Chapter 5 Spinal Cord Stimulation
General Indications
Establishing Diagnosis
Spinal cord stimulation (SCS) is an adjustable, nondestructive, mode of neuromodulation that delivers therapeutic doses of electrical current to the spinal cord for the management of neuropathic pain. The most common indications include postlaminectomy syndrome, complex regional pain syndrome (CRPS), ischemic limb pain, and angina (Table 5-1); but it has been applied to other causes of intractable pain.
Table 5-1 Spinal Cord Stimulation Indications and Probability of Success
| Indications | Probability of Success |
|---|---|
| Postlaminectomy syndrome/failed back surgery syndrome (FBSS) | 50%-60% with >50% pain relief |
| Complex regional pain syndrome (CRPS) | 67%-84% |
| Chronic critical limb ischemia (CCLI) and pain | 70%-80% with >75% pain relief |
| Angina | No data available |
| Abdominal/visceral pain syndromes | |
| Brachial plexitis/neurogenic thoracic outlet syndrome | |
| Phantom limb pain | |
| Intractable pain secondary to spinal cord injury | |
| Mediastinal pain | |
| Cervical neuritis | |
| Postherpetic neuralgia |
Anatomy
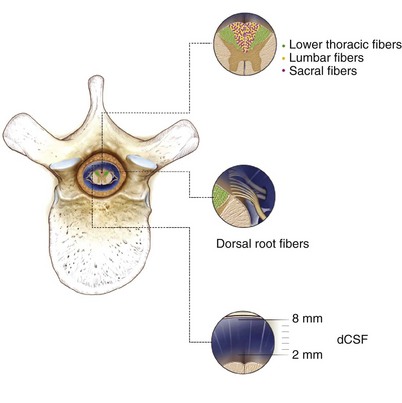
Stimulation of large myelinated afferent fibers may occur in four different areas: the dorsal root, the dorsal root entry zone (DREZ), the dorsal horn, or the dorsal columns (Fig. 5-1). Electrical stimulation of these structures results in an ipsilateral tingling paresthesia, but changes to discomfort and pain with increasing stimulation voltage. Particularly, activation of the dorsal roots causes a radicular paresthesia at a particular dermatomal level, whereas stimulation of the dorsal columns causes paresthesia in areas of the body caudal to the level of the electrode. In the cervical and thoracic cord the dorsal columns are usually about 3 to 5 mm wide, largest at the level of the cervical enlargement, and smallest in the midthoracic spine.
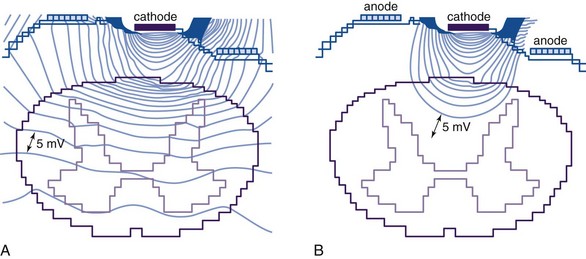
The single most important factor in determining stimulation parameters is the width of the cerebrospinal fluid (CSF) space dorsal to the spinal cord (Fig. 5-2). Increasing thickness of the dorsal CSF (dCSF) layer leads to early stimulation of the dorsal root fibers instead of the dorsal column fibers. This is largely because rootlet fibers, which are immersed in well-conducting CSF, have high conductivity at the DREZ. Therefore the stimulation threshold for the segmentary system ranges from 0.1 V to 0.5 V, whereas activation of the dorsal columns occurs at a threshold that is at least 0.5 V to 1 V higher.
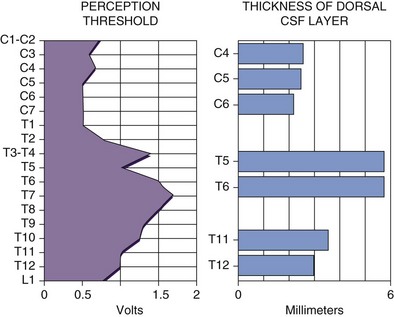
Fig. 5-2 Relationship between spinal level and perception threshold/dorsal cerebrospinal fluid thickness.
Differentiating between stimulation of the dorsal root, DREZ, or dorsal horn can be exceedingly difficult. One can expect dorsal root stimulation with laterally placed electrodes; stimulation of the DREZ or dorsal horn is more likely if the electrode is placed near midline. In the latter group stimulation leads to segmentary paresthesias that are rapidly followed by activation of the dorsal columns with a small voltage increment. Interestingly, minute changes in mediolateral position of the electrode have been shown to lead to movement of the paresthesias in a W two-dimensional pattern1 as shown in Fig. 5-3.
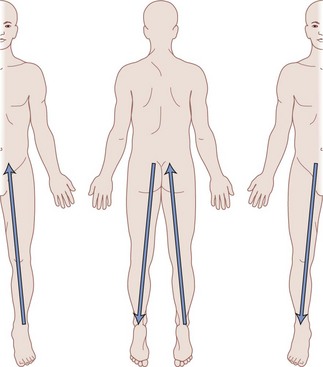
Fig. 5-3 Migration of paresthesias with mediolateral adjustment of stimulation.
Modified from Oakley JC et al: Transverse tripolar spinal cord stimulation: results of an international multicenter study, Neuromodulation 9(3):192-203, 2006.
If electrical stimulation reaches the ventral cord, motor structures are affected, and consequently muscle contractions are seen. Much like dorsal cord stimulation, activation of the ventral root or motor neurons causes muscular contractions in a myotomal distribution, whereas activation of the descending corticospinal pathways results in diffuse muscle contractions caudal to the level of the electrode. In the vast majority of patients stimulation over the dorsal columns is not complicated by simultaneous activation of the motor system unless a significantly higher voltage is implemented. Nevertheless, there have been cases in which activation of the motor structures has been seen to occur at the same threshold as the sensory system; therefore selective stimulation of the dorsal column could not be successfully maintained.2
Understanding the somatotopy of the spinal cord is paramount to knowing the technical aspects of implantation. A basic tenet of SCS is to create an area of paresthesia that overlaps the patient’s region of pain.3 Therefore correlation of the somatotopy and the level of the spinal cord is necessary. Barolat and colleagues4 have published extensively on the mapping of the spinal structures, which led to a database correlating areas of sensory response to levels dorsal SCS.4
Recently this paradigm has been challenged by case series demonstrating successful four-limb paresthesia in patients receiving only cervical stimulation.5 In general, this effect was achieved with stimulation with longer pulse widths and lower frequencies. Although the mechanism is not yet well understood, some have hypothesized that thinner dorsal CSF thickness in the cervical spine may allow for the stimulation of deeper medial and midline fibers that correspond to the lower extremities.
Basic Science
The enthusiasm with SCS began with the introduction of the gate control theory for pain control by Melzack and Wall6 in 1965. They noted that stimulation of large myelinated fibers of peripheral nerves resulted in paresthesia and blocked the activity in small nociceptive projections. In other words, appropriate stimulation of a competing afferent signal can effectively block an existing pain signal.
