Chapter 55 Spinal Cord Injury
Historical Perspective
The Edwin Smith Surgical Papyrus, written by an Egyptian physician almost 5000 years ago, vividly describes the symptoms of neurologically complete injury to the cervical spinal cord—that is, paralysis and sensory loss in the arms and legs, urinary incontinence, and priapism.79,230 Later, in ancient Greece, approximately 400 BC, Hippocrates described paraplegia caused by injury or disease as being associated with paralysis, bladder and bowel dysfunction, and pressure ulcers.3 Several ancient Roman physicians also briefly described SCI, which they invariably considered to be fatal if neurologically complete, but if incomplete, reduction of spinal deformities by traction was recommended, a practice that was continued through the Middle Ages.160
During the nineteenth century, treatment of SCI continued to be conservative and without much hope for survival. A famous anecdote reflects well the prevailing attitude in those days. In 1805, Lord Nelson, the Admiral of the British Fleet, received a gunshot wound to his thoracic spine during the battle of Trafalgar, causing paraplegia. Nelson spoke with his ship’s surgeon, Mr. Beatty, and described his loss of power of motion and feeling below the chest, and then expressed his view that he would have but a short time to live. The surgeon’s reply was, “My lord, unhappily for our Country, nothing can be done for you.”215 Within a few hours Lord Nelson was dead. In 1881 the twentieth president of the United States, James A. Garfield, was shot in the spine, causing a neurologically incomplete conus–cauda equina lesion, but even with such a lesion he was dead within 3 months.161 Despite remarkable progress in medicine and surgery during the nineteenth century—for example, Pasteur’s discoveries in bacteriology, Lister’s aseptic surgical techniques, the introduction of anesthesia, and Roentgen’s discovery of x-rays—surgical interventions for spine trauma and SCI were generally discouraged.
During the early part of the twentieth century, there was little progress made in the management of SCI, and most persons with SCI died within weeks or months. Harvey Cushing observed that during World War I, 80% of all U.S. soldiers with SCI died within 2 weeks.215 Early mortality was slightly lower in the British military, but the 3-year mortality was estimated to be 80%.215 During the 1930s and 1940s, management of SCI finally started to change. During the late 1930s, Dr. Donald Munro323–325 at Boston City Hospital developed a dedicated unit for comprehensive care of persons with SCI, and by 1943, he was able to demonstrate significant drops in both morbidity and mortality, primarily by focusing on better bladder management. A few years later, in Great Britain during World War II, it was decided to congregate all casualties with SCI in special units that were supervised by an experienced physician. These units were to be sufficiently staffed by nurses and therapists, housed in facilities with rehabilitation workshops, and organized to provide resettlement and aftercare services.215 Dr. Ludwig Guttmann was placed in charge of such a unit at Stoke Mandeville, where he introduced comprehensive care and interdisciplinary rehabilitation for persons with SCI, a program that was widely modeled around the world. Within the U.S. Veterans Affairs Hospitals, Drs. Comarr and Bors introduced new methods of urologic management and rehabilitation, which quickly improved survival rates among U.S. veterans.
Model Systems of Care
Based on this hypothesis, the U.S. government funded several SCI Model Systems of Care, first in Phoenix, Arizona, in 1970, and subsequently in several other major cities around the United States. By 1982 there were 17 such systems funded by the National Institute on Disability and Rehabilitation Research (NIDRR), U.S. Department of Education. Each funded system had to meet four basic requirements. First, it had to have several integrated clinical components: emergency medical services; level 1 trauma center; comprehensive rehabilitation services for both inpatients and outpatients, including vocational and job placement services; and lifelong follow-up and health maintenance programs. Second, each funded system had to collect data on all patients served and forward these to a National SCI Model System Database. Third, each system was required to conduct research consistent with NIDRR-announced priorities. Fourth, each system had to disseminate the research and demonstration findings as widely as possible to the appropriate audiences.
