Chapter 7 Spinal Cord Stimulation
Parameter Selection and Equipment Choices
Parameter Selection
Although spinal cord stimulation at its simplest requires only that a cathode be placed in position over cord with a closely spaced or distant anode to complete the circuit, many other factors may enter into play to change the stimulation experience for the patient. The intrinsic anatomy and physiology of a given patient is something that must be understood and considered.1 The selection of a device and its parameters for stimulation is impactful in patients. Most obvious among decisions of device selection is that of the lead or leads comprising the functional array. Array geometry and lead design have been demonstrated to be powerful in fiber selection for stimulation.2–4 In particular, much of the recent lead development has been in designing arrays and leads to exclude stimulation of certain fibers, specifically the segmental fibers, in an effort to be better able to stimulate those desired within the cord.5 The primary example of this is the ability to stimulate the back area rather than the dorsal roots representing the lower quadrants of the abdomen. Other topographic areas of desired stimulation have necessitated the use of alternate targets, such as the sacral nerve roots in patients with pelvic pain disorders.6,7
Much has been said and written about arrays. However, the parameters of stimulation have had relatively little attention.8 Parameters of stimulation include the frequency or number of pulses per second expressed in Hertz (Hz); pulse width (PW), the duration of each pulse expressed in microseconds; and the amplitude or voltage, representing the power output from the generator. Another factor with potential impact on the experience of the patient is the waveform of each pulse. Although stimulation is done through ramped currents, they are essentially square waves; but the recovery phase of each pulse varies. However, the impact of this variance is less quantifiable because waveforms vary from company to company and may also vary within a given company’s family of devices. Other factors such as constant current vs. constant voltage variances make objective comparisons between one waveform and the next difficult.
To produce paresthesia, Aβ fibers should be stimulated. These lie in the dorsal columns of the spinal cord and have a somatotopic arrangement with the most caudal segments arranged medially, whereas the more cephalad fibers present laterally (Fig. 7-1). Activation of nerve fibers depends on current density, pulse frequency, and specific fiber sensitivity to stimulation. Current density at a target is a function of power output, frequency, and distance to the target. Fiber characteristics that are most impactful to depolarization are size and resting membrane potential and curvature to or from the field.9 All other things being equal, larger fibers depolarize more readily than smaller fibers. Notably within the spinal cord, different tracts have varying sensitivities to current than others.
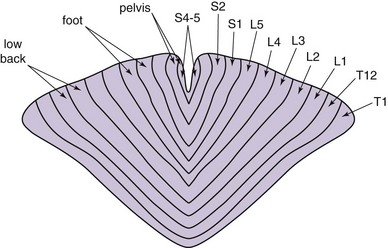
Fig. 7-1 Topographic arrangement of nerve fibers in the dorsal column.
(Adapted from Feirabend HKP et al: Morphometry of human superficial dorsal and dorsolateral column fibres: significance to spinal cord stimulation, Brain 125(5):1137-1149, 2002.)
Because the descending sympathetic tracts (intermediolateral fasciculus) are relatively sensitive to stimulation, it is reasonable to consider that pain relief secondary to stimulation may be related to direct inhibition of these pathways rather than large-fiber afferent activation. It is known that efficacious stimulation in patients treated for angina pectoris may be delivered below sensory perceptual threshold.10 Similarly, consideration for the use of stimulation below sensory threshold must be given in those patients who suffer from other conditions in which modulation of the sympathetic nervous system plays a part. Examples of this include complex regional pain syndrome (CRPS) 1 with predominant sympathetically maintained pain (SMP) and Raynaud disease. Some have theorized that attention to the patient’s response to sympathetic blocks may predict the outcome from stimulation below sensory perception. This theory has not yet been proven in clinical practice.