Although a large body of work has been published, the exact mechanisms of action of SCS remain unclear. The computer modeling work of Coburn,7,8 Coburn and Sin,9 Holsheimer and associates,10 Holsheimer and Strujik,11 and Holsheimer and Wesselink12 have shed some light, at least theoretically, on the distribution of the electrical fields within the spinal structures. It is clear that stimulation on the dorsal aspect of the epidural space creates complex electrical fields that affect a large number of structures, including afferents within the peripheral nerve, dorsal columns, or supralemniscal pathways. Mechanisms at distinct levels of the central nervous system apart from the spinal cord are believed to contribute to the effects of SCS. Such mechanisms include antidromic action potentials that pass caudally in the dorsal columns to activate spinal segmental mechanisms in the dorsal horns and action potentials ascending in the dorsal columns activating cells in the brainstem, which in turn might drive descending inhibition. Recent work by Schlaier and associates13 demonstrated changes in cortical excitability with SCS, which is believed to be N-methyl-d-aspartate (NMDA)-related neuroplasticity at the supraspinal level. At the chemical level, animal studies suggest that SCS triggers the release of serotonin, substance P, and γ-aminobutyric acid (GABA) within the dorsal horn.14–16
Indications/Contraindications
Another important consideration is the need for careful psychological screening in the selection of candidates for SCS procedure.17–24 This becomes particularly important because mood disorders can alter pain reporting and perception by a patient. Patients who have frank psychiatric disorders, excessive depression, anger, or unrealistic expectations may be inappropriate for SCS; however, some of these patients may become favorable candidates for SCS after appropriate psychiatric therapy. The most common indications for SCS follow.
Chronic Critical Limb Ischemia and Pain
In 1973 Cook and Weinstein25 were the first to suggest that the indications for SCS might extend beyond intractable pain control. They observed a group of patients with multiple sclerosis who underwent SCS to treat their chronic pain. Unexpectedly the patients experienced not only pain relief but also an improvement in mobility and sensory and bladder function. They incidentally found an apparent improvement in lower limb blood flow and subsequently used SCS in patients whose primary problem was peripheral vascular disease (PVD).26 These patients were found to have relief of rest pain, increased skin temperature, improved plethysmographic blood flow, and healing of small cutaneous ulcers.
Similarly, it is believed that SCS benefits patients with pain secondary to Raynaud phenomenon27 and diabetic neuropathy28 as well.
Angina
The role of SCS in the management of refractory angina pectoris seems to be very promising. There are well-documented reports in the literature revealing uniformly good results in the relief of angina pain.29–33 Further, the results have been maintained in long-term follow-up and have been substantiated by a reduction in the intake of nitrates as well. Interestingly, other findings have supported the evidence that SCS has effects that go beyond pain relief. The observations that there is less ST segment depression and that the exercise capacity, the time to angina, and the recovery time all improve with stimulation may suggest that there is a reduction in ischemia. In a positron emission tomography study, a redistribution of myocardial flow in favor of ischemic parts of the myocardium has been demonstrated as a long-term effect of SCS, both at rest and after pharmacological stress induction.34
Moreover, since the relation between pain and myocardial ischemia has not been fully clarified, we do not know whether the pain relief is caused by direct depression of nociceptive signals in the spinal cord or whether there is secondary gain from a reduction in the ischemia.35,36 On the one hand, a significant amount of work by Foreman14 has shown that dorsal column stimulation inhibits the activity of spinothalamic tract cells, which are typically activated by cardiac sympathetic afferents or intracardiac bradykinin. On the other hand, the effects of stimulation might be equivalent to those of a sympathectomy in that it inhibits a hyperactive sympathetic system. This latter mechanism has been shown experimentally in the rat by Linderoth and associates.37
Other Indications
Early studies in the 1980s demonstrated benefits of SCS for urinary incontinence, with consequent increases in continence and substantial reduction in residual urine volumes.38–42 These effects were found with stimulation of ventral sacral roots, a method that has also been shown to improve bowel function in patients with spinal cord injury.43 Trials in the 1970s reported initial benefits of this modality for phantom limb pain, but few of these patients experienced lasting results with long-term follow-up.44 Scattered reports also exist demonstrating the benefit of SCS in the treatment of intractable pain secondary to spinal cord injury pain, mediastinal pain, cervical neuritis, and postherpetic neuralgia.
Equipment
Electrical stimulation consists of rectangular pulses delivered to the epidural space through an implanted electrode via a power source. Electrodes were initially all unipolar in nature, but subsequently bipolar and tripolar arrays were developed. The initial radiofrequency (RF)-driven passive receivers have given way to implantable pulse generators (IPGs), which were first introduced in the mid-1970s. In 1980 the first percutaneous quadripolar electrode was produced; it could be reprogrammed noninvasively through an external transmitter.45 Subsequently IPGs that can be both transcutaneously charged and programmed have been developed. This most recent advance is now leading a renewed interest in the possibility of using special electrode arrays in the delivery of electrical stimulation to the spinal cord.
Percutaneous Electrodes
Electrode choice generally depends on how many segments of the spinal cord are to be covered. Although electrodes with larger spacing allow for broader coverage, closer spacing permits better steering and electric field shaping. The general trend is to use one or two quadripolar electrodes for limb pain and one or two octipolar electrodes for axial pain. Even insertion of three electrodes is being explored for better steering of current.46
Recently ANS (A St. Jude company, Plano, Tex) has introduced a slim-line plate type electrode that can be inserted percutaneously. The broader electrode base provides a surface in which fibrosis should lessen the risk of caudal electrode migration. The slimmer profile of the electrode might also have advantages in the cervical spine where spinal cord compression might be an issue. Finally, the design emulates that of a miniplate lead since the contacts are on one side with the other side being insulated, leading to a more energy efficient system (Fig. 5-4).
Paddle Electrodes
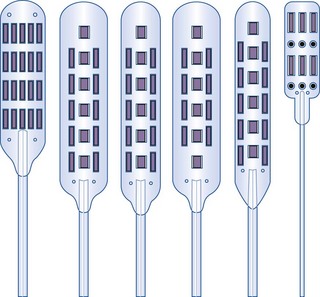
Paddle or laminotomy electrodes are offered in many sizes, shapes, lengths, spacing, and configurations of electrodes. They are primarily offered as single- and dual-column configurations; with three (tripole)- and five-column models being introduced more recently (Figs. 5-5 and 5-6). With regard to their shape, these electrodes may come with curved or hinged leads to help facilitate insertion.
The main advantage of these electrodes resides in their more inherent stability in the dorsal epidural space and lesser propensity to migrate. Some preliminary data by North and associates47 also suggest a broader stimulation pattern and lower stimulation requirements. As alluded to previously, paddle electrodes are also energy efficient in delivering electrical stimulation.