The SCI Model Systems have been instrumental in developing standards of care and new treatments, and conducting epidemiologic, health services, and outcomes research, as well as producing thousands of publications and training materials.133 The National SCI Database has been in existence since 1973, and captures data from an estimated 13% of new SCI cases in the United States. The SCI Model Systems have been directly and indirectly instrumental in a number of positive developments for persons with SCI. These include increased survival rates, reduced hospital lengths of stay, and a reduced number of rehospitalizations. In addition, the SCI Model Systems have led to the majority of persons with SCI being discharged home, and created a national database of approximately 30,000 persons with SCI.428,429,437
Subspecialty of Spinal Cord Injury Medicine
Most physicians providing nonsurgical care for people with SCI in the United States have been physiatrists. In the past, most such physicians developed their special knowledge over a lengthy period by providing care rather than by specific training. A creation of a subspecialty of SCI medicine was first advocated in the late 1970s and gained momentum in the early 1990s. Through the concerted efforts of many individuals and organizations, the American Board of Medical Specialties gave its approval in 1995 that such a subspecialty be established.127,128 Based on the work experience and training of candidates that is acceptable to the American Board of Physical Medicine and Rehabilitation, candidates can take a written examination. Those who pass receive a special SCI medicine subspecialty certificate. Several 1-year SCI fellowships have been established, each providing a structured training program approved by the Accreditation Council for Graduate Medical Education. Several hundred individuals have been certified in the subspecialty since the first examination was held in 1999.
Epidemiology
Numerous epidemiologic studies have been reported since the 1970s in various countries, and these reflect some variance in the incidence and prevalence of SCI. Data have been collected in the United States by the SCI Model Systems for its database since the early 1970s. The analysis of these data by the National Spinal Cord Injury Statistical Center (NSCISC) has provided extensive and reliable information. The epidemiologic data have been widely published, and current information can be easily accessed on the NSCISC website, which is updated annually.19,132,133
Incidence and Prevalence
The annual incidence of traumatic SCI requiring hospitalization in the United States is approximately 40 new cases per million population (or approximately 12,000 per year). These numbers are the most up to date available, but are based on data collected before 1980. These numbers do not include an unknown number of individuals with SCI who died before reaching a hospital.70,335 The incidence of traumatic SCI in other developed countries has been shown to be somewhat lower than that in the United States, often less than 20 new cases per million per year, perhaps partly because of the higher U.S. incidence of violence-related SCI.132,133
The exact number of persons with SCI currently alive in the United States (prevalence) is a matter of dispute. Two methods have been used to estimate the prevalence of SCI: mathematic calculation based on annual incidence and average duration, and counting the exact number of people with SCI within a geographic area and extrapolating this number to the population of the entire United States. With the use of the mathematic approach, the prevalence of SCI in the United States has been estimated to be 259,000 persons, but a recent population study sponsored by the Christopher and Dana Reeve Foundation concluded after surveying more than 33,000 U.S. households that nearly 1.3 million people in the United States live with paralysis caused by SCI.23,89
Age at Time of Injury, Gender, Ethnicity, and Marital Status
Almost all studies show that the incidence of SCI is lowest for persons younger than 15 years and highest for persons 16 to 30 years of age. After the age of 30, there is a consistent decline in incidence. The current average age at onset is reported to be 40.2 years.23 A rising percentage of older persons with new SCI has recently been observed. For example, those older than 60 years constituted 4.5% of all new patients with SCI in the mid 1970s, versus 11.5% in the mid 1990s.335 A simultaneous rise in the median age of persons with SCI also occurred from the mid 1970s to the 1990s, with a rise from 28.5 years to 35.9 years. This change could reflect the improved medical care for persons with SCI, as well as the rise in the median age of the U.S. general population from 28 years to 35 years during the same period.133
More than 80% of all SCI occurs in males, a figure that has remained essentially constant for more than 30 years in the United States.132,133,335 This gender difference is similar in other countries. Approximately two thirds of all persons enrolled in the National SCI Database are white, which is significantly less than the percentage of whites among the general U.S. population.335
At the time of SCI, 30.4% of individuals had intact marriages, 53% had never been married, and the remaining 26.6% were separated, divorced, or widowed.132 The unmarried rate is a relatively high figure, perhaps best explained by the fact that SCI disproportionately affects young people.23,132 For those married at the time of SCI, the divorce rate is increased after SCI, as compared with that in the general population, especially during the first 3 postinjury years. The annual marriage rate is also lower for single individuals with SCI than for nondisabled persons.132
Causes of Spinal Cord Injury
The most common causes of SCI in descending order of incidence are vehicular crashes (42.1%), falls (26.7%), violence (15.1%), and sports (7.6%).19 In recent years, there has been a gradual decline in SCIs related to vehicular crashes and sports, whereas those relating to falls have increased. The causes of SCI vary significantly between groups of different ethnicity, age, gender, and geography. For example, falls are the most common cause of SCI among the elderly and violence among African Americans, whereas women rarely sustain SCI as a result of gunshots, motorcycle crashes, or diving. Considering the causes of SCI, it is not surprising that relatively more injuries occur on weekends and during the summertime.
