Chapter 10 Spinal Cord Stimulation for Peripheral Vascular Disease
 SCS improves pain, microcirculation, limb survival, and clinical stage compared to conservative treatment.
SCS improves pain, microcirculation, limb survival, and clinical stage compared to conservative treatment. When selecting patients, the improvement in the transcutaneous partial pressure of oxygen (TcpO2) and pain relief during trial stimulation is a predictor of outcome.
When selecting patients, the improvement in the transcutaneous partial pressure of oxygen (TcpO2) and pain relief during trial stimulation is a predictor of outcome. Increase of TcpO2 of 10 mm Hg (minimum), preferably >15 mmHg, is a prognostic indicator for both limb salvage and pain relief; however, there is no linear correlation.
Increase of TcpO2 of 10 mm Hg (minimum), preferably >15 mmHg, is a prognostic indicator for both limb salvage and pain relief; however, there is no linear correlation. If the regional perfusion index (RPI) improves 0.2 or more from the baseline and is sustained, the limb salvage rate could reach up to 90%.
If the regional perfusion index (RPI) improves 0.2 or more from the baseline and is sustained, the limb salvage rate could reach up to 90%. If SCS is implanted in cases where trial stimulation produces less than 80% coverage of the area of pain, results will be less than satisfactory.
If SCS is implanted in cases where trial stimulation produces less than 80% coverage of the area of pain, results will be less than satisfactory.Establishing Diagnosis
Peripheral vascular disease (PVD) results from progressive atherosclerosis of the arteries of the lower extremities. The presence of PVD signals an increased likelihood of disease in other regions of the body, resulting in cardiovascular and cerebrovascular morbidity and mortality. PVD affects 10% to15% of the U.S. population; the prevalence increases with advancing age.1 Risk factors for PVD include smoking, diabetes, hypertension, dyslipidemia, age greater than 40 years, being of African origin, and previous cardiac and cerebrovascular disease.
 The individual with neurogenic claudication who walks for a long time is able to walk shorter and shorter distances at the expense of longer and longer periods of rest. This is in contrast to the patient with vascular claudication, who can walk the same distance with equal rest periods in between.
The individual with neurogenic claudication who walks for a long time is able to walk shorter and shorter distances at the expense of longer and longer periods of rest. This is in contrast to the patient with vascular claudication, who can walk the same distance with equal rest periods in between.The severity of the symptoms in vascular claudication is governed by the amount of stenosis, presence of collateral circulation, and vigor of exercise. The classification systems used to stage severity of disease are the Fontaine and the Rutherford systems, with the Fontaine system used more widely (Table 10-1). Stenoses in the arterial tree secondary to atherothrombosis are responsible for the underlying pathophysiology of this disease. As the disease advances, the resistance in the vascular system increases, and the system is unable to provide oxygenation to the muscles, especially during exercise. This results in claudication, ischemia, ulceration, and eventual loss of limb if left untreated.2 Skeletal muscle ischemia affects muscle metabolism with the accumulation of lactate and intermediates of oxidative metabolism (acylcarnitines). This in turn causes muscle deterioration, denervation, and atrophy.3 Chronic ischemia leads to ulceration and gangrene. Pain resulting from ulcers and gangrene is caused by ischemic neuropathy and necrosis of the sensory nerves at the site of the lesion. Severe necrosis of the sensory nerves can paradoxically make gangrenous lesions insensate and anesthetic.
| Stage | Clinical |
|---|---|
| I | Asymptomatic |
| IIa | Mild claudication |
| IIb | Moderate-severe claudication |
| III | Ischemic rest pain |
| IV | Ulceration or gangrene |
Investigations
In the initial stages of PVD clinical history and physical examination are generally unreliable, with the diagnosis being missed more than 90% of the time based on these two factors alone. To make an early diagnosis of PVD, measurement of the ankle-brachial index (ABI) is helpful. The ABI is a ratio of the systolic blood pressure in the ipsilateral dorsalis pedis and posterior tibial arteries to that in the brachial artery (higher of bilateral brachial pressures), measured with a handheld continuous wave Doppler in the supine position. Normal ABI ranges between 1.0 and 1.3. An ABI <0.9 is 95% sensitive and 100% specific for PVD. Angiograms completed in these patients show more than 50% stenosis in one or more major blood vessels.4 The lower the ABI score, the more severe the PVD, with ABI <0.4 representing advanced ischemia. Segmental limb pressure, segmental volume plethysmography, duplex ultrasonography, computed tomography (CT) angiography, and magnetic resonance (MR) angiography are additional modalities for evaluation of the level and extent of disease (Fig. 10-1).
