel approach in the management of peripheral vascular disease. Tech Regional Anesthes Pain Management. 2006;10:3-6.
7 Second European Consensus Document on chronic critical leg ischemia. Eur J Vasc Surg. 1992;6(suppl A):1-32.
8 Gibbons RJ, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol. 1999;33(7):2092-2197.
9 Buchser E, Durrer A, Albrecht E. Spinal cord stimulation for the management of refractory angina pectoris. J Pain Symptom Manage. 2006;31(4 Suppl):S36-S42.
10 Erdek MA, Staats PS. Spinal cord stimulation for angina pectoris and peripheral vascular disease. Anesthesiol Clin North Am. 2003;21(4):797-804.
11 Sestito A, et al. Spinal cord stimulation normalizes abnormal cortical pain processing in patients with cardiac syndrome X. Pain. 2008;139(1):82-89.
12 Panting JR, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346(25):1948-1953.
13 Latif OA, Raj PP. Spinal cord stimulation: a comparison of efficacy versus other novel treatments for refractory angina pectoris. Pain Pract. 2001;1(1):36-45.
14 Campeau L. Letter: Grading of angina pectoris. Circulation. 1976;54(3):522-523.
15 Management of stable angina pectoris: recommendations of the Task Force of the European Society of Cardiology. Eur Heart J. 1997;18(3):394-413.
16 Eckert S, Horstkotte D. Management of angina pectoris: the role of spinal cord stimulation. Am J Cardiovasc Drugs. 2009;9(1):17-28.
17 Bolser DC, et al. Effects of intracardiac bradykinin and capsaicin on spinal and spinoreticular neurons. Am J Physiol. 1989;257(5 Pt 2):H1543-H1550.
18 Selzer M, Spencer WA. Interactions between visceral and cutaneous afferents in the spinal cord: reciprocal primary afferent fiber depolarization. Brain Res. 1969;14(2):349-366.
19 Jolicoeur EM, et al. Clinical and research issues regarding chronic advanced coronary artery disease: part I: Contemporary and emerging therapies. Am Heart J. 2008;155(3):418-434.
20 Mannheimer C, et al. The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J. 2002;23(5):355-370.
21 Chaitman BR et al: Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 43(8):1375-1382, 1004.
22 Chaitman BR, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291(3):309-316.
23 Abbott JD, et al. Recent trends in the percutaneous treatment of chronic total coronary occlusions. Am J Cardiol. 2006;97(12):1691-1696.
24 Olivari Z, et al. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). J Am Coll Cardiol. 2003;41(10):1672-1678.
25 Suero JA, et al. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol. 2001;38(2):409-414.
26 Sinvhal RM, Gowda RM, Khan IA. Enhanced external counterpulsation for refractory angina pectoris. Heart. 2003;89(8):830-833.
27 Soran O, et al. Two-year clinical outcomes after enhanced external counterpulsation (EECP) therapy in patients with refractory angina pectoris and left ventricular dysfunction (report from The International EECP Patient Registry). Am J Cardiol. 2006;97(1):17-20.
28 De Vries J, et al. Spinal cord stimulation for ischemic heart disease and peripheral vascular disease. Adv Tech Stand Neurosurg. 2007;32:63-89.
29 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971-979.
30 Chandler MJ, et al. A mechanism of cardiac pain suppression by spinal cord stimulation: implications for patients with angina pectoris. Eur Heart J. 1993;14(1):96-105.
31 Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138(1-2):9-23.
32 Cui JG, et al. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73(1):87-95.
33 Oldroyd KG, et al. Beta endorphin release in patients after spontaneous and provoked acute myocardial ischaemia. Br Heart J. 1992;67(3):230-235.
34 Eliasson T, et al. Myocardial turnover of endogenous opioids and calcitonin-gene-related peptide in the human heart and the effects of spinal cord stimulation on pacing-induced angina pectoris. Cardiology. 1998;89(3):170-177.
35 Hautvast RW, et al. Relative changes in regional cerebral blood flow during spinal cord stimulation in patients with refractory angina pectoris. Eur J Neurosci. 1997;9(6):1178-1183.
36 Zonenshayn M, Mogilner AY, Rezai AR. Neurostimulation and functional brain imaging. Neurol Res. 2000;22(3):318-325.
37 Mobilia G, et al. Effects of spinal cord stimulation on regional myocardial blood flow in patients with refractory angina: a positron emission tomography study. G Ital Cardiol. 1998;28(10):1113-1119.
38 Norrsell H, et al. Effects of spinal cord stimulation on coronary blood flow velocity. Coron Artery Dis. 1998;9(5):273-278.