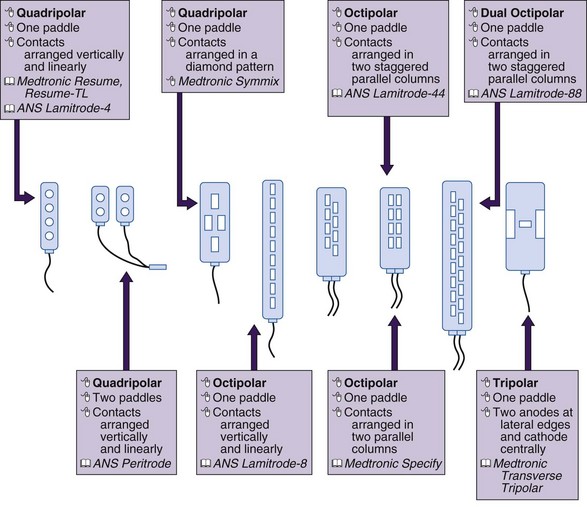
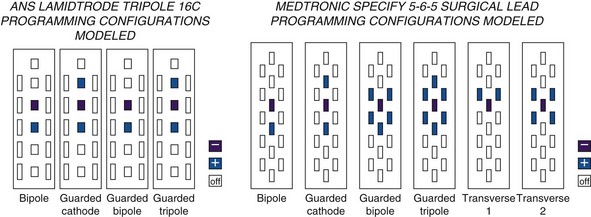
There is some theoretical evidence that shaping of the electrical field is possible with more complex electrode arrays. Current available tripole electrodes and their dimensions are shown in Table 5-2. Holsheimer and colleagues48 concluded that the transverse tripolar system enabled finer control of paresthesia because of its ability to more accurately shape the stimulating electromagnetic field. Specifically the transverse tripolar configurations allow for stimulation of the dorsal columns at higher amplitudes before the dorsal roots begin experiencing clinically significant stimulation. These electrodes are composed of a central cathode flanked by lateral anodes, which modulate the field medially or laterally, depending on their programmed voltage. In other words, the current delivered to the spinal cord can be steered not by moving the electrode but instead by simply modifying the voltage ratio between the central cathode and two flanking anodes.1 The lateral anodes create a shielding effect such that the dorsal roots are protected from stimulation; deeper activation of the dorsal columns is achieved with higher voltages (Fig. 5-7). This feature ultimately results in a wider therapeutic range, wider paresthesia coverage, and a greater probability to fully cover the painful area with paresthesia. Possible programming configurations of these tripole electrodes are shown in Fig. 5-8.
MDT Specify 5-6-5  |
ANS Lamitrode Tripole 16C  |
|
|---|---|---|
| Electrode length (mm) | 4.0 | Mid: 4.1 Out: 5.8 |
| Electrode width (mm) | 1.5 | Mid: 2.6 Out: 1.8 |
| Longitudinal ctr. to ctr. (mm) | 9.0 | Mid: 7.0 Out: 7.0 |
| Lateral ctr. to ctr. (mm) | 3.0 | 3.2 |
| Electrode area (mm2) | 6.0 | Mid: 10.7 Out: 10.4 |
Rechargeable Implantable Pulse Generators
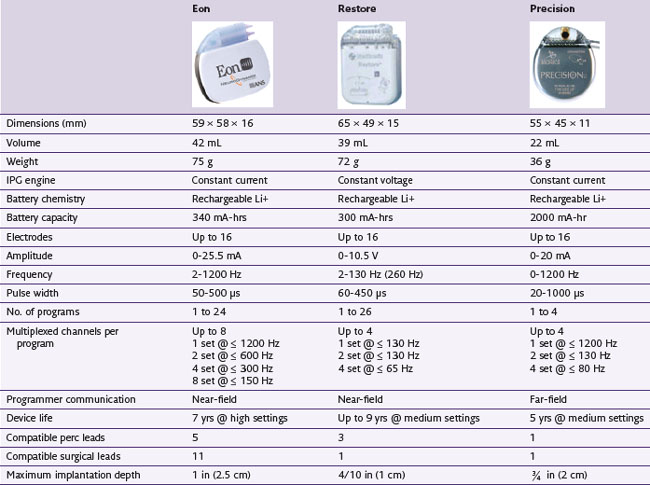
Rechargeable systems have now become available. Medtronic’s device, known as the Restore Rechargeable Neurostimulation System, uses a battery with an estimated 9-year total life span and takes about 6 hours to fully recharge. Advanced Neuromodulation Systems’ Eon device has a battery life that is currently estimated to be 7 years. Boston Scientific’s Precision device has a battery life estimated to be 5 years. A detailed comparison of the features of these rechargeable batteries is shown in Table 5-3.
In an analysis of cost between rechargeable and nonrechargeable systems, Hornberger and associates49 found that the former would require 2.6 to 4.2 fewer replacement procedures, resulting in a total lifetime savings ranging from $104,000 to $168,833. With regard to overall efficacy, a 12-month study looking specifically at the Restore rechargeable IPG demonstrated that the system was easy to use and that all patients were able to recharge it successfully. Moreover, both patient and physician satisfaction was high, with significant improvements in pain, quality of life, and functional status.50
Technique
In 1967 Shealy and Cady51 and Shealy, Mortimer, and Reswick52 inserted the first dorsal column stimulator in a human suffering from terminal metastatic cancer. Subsequently electrodes have been implanted using a variety of techniques: via a laminectomy in the subarachnoid space52; between the two layers of the dura, or in the epidural space, either dorsal or ventral to the spinal cord.53–55 Subsequently less invasive percutaneous techniques were introduced.56 Current practice indicates the placement of electrodes in the dorsal epidural space and connecting them to an implantable pulse generator usually placed in the buttock. However, before a permanent system is implanted, patients typically undergo a trial period of 3 to 10 days, during which time stimulation is provided by percutaneous electrodes to determine their overall efficacy.57
Placement of Percutaneous Electrodes
Once in place, the electrode must be secured to the interspinous ligament to minimize dislodgment. An important measure is to secure the wire as loops at the electrode insertion site to relieve the strain and reduce migration during bending. Applying suture directly to the leads has been shown to be safe since it does not cause substantial physical damage or any electrical impairment.58 Nevertheless, manufacturers still do not recommend tying directly to the lead and continue to recommend the use of anchors to secure leads. When anchors are used to secure the electrode, one must remember to secure the anchor to both the electrode and the fascia.
Placement of Paddle Electrodes
Paddle electrodes require a surgical procedure for implantation under direct vision.59 The patient may be placed in one of two basic positions: prone or semilateral. The prone position allows a more intuitive understanding of the spatial relations, which is more familiar to surgeons. At the same time, it can be difficult to obtain adequate sedation and maintain the airway in this position. In the semilateral position the surgeon has access to the spine and the flank, abdomen, or buttock for the implant of the pulse generator. The patient is asked to place himself or herself in the most comfortable position. If the pain is predominantly on one side, the patient is asked to lie on the less affected side. In this position airway management is safer than in the prone position, and the anesthesiologist is more comfortable in keeping the patient deeply sedated. For cervical placement the neck is slightly flexed but not excessively rotated laterally because extreme rotation substantially increases the difficulty of electrode placement. In general the variable degree of rotation with the patient in the semilateral position makes it more difficult to determine the location of the midline, which may be a significant problem in the cervical area.