Neurologic Level and Extent of Neurologic Deficit
According to the National SCI Database, tetraplegia is more common than paraplegia (50.5% vs. 44.1%). These are subdivided into the following neurologic categories: incomplete tetraplegia (30.1%), complete tetraplegia (20.4%), complete paraplegia (25.6%), and incomplete paraplegia (18.5%). Recent trends show an increase in incomplete tetraplegia and a slight reduction in complete paraplegia.23
Length of Stay, Rehospitalization, and Discharge Destination
The average length of stay for patients with SCI has declined dramatically over the years, according to the National SCI Database. This is true for both acute and rehabilitation hospitalizations, from 25 acute days in 1974 to 12 days in 2008, and from 115 rehabilitation days to 37 days.23 The number of days hospitalized is greater for persons with complete SCI compared with those with incomplete SCI. The number of rehospitalizations during the first year has steadily decreased as well.175 Among persons listed in the National SCI Database and discharged alive, 88.3% went to a private noninstitutional residence, 5% went to group living situations, 5.1% went to nursing homes, and the remaining 1.6% went to acute hospitals. Approximately 3% died during the initial hospitalization.132
Life Expectancy and Causes of Death
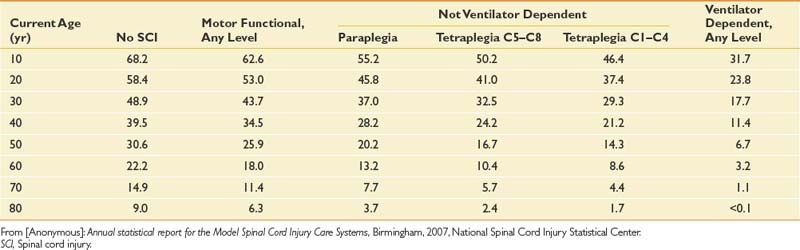
Life expectancy for persons with SCI has increased steadily for many decades but still remains below that of able-bodied individuals. The mortality rate is highest during the first postinjury year, at 6.3%, but declines significantly thereafter.132,140 The first-year mortality rate declined by 67% from the 1970s to the 1990s.132,138 Significant predictors of mortality include being older, male, injured by acts of violence, neurologically complete, and ventilator dependent, and having a high neurologic level.138 Additional factors that affect longevity after the first postinjury year include low life satisfaction, poor health, emotional distress, functional dependency, and poor adjustment to disability.256 The NSCISC website provides annual updates on life expectancy after the onset of SCI, based on neurologic level and ventilatory dependency (Table 55-1). These life expectancy estimates do not include many important variables that can also significantly affect survival, such as gender, ethnicity, preexisting medical conditions, access to medical and nursing care, and social support.
