CHAPTER 18 Spinal cord: internal organization
The spinal cord provides innervation for the trunk and limbs via spinal nerves and their peripheral ramifications. It receives primary afferent fibres from peripheral receptors located in widespread somatic and visceral structures, and sends motor axons to skeletal muscle. It also contains the cell bodies of all the preganglionic neurones responsible for the sympathetic innervation of cardiac and smooth muscle and secretory glands, and for the parasympathetic innervation of smooth muscle in the distal part of the hind gut, the pelvic viscera and the erectile tissues of the external genitalia. Many bodily functions are regulated at an unconscious level by intraspinal reflex connections between afferent and efferent neurones. Profuse ascending and descending pathways link the spinal cord with the brain, allowing higher centres to monitor and perceive external and internal stimuli and to modulate and control spinal efferent activity.
EXTERNAL FEATURES AND RELATIONS
The topographical anatomy of the spinal cord, its external features and relations are described in more detail in Chapter 43. In brief, the cord lies within the vertebral canal. It is continuous rostrally with the medulla oblongata, just below the level of the foramen magnum (Fig. 28.11) and it terminates caudally as the conus medullaris, which is continuous with the filum terminale and is anchored to the dorsum of the coccyx. The cord is ensheathed by spinal meninges which are continuous with the cranial meninges through the foramen magnum. Although it is approximately circular in cross-section, the diameter of the spinal cord varies according to level; it bears two enlargements, cervical and lumbar.
The spinal cord is essentially a segmental structure, giving rise to 31 bilaterally-paired spinal nerves. These attach to the cord as linear series of smaller dorsal and ventral nerve rootlets. Dorsal rootlets contain afferent nerve fibres and ventral rootlets contain efferent fibres (see Fig. 15.4). Groups of adjacent rootlets coalesce to form dorsal or ventral nerve roots that cross the subarachnoid space and unite to form functionally mixed spinal nerves as they pass through the intervertebral foramina. The dorsal roots bear dorsal root ganglia that contain the cell bodies of primary afferent neurones.
INTERNAL ORGANIZATION
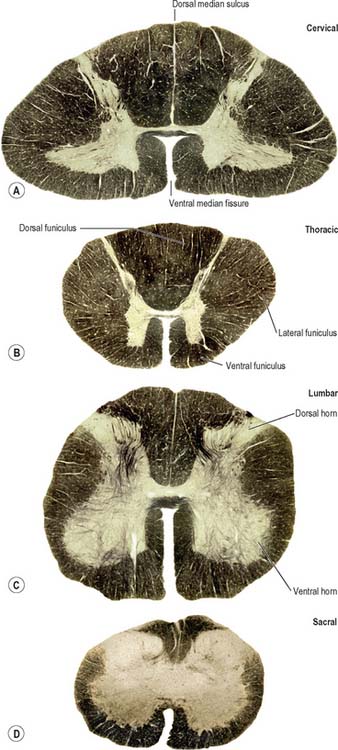
In transverse section, the spinal cord is incompletely divided into symmetrical halves by a dorsal (posterior) median septum and a ventral (anterior) median sulcus (Fig. 18.1). It consists of an outer layer of white matter and an inner core of grey matter; their relative sizes and configuration vary according to level. The amount of grey matter reflects the number of neurones present; it is proportionately largest in the cervical and lumbar enlargements, which contain the neurones that innervate the limbs. The absolute amount of white matter is greatest at cervical levels, and decreases progressively at lower levels, because descending tracts shed fibres as they descend and ascending tracts accumulate fibres as they ascend.
SPINAL GREY MATTER
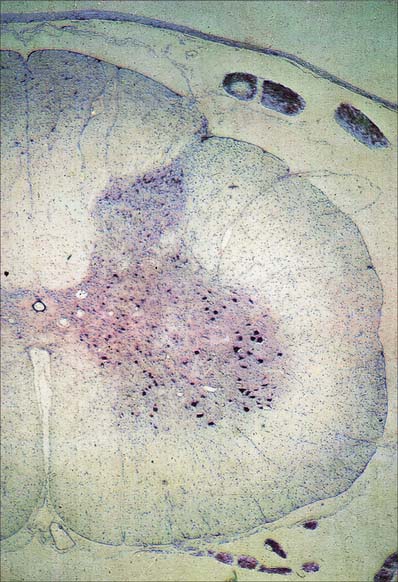
In three dimensions, the spinal grey matter is shaped like a fluted column (Fig. 43.1F). In transverse section the column is often described as being ‘butterfly-shaped’ or resembling the letter ‘H’ (Fig. 18.1). It consists of four linked cellular masses, the right and left dorsal and ventral horns, that project dorsolaterally and ventrolaterally towards the surface respectively. The grey matter that immediately surrounds the central canal and unites the two sides constitutes the dorsal and ventral grey commissures. The dorsal horn is the site of termination of the primary afferent fibres that enter the cord via the dorsal roots of spinal nerves. The tip of the dorsal horn is separated from the dorsolateral surface of the cord by a thin fasciculus or tract (of Lissauer) in which primary afferent fibres ascend and descend for a short distance before terminating in the subjacent grey matter. The ventral horn contains efferent neurones whose axons leave the spinal cord in ventral nerve roots. A small intermediate, or lateral, horn is present at thoracic and upper lumbar levels; it contains the cell bodies of preganglionic sympathetic neurones.
Spinal grey matter (Fig. 18.2) is a complex mixture of neuronal cell bodies, their processes and synaptic connections, neuroglia and blood vessels. Neurones in the grey matter are multipolar. They vary in size and features such as the length and the arrangement of their axons and dendrites. Neurones may be intrasegmental, i.e. contained within a single segment, or intersegmental, i.e. their ramifications spread through several segments.
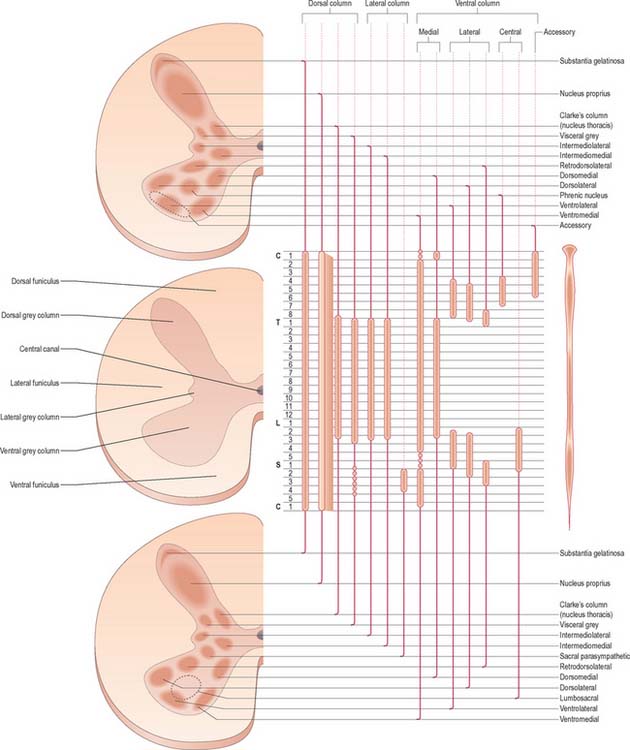
Neuronal cell groups of the spinal cord
Laminae V and VI lie at the base of the dorsal horn. They receive most of the terminals of proprioceptive primary afferents, profuse corticospinal projections from the motor and sensory cortex and input from subcortical levels, suggesting their involvement in the regulation of movement. Lamina V is a thick layer, divisible into a lateral third and medial two-thirds. Both have a mixed cell population but the former contains many prominent well-staining somata interlaced by numerous bundles of transverse, dorsoventral and longitudinal fibres. Lamina VI is most prominent in the limb enlargements. It has a densely staining medial third of small, densely packed neurones and a lateral two-thirds containing larger, more loosely packed, triangular or stellate somata.