Such testing previously consisted of waking the patient and determining efficacy of stimulation from patient responses. This has gradually been replaced with intraoperative stimulation with electromyography (EMG) stimulation coupled with somatosensory evoked potential (SSEP) testing, which helps detect proper positioning of the electrode. EMG stimulation is performed with a signal consisting of a greater than 310 microseconds pulse width at a frequency of 5 Hz; the amplitude is gradually increased until EMG signal changes are detected. Alternatively, awake testing is commonly performed with 50-Hz stimulation. As one would expect, bilateral extremity stimulation suggests midline placement, and early root onset implies too lateral of a placement. In addition, stimulation of the dorsal columns results in a greater area of coverage with paresthesia—the percentage of body surface covered is inversely proportional to the distance between two electrodes.60 To achieve this, most of the current has to be directed to an area within 2 to 3 mm on each side of the physiological midline. It is important to note that the physiological midline may differ from its anatomic counterpart by up to 2 mm1; in such instances one should rely on the former since it has a higher clinical correlation. With this feedback, the implant should be placed midline in cases of axial symptomatology or with a bias to one side for patients with unilateral pain. By correlating paddle position to the prior position of trial electrodes and testing motor stimulation responses, one can effectively find the proper location for implantation without waking the patient.
Implantable Pulse Generator Placement
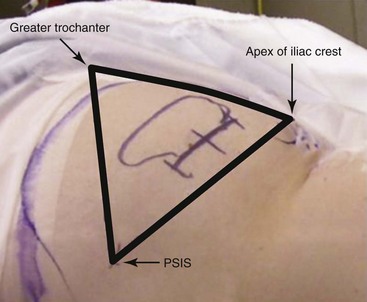
The IPG should be placed in an area where the unit will have enough overlying soft tissue but also not be pressing against bone, usually the abdomen or buttock. To find the appropriate area of implantation in the latter, three boney prominences are used as landmarks. The posterior superior iliac crest marks the medial border; the greater trochanter of the femur and the apex of the iliac crest define the lateral border. The IPG is implanted approximately 2 to 3 cm deep in the lateral aspect of this triangle (Fig. 5-9).
Postlaminectomy Syndrome/Failed Back Surgery Syndrome
In the treatment of postlaminectomy syndrome, a great challenge has been to obtain stimulation in the low back. Even with direct stimulation to the low back, the pattern of paresthesia is often replaced in time by an unpleasant segmental band of stimulation from the thoracic roots, which negates the benefits of the procedure. Previous pioneering work by Jay Law61,62 has shown that stimulation in the low back can be obtained only if one uses multiple arrays of closely spaced bipoles at T9-T10. North and associates63 have challenged the concept of the superiority of centered dual electrodes by showing that one single quadripolar electrode in midline has the ability to stimulate the axial low back. These were acute observations, and no data exist as to the long-term behavior of single vs. dual electrodes. The advent of the tripole electrodes and the ability to steer current has made it more plausible to aim for low-back paresthesia. This technology, which consists of a cathode flanked by lateral anodes, also theoretically increases the discomfort threshold.46
Angina
The most appropriate electrode location for the treatment of angina pectoris is most likely the lower cervical and upper thoracic region, although some have reported successful higher cervical placements.64 In terms of its daily use, most patients use a low-intensity stimulation for several hours per day for prophylactic purposes.65
Patient Management/Evaluation
Careful follow-up of the patients is necessary for successful long-term satisfaction. Equipment-related problems can arise at any time after implantation. Such problems include discomfort at the pulse generator/radio receiver site, electrode breakage or migration, and infection. Titration of the electrical parameters and careful follow-up of the patients is just as important as a technically correct surgical implant procedure. This process can be time consuming. The use of computer-assisted and patient interactive programming is showing great promise;66,67 the ability to download several programs into the pulse generator/receiver, which can later be recalled by the patient at the push of a button, has greatly facilitated the management of the implanted stimulation systems.
There are three very important electrical parameters: the perception threshold, the discomfort threshold, and the usage range. The perception threshold is the voltage where the patient begins to perceive paresthesia;68 the discomfort threshold is the voltage where the patient perceives the stimulation as uncomfortable, either because the paresthesias are too strong or because it elicits motor contractions; and the usage range is the difference between these two parameters. As previously indicated, the perception threshold is the lowest in the cervical area and highest in the midthoracic area because of the increasing thickness of CSF dorsal to the spinal cord in the midthoracic spine. For this reason electrode placement in the midthoracic spine favors more selective activation of the segmentary sensory system and seldom results in satisfactory long-term stimulation of the dorsal columns. The distance between electrode and nerve fibers is also affected by patient posture69 and thus may require use of different programs throughout the day for such variations. This can be cumbersome, and no technology yet exists that is capable of adapting to these changes.
Once the optimal combination and stimulation voltage have been established, other electrical parameters must also be assessed to optimize the stimulation. The rate of stimulation is very important. Although most patients choose rates between 20 and 100 Hz, there is a high individual variability, and several different rates must be attempted before settling on the optimal one. Some evidence exists that patients with specific neuropathic pain syndromes may respond better to higher stimulation rates (>250 Hz).66 The other parameter of pulse width provides a finer adjustment for the current intensity; and other features such as cycling, ramping, and biphasic pulses should be explored to promote the most effective delivery of stimulation. It is not unusual for optimal stimulation parameters not to be fully established for several weeks following implantation.
Outcomes Evidence
Postlaminectomy Syndrome/Failed Back Surgery Syndrome
Longitudinal studies by North and associates70 evaluated 50 patients with postsurgical lumbar arachnoid or epidural fibrosis without surgically remediable lesions. In this group of patients SCS was superior to repeated surgical interventions on the lumbar spine for back and leg pain and superior to dorsal ganglionectomy for leg pain. Specifically a reduction in pain by at least 50% and patient satisfaction were obtained in 53% of patients at 2.2 years. These results are consistent with a 1995 systematic review of 41 studies,71 in which approximately 50% to 60% of patients with failed back surgery syndrome (FBSS) had greater than 50% pain relief from SCS. Similarly, Burchiel and associates72 conducted a prospective multicenter study in 1996 with 1-year follow-up and also reported 55% successful stimulation; but unfortunately medication usage and work status were not significantly changed. In 2007 a small retrospective series also demonstrated patient satisfaction of 73.5% with reduction in visual analog scale (VAS) scores and amount of additional medication required.73
North, Kidd, and Piantadosi74 also conducted a prospective study randomizing patients with FBSS to repeat back surgery or SCS surgery; but allowing patients to cross over after 6 months. Although 10 of 15 patients crossed over from back surgery to SCS, only two of 12 patients crossed over from SCS to back surgery. North and colleagues47 also compared the difference between paddle and percutaneous electrodes. Although laminectomy electrode placement is more invasive than percutaneous placement, it yielded significantly better clinical results in patients with FBSS at up to 3-year follow-up.
The PROCESS trial, published in 2008, involved randomizing 100 patients with FBSS to medical management alone or to SCS in addition to medical management.75 In this 2-year study, the authors found that patients randomized to SCS demonstrated >50% leg pain relief, improved quality of life, and greater functional capacity more frequently (37%) than those randomized to medical management alone (2%). However, once again no difference was noted in the amount of opioid use between the two groups.