Frequency is the parameter that has been easiest to study. Case studies report benefit in many patients achieved by using “high frequencies” to treat when “normal frequencies” have failed.8 Anecdotal experience shows that most patients prefer frequencies of stimulation of 50 Hz ± 10 Hz. This perception has been supported by Gordon and associates.11 In some patients with CRPS 1, frequencies have been required that exceed 250 Hz to achieve and or maintain satisfactory outcome. The physiological impact of stimulations as high as 1200 Hz is not well understood. It is conceivable that high-frequency stimulation functions by blocking transmission, perhaps by keeping neurons in their relative refractory period. This would be a frequency-dependent mechanism, as opposed to increasing signal throughput at more conventional frequency ranges (100 ± 50 Hz). The need in some patients for high-frequency stimulation, which is not necessarily predictable based on a trial of stimulation, presents a potential problem if a properly selected generator has not been implanted. Fortunately most current-generation rechargeable generators and some conventional batteries and radiofrequency units are capable of producing high-frequency rates. The obvious disadvantage of conventional batteries in a high-frequency setting is that of early depletion. It would be advisable to consider rechargeable power sources or transcutaneous induction generators that are capable of high-frequency stimulation in patients who suffer with pain from CRPS 1.
Equipment Choices
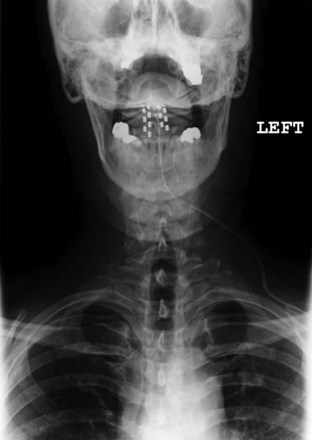
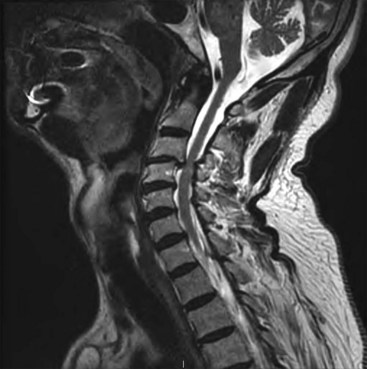
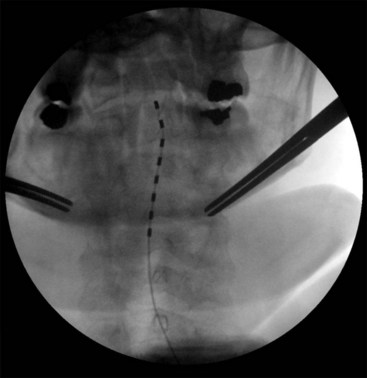
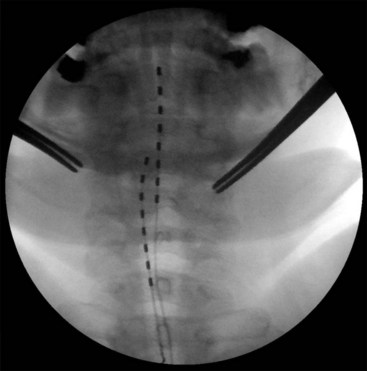
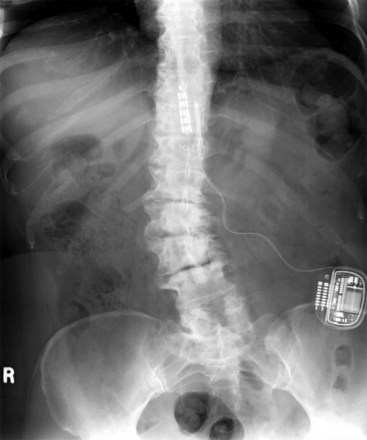
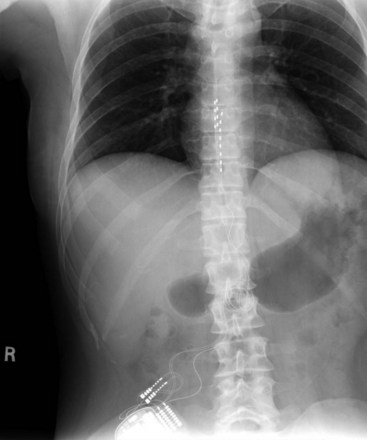
Migration and positional stimulation is problematic in the cervical spine; thus it is advantageous to use retrograde paddle leads under C1-C2 in these cases (Fig. 7-2).12 The use of paddle leads below the disc space of C2 should be avoided if possible because of concern for both acute and subacute acquired spinal stenosis and resultant paresis or plegia. Preoperative evaluation with magnetic resonance imaging or myelography is essential to the proper evaluation of the cervical canal both before placing a paddle lead and before placing a percutaneous lead (Fig. 7-3). In certain cases percutaneous implantation in the cervical spine may be beneficial for another reason. The patient may prefer stimulation carefully limited to his or her pain area but physiologically may also need a centrally placed lead to help with his or her underlying pathophysiology (Figs. 7-4 and 7-5).