Laminae VII–IX show a variety of forms in the limb enlargements. Lamina VII includes much of the intermediate (lateral) horn. It contains prominent neurones of Clarke’s column (nucleus dorsalis, nucleus thoracis, thoracic nucleus) and intermediomedial and intermediolateral cell groupings (Fig. 18.3). The lateral part of lamina VII has extensive ascending and descending connections with the midbrain and cerebellum (via the spinocerebellar, spinotectal, spinoreticular, tectospinal, reticulospinal and rubrospinal tracts) and is thus involved in the regulation of posture and movement. Its medial part has numerous propriospinal reflex connections with the adjacent grey matter and segments concerned both with movement and autonomic functions. Lamina VIII spans the base of the thoracic ventral horn but is restricted to its medial aspect in limb enlargements. Its neurones display a heterogeneous mixture of sizes and shapes from small to moderately large. Lamina VIII is a mass of propriospinal interneurones. It receives terminals from the adjacent laminae, many commissural fibres from the contralateral lamina VIII, and descending connections from the interstitiospinal, reticulospinal and vestibulospinal tracts and the medial longitudinal fasciculus. The axons from these interneurones influence α motor neurone activity bilaterally, perhaps directly but more probably by excitation of small γ motor neurones supplying efferent fibres to muscle spindles. Lamina IX is a complex array of cells consisting of α and γ motor neurones and many interneurones. The large α motor neurones supply motor end-plates of extrafusal muscle fibres in striated muscle. Recording techniques have demonstrated tonic and phasic α motor neurones. The former have a lower rate of firing and lower conduction velocity and tend to innervate type S muscle units. The latter have higher conduction velocity and tend to supply fast twitch (type FR, FF) muscle units. The smaller γ motor neurones give rise to small-diameter efferent axons (fusimotor fibres), which innervate the intrafusal muscle fibres in muscle spindles. There are several functionally distinct types of γ motor neurone. The ‘static’ and ‘dynamic’ responses of muscle spindles have separate controls mediated by static and dynamic fusimotor fibres, which are distributed variously to nuclear chain and nuclear bag fibres.
Lamina X surrounds the central canal and consists of the dorsal and ventral grey commissures.
Dorsal horn
The dorsal horn is a major zone of termination of primary afferent fibres, which enter the spinal cord through the dorsal roots of spinal nerves. Dorsal root fibres contain numerous molecules, which are either known, or suspected, to fulfil a neurotransmitter or neuromodulator role. These include glutamic acid, substance P, calcitonin gene-related peptide (CGRP), bombesin, vasoactive intestinal polypeptide (VIP), cholecystokinin (CCK), somatostatin, dynorphin and angiotensin II. Dorsal root afferents carry exteroceptive, proprioceptive and interoceptive information. Laminae I–IV are the main cutaneous receptive areas; lamina V receives fine afferents from the skin, muscle and viscera; lamina VI receives proprioceptive and some cutaneous afferents. Most, if not all, primary afferent fibres divide into ascending and descending branches on entering the cord. These then travel for variable distances in the tract of Lissauer, near the surface of the cord, and send collaterals into the subjacent grey matter. The formation, topography and division of dorsal spinal roots have all been confirmed in man.
The lamina marginalis is a thin lamina of neurones at the dorsolateral tip of the dorsal horn, deep to the tract of Lissauer. Beneath it lies the substantia gelatinosa (laminae II and III), which is present at all levels, and consists mostly of small neurones, together with some larger neurones. The substantia gelatinosa receives afferents via the dorsal roots, and its neurones give rise to fibres that form the contralateral spinothalamic tract. The large propriospinal neurones of the nucleus proprius lie ventral to the substantia gelatinosa; they link segments for the mediation of intraspinal coordination (Fig. 18.3).
Ventral horn
Considered longitudinally, ventral horn neurones are arranged in elongated groups, and form a number of separate columns, which extend through several segments. These are seen most easily in transverse sections. The ventral horn may be divided into medial, central and lateral cell columns, which all exhibit subdivision at certain levels, usually into dorsal and ventral parts (Fig. 18.3). The medial group extends throughout the cord, but may be absent in the fifth lumbar and first sacral segments. In the thoracic and the upper four lumbar segments, it is subdivided into ventromedial and dorsomedial groups. In segments cranial and caudal to this region, the medial group has only a ventromedial moiety, except in the first cervical segment, where only the dorsomedial group exists.
The central group of cells is the least extensive, and is found only in some cervical and lumbosacral segments. The centrally situated phrenic nucleus, containing the motor neurones that innervate the diaphragm, lies in the third to seventh cervical segments. An irregular accessory group of neurones in the upper five or six cervical segments at the ventral border of the ventral horn give rise to axons that are thought to enter the spinal accessory nerve (Fig. 18.3).
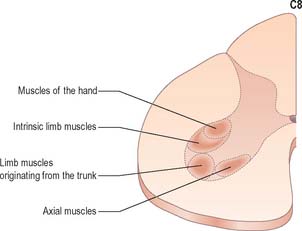
The motor neurones of the ventral horn are somatotopically organized. The basic arrangement is that medial cell groups innervate the axial musculature, and lateral cell groups innervate the limbs. The basic building block of the somatic motor neuronal populations is represented by a longitudinally disposed group of neurones, which innervate a given muscle, and in which the α and γ motor neurones are intermixed. The various groups innervating different muscles are aggregated into two major longitudinal columns, medial and lateral. In transverse section these form the medial and lateral cell groups in the ventral horn (Fig. 18.4).
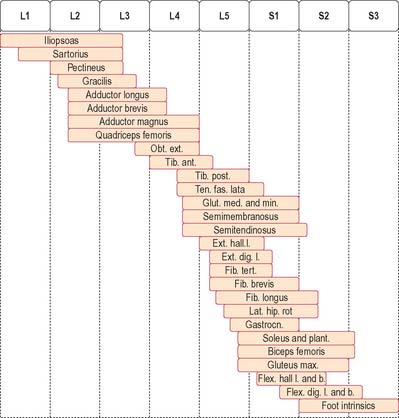
The lateral longitudinal motor column is found only in the enlargements of the spinal cord. The motor neurones in this column in the cervical and lumbar enlargements innervate muscles of the upper and lower limbs, respectively. In the cervical enlargement, motor neurones which supply muscles intrinsic to the upper limb are situated dorsally in the ventral grey column, and those innervating the most distal (hand) muscles are sited further dorsally. Motor neurones of the girdle muscles lie in the ventrolateral part of the ventral horn. There is a further somatotopic organization in that the proximal muscles of the limb are supplied from motor cell groups located more rostrally in the enlargement than those supplying the distal muscles. For example, motor neurones innervating intrinsic muscles of the hand are sited in segments C8 and T1, while motor neurones of shoulder muscles are in segments C5 and 6. A similar overall arrangement of motor neurones innervating lower limb muscles applies in the lumbosacral cord (Fig. 18.5).
