Spinal Cord Stimulation for Heart Failure and Arrhythmias
Spinal Cord Stimulation: Background and Current Clinical Uses
Treatment of Chronic Pain Syndromes
Spinal cord stimulation was first used clinically to treat complex pain syndromes refractory to medical therapy. It is approved by the U.S. Food and Drug Administration and is used widely for the treatment of complex regional pain syndromes in the cervical and lumbar distributions and the extremities. A recent metaanalysis found that SCS is a cost-effective and acceptable therapy in patients who have persistent neuropathic pain despite adequate conventional medical therapy.1 Randomized studies have found that SCS provides additional benefits over conventional medical therapy for treatment of chronic pain.2 The mechanism by which it treats chronic pain is widely thought to be due to interruption of the ascending pain signals within the spinal cord, although efficacy is also linked to placement of the electrodes in a position that produces mild paresthesia in the dermatomal location of the chronic pain. This finding suggests that the mechanism of pain suppression is highly complex and involves efferent and afferent limbs of the nervous system.
Treatment of Cardiac Angina
Spinal cord stimulation was first used in the late 1980s for the treatment of chronic angina refractory to medical or revascularization therapy. It is an approved therapy for this condition in many European countries. Many case reports and two randomized studies have reported that SCS is more efficacious than medical therapy alone in this population that is difficult to treat.3 The placement of the SCS electrode is usually at spinal segments C8 to T1 and slightly left of midline. In this location, SCS output settings are generally set to produce a slight paresthesia over the left precordium, which somehow blunts the chronic angina symptoms.4 The mechanism by which SCS produces analgesia and antiischemia effects is not clear, but some evidence suggests it is a vagal-dependent process.5 Others have theorized that it produces direct effects on the coronary vasculature by promoting vasodilatation.3,6
Treatment of Peripheral Vascular Disease
Spinal cord stimulation is also approved by the U.S. Food and Drug Administration for the treatment of limb pain resulting from peripheral vascular disease refractory to standard therapies. Many patients experience significant relief with SCS, showing improvement in symptoms and decreased claudication.6 The epidural stimulation site is often in the lumbar region for the more common lower extremity symptoms. The beneficial effect is thought to be manifest via improved peripheral blood flow.
Spinal Cord Stimulation: Cardiac Effects from Preclinical Studies in Animal Models
Modulation of Cardiac Autonomic Nerve Activity
In anesthetized canines, Foreman et al.7 demonstrated that provocative coronary artery occlusion was associated with increased intracardiac nerve firing. Interestingly, this nerve activity was suppressed by active SCS at spinal segment T1, with no change in other cardiac parameters such as heart rate or blood pressure.7 They concluded that this finding could provide insight into the beneficial effect of SCS in patients with chronic angina pectoris. In another study in a rabbit cardiac ischemia model, preemptive SCS delivered at spinal segment C8-T2 (delivered before and during coronary artery occlusion) reduced subsequent cardiac infarct size. In this study, SCS initiated after the onset of coronary artery occlusion had no effect on subsequent infarct size. The reduction in infarct size by preemptive SCS was blocked by treatment with α- or β-adrenergic receptor blockers, leading the authors to conclude that this cardioprotective effect was mediated by adrenergic neurons of the ANS.8
Effects on Cardiac Electrophysiology and Electrical Conduction
Olgin et al.9 examined the effects of SCS on cardiac electrophysiology in anesthetized normal canines. In this work, acute epidural spinal cord stimulation at segment T1 significantly increased spontaneous sinus cycle length and the AH interval. Vagal nerve transection, but not ansae subclaviae transection, eliminated the effects of SCS on sinus rate and AH interval. The authors concluded that SCS enhanced parasympathetic activity via a vagus nerve–dependent mechanism.9 In a canine model of established postinfarction heart failure, subsequent coronary artery balloon occlusion produced ventricular arrhythmias.10 Acute SCS at T1 before balloon inflation greatly suppressed the number of ventricular arrhythmias during acute coronary occlusion. In these experiments, acute SCS also significantly decreased sinus rate, increased the PR interval, and reduced systolic blood pressure.10 In a similar manner, significant effects of SCS on ischemic ventricular