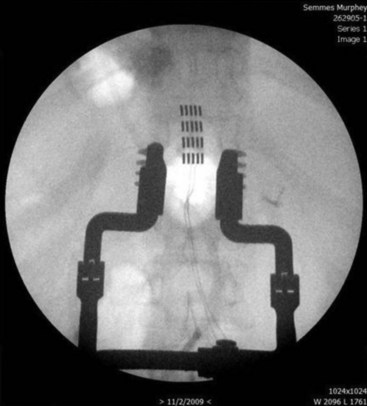
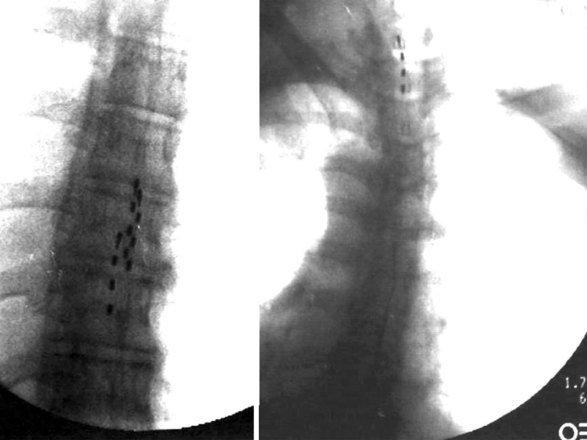
Strategies to improve coverage of the back include multilead methods such as tripolar lead configurations (Fig. 7-6). This helps the implanter produce low back coverage by truncating the field between the dorsal rootlets and hyperpolarizing the nerve roots, thus reducing radicular stimulation. Although these arrays may be created with percutaneous leads, maintaining proper alignment of three independent percutaneous catheters can be quite challenging (Fig. 7-7). In contrast, creating the same array with a tripolar paddle lead is straightforward, with the caveat that the lead must be placed so it properly straddles the midline. Recently a five-column lead has become available that offers the user the ability to selectively stimulate dermatomes in the low back and lower extremities through the use of small textured contacts that allow both aggressive field truncation and anodal hyperpolarization (Fig. 7-8).2 In spite of the sophistication of these paddle leads, without question the percutaneous array has the edge in treating distant multifocal pain topographies (Fig. 7-9).
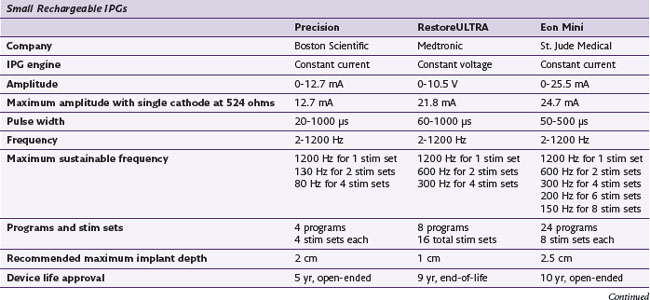
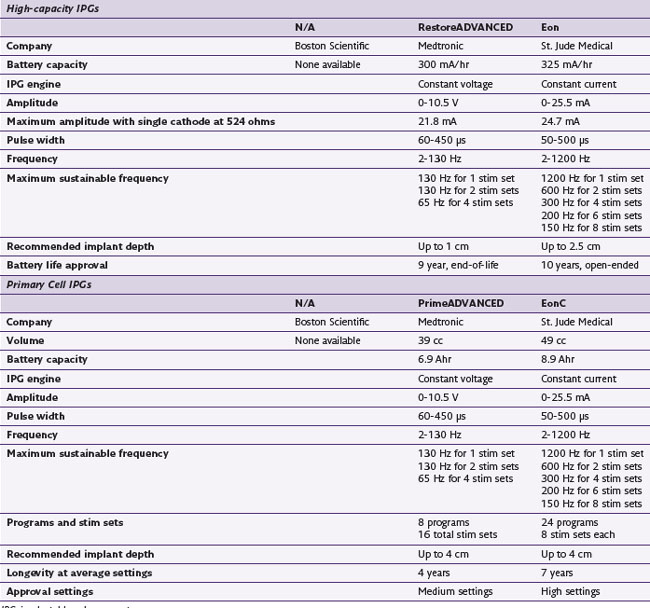
The history of generators available for SCS is relevant to the topic of parameters because not all generators have historically been capable of the same parametric variance. Frequencies in particular have varied substantially from device to device. Some generators have been limited to frequencies less than 130 Hz, excluding the possibility of high-frequency stimulation as an option in patients who are not responding favorably to permanent implants (Table 7-1). One would assume that frequency limitations are related to the use of conventional batteries and that high frequencies would be available with rechargeable devices, but neither of these assumptions is correct. Furthermore, frequency capability changes with the addition of programs or stim sets (depending on the manufacturer’s terminology). An implanter should know the capabilities of the devices that they implant so they may provide the most appropriate care for their patients.