Spinal reflexes
Stretch reflex
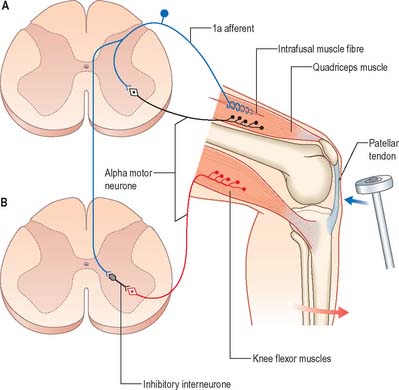
The stretch reflex is the mechanism by which stretch applied to a muscle elicits its reflex contraction. It is essential for the maintenance of both muscle tone and an upright stance (via the innervation of the postural muscles of the neck, back and lower limbs). Anatomically it is the simplest of reflexes, since it is mediated solely by an afferent and an efferent neurone. The afferent component arises from stretch receptors associated with intrafusal muscle fibres located within muscle spindles. The primary or annulospiral endings of these receptive cells give rise to primary afferent fibres which enter the spinal cord, where they make excitatory synaptic contact directly onto α motor neurones innervating the same muscle (Fig. 18.6). The α motor neurones of antagonistic muscles are simultaneously inhibited via collateral connections to inhibitory interneurones.
Gamma reflex
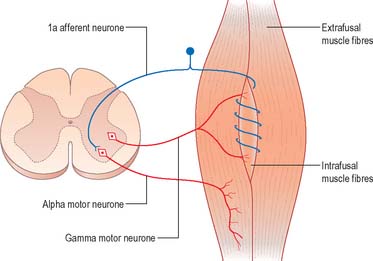
As well as α motor neurones innervating extrafusal muscle fibres, muscles also receive γ motor neurones, which innervate intrafusal muscle fibres. Activation of γ motor neurones increases the sensitivity of the intrafusal fibres to stretch (Fig. 18.7). Therefore, changes in γ activity have a profound effect upon the stretch reflex and upon muscle tone. Like α motor neurones, γ motor neurones are under the influence of descending pathways from the brain stem and cerebral cortex. Changes in the activity of the stretch reflex and of muscle tone are commonly found in disorders of the CNS as well as the PNS.
Flexor reflex
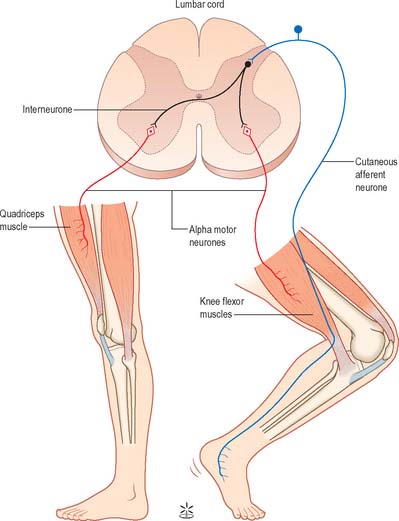
Painful stimulation of the limbs leads to flexion withdrawal, that is mediated by a polysynaptic reflex (Fig. 18.8) in which interneurones link the afferent and efferent neurones. Thus, activation of nociceptive primary afferents indirectly causes activation of limb flexor motor neurones. Collateralization of fibres to nearby spinal segments mediates flexion of a limb at several joints, depending on the intensity of the stimulus. Decussating connections to the contralateral side of the cord activate α motor neurones innervating corresponding extensor muscles, which produces the so-called crossed extensor reflex. In principle, virtually any cutaneous stimulus has the potential to induce a flexor reflex, but, other than in the case of noxious stimuli, this response is normally inhibited by descending pathways. When descending influences are lost, even harmless cutaneous stimulation can elicit flexion of the limbs. The Babinski (extensor plantar) reflex, which is generally regarded as pathognomonic of damage to the corticospinal tract, is part of a flexion withdrawal of the lower limb in response to stimulation of the sole of the foot.
SPINAL WHITE MATTER
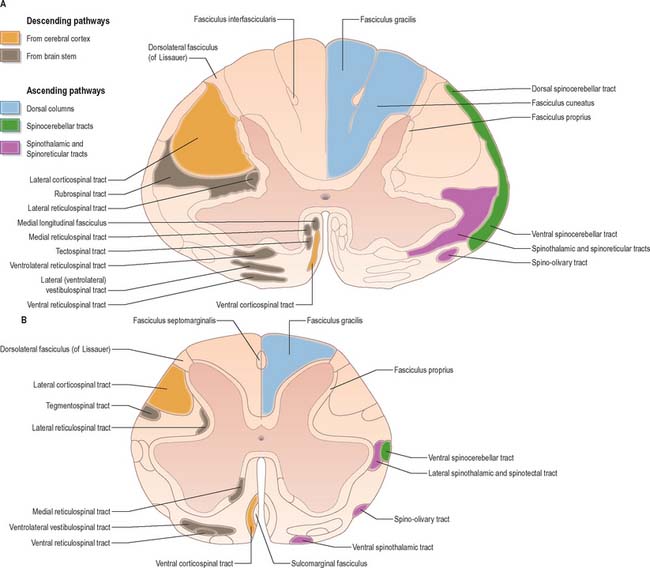
The spinal white matter is conventionally described as being arranged into three large, bilaterally paired masses, the dorsal, lateral and ventral funiculi, each of which contains a number of predominantly specific tracts (see Fig. 18.1 and Fig. 18.9). Narrow dorsal and ventral white commissures run between the two halves of the cord.
Whilst the ascending and descending tracts are to a large extent discrete and regularly located, significant overlap between adjacent tracts does occur. Their general disposition is shown in Fig. 18.9.
Ascending pathways
Dorsal columns
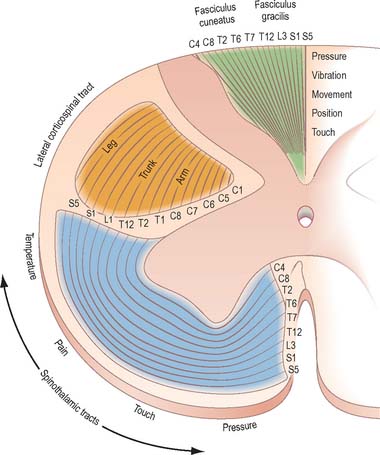
The dorsal funiculus consists of two large ascending tracts, the fasciculus gracilis and fasciculus cuneatus (Fig. 18.10), that are also known as the dorsal columns. They are separated by a posterointermediate septum. The dorsal columns contain a high proportion of myelinated fibres carrying proprioceptive (position sense and kinaesthesia), exteroceptive (touch-pressure) and vibratory sensation to higher levels. These fibres come from several sources: long primary afferent fibres which enter the cord in the dorsal roots of spinal nerves and ascend to the dorsal column nuclei in the medulla oblongata; shorter primary afferent fibres projecting to neurones of Clarke’s column and other spinal neurones; axons from secondary neurones of the spinal cord ascending to the dorsal column nuclei. The dorsal columns also contain axons of propriospinal neurones.