tachyarrhythmia reduction were seen in porcine ischemia studies.11 Interestingly, electrocardiographic vectorcardiography demonstrated a reduction in ST changes and T wave repolarization alterations by SCS in this porcine study, suggesting a potential myocardial mechanism for arrhythmia suppression. In contrast to the rabbit studies cited above, SCS had no effect on subsequent infarct size in this porcine study.8,11 A small study in humans was performed in three patients with ischemic cardiomyopathy and unrevascularizable refractory angina to determine the effect of SCS on microvolt T wave alternans, which is thought to be a potential marker of arrhythmic ventricular substrate.12 All patients had both implanted ICDs and spinal cord stimulation systems. Studies were performed with SCS off for 24 hours, SCS on for 2 hours, and SCS on for 24 hours. SCS was applied at standard clinical settings. No electrical interactions were noted between the two systems. T wave alternans testing results were positive under the conditions of SCS off for 24 hours and SCS on 2 hours, but became negative when SCS was on or 24 hours, leading the authors to suggest that SCS has an acute, time-dependent effect on the ventricular arrhythmic substrate.12
Effects on Atrial and Ventricular Arrhythmias and Left Ventricle Systolic Function
Additional experimental studies have specifically examined the effects of SCS on cardiac arrhythmias. In a canine atrial tachypacing model, Morley et al.13 examined the effect of chronic SCS at reducing atrial fibrillation (AF) episodes. Before atrial tachypacing, SCS significantly increased atrial effective refractory periods. SCS that was started at time of tachypacing initiation (early SCS) significantly decreased AF episodes and total AF burden, compared with the control group (no SCS). A group in which SCS was turned on 8 weeks after tachypacing initiation produced no significant change in AF episodes or burden compared with the control group. Interestingly, there were no changes noted in myocardial fibrosis in any group; however, there were significant SCS-induced changes in right atrial nerve sprouting and sympathetic and parasympathetic innervation in this study.13
Chronic SCS has been shown to suppress ventricular arrhythmias in a chronic canine postinfarction HF model.14 In this study, canines underwent LAD foam embolization to induce myocardial infarction followed by ventricular high-rate pacing for 3 weeks to induce HF. Animals were randomized to control (no SCS), chronic SCS at spinal segment T4 (delivered at 90% motor threshold, 50 Hz, 0.2-ms pulse duration, for 2 hours tid), standard medical therapy (carvedilol plus ramipril), or SCS plus medical therapy. After 10 weeks, significant decreases in spontaneous and ischemic ventricular arrhythmias, compared with the control group, were noted in animals assigned to the SCS, medical therapy, and SCS plus medical therapy groups, with the SCS group showing the greatest decrease in ventricular tachyarrhythmias.14 Interestingly, there were significant improvements in clinical HF parameters (resting heart rate, systolic blood pressure, oxygen saturation) in the SCS and SCS plus medical therapy groups compared with the medical therapy and control groups.14 This clinical improvement was associated with recovery of left ventricular ejection fraction (Figure 131-1). In addition, significant reversal of left ventricle (LV) dilatation was noted in these SCS-treated groups, but not in the control or medical therapy groups. Furthermore, serum norepinephrine and B-type natriuretic peptide levels were reduced after completion of the treatment interval in both SCS-treated groups compared with the standard medical therapy group, which showed no significant changes in serum neurohormone levels compared to animals with untreated HF (Figure 131-2). This finding suggests that SCS must be acting via a unique or discrete mechanism, and not by just providing additive effects on the same molecular targets as standard HF medical therapy. It was hypothesized that normalization of autonomic tone by SCS therapy led to the improvement in LV contractile function and suppression of ventricular tachyarrhythmias.14
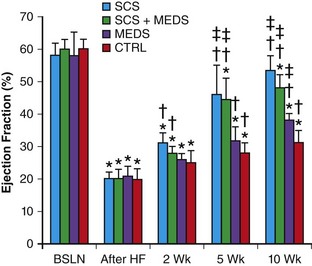
Figure 131-1 Spinal cord stimulation improves left ventricle function in experimental heart failure. BSLN, Baseline; After HF, after HF induction; 2 wk, after 2 weeks of neuromodulation stage; 5 wk, after 5 weeks of neuromodulation stage; 10 wk, after 10 weeks (completion) of neuromodulation stage. *P < 0.05 versus group baseline. †P < 0.05 versus group after heart failure induction, ‡P < 0.05 versus control group at the same time point. (Used with permission from Lopshire JC, Zhou X, Dusa C, et al: Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation 120:286–294, 2009.)