In a recent prospective cohort study of workers’ compensation recipients who underwent SCS for FBSS, no significant difference was detected with regard to pain, function, medication use, and work time loss after 6 months of follow-up.76 This study emphasizes the importance of patient screening to increase chances of a favorable outcome since those who underwent SCS in this study had low average mental health scores, poor baseline functioning, and axial back pain in addition to radicular leg pain.77
Complex Regional Pain Syndrome
In 1989 Barolat, Schwartzman, and Woo78 reported reduction of pain in 10 of 13 patients implanted. No patients in that series were made pain free, but all 10 reported a definitive difference when the stimulation was stopped. In 1997 Kumar, Nath, and Toth79 presented a median follow-up of 41 months on 12 patients with permanently implanted leads. Eight patients reported near complete resolution of their symptoms, and four also maintained good relief.
Kemler and associates80 reported 23 cases with 78% of the patients reporting improvement. In 2000 this group published another series of 54 patients who underwent randomization to SCS with physical therapy or to physical therapy alone.81 Using an intent-to-treat analysis, in the SCS-randomized group 67% of patients experienced significant pain relief, which persisted at 6 months. Specifically 2.4 cm decrease in VAS for pain was noted in the SCS group; compared to 0.2 cm increase in the group undergoing physical therapy alone. At the same time, no functional improvement was observed in either group. However, in subsequent publications Kemler and associates82 noted that the effects of SCS that were maintained at 2-year follow-up, diminished over time for these patients such that, at 5-year follow-up, results were no longer statistically significant in favor of SCS. Nevertheless an as-treated analysis revealed near statistical significance for pain relief (p = 0.06) and statistically significant improvement in global perceived effect. When interviewed, 95% of patients with an implant reported that they would repeat treatment for the same result.83
Oakley and Weiner84 reported a prospective study of 19 patients with CRPS implanted with SCS systems. Of the 10 patients about whom detailed long-term efficacy data were available, three reported full relief from their pain, and seven reported partial relief.
Three additional prospective studies without matched controls have been reported (total of 50 subjects).59,84,85 Two of the studies reported success rates with an 84% overall success rate. The third study by Calvillo and colleagues85 reported a significant improvement in pain scores (VAS) and a >50% reduction in narcotic use by 44% of subjects. In eight retrospective studies the overall success rate was 84% (192 patients).86
Chronic Critical Limb Ischemia (CCLI) and Pain
After the work by Cook and Weinstein,26 in 1981 Meglio and associates87 reported pain relief and ulcer healing in a patient with advanced peripheral arterial insufficiency. In 1988 Jacobs and colleagues88 published clinical evidence that SCS improved the microcirculation as measured by capillary microscopy.
An early trial was performed by Klomp and associates,89 who randomized 120 patients with critical painful limb ischemia to receive medical therapy alone or SCS in conjunction with medical therapy. At a mean follow-up of 19 months there was no significant difference in pain score improvement between the two groups. Yet Jivegard and colleagues90 found benefits of SCS when they studied 51 patients who were randomized to receive either oral medication alone or SCS with oral medication. This group reported a significant improvement in pain scores of the SCS-treated group over the non-SCS group (p < 0.01). Similarly, a prospective randomized trial by Guarnera, Furgiuele, and Camilli91 demonstrated superior pain control and increased ulcer healing of SCS (72%) compared to distal arterial reconstruction (40%).
Four prospective studies without matched controls reveal an overall success rate of 78% (n = 271); and an analysis of seven retrospective studies found an overall success rate of 76% (n = 308).86 A review of the European literature demonstrates that 70% to 80% of patients achieved significant (>75%) pain relief. Moreover, pain relief with SCS was found to have more persistent effects than medical treatment alone. Cochrane reviewers have found evidence to favor SCS over conservative treatment to improve limb salvage and achieve pain relief in those unfit for surgery or who have persistent distal ischemia and pain despite revascularization.92,93
Angina
Vulinkand colleagues94 conducted a prospective study on quality of life changes in patients with refractory angina pectoris implanted with SCS. They found that both the pain and the health aspects of quality of life improved significantly after 3 months of SCS. Further, social, mental, and physical aspects of quality of life were found improved after 1 year of SCS.
Hautvast and associates95 implanted SCS in patients with stable angina pectoris and randomized them. One group remained inactivated, and the other group was instructed to use the stimulator three times per day for 1 hour and with any angina attack. At 6 weeks the treatment group had increased exercise duration and time to angina and decreased angina attacks and sublingual nitrate consumption. Also observed was a decrease in ischemic episodes on electrocardiogram (ECG), with a decrease in observed ST segment depressions on exercise ECG. There was an increase in perceived quality of life and decrease in pain. It was shown that a placebo effect from surgery in the treatment group was unlikely because all patients had implantation surgery at baseline.
In another prospective study of 104 patients who underwent SCS implantation for refractory angina pectoris there was a significant decrease in angina episodes at rest, angina episodes with activity, and total angina episodes.96 Five additional studies are reported to be prospective but without matched controls.94,97–100 Each of these also revealed significant benefit from SCS. The benefit indices ranged from reduction in angina attacks, decreased nitrate consumption, decrease in New York Heart Association (NYHA) grade, and improvement in Nottingham Health Profile (NHP) grade.
A recent randomized controlled study in patients already implanted with SCS for angina, demonstrated improvement in functional status and symptoms in treatment arms with conventional or subthreshold stimulation in comparison to a low-output placebo treatment arm.101 This is the first blinded study in which stimulation below the sensory threshold for paresthesia has demonstrated therapeutic efficacy while eliminating the possibility of a placebo effect.
To compare SCS to coronary artery bypass graft (CABG), Mannheimer and associates64 randomized 104 patients accepted for CABG to receive either CABG (n = 51) or SCS (n = 53) in the electrical stimulation versus coronary artery bypass surgery in severe angina pectoris (ESBY) study. This study demonstrated that patients randomized to SCS showed a greater than 30% improvement in Nottingham Health Profile (NHP) scores when compared with their baseline. This difference was significant and comparable to that shown by patients randomized to CABG102 and were consistent on follow-up after 4 years. More important, the 5-year mortality of 27.9% in the ESBY study was similar between those receiving SCS and those who received CABG, with no difference in the percentage of cardiac deaths. In addition, whereas cardiac events were similar across the groups, significantly more cerebrovascular events were observed in the CABG group. Both groups experienced a significant and similar reduction in the number of angina attacks and the consumption of nitrates. Ultimately SCS appears to be equivalent to CABG in terms of symptom relief and may be an alternative to patients with increased surgical risk.
The concern of whether or not stimulation can conceal an acute myocardial infarction was directly addressed by Andersen, Hole, and Oxhoj.103 They reported on 3 out of 45 patients treated with SCS for angina pain who survived a myocardial infarction (MI). All three patients noticed the pain to be different and unrelieved with SCS, and all patients correctly guessed that the pain was caused by a myocardial infarction. Therefore it was believed that SCS does not seem to conceal an acute MI.104 Instead, SCS is believed to reduce the severity of angina attacks but not be able to suppress the conduction and perception of cardiac pain signals that act as indicators of cardiac distress.105 Similarly, Murray and associates106 have shown that SCS for refractory angina is effective in preventing hospital admissions without masking the ischemic symptoms or leading to silent infarction.