39 Kingma JGJr, et al. Neuromodulation therapy does not influence blood flow distribution or left-ventricular dynamics during acute myocardial ischemia. Auton Neurosci. 2001;91(1-2):47-54.
40 Armour JA. Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res. 1999;41(1):41-54.
41 Lathrop DA, Spooner PM. On the neural connection. J Cardiovasc Electrophysiol. 2001;12(7):841-844.
42 Hautvast RW, et al. Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol. 1996;77(7):462-467.
43 Ardell JL. Intrathoracic neuronal regulation of cardiac function. In: Armour JA, Ardell JL, editors. Basic and clinical neurocardiology. Oxford University Press; 2004:118-152.
44 Cardinal R, et al. Spinal cord stimulation suppresses bradycardias and atrial tachyarrhythmias induced by mediastinal nerve stimulation in dogs. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1369-1375.
45 Mannheimer C, et al. Effects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. Br Med J. 1993;307(6902):477-480.
46 Eliasson T, Augustinsson LE, Mannheimer C. Spinal cord stimulation in severe angina pectoris–presentation of current studies, indications and clinical e experience. Pain. 1996;65(2-3):169-179.
47 Mannheimer C, et al. The effects of transcutaneous electrical nerve stimulation in patients with severe angina pectoris. Circulation. 1985;71(2):308-316.
48 Foreman RD, et al. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res. 2000;47(2):367-375.
49 Murray S, et al. Spinal cord stimulation significantly decreases the need for acute hospital admission for chest pain in patients with refractory angina pectoris. Heart. 1999;82(1):89-92.
50 Ganz P, Braunwald E. Coronary blood flow and myocardial ischemia. In: Braunwald E, editor. Heart Disease. Philadelphia: Saunders; 1997:1168-1174.
51 Deer TR. Spinal cord stimulation for the treatment of angina and peripheral vascular disease. Curr Pain Headache Rep. 2009;13(1):18-23.
52 Hautvast RW, et al. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study. Am Heart J. 1998;136(6):1114-1120.
53 Mannheimer C, et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris: the ESBY study. Circulation. 1998;97(12):1157-1163.
54 Taylor RS, et al. Spinal cord stimulation in the treatment of refractory angina: systematic review and meta-analysis of randomised controlled trials. BMC Cardiovasc Disord. 2009;9:13.
55 McNab D, et al. An open label, single-centre, randomized trial of spinal cord stimulation vs. percutaneous myocardial laser revascularization in patients with refractory angina pectoris: the SPiRiT trial. Eur Heart J. 2006;27(9):1048-1053.
56 de Jongste MJ, et al. Stimulation characteristics, complications, and efficacy of spinal cord stimulation systems in patients with refractory angina: a prospective feasibility study. Pacing Clin Electrophysiol. 1994;17(11 Pt 1):1751-1760.
57 Di Pede F, et al. Long-term effects of spinal cord stimulation on myocardial ischemia and heart rate variability: results of a 48-hour ambulatory electrocardiographic monitoring. Ital Heart J. 2001;2(9):690-695.
58 Eddicks S, et al. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomised study. Heart. 2007;93(5):585-590.
59 Jessurun GA, et al. Clinical follow-up after cessation of chronic electrical neuromodulation in patients with severe coronary artery disease: a prospective randomized controlled study on putative involvement of sympathetic activity. Pacing Clin Electrophysiol. 1999;22(10):1432-1439.
60 Gibbons RJ, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina–summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). J Am Coll Cardiol. 2003;41(1):159-168.
61 Simpson EL, et al. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess. 2009;13(17):1-154. iii, ix-x
62 Rasmussen MB, et al. Cost-benefit analysis of electric stimulation of the spinal cord in the treatment of angina pectoris. Ugeskr Laeger. 1992;154(17):1180-1184.
63 Yu W, et al. Spinal cord stimulation for refractory angina pectoris: a retrospective analysis of efficacy and cost-benefit. Coron Artery Dis. 2004;15(1):31-37.
64 TenVaarwerk IA, et al. Clinical outcome of patients treated with spinal cord stimulation for therapeutically refractory angina pectoris: the working group on neurocardiology. Heart. 1999;82(1):82-88.
65 Ubbink DT, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2009;1:1-35.
66 Hirsch AT, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463-e654.
67 Augustinsson LE, Linderoth B, Mannheimer C, Eliasson T. Spinal cord stimulation in cardiovascular disease. Functional Neurosurgery. 1995;6(1):157-165.
68 Jacobs MJ, Jorning PJ. Is epidural spinal cord electrical stimulation indicated in patients with severe lower limb ischaemia? Eur J Vasc Surg. 1988;2(4):207-208.