The fasciculus gracilis begins at the caudal end of the spinal cord. It contains long ascending branches of primary afferents, which enter the cord through ipsilateral dorsal spinal roots and ascending axons of secondary neurones in laminae IV to VI of the ipsilateral dorsal horn. As the fibres ascend, they are joined by axons of successive dorsal roots. Fibres entering in coccygeal and lower sacral regions are shifted medially by successive additions of fibres entering at higher levels.
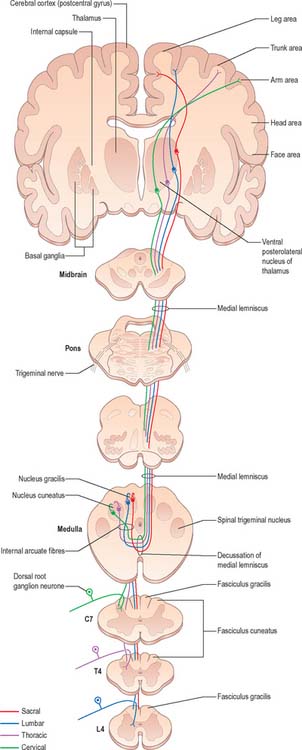
The fasciculus gracilis lies medial to the fasciculus cuneatus in the upper spinal cord (Fig. 18.9). At upper cervical levels the fasciculus gracilis contains a larger proportion of afferents from cutaneous receptors than from deep proprioceptors because many of the latter leave the fasciculus at lower segments to synapse in Clarke’s column. Indeed, proprioception from the lower limb mostly reaches the thalamus by relaying in Clarke’s column and then again in nucleus Z (p. 280). Axons of the fasciculus gracilis, from both primary and secondary neurones, terminate in the nucleus gracilis of the dorsal medulla.
The fasciculus cuneatus (Fig. 18.9) begins at midthoracic level and lies lateral to the fasciculus gracilis. It is composed mostly of primary afferent fibres of the upper thoracic and cervical dorsal roots. At upper cervical levels it contains a large population of afferents from both deep and cutaneous receptors of the upper limb. In addition, some of its axons arise from secondary neurones in laminae IV–VI of the ipsilateral dorsal horn. Many axons (both primary and secondary) that ascend in the fasciculus cuneatus terminate in the nucleus cuneatus of the dorsal medulla. Some also end in the lateral (external or accessory) cuneate nucleus; neurones in this nucleus project to the cerebellum via the cuneocerebellar pathway.
Many ascending fibres of the fasciculus gracilis and fasciculus cuneatus terminate by synapsing on neurones of the dorsal column nuclei (nucleus gracilis and nucleus cuneatus, respectively) in the medulla oblongata. (The connections of the dorsal column nuclei are described further with the medulla oblongata, p. 280.) Axons arising from neurones in the dorsal column nuclei arch ventromedially round the central grey matter of the medulla as internal arcuate fibres (Fig. 19.5) and decussate in the great sensory decussation (decussation of the medial lemniscus) to form the medial lemniscus itself. They ascend to the ventral posterolateral nucleus of the thalamus, from where neurones project to the somatosensory cortex in the postcentral gyrus of the parietal lobe (areas 3, 1 and 2). Some neurones of the dorsal column nuclei form posterior external arcuate fibres which enter the cerebellum.
Spinocerebellar tracts
There are two principal spinocerebellar tracts: dorsal (posterior) and ventral (anterior). They occupy the periphery of the lateral aspect of the spinal white matter (Fig. 18.9, Fig. 18.11) and carry proprioceptive and cutaneous information to the cerebellum for the coordination of movement. Both tracts contain large-diameter myelinated fibres, but there are more in the dorsal tract. Finer-calibre fibres are associated with the ventral tract.
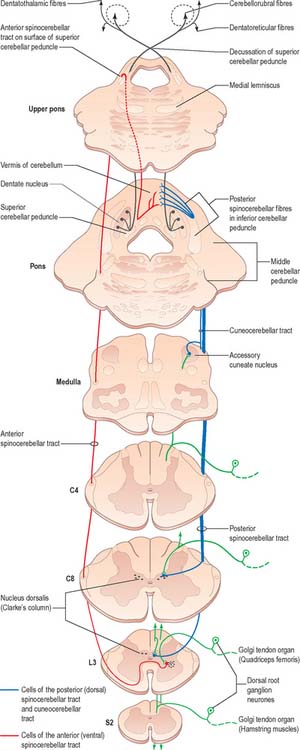
Fig. 18.11 The spinocerebellar tracts.
(Redrawn from Carpenter MB 1991 Core Text of Neuroanatomy, 4th edn. Baltimore: Williams and Wilkins, by permission of author and publisher.)
The spinocerebellar tracts are organized such that fibres from lower spinal segments are most superficial. Both tracts convey proprioceptive and exteroceptive information, but they are functionally different. Neurones of Clarke’s column are excited monosynaptically by Ia and Ib primary afferent fibres (from muscle spindles and tendon organs, respectively) and also by group II muscle afferents, and cutaneous touch and pressure afferents. The proprioceptive impulses often arise from a single muscle or from synergistic muscles acting at a common joint. Thus, the dorsal spinocerebellar tract transmits modality-specific and space-specific information that is used in the fine coordination of individual limb muscles. On the other hand, the cells of the ventral tract are activated monosynaptically by Ib afferents and transmit information from large receptive fields that include different segments of a limb. The ventral tract lacks subdivisions for different modalities and transmits information for the coordinated movement and posture of the entire lower limb.
Since Clarke’s column diminishes rostrally (Fig. 18.3) and does not extend above the lowest cervical segment, it follows that the dorsal spinocerebellar tract carries information from the trunk and lower limb. Proprioceptive and exteroceptive information from the upper limb travel in primary afferent fibres of the fasciculus cuneatus. These fibres end somatotopically in the accessory (external or lateral) cuneate nucleus and the adjoining part of the cuneate nucleus situated in the medulla oblongata. Cells of these nuclei give rise to the posterior external arcuate fibres that form the cuneocerebellar tract (Fig. 18.11), which enters the cerebellum via the ipsilateral inferior cerebellar peduncle. The accessory cuneate nucleus and the lateral part of the cuneate nucleus are considered to be homologous to the cells of Clarke’s column. The cuneocerebellar tract is, therefore, functionally allied to the dorsal spinocerebellar tract, and is its upper limb equivalent.
Axons of all the spinocerebellar tracts and the cuneocerebellar tract form part of the ‘mossy-fibre system’. They end in the cerebellar cortex in a highly organized, somatotopical and functional pattern (p. 302).