Abdominal/Visceral Pain Syndromes
Several early studies have since demonstrated the benefit of SCS in abdominal visceral disease. There was an initial hesitancy toward the application of SCS for visceral and somatic pain secondary to the belief that nociceptive pain could not be modulated via stimulation. However, Ceballos and colleagues107 demonstrated reduction in pain scores and decrease in narcotic use in a patient treated for mesenteric ischemia with stimulation at T6. Similarly, Krames and Mousad108 described a patient treated for irritable bowel syndrome who was developing escalating pain and diarrhea. After a stimulator was placed at T8, there was a subjective decrease in pain from 9/10 to 2/10 with only two diarrhea episodes and significant reduction in pain medications in the first 6 months. Even though the patient’s pain recurred after 10 months, the significant reduction in the amount of diarrhea at that time persisted.
Kha, Raza, and Khan109 reported the largest series of nine patients with refractory abdominal pain. Five of the nine patients had nonalcoholic pancreatitis, three had a presumed abdominal wall neuroma from frequent abdominal surgery, and another had postsplenectomy pain after trauma. All patients had a significant improvement in VAS scores and decreased narcotic use with placement of the leads at the T5-T7 level at 6- to 8-month follow-up.
Tiede and associates110 reported on multiple abdominal surgeries and failed conservative measures. Each patient had an element of postprandial abdominal pain associated with nausea and vomiting. In both patients the leads were placed at the T2 level with significant improvement in pain, decreased narcotic use, and increased functioning such as return to work.
More recent studies have looked at the treatment of visceral pelvic pain specifically with SCS applied to the dorsal columns. Kapural and colleagues111 reported on the value of neurostimulation for chronic visceral pelvic pain in six female patients with long-standing pelvic pain secondary to endometriosis, multiple surgical explorations, and dyspareunia. At an average follow-up of 30 months, they found a significant decrease in the VAS score with an average of more than 50% pain relief, accompanied with a decrease in opiate use.
Additional case reports have also demonstrated benefit of SCS for treatment of intractable abdominal or other etiologies, including familial Mediterranean fever and Bannayan-Riley-Ruvalcaba syndrome.112,113
Risk and Complication Avoidance
With the proper expertise, permanent complications are rare.88 The most serious complication, which is shared with any type of spine surgery, is paralysis or severe neurological deficits, which are more likely during the dissection of epidural adhesions. Fortunately the number of cases of neurological injury involves far less than 1% of implants. An epidural hematoma is another rare but serious complication that can result from venous plexus damage on electrode insertion.
Electrode migration is not an uncommon problem and may require a reoperation to recapture adequate paresthesia. This phenomenon tends to occur most commonly in the first few days since it takes approximately 3 to 4 weeks for scar tissue to form around the plate electrode. In a retrospective review performed in 2006, lead migration was found to be slightly more common with plate electrodes (11.4%) than with percutaneous electrodes (9.8%); and this complication was also found to occur sooner in the former.114 Close attention to programming is essential since a change in voltage requirements or sudden loss in previously captured paresthesia may hint at this problem. The incidence of electrode migration has varied significantly in the literature but is more accurately between 1% and 15%.
As technology continues to improve, hardware-related complications continue to decrease. Overall complications with the equipment include a 1% to 4% incidence of unspecified hardware failure or <2% incidence of battery failure. There is also a 1% to 8% incidence of lead breakage, which is common in the cervical spine and with plate electrodes.114 An x-ray film may not reveal the break—particularly if the break is limited to a small number of filaments—and high impedance is the only indication of this diagnosis. Most hardware-related problems are simply corrected with replacement.
1 Oakley JC, et al. transverse tripolar spinal cord stimulation: results of an international multicenter study. Neuromodulation. 2006;9(3):192-203.
2 Barolat G, Sharan A. Spinal cord stimulation for chronic pain management. Arch Med Res. 2000;31:258-262.
3 North RB, et al. Spinal cord stimulation for chronic, intractable pain: superiority of “multi-channel” devices. Pain. 1991;44(2):119-130.
4 Barolat G, et al. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J Neurosurg. 1993;78:233-239.
5 Hayek SM, Veizi IE, Stanton-Hicks M. Four-limb neurostimulation with neuroelectrodes placed in the lower cervical epidural space. Anesthesiology. 2009;110(3):681-684.
6 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
7 Coburn B. Electrical stimulation of the spinal cord: two-dimensional finite element analysis with particular reference to epidural electrodes. Med Biol Eng Comput. 1980;18:573-584.
8 Coburn B. A theoretical study of epidural electrical stimulation of the spinal cord–Part II: Effects on long myelinated fibers. IEEE Trans Biomed Eng. 1985;32:978-986.
9 Coburn B, Sin WK. A theoretical study of epidural electrical stimulation of the spinal cord–Part I: Finite element analysis of stimulus fields. IEEE Trans Biomed Eng. 1985;32:971-977.
10 Holsheimer J, et al. Significance of the spinal cord position in spinal cord stimulation. Acta Neurochir Suppl. 1995;64:119-124.
11 Holsheimer J, Struijk JJL. How do geometric factors influence epidural spinal cord stimulation? A quantitative analysis by computer modeling. Stereotact Funct Neurosurg. 1991;56:234-249.
12 Holsheimer J, Wesselink WA. Effect of anode-cathode configuration on paresthesia coverage in spinal cord stimulation. Neurosurgery. 1997;41:654-659. discussion 659-660
13 Schlaier JR, et al. Effects of spinal cord stimulation on cortical excitability in patients with chronic neuropathic pain: a pilot study. Eur J Pain. 2007;11(8):863-868.
14 Foreman RD, et al. Effects of dorsal column stimulation on primate spinothalamic tract neurons. J Neurophysiol. 1976;39:534-546.
15 Linderoth B, et al. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31:289-296. discussion 296-297
16 Linderoth B, Stiller CO, Gunasekera L, et al. Gamma-aminobutyric acid is released in the dorsal horn by electrical spinal cord stimulation: an in vivo microdialysis study in the rat. Neurosurgery. 1994;34:484-488. discussion 488-489
17 Brandwin MAK, Kewman DG. MMPI indicators of treatment response to spinal epidural stimulation in patients with chronic pain and patients with movement disorders. Psychological Rep. 1982;51(3 Pt 2):1059-1064.
18 Kupers RC, et al. Spinal cord stimulation: a nation-wide survey on the incidence, indication, and therapeutic efficacy by health insurer. Pain. 1994;56:211-216.
19 Nielson KD, Adams JE, Hosobuchi Y. Experience with dorsal column stimulation for relief of chronic intractable pain: 1968-1973. Surg Neurol. 1975;4(1):148-152.
20 Monsalve V, de Andres JA, Valia JC. Application of a psychological decision algorithm for the selection of patients susceptible to implantation of neuromodulation systems for the treatment of chronic pain. a proposal. Neuromodulation. 2000;3(4):191-200.
21 Olson KA, et al. Psychological variables associated with outcome of spinal cord stimulation trials. Neuromodulation. 1998;1(1):6-13.
22 Burchiel KJ, et al. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery. 1995;36(6):1101-1110.
23 Ruchinskas R, O’Grady T. Psychological variables predict decisions regarding implantation of a spinal cord stimulator. Neuromodulation. 2000;3(4):183-189.