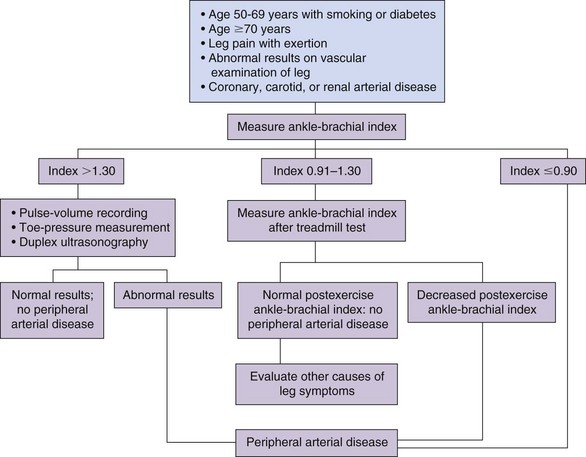
Fig. 10-1 Evaluation of patients with suspected peripheral vascular disease.
Adapted from Hiatt WR et al.: Medical treatment of peripheral arterial disease and claudication, N Engl J Med 344:1608, 2001.
The goals of treatment for patients with PVD are to relieve claudication, improve walking capacity, and improve quality of life. Initial management of PVD includes modification of risk factors such as smoking cessation, glycemic control, blood pressure normalization, and dyslipidemia management. Exercise rehabilitation has also been shown to be beneficial in reducing the symptoms of intermittent claudication by improving collateral circulation and thus improving functional status. Medical treatment of PVD includes antiplatelet and vasodilatory drugs. Revascularization is indicated when ischemic pain is severe, indicated by disabling claudication that prevents the patient from performing daily activities of living, rest pain, ischemic ulcers, or gangrene. Amputation is indicated in approximately 5% of patients with nonreconstructible critical limb ischemia and extensive tissue necrosis or life-threatening infection.5
Spinal cord stimulation (SCS) was first shown to be effective in relieving claudication symptoms and increasing blood flow to lower extremities by Cook and associates6 in 1976, who were using SCS to treat limb pain in patients with multiple sclerosis. During the late 1980s to early 1990s, the use of SCS as an alternative treatment measure for PVD rapidly advanced, especially in Europe. Currently SCS is the most promising neuromodulatory treatment for ischemic pain, and the overall beneficial effects last for at least 1 year in 80% of patients and for up to 5 years in 60% of patients.7 The beneficial effects of SCS in the treatment of ischemic pain include pain relief, ulcer healing, decreased oxygen requirement, and increased claudication distance.
Basic Science
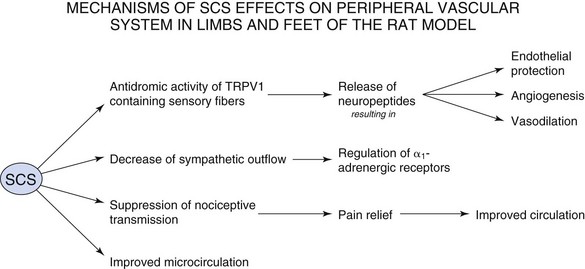
There are no established animal models with PVD that give rise to ischemic pain. Therefore normal Sprague-Dawley rats are used for research into the mechanisms of action of SCS for PVD. These rats are used to study acute changes in peripheral blood flow during SCS. SCS intensity in the rat is determined by the motor threshold (MT), which is the stimulation required for muscle contraction to be observed. Experimental SCS is performed at 30%, 60%, 90%, and 300% of MT. Stimulation at 30% MT is the minimum stimulus that produces vasodilation, with 60% MT being the level that approximates the stimulation parameters in clinical applications in humans. SCS at the upper lumbar spinal segments such as L2-L3 produces the largest increase in cutaneous blood flow in the lower limbs in the rat.8 There are several proposed theories as to the mechanism of action of SCS in the treatment of PVD:
 With regard to pain control, the action of SCS falls back on the Wall-Melzack gate control theory of pain, which proposes that stimulation of large diameter afferent fibers such as those in the dorsal columns of the spinal cord would close notional gates in lamina V of the dorsal horn. This would prevent the ascent of impulses that mediate pain and that originate in small-diameter afferents from ascending to higher levels.9 Relief from pain decreases the vasoconstriction that occurs as a reflex response.