Spinothalamic tracts
The spinothalamic tracts (Fig. 18.9) consist of second-order neurones which convey pain, temperature, coarse (non-discriminative) touch and pressure information to the somatosensory region of the thalamus. The cells of origin lie in various laminae of all segments of the spinal cord. Fibres decussate in the ventral white commissure to reach the contralateral spinothalamic tracts: pain and temperature fibres do so promptly, within about one segment of their origin, whilst fibres carrying other modalities may ascend for several segments before crossing. Spinothalamic fibres mostly ascend in the white matter ventrolateral to the ventral horn, partly intermingled with ascending spinoreticular fibres and descending reticulospinal fibres. Some authorities describe two spinothalamic tracts (lateral and ventral) with more-or-less distinct anatomical locations and functions. However, physiological studies in animals support the notion that these tracts may be best considered as a structural and functional continuum.
The lateral spinothalamic tract (Fig. 18.12) is sited in the lateral funiculus, lying medial to the ventral spinocerebellar tract. Clinical evidence indicates that it subserves pain and temperature sensations. The ventral spinothalamic tract (Fig. 18.13) lies in the anterior funiculus medial to the point of exit of the ventral spinal nerve roots and dorsal to the vestibulospinal tract, which it overlaps. On the basis of clinical evidence, it subserves coarse tactile and pressure modalities.
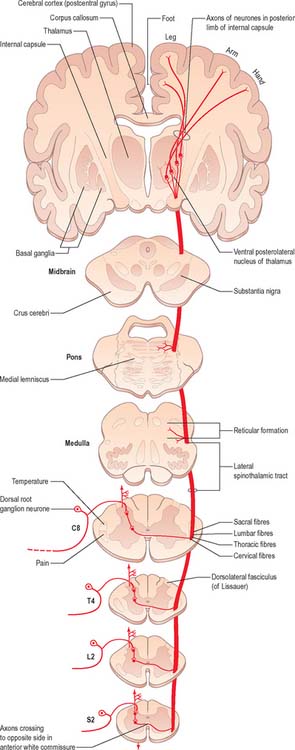
Fig. 18.12 The lateral spinothalamic tract.
(Redrawn from Carpenter MB 1991 Core Text of Neuroanatomy, 4th edn. Baltimore: Williams and Wilkins, by permission of author and publisher.)
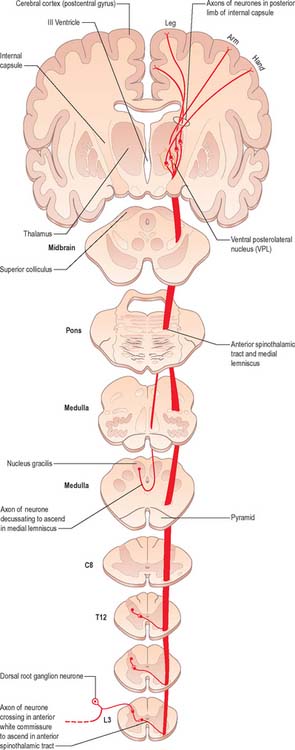
Fig. 18.13 The ventral (anterior) spinothalamic tract.
(Redrawn from Carpenter MB 1991 Core Text of Neuroanatomy, 4th edn. Baltimore: Williams and Wilkins, by permission of author and publisher.)
There is clear somatotopic organization of the fibres in the spinothalamic tracts throughout their extent. Fibres crossing at any cord level join the deep aspect of those that have already crossed, which means that both tracts are segmentally laminated (Fig. 18.14). Somatotopy is maintained throughout the medulla oblongata and pons. In the midbrain, fibres in the spinal lemniscus conveying pain and temperature sensation from the lower limb extend dorsally, while those from the trunk and upper limb are more ventrally placed. Both lemnisci ascend to end in the thalamus. The major spinothalamic projections in man are to the ventral posterolateral nucleus, and also to the centrolateral intralaminar nucleus.
Neurones of the spinothalamic tracts
Neurones of the spinothalamic tracts have very different receptive fields. Specificity of separate channels, as it exists in the dorsal column nuclei, is absent in the laminae of the cord. Convergence of different functional types of afferent fibres onto an individual tract cell is a common feature in the cord. On the basis of laminar site, functional properties, and specific thalamic termination of their axons, spinothalamic tract neurones may be divided into three separate groups. These are the apical cells of the dorsal grey column (lamina I), deep dorsal column cells (laminae IV–VI), and cells in the ventral grey column (laminae VII, VIII). There are species differences and the description below is derived from studies in non-human primates.
Lamina I cells which project to the thalamus show the following characteristics. In essence they respond maximally to noxious or thermal cutaneous stimulation, and consist mainly of high-threshold, but also some wide-dynamic-range, units. Their receptive fields are usually small, representing a part of a digit or a small area of skin involving several digits. Lamina I spinothalamic tract neurones receive input from Aδ and C fibres, and some respond to convergent input from deep somatic and visceral receptors. Spinothalamic tract cells in the thoracic cord display marked viscerosomatic convergence. Lamina I spinothalamic tract neurones project preferentially to the ventral posterolateral nucleus of the thalamus, with limited projections to the centrolateral and mediodorsal thalamic nuclei. The population of deep dorsal column (laminae IV–VI) spinothalamic neurones of the lumbar cord contains wide-dynamic-range (60%), high-threshold (30%), and low-threshold (10%) type units. They can code accurately both innocuous and noxious cutaneous stimuli. Some cells also respond to input from deep somatic and visceral receptors. In the lumbar cord their receptive fields are small or medium sized; they are larger than the area of the foot, but smaller than the entire leg. In the thoracic cord the fields of these laminar cells are larger, and often include the entire upper limb plus part of the chest. Many of the deep dorsal grey column spinothalamic tract neurones in the thoracic segments receive convergent input from sympathetic afferent fibres. Laminae IV–VI spinothalamic tract units project either to the ventral posterolateral nucleus or to the centrolateral nucleus of the thalamus, and sometimes to both. Units projecting to the ventral posterolateral nucleus receive input from all classes (Aβ, A δ and C) of cutaneous fibres.
Pain mechanisms
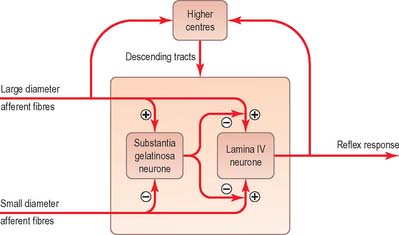
The ‘gate control theory’ (Melzack & Wall 1965) proposed a mechanism for the modulation of inflow of information along nociceptive and other afferent pathways (Fig. 18.15). The hypothesis was that large-diameter afferents (e.g. from hairs and touch corpuscles) are excitatory to the large neurones of lamina IV, from which spinothalamic fibres arise, and to interneurones in the substantia gelatinosa. In contrast, fine non-myelinated afferents are excitatory to tract cells but inhibitory to the interneurones. The axons of substantia gelatinosa interneurones are presumed to inhibit presynaptically the terminals of all afferents that synapse with tract cells. In such a system, low activity in the fine afferents inhibits the interneurones, and so prevents them inhibiting tract cells. Hence the ‘gate’ to cells in lamina IV is opened to transmit intermittent, small volleys of impulses from the large fibres. A prolonged high-frequency volley of impulses in the large-diameter afferents would be transmitted to lamina IV tract cells initially, but this would soon cease as activity in the interneurones closed the gate. Conversely, a persistent high activity in the fine afferents would open the gate resulting in massive bombardment of neurones of lamina IV (which include some neurones of high threshold that are only activated by such bombardment). It was assumed that onward transmission in the lateral spinothalamic tract would evoke the perception of pain at supraspinal levels. Pain, therefore, would result from an imbalance between the varieties of afferent impulses when there was a disproportionately large traffic along the fine afferents. In fact, inhibition of spinothalamic tract neurones can be produced by electrical stimulation of peripheral nerves, the most effective being volleys which activate Aδ fibres.