Patient Management/Evaluation
It would seem empirically true that prediction of a patient’s need for high frequency stimulation would be reliable based on that given patient’s trial of SCS. However, this is not the case. During the trial, patients usually do not have their stimulation optimized, and trials are rarely long enough for a patient to truly understand the limitations of conventional frequencies in their pain modulation. Optimization and realization take time. On the basis of the literature, it is reasonable to use a high-frequency system in patients who are being treated for CRPS 1. These patients in particular may need this system capability. Not having the opportunity to stimulate at high frequency may result in a suboptimal or nonexistent response to stimulation. There are less data regarding CRPS 2 patients. Bennett and associates8 report the use of frequencies greater than 250 Hz but do not identify the treatment population physiologically. It is not unreasonable to hypothesize that some of these patients might have CRPS 2. While SCS as a modality to help patients with mechanical back pain is not well supported,13 consideration must be given to a trial of high-frequency stimulation in these patients to potentially block C-fiber transmission rather than the more conventional mechanistic concept of large-fiber activation to reduce throughput at the level of the gate.
1 Barolat G. Epidural spinal cord stimulation: anatomical and electrical properties of the intraspinal structures relevant to spinal cord stimulation and clinical correlations. Neuromodulation. 1998;1:63-71.
2 Feler C, Garber J: Selective dermatome activation using a novel five-column spinal cord stimulation paddle lead: a case series, NANS poster presentation, 2009.
3 Struijk JJ, Holsheimer J, Boom HBK. Excitation of dorsal root fibers in spinal cord stimulation: a theoretical study. IEEE Trans Biomed Eng. 1993;40:632-639.
4 Struijk JJ, et al. Theoretical performance and clinical evaluation of transverse tripolar spinal cord stimulation. IEEE Trans Rehab Eng. 1998;6:277-285.
5 Smith S: Stimulation coverage of transverse tripole programming using the Lamitrode Tripole 16c surgical lead: preliminary evaluation from one clinic in a prospective, multi-centered, post-market study, NANS 2007.
6 Feler C, et al. Recent advances: sacral nerve root stimulation using a retrograde method of lead insertion for the treatment of pelvic pain due to interstitial cystitis. Neuromodulation. 1999;2:211-216.
7 Feler C, Whitworth LA, Fernandez J. Sacral neuromodulation for chronic pain conditions. Anesth Clin North Am. 2003;21:785-791.
8 Bennett DS, et al. Spinal cord stimulation for complex regional pain syndrome I (RSD): a retrospective multicenter experience from 1995 to 1998 of 101 patients. Neuromodulation. 1999;2:202-210.
9 Holsheimer J, Struijk JJ, Tas NR. Effects of electrode geometry and combination on nerve fibre selectivity in spinal cord stimulation. Med Biol Eng Comput. 1995;33(5):676-682.
10 Wesselink W, Holsheimer J, Boom H. Analysis of current density related parameters in spinal cord stimulation. IEEE Trans Rehab Eng. 1998;6(2):200-220.
11 Gordon A, et al. Challenges to setting spinal cord stimulator parameters during intraoperative testing: factors affecting coverage of low back and leg pain. Neuromodulation. 2007;7(10):133-141.
12 Whitworth LA, Feler CA. C1-C2 Sublaminar insertion of paddle leads for the management of chronic painful conditions of the upper extremity. Neuromodulation. 2003;6(3):153-157.
13 Barolat G, et al. Epidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back pain. Neuromodulation. 2001;4(2):59-66.