With regard to pain control, the action of SCS falls back on the Wall-Melzack gate control theory of pain, which proposes that stimulation of large diameter afferent fibers such as those in the dorsal columns of the spinal cord would close notional gates in lamina V of the dorsal horn. This would prevent the ascent of impulses that mediate pain and that originate in small-diameter afferents from ascending to higher levels.9 Relief from pain decreases the vasoconstriction that occurs as a reflex response. SCS-induced vasodilation occurs via suppression of sympathetic activity. The sympathetic nervous system causes vasoconstriction via stimulation of α1– and α2-adrenoreceptors. Linderoth, Herregodts, and Meyerson10 observed that cutaneous vasodilation after SCS in the rat hind paw was eliminated by complete surgical sympathectomy. Administration of ganglionic blocker, hexamethonium, or neuronal nicotinic ganglionic blocker, chlorisondamine, had the same effect. High-dose adrenergic receptor blockers phentolamine and prazosin also suppressed SCS-induced vasodilation. Inhibition of vasodilation was not observed after administration of muscarinic receptor antagonists. However, there are conflicting data with the sympathetic mechanism because some patients demonstrate vasodilation with SCS even after chemical or surgical sympathectomy11 and some incompletely sympathectomized rats in Linderoth, Herregodts, and Meyerson’s study still retained the effects of SCS.
SCS-induced vasodilation occurs via suppression of sympathetic activity. The sympathetic nervous system causes vasoconstriction via stimulation of α1– and α2-adrenoreceptors. Linderoth, Herregodts, and Meyerson10 observed that cutaneous vasodilation after SCS in the rat hind paw was eliminated by complete surgical sympathectomy. Administration of ganglionic blocker, hexamethonium, or neuronal nicotinic ganglionic blocker, chlorisondamine, had the same effect. High-dose adrenergic receptor blockers phentolamine and prazosin also suppressed SCS-induced vasodilation. Inhibition of vasodilation was not observed after administration of muscarinic receptor antagonists. However, there are conflicting data with the sympathetic mechanism because some patients demonstrate vasodilation with SCS even after chemical or surgical sympathectomy11 and some incompletely sympathectomized rats in Linderoth, Herregodts, and Meyerson’s study still retained the effects of SCS. The antidromic mechanism was first proposed by Bayliss in 1901,12 who noted that dorsal root stimulation at high intensity induced peripheral vasodilation mediated by thin fibers. SCS antidromically activates afferent fibers in dorsal roots, causing the peripheral release of calcitonin gene-related peptide (CGRP), a powerful microvascular vasodilator. Since this time there has been intensive research into the types of fibers that mediate vasodilation and the vasodilators that are released during SCS. CGRP is found in small, myelinated Aδ fibers and unmyelinated C fibers in dorsal root ganglia. By stimulating at various thresholds and blocking C-fiber conduction with the application of capsaicin, Tanaka and colleagues13 determined that SCS-induced vasodilation at ≤60% MT is mediated by antidromic activation of myelinated fibers, whereas vasodilation at ≥90% is mediated by both myelinated and unmyelinated C-fibers.13 Wu and associates14 further characterized that SCS-induced vasodilation is predominantly mediated by those sensory fibers that contain transient receptor potential vanilloid-1 (TRPV1).14
The antidromic mechanism was first proposed by Bayliss in 1901,12 who noted that dorsal root stimulation at high intensity induced peripheral vasodilation mediated by thin fibers. SCS antidromically activates afferent fibers in dorsal roots, causing the peripheral release of calcitonin gene-related peptide (CGRP), a powerful microvascular vasodilator. Since this time there has been intensive research into the types of fibers that mediate vasodilation and the vasodilators that are released during SCS. CGRP is found in small, myelinated Aδ fibers and unmyelinated C fibers in dorsal root ganglia. By stimulating at various thresholds and blocking C-fiber conduction with the application of capsaicin, Tanaka and colleagues13 determined that SCS-induced vasodilation at ≤60% MT is mediated by antidromic activation of myelinated