In the brain stem, the regions inducing such effects correspond to a number of midbrain and rhombencephalic nuclei which, with their connections, constitute an endogenous analgesic system. In the midbrain, these regions are the periaqueductal grey matter, dorsal raphe nucleus and part of the cuneiform nucleus. Neurones in these sites contain serotonin (5-HT), γ-aminobutyric acid (GABA), substance P, CCK, neurotensin, enkephalin and dynorphin. The periaqueductal grey matter receives afferents from the frontal somatosensory and cingulate neocortex, the amygdala, numerous local reticular nuclei and the hypothalamus. Afferents from the latter are separate bundles, which carry histamine, luteinizing hormone-releasing hormone (LHRH), vasopressin, oxytocin, adrenocorticotrophic hormone (ACTH), melanocyte-stimulating hormone (γ-MSH), endorphin, and angiotensin II. Some fibres descend from the periaqueductal grey matter to rhombencephalic centres, others pass directly to the spinal cord.
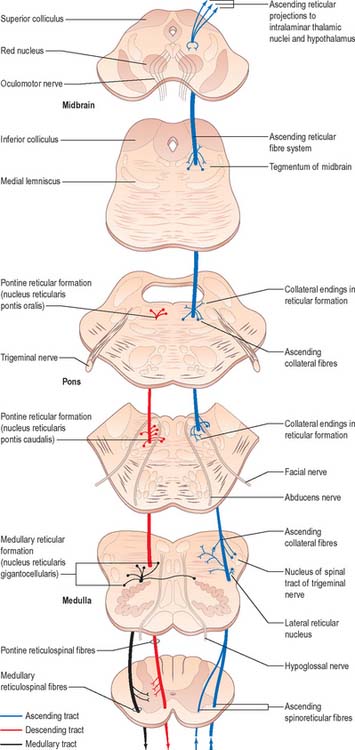
Spinoreticular pathway
Spinoreticular fibres are intermingled with those of the spinothalamic tracts, and ascend in the ventrolateral quadrant of the spinal cord (Fig. 18.16). Evidence from animal studies suggests that cells of origin occur at all levels of the spinal cord, particularly in the upper cervical segments. Most neurones are in lamina VII, some are in lamina VIII, and others are in the dorsal horn, especially lamina V. Most axons in the lumbar and cervical enlargements cross the midline, but there is a large uncrossed component in cervical regions. Most axons are myelinated. The pattern of anterograde degeneration, in both human postmortem studies and in experimental animals following anterolateral cordotomy, indicates the existence of spinoreticular projections to many nuclei of the medial pontomedullary reticular formation. There is also a projection to the lateral reticular nucleus (a precerebellar relay nucleus). These projections do not appear to be somatotopically organized. Spinoreticular neurones respond to inputs from the skin or deep tissues. Innocuous cutaneous stimuli may inhibit or excite a particular cell, whereas noxious stimuli are often excitatory. A spino-reticulo-thalamo-cortical pathway has been proposed as an important route serving pain perception. Like other ascending pathways, the tract cells are influenced by descending control. For example, electrical stimulation of the periaqueductal grey matter inhibits the responses of certain spinoreticular cells to input from cardiopulmonary afferents. Stimulation of the reticular formation also alters the activity of spinoreticular neurones.
Spino-olivary tract
The spino-olivary tract is described in animals as arising from neurones in the deeper laminae of grey matter. Axons forming the tract cross and then ascend superficially at the junction of the anterior and lateral white funiculi, to end in the dorsal and medial accessory olivary nuclei. The tract carries information from muscle and tendon proprioceptors, and also from cutaneous receptors. A functionally similar route, the dorsal spino-olivary tract, ascends in the dorsal white funiculi, and relays in the dorsal column nuclei to the contralateral inferior olivary nucleus. Information on these tracts in primates is scant, but postmortem evidence following cordotomies in man has revealed degenerating axonal terminals in the inferior olivary nucleus.
Descending tracts
Descending pathways to the spinal cord originate primarily from the cerebral cortex and from numerous sites within the brain stem (Fig. 18.16, Fig. 18.17, Fig. 18.18). They are concerned with the control of movement, muscle tone and posture, the modulation of spinal reflex mechanisms and the transmission of afferent information to higher levels. They also mediate control over spinal autonomic neurones.
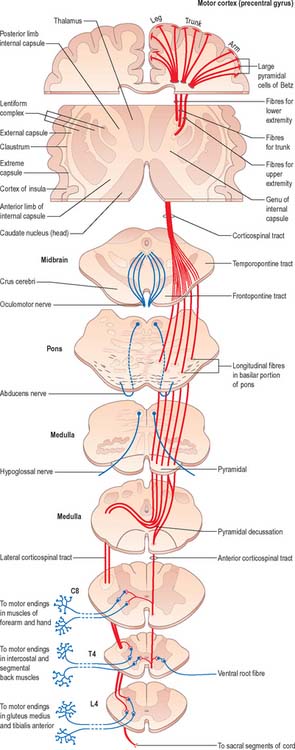
Fig. 18.17 The corticospinal tracts.
(Redrawn from Carpenter MB 1991 Core Text of Neuroanatomy, 4th edn. Baltimore: Williams and Wilkins, by permission of author and publisher.)
Corticospinal and corticobulbar tracts
Corticospinal and corticobulbar fibres arise from neurones in the cerebral cortex. They project, in a somatotopically organized fashion, to neurones that are mostly located in the contralateral side of the spinal cord or brain stem respectively (Fig. 18.17). The majority of corticospinal and corticobulbar fibres arise from cells situated in the primary motor cortex (area 4) and the premotor cortex (area 6). A small contribution comes from cells in the postcentral gyrus (somatosensory cortex; areas 3, 1, and 2) and the adjacent parietal cortex (area 5). In the monkey, 30% of corticospinal fibres arise from area 4, 30% from area 6, and 40% from the parietal regions. Cells of origin of corticospinal and corticobulbar fibres vary in size in the different cortical areas and are clustered into groups or strips. The largest cells (giant pyramidal neurones, or Betz cells) are located in the primary motor cortex of the precentral gyrus.