69 Ubbink DT, et al. Prediction of imminent amputation in patients with non-reconstructible leg ischemia by means of microcirculatory investigations. J Vasc Surg. 1999;30(1):114-121.
70 Petrakis IE, Sciacca V. Epidural spinal cord electrical stimulation in diabetic critical lower limb ischemia. J Diabetes Complications. 1999;13(5-6):293-299.
71 Ubbink DT, et al. Systematic review and meta-analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischaemia. Br J Surg. 2004;91(8):948-955.
72 Cook AW, et al. Vascular disease of extremities. Electric stimulation of spinal cord and posterior roots. NY State J Med. 1976;76(3):366-368.
73 Dooley DM, Kasprak M. Modification of blood flow to the extremities by electrical stimulation of the nervous system. South Med J. 1976;69(10):1309-1311.
74 Bayliss WM. On the origin from the spinal cord of the vasodilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901;26(3-4):173-209.
75 Tanaka S, et al. Role of primary afferents in spinal cord stimulation-induced vasodilation: characterization of fiber types. Brain Res. 2003;959(2):191-198.
76 Croom JE, et al. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am J Physiol. 1997;272(2 Pt 2):H950-957.
77 Tanaka S, et al. Low intensity spinal cord stimulation may induce cutaneous vasodilation via CGRP release. Brain Res. 2001;896(1-2):183-187.
78 Wu M, et al. Sensory fibers containing vanilloid receptor-1 (VR-1) mediate spinal cord stimulation-induced vasodilation. Brain Res. 2006;1107(1):177-184.
79 Wu M, et al. Roles of peripheral terminals of transient receptor potential vanilloid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res. 2007;1156:80-92.
80 Linderoth B, Gunasekera L, Meyerson BA. Effects of sympathectomy on skin and muscle microcirculation during dorsal column stimulation: animal studies. Neurosurgery. 1991;29(6):874-879.
81 Linderoth B, Herregodts P, Meyerson BA. Sympathetic mediation of peripheral vasodilation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery. 1994;35(4):711-719.
82 Tanaka S, et al. Local cooling alters neural mechanisms producing changes in peripheral blood flow by spinal cord stimulation. Auton Neurosci. 2003;104(2):117-127.
83 Ubbink DT, Vermeulen H. Spinal cord stimulation for critical leg ischemia: a review of effectiveness and optimal patient selection. J Pain Symptom Manage. 2006;31(4 Suppl):S30-S35.
84 Amann W, et al. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur J Vasc Endovasc Surg. 2003;26(3):280-286.
85 Claeys LG, Horsch S. Transcutaneous oxygen pressure as predictive parameter for ulcer healing in endstage vascular patients treated with spinal cord stimulation. Int Angiol. 1996;15(4):344-349.
86 Jivegard LE, et al. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischaemia: a prospective randomised controlled study. Eur J Vasc Endovasc Surg. 1995;9(4):421-425.
87 Klomp HM, et al. Design issues of a randomised controlled clinical trial on spinal cord stimulation in critical limb ischaemia: ESES Study Group. Eur J Vasc Endovasc Surg. 1995;10(4):478-485.
88 Klomp HM, et al. Spinal-cord stimulation in critical limb ischaemia: a randomised trial: ESES Study Group. Lancet. 1999;353(9158):1040-1044.
89 Klomp HM, et al. Spinal cord stimulation is not cost-effective for non-surgical management of critical limb ischaemia. Eur J Vasc Endovasc Surg. 2006;31(5):500-508.
90 Spincemaille GH, et al. Pain and quality of life in patients with critical limb ischaemia: results of a randomized controlled multicentre study on the effect of spinal cord stimulation: ESES Study Group. Eur J Pain. 2000;4(2):173-184.
91 Spincemaille GH, et al. Spinal cord stimulation in patients with critical leg ischemia: a preliminary evaluation of a multicenter trial. Acta Chirurgica Austriaca. 2000;32:49-51.
92 Spincemaille GH, et al. Technical data and complications of spinal cord stimulation: data from a randomized trial on critical limb ischemia. Stereotact Funct Neurosurg. 2000;74(2):63-72.
93 Suy R, et al. Spinal cord stimulation for ischemic rest pain. The Belgian randomized study. Steinhof: Darmstadt; 1994.
94 Ubbink DT, Vermeulen H: Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev (3):CD004001, 2005.
95 Ubbink DT, et al. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the Dutch multicenter randomized controlled trial. J Vasc Surg. 1999;30(2):236-244.