fibers, whereas vasodilation at ≥90% is mediated by both myelinated and unmyelinated C-fibers.13 Wu and associates14 further characterized that SCS-induced vasodilation is predominantly mediated by those sensory fibers that contain transient receptor potential vanilloid-1 (TRPV1).14Several vasodilators, including CGRP, are contained within the terminals of TRPV1 sensory nerve endings. Antidromic activation and depolarization of these nerve endings cause release of vasodilators into muscle tissue. CGRP is a potent vasodilator that is tenfold more powerful than prostaglandins and 100 to 1000 times more effective than other typical vasodilators such as acetylcholine, adenosine, and substance P.15 CGRP binds to CGRP-1 receptor of smooth muscle cells and causes direct relaxation or can bind to CGRP-1 receptor of endothelial cells, which causes release of nitric oxide, which leads to vasodilation. Adrenomedullin is a peptide that co-localizes with CGRP in perivascular nerves and dorsal root ganglia and is involved with angiogenesis and endothelial protection.8
Despite the intense research into the mechanisms of action of SCS for the treatment of PVD, the theories are incompletely understood, and there is much more to explore. It is very likely that reduction in pain transmission, the sympathetic theory, and the antidromic theory act in concert to provide pain relief and vasodilation in patients suffering from PVD. The relative weight of one mechanism over another may depend on the patient’s personal set of risk factors, sympathetic activity, and stimulation parameters. The mechanisms of SCS on the peripheral vascular system are summarized in Fig. 10-2.
Indications and Contraindications
General criteria for consideration of a spinal cord stimulator include16:
 Absence of unresolved issues of secondary gain or litigation that potentially could be central to the propagation of the pain complaint.
Absence of unresolved issues of secondary gain or litigation that potentially could be central to the propagation of the pain complaint.SCS is not indicated in PVD for patients who have17:
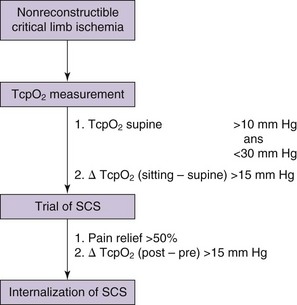
Based on a critical review of available literature on the topic of SCS for PVD, Spincemaille and associates18 have proposed an algorithm for successful patient selection. This has been adapted and modified (Fig. 10-3). The patient selection algorithm has not been tested by a randomized controlled trial.
Technique
 Cylindrical electrodes with multiple contact points can be introduced in the epidural space percutaneously using a Tuohy type needle. The commonly used percutaneous leads have four or eight contact points.
Cylindrical electrodes with multiple contact points can be introduced in the epidural space percutaneously using a Tuohy type needle. The commonly used percutaneous leads have four or eight contact points. Because of their larger size, surgical or paddle electrodes need a small laminotomy for the introduction into the epidural space. These electrodes also can have multiple contact points in various types of configurations. These electrodes can have 4, 8, or 16 contact points (Fig. 10-4).
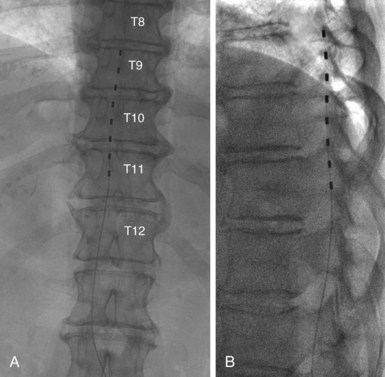
Because of their larger size, surgical or paddle electrodes need a small laminotomy for the introduction into the epidural space. These electrodes also can have multiple contact points in various types of configurations. These electrodes can have 4, 8, or 16 contact points (Fig. 10-4).Positioning of the lead must be performed under fluoroscopic control with lead placement either in the midline if the pain is bilateral, or slightly off of midline to the symptomatic side (Fig. 10-5). Intraoperative stimulation is then carried out. The tip of the electrode position should be such that stimulation induces paresthesias. For best results the stimulation-induced paresthesias should cover the entire territory of the pain. Results may be less than satisfactory if stimulation covers <80% of pain territory. The Tuohy needle and the stylet of the electrode are removed, and the lead is then fixed into the deep fascia. For this purpose an anchoring device is used. The lead is then externalized, and a trial of stimulation is initiated. The trial period in North America usually lasts approximately 1 week. In certain European countries (e.g., Belgium) by law the trial must last for 4 weeks.