Corticospinal and corticobulbar fibres descend through the subcortical white matter to enter the genu and posterior limb of the internal capsule. They then pass through the ventral part of the midbrain in the crus cerebri. As they continue caudally through the pons they are separated from its ventral surface, and fragmented into fascicles, by transversely running pontocerebellar fibres. Corticobulbar fibres leave to terminate in association with the cranial nerve motor nuclei of the midbrain, pons and medulla. In the medulla oblongata, the residual corticospinal fibres form a discrete bundle, the pyramid (Fig. 19.2) which forms a prominent longitudinal column on the ventral surface of the medulla. The corticospinal tract is, therefore, also referred to as the pyramidal tract. Each pyramid contains about a million axons of varying diameter. The majority (70%) are myelinated: most (90%) have a diameter of 1–4 μm; 9% have diameters of 5–10 μm; and less than 2% have diameters of 11–22 μm. The largest diameter axons arise from the giant Betz cells.
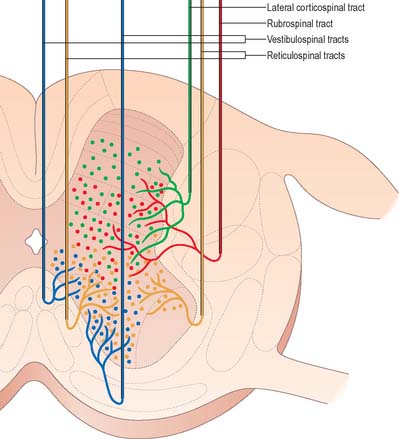
Just rostral to the level of the spinomedullary junction, approximately 75–90% of the corticospinal fibres in the pyramid cross the median plane in the pyramidal decussation (decussation of the pyramids) and continue caudally as the lateral corticospinal tract. The rest of the fibres continue uncrossed as the ventral corticospinal tract. The lateral tract also contains some uncrossed corticospinal fibres. The lateral corticospinal tract (Fig. 18.17) descends in the lateral funiculus throughout most of the length of the spinal cord. It occupies an oval area, ventrolateral to the dorsal horn and medial to the dorsal spinocerebellar tract (Fig. 18.9). In the lumbar and sacral regions, where the dorsal spinocerebellar tract is absent, the lateral corticospinal tract reaches the dorsolateral surface of the cord. As it descends, the tract diminishes in size as its fibres terminate in progressively lower spinal segments until about the fourth sacral segment: its axons terminate on ipsilateral spinal neurones.
The smaller ventral corticospinal tract (Fig. 18.17) descends in the ventral funiculus. It lies close to the ventral median fissure, and is separated from it by the sulcomarginal fasciculus (Fig. 18.9). The ventral corticospinal tract diminishes as it descends and usually disappears completely at midthoracic cord levels. It may either be absent or, very rarely, contain almost all the corticospinal fibres. Near their termination, most fibres of the tract cross the median plane in the ventral white commissure to synapse on contralateral neurones. The vast majority of corticospinal fibres, irrespective of the tract in which they descend, therefore terminate in the spinal cord on the side contralateral to their cortical origin.
Knowledge of the detailed termination of corticospinal fibres is based largely upon animal studies, but is supplemented by data from postmortem studies on human brains using anterograde degeneration methods. Most corticospinal fibres are believed to terminate contralaterally on interneurones in the lateral parts of laminae IV–VI and both lateral and medial parts of lamina VII. Some are also distributed to lamina VIII bilaterally. Terminals are also associated with contralateral motor neuronal cell groups in lamina IX, in the dorsolateral group and the lateral parts of both central and ventrolateral groups (Fig. 18.18).
Rubrospinal tract
The rubrospinal tract arises from neurones in the caudal magnocellular part of the red nucleus (an ovoid mass of cells situated centrally in the midbrain tegmentum (p. 290)). This part of the nucleus contains some 150–200 large neurones, interspersed with smaller neurones.
The origin, localization, termination and functions of rubrospinal connections are poorly defined in man, and the tract appears to be rudimentary. Rubrospinal fibres cross in the ventral tegmental decussation and descend in the lateral funiculus of the cord, where they lie ventral to, and intermingled with, fibres of the lateral corticospinal tract (Fig. 18.9). In animals, the tract descends as far as lumbosacral levels, whereas in man it appears to project only to the upper three cervical cord segments. Rubrospinal fibres are distributed to the lateral parts of laminae V–VI and the dorsal part of lamina VII of the spinal grey matter. The terminal zones of the tract correspond to those of corticospinal fibres from the motor cortex. Animal studies demonstrate that the effects of rubrospinal fibres on α and γ motor neurones are similar to those of corticospinal fibres.
Tectospinal tract
The tectospinal tract arises from neurones in the intermediate and deep layers of the superior colliculus of the midbrain. It crosses ventral to the periaqueductal grey matter in the dorsal tegmental decussation and descends in the medial part of the ventral funiculus of the spinal cord (Fig. 18.9). Fibres of the tract project only to the upper cervical cord segments, ending in laminae VI–VIII. They make polysynaptic connections with motor neurones serving muscles in the neck, facilitating those that innervate contralateral muscles and inhibiting those that innervate ipsilateral ones. In animals, unilateral electrical stimulation of the superior colliculus causes turning of the head to the contralateral side, an effect mainly mediated through the tectospinal tract.
Vestibulospinal tracts
The vestibular nuclear complex lies in the lateral part of the floor of the fourth ventricle, at the level of the pontomedullary junction. It gives rise to the lateral and medial vestibulospinal tracts, which are functionally and topographically distinct (Fig. 18.19).
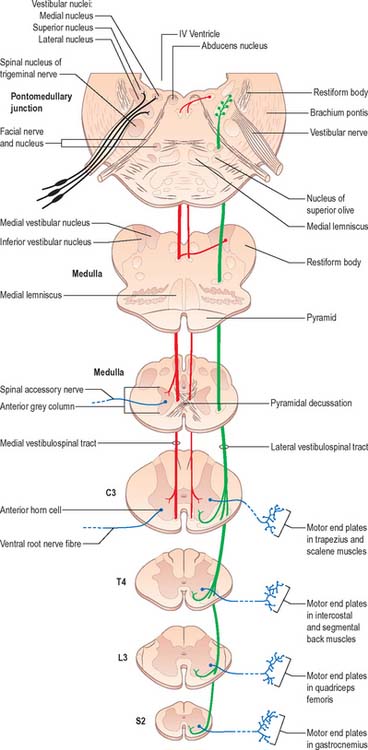
Fig. 18.19 The vestibulospinal tracts.
(Redrawn from Carpenter MB 1991 Core Text of Neuroanatomy, 4th edn. Baltimore: Williams and Wilkins, by permission of author and publisher.)
The medial vestibulospinal tract (Fig. 18.19) arises mainly from neurones in the medial vestibular nucleus, but some are also located in the inferior and lateral vestibular nuclei. The medial vestibulospinal tract descends in the medial longitudinal fasciculus into the ventral funiculus of the spinal cord where it lies close to the midline in the so-called sulcomarginal fasciculus (Fig. 18.9). Unlike the lateral tract it contains both crossed and uncrossed fibres, and does not extend beyond the midthoracic cord level. Fibres of the medial tract project mainly to the cervical cord segments, ending in lamina VIII and the adjacent dorsal part of lamina VII. Data from stimulation of the vestibular nuclei in animals indicate that axons of the medial tract monosynaptically inhibit the motor neurones that innervate axial muscles of the neck and upper part of the back.