24 Daniel MSL, et al. Psychological factors and outcome of electrode implantation for chronic pain. Neurosurgery. 1985;17(5):773-777.
25 Cook AW, Weinstein SP. Chronic dorsal column stimulation in multiple sclerosis; preliminary report. N Y State J Med. 1973;73:2868-2872.
26 Cook AW, et al. Vascular disease of extremities: electric stimulation of spinal cord and posterior roots. NY State J Med. 1976;76:366-368.
27 Benyamin R, Kramer J, Vallejo R. A case of spinal cord stimulation in Raynaud’s Phenomenon: can subthreshold sensory stimulation have an effect? Pain Physician. 2007;10(3):473-478.
28 de Vos CC, et al. Effect and safety of spinal cord stimulation for treatment of chronic pain caused by diabetic neuropathy. J Diabetes Complications. 2009;23(1):40-45. Epub Apr 16, 2008
29 Augustinsson LE. Spinal cord electrical stimulation in severe angina pectoris: surgical technique, intraoperative physiology, complications, and side effects. Pacing Clin Electrophysiol. 1989;12:693-694.
30 de Jongste MJ, et al. Effects of spinal cord stimulation on myocardial ischaemia during daily life in patients with severe coronary artery disease. A prospective ambulatory electrocardiographic study. Br Heart J. 1994;71:413-418.
31 de Jongste MJ, et al. Efficacy of spinal cord stimulation as adjuvant therapy for intractable angina pectoris: a prospective, randomized clinical study: Working Group on Neurocardiology. J Am Coll Cardiol. 1994;23:1592-1597.
32 Mannheimer C, et al. Epidural spinal electrical stimulation in severe angina pectoris. Br Heart J. 1988;59:56-61.
33 Sanderson JE, et al. Epidural spinal electrical stimulation for severe angina: a study of its effects on symptoms, exercise tolerance and degree of ischaemia. Eur Heart J. 1992;13:628-633.
34 Hautvast RW, et al. Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol. 1996;77:462-467.
35 Meller ST, Gebhart GF. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience. 1992;48:501-524.
36 Thamer V, et al. Pain and myocardial ischemia: the role of sympathetic activation. Basic Res Cardiol. 1990;85(suppl 1):253-266.
37 Linderoth B, Fedorcsak I, Meyerson BA. Is vasodilatation following dorsal column stimulation mediated by antidromic activation of small diameter afferents? Acta Neurochir Suppl (Wien). 1989;46:99-101.
38 Brindley GS, et al. Sacral anterior root stimulators for bladder control in paraplegia: the first 50 cases. J Neurol Neurosurg Psych. 1986;49:1104-1114.
39 Brindley GS, Rushton DN. Long term follow-up of patients with sacral anterior root stimulator implants. Paraplegia. 1990;28:469-475.
40 Van Kerrebroeck PEV, et al. Results of the treatment of neurogenic bladder dysfunction in spinal cord injury by sacral posterior root rhizotomy and anterior sacral root stimulation. J Urol. 1996;155:1378-1381.
41 Van Kerrebroeck PCV, et al. Urodynamic evaluation before and after intradural posterior sacral rhizotomies and implantation of the Finetech-Brindley anterior sacral root stimulator. Urodinamica. 1992;1:7.
42 O’Flynn KJ, et al. The effect of sacral rhizotomy on lower urinary tract function in spinal injury patients. Eur Urol. 1990;18:8.
43 Vallès M, et al. Effect of sacral anterior root stimulator on bowel dysfunction in patients with spinal cord injury. Dis colon rectum. 2009;52(5):986-992.
44 Krainick JU, Thoden U, Riechert T. Pain reduction in amputees by long-term spinal cord stimulation: long-term follow-up study over 5 years. J Neurosurg. 1980;52:346-350.
45 Waltz JM. Computerized percutaneous multi-level spinal cord stimulation in motor disorders. Appl Neurophysiol. 1982;45:73-92.
46 Sharan A. Selective dorsal column activation with three column electrode arrays using percutaneous and paddle leads. Washington, DC: AANS; 2007.
47 North R et al: Spinal cord stimulation electrode design: a prospective randomized comparison of percutaneous and insulated paddle electrodes. Fourth International Congress of the INS, 1998, p 211.
48 Holsheimer J, et al. Clinical evaluation of paresthesia steering with a new system for spinal cord stimulation. Neurosurgery. 1998;42:541-547. discussion 7-9
49 Hornberger J, et al. Rechargeable spinal cord stimulation versus non-rechargeable system for patients with failed back surgery syndrome: a cost-consequences analysis. Clin J Pain. 2008;24(3):244-252.
50 Van Buyten JP, et al. The restore rechargeable, implantable neurostimulator: handling and clinical results of a multicenter study. Clin J Pain. 2008;24(4):325-334.
51 Shealy CN, Cady RK. Historical perspective of pain management. In: Weiner RS, editor. Pain management: a practical guide for clinicians. ed 5. Florida: St Lucie Press; 1998:7-15.
52 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489-491.
53 Hoppenstein R. A device for measuring intracranial pressure. Lancet. 1965;1:90-91.
54 Larson SJ, et al. A comparison between anterior and posterior spinal implant systems. Surg Neurol. 1975;4:180-186.
55 Lazorthes Y, Verdie JC, Arbus L. Anterior and posterior medullary analgesic stimulation, using a percutaneous implantation technic. Acta Neurochir (Wien). 40, 1978. 277–223
56 Dooley DM. Percutaneous electrical stimulation of the spinal cord. Bal Harbour, Fla: Associations of Neurology Surgeons; 1975.
57 Cepeda MS, et al. An N-of-1 trial as an aid to decision-making prior to implanting a permanent spinal cord stimulator. Pain Med. 2008;9(2):235-239.
58 Kreis PG, Fishman SM, Chau K. Impact to spinal cord stimulator lead integrity with direct suture loop ties. Pain Med. 2009;10(3):495-500.
59 Ebel H, et al. Augmentative treatment of chronic deafferentation pain syndromes after peripheral nerve lesions. Minimally Invasive Neurosurg. 2000;43:44-50.
60 Barolat G, Zeme S, Ketcik B. Multifactorial analysis of epidural spinal cord stimulation. Stereotact Funct Neurosurg. 1991;56:77-103.
61 Law JD. Targeting a spinal stimulator to treat the “failed back surgery syndrome”. Appl Neurophysiol. 1987;50:437-438.
62 Law JD. Spinal stimulation in the “failed back surgery syndrome”: comparison of technical criteria for palliating pain in the leg vs. in the low back. Acta Neurochir. 1992;117:95.
63 North R et al: Spinal cord stimulation for axial low back pain: single versus dual percutaneous electrodes, Fourth International Congress of the INS, 1998, p 212.
64 Mannheimer C, et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris: the ESBY study. Circulation. 1998;97:1157-1163.
65 Norrsell H, et al. Effects of spinal cord stimulation and coronary artery bypass grafting on myocardial ischemia and heart rate variability: further results from the ESBY study. Cardiology. 2000;94:12-18.