There is controversy in the literature whether or not a trial of stimulation is beneficial. An analysis of multiple pathologies treated with SCS showed that approximately 18% to 20% of patients fail trial stimulation in spite of the best efforts in selecting candidates for SCS therapy.16 This emphasizes the importance of screening with trial stimulation before permanent implantation. This approach reduces the rate of failed permanent implants and improves cost-effectiveness. The trial stimulation also allows for a period of patient adjustment to stimulation-induced paresthesia and counseling by the neurosurgical team. The main disadvantages of this process are that it is an added procedure with associated costs and minimal risks such as root irritation, hematoma, or infection, which may add to the hospital stay.
Effective stimulation parameters vary with each patient and can range from amplitudes between 1.5 and 6 V, frequencies of 55 to 60 Hz, and pulse widths of 210 to 300 ms. Stimulation can be performed continuously or with cyclical use to preserve the life of the pulse generator battery. Kumar and colleagues19 found that optimal results were achieved with a cycling mode of 1 minute on, 2 minutes off.19 With the advent of rechargeable pulse generators, this issue has been resolved. The new pulse generators also allow for multiple programming.
Patient Management and Evaluation
A review of the literature fails to provide an adequate explanation or guidance in treatment of tolerance. Stimulation “holidays” of up to 6 weeks have met with minimal success, and addition of adjuvant medications, such as amitriptyline or L-tryptophan, has also been met with equally poor results.16
Outcome Evidence
Pain
Several studies show a marked decrease in pain symptoms following SCS (Table 10-2). In two separate randomized controlled trials by Spincemaille and associates20 and Jivegard and colleagues,21 pain relief is significantly better in the SCS group compared to nontreatment groups at 3 and 12 months. A critical review of the European literature showed that 70% to 80% of patients achieve >75% of pain relief, which is lost with lead displacement, fracture, or depletion of the pulse generator battery, indicating that pain relief is unlikely a placebo effect.22
Macrocirculation
Kumar and associates19 studied improvement in macrocirculation by measuring blood flow velocities, pulse volume recordings, claudication distance, and ABI. Blood flow velocities at the common femoral artery were most valid in patients with PVD who had undergone previous vascular surgery. In patient in whom SCS was a success, the blood flow velocity increased on average by 40.4 cm/second. The increase in blood flow velocity was directly proportional to increases in TcpO2.
Microcirculation
Since increased circulation is one of the most desirable and beneficial effects of SCS for the disease process, investigators measure improvement in microcirculation using TcpO2. In a randomized controlled trial, Claeys and Horsch23 compared SCS with optimal medical treatment (OMT) with prostaglandin. There was no significant difference between the initial mean value of TcpO2 between SCS and OMT (10 vs. 11 mm Hg). At 12 months TcpO2 was 21 vs. 11.4 mm Hg (p < 0.0001) between the SCS and OMT, respectively. An increase in TcpO2 greater than 10 mm Hg was a predictor of long-term success. Table 10-3 summarizes the results of several studies on the measurement of TcpO2.