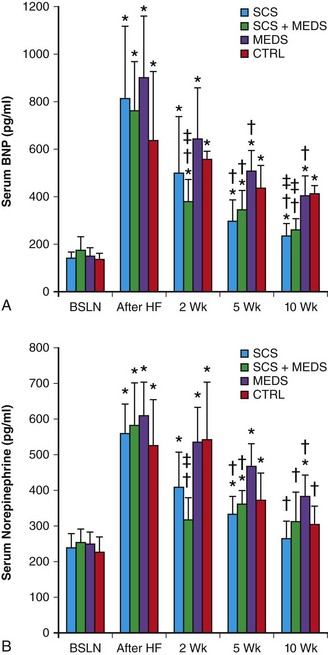
Figure 131-2 Spinal cord stimulation improves neurohumoral function in experimental heart failure. BNP, B-type natriuretic peptide; BSLN, baseline; After HF, after HF induction; 2 wk, after 2 weeks of neuromodulation stage; 5 wk, after 5 weeks of neuromodulation stage; 10 wk, after 10 weeks (completion) of neuromodulation stage. *P < .05 versus group baseline; †P < .05 versus group after heart failure induction; ‡P < .05 versus control group at the same time point. (Used with permission from Lopshire JC, Zhou X, Dusa C, et al: Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation 120:286–294, 2009.)
A follow-up study using the same canine HF model determined the optimal site and intensity of SCS that produced maximal VT-suppressing and LV-remodeling effects.15 After HF induction, SCS leads were placed in one of three spinal locations (T1, T4, or T8); all locations received SCS at 90% motor threshold. Two additional T4 groups received SCS at 60% or 30% motor threshold (the other stimulation parameters of stimulation frequency, pulse width, and delivery intervals were unchanged from the prior study). Significant beneficial effects on VT suppression and LV ejection fraction improvement were noted with 90% stimulation at T1 or T4 and 60% stimulation at T4, with the most profound effects on both parameters noted in the 90% T4 group. The other groups showed no change in these parameters relative to HF control (untreated) animals.15 Interestingly, in HF models in other species, SCS has also been shown to have a beneficial therapeutic effect. In a porcine ischemic HF model, SCS treatment improved echocardiographic regional myocardial strain and LV function. SCS also decreased myocardial oxygen consumption with no change in serum NE levels in this porcine model.16 Another recent study in pigs with ischemic heart failure suggested that continuous spinal cord stimulation was more effective than intermittent stimulation.17 In human patients with HF, a recent study showed that SCS reduced life-threatening ventricular tachyarrhythmias in two patients with frequent ventricular arrhythmias.18
Spinal Cord Stimulation in Heart Failure: Ongoing Human Clinical Trials
The studies discussed in this chapter suggest that SCS can ameliorate excessive autonomic nervous system activation, decrease atrial and ventricular arrhythmias, and improve HF parameters in animal models. Because of apparent vagal effects,19 SCS could also have an antiinflammatory action. These studies provide compelling support for human trials of SCS in the HF population. In fact, human trials are currently underway to evaluate the safety and efficacy of these device-based neuromodulatory modalities in the heart failure population.
Studies Evaluating Effects in Human Heart Failure
Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Heart Failure (DEFEAT-HF) is a multisite (Europe and North America), industry-sponsored, phase II clinical trial to determine the safety and efficacy of SCS in advanced heart failure patients.20,21 Inclusion criteria are patients with NHYA class III-IV symptoms, an LVEF ≤ 35% and LV end-diastolic diameter between 55 and 80 mm, no implanted cardiac resynchronization device, and currently receiving stable medical HF therapy. All enrolled patients receive an implanted SCS device with randomization to active treatment with SCS or no therapy, in a 2 : 1 scheme favoring active SCS therapy. The no-therapy group will cross over to active SCS treatment at 6 months. The primary endpoint is change in LV volumes as measured by cardiac echocardiography at 6 and 12 months with secondary outcomes of changes in blood chemistry (pro–B-type natriuretic peptide) and exercise capacity (peak oxygen uptake). This trial started in 2010, with actively enrolled patients in the United States and Europe, and has a targeted completion date in 2014.20 Smaller human trials are also investigating the utility of SCS in patients with patients. A study in Hong Kong and Australia (SCS-Heart)22 will enroll 20 patients with NYHA class III HF. In addition a phase I safety and efficacy trial is underway in Texas.23
References
1. Ades, A, Adler, A, Allison, A, et al, Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin for the NICE Project Appraisal Committee London (UK). National Institute for Health and Clinical Excellence (NICE) Technology Appraisal Guidance No. 159. 2008:1–33.