66 Alò K, et al. Computer assisted and patient interactive programming of dual octrode spinal cord stimulation in the treatment of chronic pain. Neuromodulation. 1998;1:30-45.
67 North R, et al. Patient-interactive microprocessor-controlled neurological stimulation system. Neuromodulation. 1998;1:185-193.
68 He J, et al. Perception threshold and electrode position for spinal cord stimulation. Pain. 1994;59:55-63.
69 Abejón D, et al. Effect of posture on spinal cord stimulation in patients with chronic pain syndromes: analysis of energy requirements in different patient postures. Rev Esp Anestesiol Reanim. 2009;56(5):292-298.
70 North RB, et al. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28:692-699.
71 Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery. 1995;37:1088-1095. discussion 1095-1096
72 Burchiel KJ, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21:2786-2794.
73 De Andrés J, et al. Patient satisfaction with spinal cord stimulation for failed back surgery syndrome. Rev Esp Anestesiol Reanim. 2007;54(1):17-22.
74 North RB, Kidd DH, Piantadosi S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochir Suppl. 1995;64:106-108.
75 Kumar K, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762-770.
76 Turner JA, et al. Spinal cord stimulation for failed back surgery syndrome: outcomes in a workers’ compensation setting. Pain. 2010;148(1):14-25.
77 Wasan AD. Spinal cord stimulation in a workers’ compensation population: how difficult it can be to interpret a clinical trial. Pain. 2010;148(1):3-4.
78 Barolat G, Schwartzman R, Woo R. Epidural spinal cord stimulation in the management of reflex sympathetic dystrophy. Stereotact Funct Neurosurg. 1989;53:29-39.
79 Kumar K, Nath RK, Toth C. Spinal cord stimulation is effective in the management of reflex sympathetic dystrophy. Neurosurgery. 1997;40:503-508. discussion 508-509
80 Kemler MA, et al. Electrical spinal cord stimulation in reflex sympathetic dystrophy: retrospective analysis of 23 patients. J Neurosurg. 1999;90:79-83.
81 Kemler MA, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618-624.
82 Kemler MA, et al. Spinal cord stimulation for chronic reflex sympathetic dystrophy—five-year follow-up. N Engl J Med. 2006;354:2394-2396.
83 Kemler MA, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108(2):292-298.
84 Oakley J, Weiner RL. Spinal cord stimulation for complex regional pain syndrome: a prospective study of 19 patients at two center. Neuromodulation. 1999;2:47-50.
85 Calvillo O, et al. Neuroaugmentation in the treatment of complex regional pain syndrome of the upper extremity. Acta Orthop Belg. 1998;64:57-63.
86 Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254-267.
87 Meglio M, et al. Pain control and improvement of peripheral blood flow following epidural spinal cord stimulation: case report. J Neurosurgery. 1981;54:821-823.
88 Jacobs MJ, et al. Epidural spinal cord electrical stimulation improves microvascular blood flow in severe limb ischemia. Ann Surg. 1988;207:179-183.
89 Klomp HM, et al. Spinal-cord stimulation in critical limb ischaemia: a randomised trial: ESES Study Group. Lancet. 1999;353:1040-1044.
90 Jivegard LE, et al. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischaemia: a prospective randomised controlled study. Eur J Vasc Endovasc Surg. 1995;9:421-425.
91 Guarnera G, Furgiuele S, Camilli S. Spinal cord electric stimulation vs. femoro-distal bypass in critical ischemia of the legs: preliminary results in a randomized prospective study. Minerva Cardioangiol. 1994;42:223-227.
92 Ubbink DT, Vermeulen H: Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia (update of Cochrane Database Syst Rev. 2003;(3):CD004001; PMID: 12917998) Cochrane Database Systematic Rev CD004001, 2005.
93 Pedrini L, Magnon F. Spinal cord stimulation for lower limb ischemic pain treatment. Interactive Cardiovasc Thorac Surg. 2007;6:495-500.
94 Vulink N, et al. The effects of spinal cord stimulation on quality of life in patients with therapeutically chronic refractory angina pectoris. Neuromodulation. 1999;2:33-34.
95 Hautvast RW, et al. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study [see comment]. Am Heart J. 1998;136:1114-1120.
96 Di Pede F, et al. Immediate and long-term clinical outcome after spinal cord stimulation for refractory stable angina pectoris. Am J Cardiol. 2003;91:951-955.
97 Andersen C. Complications in spinal cord stimulation for treatment of angina pectoris: differences in unipolar and multipolar percutaneous inserted electrodes. Acta Cardiol. 1997;52:325-333.
98 Bagger JP, Jensen BS, Johannsen G. Long-term outcome of spinal cord electrical stimulation in patients with refractory chest pain. Clin Cardiol. 1998;21:286-288.
99 Eliasson T, et al. Safety aspects of spinal cord stimulation in severe angina pectoris. Coron Artery Dis. 1994;5:845-850.
100 Sanderson JE, et al. Spinal electrical stimulation for intractable angina–long-term clinical outcome and safety. Eur Heart J. 1994;15:810-814.
101 Eddicks S, et al. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomised study. Heart. 1997;93:585-590.
102 Ekre O, et al. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study (see comment). Eur Heart J. 2002;23:1938-1945.
103 Andersen C, Hole P, Oxhoj H: Will SCS treatment for angina pectoris pain conceal myocardial infraction? Abstracts of the First Meeting of the International Neuromodulation Society. Rome, 1992.
104 Andersen C, Hole P, Oxhoj H. Does pain relief with spinal cord stimulation for angina conceal myocardial infarction? Br Heart J. 1994;71:419-421.
105 Hautvast R. Cardiac nociception in rats-neuronal pathways and the influence of dermal stimulation on conveyance to the central nervous system. Netherlands: Kader, Groningen; 1997.
106 Murray S, et al. Spinal cord stimulation significantly decreases the need for acute hospital admission for chest pain in patients with refractory angina pectoris. Heart. 1999;82:89-92.
107 Ceballos A, et al. Spinal cord stimulation: a possible therapeutic alternative for chronic mesenteric ischaemia. Pain. 2000;87:99-101.
108 Krames E, Mousad DG. Spinal cord stimulation reverses pain and diarrheal episodes of irritable bowel syndrome: a case report. Neuromodulation. 2004;7:82-88.
109 Khan Y, Raza S, Khan E. Application of spinal cord stimulation for the treatment of abdominal visceral pain syndromes: case reports. Neuromodulation. 2005;8:14-27.
110 Tiede JM, et al. The use of spinal cord stimulation in refractory abdominal visceral pain: case reports and literature review. Pain Pract. 2006;6:197-202.
111 Kapural L, et al. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7:440-443.
112 Kapur S, Mutagi H, Raphael J. Spinal cord stimulation for relief of abdominal pain in two patients with familial Mediterranean fever. Br J Anaesth. 2006;97:866-868.
113 Yakovlev AE, Resch BE. Treatment of intractable abdominal pain patient with Bannayan-Riley-Ruvalcaba syndrome using spinal cord stimulation. WMJ. 2009;108(6):323-326.
114 Rosenow JM, et al. Failure modes of spinal cord stimulation hardware. J Neurosurg Spine. 2006;5(3):183-190.