| Prospective Studies | ||
| Author | Mean Baseline TcpO2 at Rest—Supine (mm Hg) | Mean Change in TcpO2 at Last Follow-up (mm Hg) |
| Horsch | 16 | >12 |
| Gersbach | 19.5 | >16 |
| Kumar | >30 | 7.2 |
| <30 | 45.5 | |
| Amann | 36 | 18.4 |
| Randomized-Controlled Trials | ||
| Author | Mean Baseline TcPO2 at Rest —Supine (mm Hg) | Percent Change in TcpO2 at Last Follow-up |
| Claeys | ||
| SCS | 10 | +213% |
| Control | 11 | −2% |
| Ubbink | ||
| SCS | 10 | +70% |
| Control | 9 | 0 |
Limb Salvage
A meta-analysis on SCS for PVD by Ubbink and colleagues24 revealed significant effects on limb salvage. Pooled results of all trials showed favorable results for SCS after 12 months. The calculated number needed to treat to prevent one major amputation was eight (CI95% 5 to 25). Proper selection and screening of patients is helpful in predicting successful outcomes with respect to limb salvage. In the study by Gersbach and associates,25 patients who had excellent pain relief and a change in TcpO2 >15 mm Hg after 1 week of trial stimulation had an 83% rate of limb salvage vs. 63% for the overall patient population. Amann and associates26 also found that SCS treatment of nonreconstructible critical leg ischemia provides a significantly better limb survival rate compared with conservative treatment. Patient selected based on TcpO2 and the results of trial screening further increased the probability of limb survival after SCS therapy. Individual data of several studies with respect to limb salvage is presented in Table 10-4.
| Author | Limb Survival (%) |
|---|---|
| Horsch | 66 |
| Gersbach | 63 |
| Ubbink | 60 |
| Jivegard | |
| SCS | 62 |
| Control | 45 |
| Klomp | |
| SCS | 55 |
| Control | 46 |
| Amann | |
| SCS | 78 |
| Control | 45 |
Clinical Improvement
Using the Fontaine classification, SCS has been shown to improve a patient’s clinical status. A number needed to treat analysis performed by Ubbink and colleagues24 showed that one needs to treat three patients for one patient to improve to Fontaine stage II from Fontaine stage IV (CI95% 2 to 5).
The most recent Cochrane review of 2009 analyzed six studies comprising nearly 450 patients.27 The study concluded that limb salvage after 12 months was significantly higher in the SCS group (CI95% 0.56 to 0.90). Pain relief was more impressive in the SCS group, and patients required significantly fewer analgesics. More patients reached Fontaine stage II in the SCS group than in the conservative group (CI95% 2.0 to 11.9). Two studies reported on the healing of ischemic ulcers.23,28 Claeys23 found that SCS had a significantly better effect on wound healing than conservative treatment (p = 0.013). However, Klomp and Cochrane28 pooled data showed no significant difference on ulcer healing between the two treatment groups.
Spinal Cord Stimulation for Nonatherosclerotic Peripheral Vascular Disease
SCS also has beneficial effects in the treatment of nonatherosclerotic PVD such as Buerger disease or Raynaud phenomenon. Unfortunately there are only a few published studies on this topic, and the studies do not have large samples of patients. In a prospective study by Donas and colleagues,29 patients with Buerger disease received SCS treatment. Microcirculation was measured using the regional perfusion index (RPI), which is the ratio between the foot and chest transcutaneous oxygen pressure. Baseline RPI was recorded to be 0.27, which improved to 0.41 at 3 months, and was sustained at 1 and 3 years at 0.49 and 0.52, respectively. Limb survival rate was 93.1%. Claudication symptoms were also improved, and healing of trophic lesions was noted. Thus SCS can also be considered as an alternative treatment for patients with vascular disorders of nonatherosclerotic origin.
Risk and Complication Avoidance
Complications with SCS are generally related to the hardware and are similar, regardless of the indication for treatment. In a retrospective analysis of 410 patients over a period of 22 years, Kumar, Hunter, and Demeria16 found the following types and incidence of complications: displaced electrode (21.5%), fractured electrode (5.9%), other hardware malfunction (8.1%), subcutaneous hematomas (4.4%), infection (3.4%), cerebrospinal fluid leak (0.5%), rotation of the pulse generator (0.7%), and discomfort at the pulse generator site (1.2%). The most common fracture site was distal to the point of anchor where the lead exits from the deep fascia; a bend or kink is created at this point, increasing the stress on the lead. In cases of lead migration, the need for surgical revision is decreasing because of the use of octipolar leads and multichannel pulse generators.
1 Aslam F, et al. Peripheral arterial disease: current perspectives and new trends in management. South Med J. 2009;102:1141-1149.