2. Kumar, K, Taylor, R, Jacques, L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007; 132(1):179–188.
3. Borjesson, M, Andrell, P, Mannheimer, C. Spinal cord stimulation for long-term treatment of severe angina pectoris: what does the evidence say? Future Cardiology. 2011; 7(6):825–833.
4. Zipes, DP, Svorkdal, N, Berman, D, et al. Spinal cord stimulation therapy for patients with refractory angina who are not candidates for revascularization. Neuromodulation. 2012.
5. Foreman, RD, Qin, C. Neuromodulation of cardiac pain and cerebral vasculature: neural mechanisms. Cleve Clin J Med. 2009; 76(Suppl 2):S75–S79.
6. Deer, T. Spinal cord stimulation for the treatment of angina and peripheral vascular disease. Current Pain Reports. 2009; 13(1):18–23.
7. Foreman, R, Linderoth, B, Ardelt, J, et al. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res. 2000; 47(2):367–375.
8. Southerland, EM, Milhorn, DM, Foreman, RD, et al. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol. 2007; 292:H311–H317.
9. Olgin, JE, Takahashi, T, Wilson, E, et al. Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and atrioventricular nodes. J Card Electrophysiol. 2002; 13(5):475–481.
10. Issa, ZF, Zhou, X, Ujhelyi, MR, et al. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a post-infarction heart failure canine model. Circulation. 2005; 111(24):3217–3220.
11. Odenstedt, J, Linderoth, B, Bergfeldt, L, et al. Spinal cord stimulation effects on myocardial ischemia, infarct size, ventricular arrhythmia, and noninvasive electrophysiology in a porcine ischemia–reperfusion model. Heart Rhythm. 2011; 8(6):892–898.
12. Ferraro, P, Castogno, D, Massa, R, et al. Spinal cord stimulation affects T-wave alternans in patients with ischaemic cardiomyopathy: a pilot study. Europace. 2008; 10:506–508.
13. Bernstein, SA, Wong, B, Vasquez, C, et al. Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm. 2012; 9(9):1426–1433.
14. Lopshire, JC, Zhou, X, Dusa, C, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009; 120:286–294.
15. Lopshire, JC, Zhou, X, Mullen, T, et al. The site and intensity of spinal cord stimulation determines remodeling response in canine heart failure. Heart Rhythm. 2011; 8(5):S78.
16. Liu, Y, Yue, WS, Liao, SY, et al. Thoracic spinal cord stimulation improves cardiac contractile function and myocardial oxygen consumption in a porcine model of ischemic heart failure. J Cardiovasc Electrophysiol. 2012; 23(5):534–540.
17. Tse, H-F, Lie, Y, Zuo, M, et al. Intermittent versus continuous spinal cord stimulation for treatment of ischemic heart failure. Eur Heart J. 2012; 33:963–964.
18. Grimaldi, R, de Luca, A, Kornet, L, et al. Can spinal cord stimulation reduce ventricular arrhythmias? Heart Rhythm. 2012; 9(11):1884–1887.
19. Borovikova, LV, Ivanova, S, Zhang, M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000; 405:458–462.
20. Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Heart Failure (DEFEAT-HF). Available at: http://clinicaltrials.gov/ct2/show/NCT01112579. Last accessed Aug 6, 2013.
21. Cornelussen, RN, Splett, V, Klepfer, RN, et al. Electrical modalities beyond pacing for the treatment of heart failure. Heart Fail Rev. 2011; 16:315–325.
22. Spinal Cord Stimulation For Heart Failure (SCS-HEART). Available at: http://clinicaltrials.gov/ct2/show/NCT01362725. Last accessed Aug 6, 2013.
23. Neurostimulation of Spinal Nerves That Affect the Heart. Available at: >http://clinicaltrials.gov/ct2/show/NCT01124136. Last accessed Aug 6, 2013.