Reticulospinal tracts
The medial reticulospinal tract (Fig. 18.16) originates from the medial tegmental fields of the pons and medulla. The main sources are the oral and caudal pontine reticular nuclei and the gigantocellular reticular nucleus in the medulla. Pontine fibres descend, mainly ipsilaterally, in the ventral funiculus of the cord. Medullary fibres descend, both ipsilaterally and contralaterally, in the ventral funiculus and the ventral part of the lateral funiculus. These fibres have many collaterals, and two-thirds of the reticulospinal neurones that reach the cervical cord also descend to lumbosacral levels. The terminals of reticulospinal fibres are distributed to lamina VIII, and the central and medial parts of lamina VII. The medullary reticulospinal terminals are more widely distributed, ending additionally in the lateral parts of laminae VI and VII and also directly on motor neurones. Terminations of reticulospinal fibres that originate in the medulla are, in general, more dorsally placed than those that originate in the pons, although there is considerable overlap.
The lateral reticulospinal tract lies in the lateral funiculus of the spinal cord, closely associated with the rubrospinal and lateral corticospinal tracts (Fig. 18.9). Its fibres arise from neurones of the ventrolateral tegmental field of the pons. The fibres cross in the rostral medulla oblongata and project, with a high degree of collateralization, throughout the length of the spinal cord. Axons of this tract terminate in laminae I, V and VI, and also bilaterally in the lateral cervical nucleus. Evidence suggests that this pathway is involved in the control of pain perception and in motor functions.
Monoaminergic spinal pathways
Monoaminergic cell groups utilize dopamine, adrenaline (epinephrine), noradrenaline (norepinephrine) and 5-HT (5-hydroxytryptamine, serotonin) as neurotransmitters. They occur widely throughout the brain stem and in the hypothalamus. They project rostrally to many forebrain areas and caudally to the spinal cord and appear to be concerned with the modulation of sensory transmission, and the control of autonomic and somatic motor neuronal activities.
Propriospinal pathways
Propriospinal pathways (fasciculi proprii) consist of the ascending and descending fibres of intrinsic spinal neurones. They contact other neurones within the same segment and/or in more distant segments of the spinal cord and so subserve intrasegmental and intersegmental integration and coordination. The majority of spinal neurones are propriospinal neurones, most of which lie in laminae V–VIII. Propriospinal fibres are mainly concentrated around the margins of the grey matter (Fig. 18.9), but are also dispersed diffusely in the white funiculi.
SPINAL CORD LESIONS
Mechanical compression and secondary ischaemic damage to underlying nervous tissue cause surgically relevant spinal cord disease (myelopathy). The site and the level of damage to the cord determine the particular clinical syndrome, e.g. whether the lesion involves the upper or lower cervical, thoracic or lumbosacral spinal cord. At each of these levels, symptoms and signs are determined by direct destruction of segmental tissue, i.e. transversely distributed damage, and disconnection of suprasegmental ascending and descending tracts above and below the level of a lesion, i.e. longitudinally distributed damage (Fig. 18.20). For example, a lower cervical spinal cord lesion damages the segmental sensory and motor contributions to the nerve roots and brachial plexus causing sensory loss, weakness and wasting of the muscles and loss of tendon reflexes in the upper limbs. Disruption of the ascending sensory pathways in the lateral and dorsal columns of the cervical spinal cord leads to loss of sensation to pain and temperature (lateral spinothalamic tracts) and touch and proprioception (dorsal fasciculi) below the ‘sensory level’ corresponding to the segment of the spinal cord.
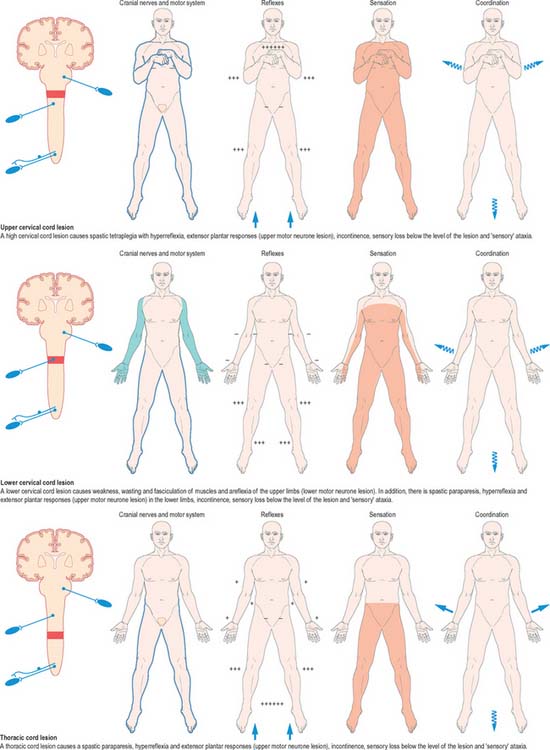
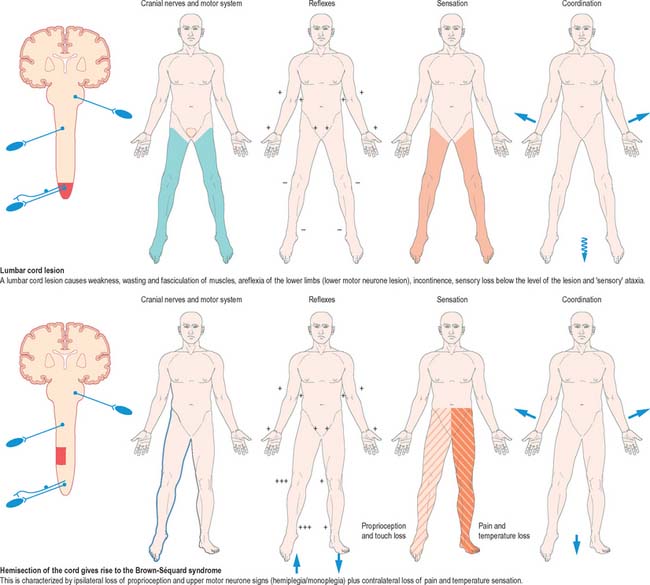
Fig. 18.20 Lesions of the spinal cord.
(By permission from Crossman AR, Neary D 2000 Neuroanatomy, 2nd edn. Edinburgh: Churchill Livingstone.)
The same principles apply to lesions at other levels of the spinal cord and they are illustrated in diagrammatic form in Fig. 18.20.
| Extradural lesions |
Boyd IA, Gladden MH. The Muscle Spindle. London: Macmillan, 1985.
Kuypers HGJM. Anatomy of descending pathways. In: Brookhart JM, Mountcastle VB, et al. Handbook of Physiology, vol 2 Motor Control, pt 1. Bethesda, MD: Am Physiol Soc; 1981:597-666.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952;96:415-495.
Schoenen J, Faull RLM. Spinal cord: cytoarchitectural, dendroarchitectural and myeloarchitectural organization. In: Paxinos G, editor. The Human Nervous System. San Diego, CA: Academic Press; 1990:19-53.