2 Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257-1264.
3 Levy PJ. Epidemiology and pathophysiology of peripheral arterial disease. Clin Cornerstone. 2002;4:1-15.
4 Belch JJ, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163:884-892.
5 Imparato AM, et al. Intermittent claudication: its natural course. Surgery. 1975;78:795-799.
6 Cook AW, et al. Vascular disease of extremities. electric stimulation of spinal cord and posterior roots. NY State J Med. 1976;76:366-368.
7 Deer TR, Raso LJ. Spinal cord stimulation for refractory angina pectoris and peripheral vascular disease. Pain Physician. 2006;9:347-352.
8 Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138:9-23.
9 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
10 Linderoth B, Herregodts P, Meyerson BA. Sympathetic mediation of peripheral vasodilation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery. 1994;35:711-719.
11 Jacobs MJ, et al. Epidural spinal cord electrical stimulation improves microvascular blood flow in severe limb ischemia. Ann Surg. 1988;207:179-183.
12 Bayliss WM. On the origin from the spinal cord of the vasodilator fibers of the hind-limb and on the nature of these fibers. J Physiol. 1901;26:173-209.
13 Tanaka S, et al. Role of primary afferents in spinal cord stimulation-induced vasodilation: characterization of fiber types. Brain Res. 2003;959:191-198.
14 Wu M, et al. Sensory fibers containing vanilloid receptor-1 (VR-1) mediate spinal cord stimulation-induced vasodilation. Brain Res. 2006;1107:177-184.
15 Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903-934.
16 Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58:481-496.
17 De Vries J, et al. Spinal cord stimulation for ischemic heart disease and peripheral vascular disease. Adv Tech Stand Neurosurg. 2007;32:63-89.
18 Spincemaille GH, et al. The results of spinal cord stimulation in critical limb ischaemia: a review. Eur J Vasc Endovasc Surg. 2001;21:99-105.
19 Kumar K, et al. Improvement of limb circulation in peripheral vascular disease using epidural spinal cord stimulation: a prospective study. J Neurosurg. 1997;86:662-669.
20 Spincemaille G, et al. Spinal cord stimulation in patients with critical limb ischemia: A preliminary evaluation of a multicentre trial. Acta Chir Austriaca. 2000;32:49-51.
21 Jivegard LE, et al. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischaemia: a prospective randomised controlled study. Eur J Vasc Endovasc Surg. 1995;9:421-425.
22 Claeys L. Spinal cord stimulation for peripheral vascular disease: A critical review—European series. Pain Digest. 1999;9:337-341.
23 Claeys LG, Horsch S. Transcutaneous oxygen pressure as predictive parameter for ulcer healing in endstage vascular patients treated with spinal cord stimulation. Int Angiol. 1996;15:344-349.
24 Ubbink DT, et al. Systematic review and meta-analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischaemia. Br J Surg. 2004;91:948-955.
25 Gersbach P, et al. Discriminative microcirculatory screening of patients with refractory limb ischaemia for dorsal column stimulation. Eur J Vasc Endovasc Surg. 1997;13:464-471.
26 Amann W, et al. Spinal cord stimulation in the treatment of nonre-constructible stable critical leg ischaemia: Results of the European peripheral vascular disease outcome study (SCS-EPOS). Eur J Vasc Endovasc Surg. 2003;26:280-286.
27 Ubbink DT, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2005:004001.
28 Klomp HM, et al. Spinal-cord stimulation in critical limb ischaemia: a randomised trial. ESES study group. Lancet. 1999;353:1040-1044.
29 Donas KP, et al. The role of epidural spinal cord stimulation in the treatment of Buerger disease. J Vasc Surg. 2005;41:830-836.
30 Augustinsson LE, et al. Epidural electrical stimulation in severe limb ischemia. pain relief, increased blood flow, and a possible limb-saving effect. Ann Surg. 1985;202:104-110.
31 Horsch S, Claeys L. Epidural spinal cord stimulation in the treatment of severe peripheral arterial occlusive disease. Ann Vasc Surg. 1994;8:468-474.
32 Ubbink DT, et al. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the Dutch multicenter randomized controlled trial. J Vasc Surg. 1999;30:236-244.