Table 55-1 Life Expectancy (Years) for Persons With Spinal Cord Injury Surviving at Least 1 Year Postinjury
Diseases of the respiratory system, especially pneumonia, are the leading cause of death both during the first postinjury year and during subsequent years (Table 55-2). The second most common cause of death, “other heart disease,” is thought to reflect deaths that are apparently caused by heart attacks in younger persons without apparent underlying heart or vascular disease and cardiac dysrhythmia.132 Interestingly, diseases of the genitourinary system are currently the cause of death in only 3.7% of patients with SCI. In the past, renal failure was by far the leading cause of death after SCI. This is truly a great testament to the advances in urologic management during the past several decades.
| Primary Cause of Death | Percentage |
|---|---|
| Diseases of the respiratory system (70% pneumonia) | 22 |
| Other heart disease (likely overreported representing poor quality of cause-of-death data after spinal cord injury) | 12 |
| Infective and parasitic diseases (94% septicemia usually associated with pressure ulcers or urinary tract or respiratory tract infections) | 10 |
| Hypertensive and ischemic heart disease | 8 |
| Neoplasms | 7 |
| Diseases of pulmonary circulation (96% pulmonary emboli) | 5 |
| Diseases of the genitourinary system | 4 |
| Suicides | 4 |
| Other causes | 28 |
From [Anonymous]: Annual statistical report for the Model Spinal Cord Injury Care Systems, Birmingham, 2007, National Spinal Cord Injury Statistical Center.
Anatomy, Mechanics, and Syndromes of Traumatic Injury
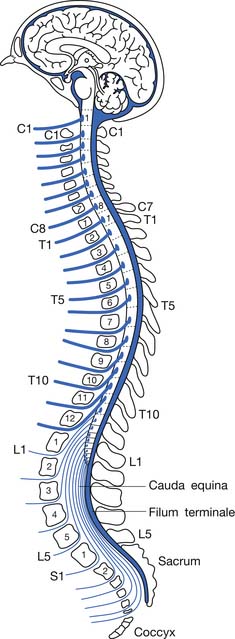
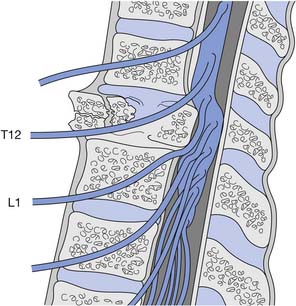
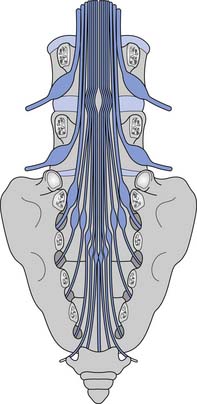
Because the bony vertebral column elongates more than the spinal cord during embryologic development, the spinal cord terminates at the level of the L1–L2 intervertebral disk. Due to natural variation, the spinal cord termination can be as high as the T12 or as low as the L3 vertebral body. The individual spinal cord segments do not line up with the corresponding bony levels of the same number (Figure 55-1). This is especially evident in the lower thoracic and lumbar spine, where the L1–L5 spinal segments are adjacent to the T11–T12 vertebrae, and the S1–S5 spinal segments are adjacent to the L1 vertebra. This concept can also be used when evaluating radiologic studies to correlate the neurologic level of injury (NLI) to the appropriate bony level of damage (e.g., a T11 burst fracture with cord compression would be expected to cause an NLI at L1 or L2 rather than at T11).
The tapered end of the spinal cord, which contains the sacral cord segments, is called the conus medullaris. The collection of long lumbar and sacral roots found in the canal, distal to the conus medullaris, is called the cauda equina, because it resembles a horse’s tail. The meninges of the spinal cord include the pia matter, a vascular membrane covering the spinal cord, the arachnoid membrane, and the dura mater. The subarachnoid space, also called the intrathecal space, contains cerebrospinal fluid (CSF). The CSF pushes the arachnoid directly against the dura mater. The caudal margin of the dura mater and arachnoid, the inferior extent of the intrathecal space, is the second sacral vertebrae (Figure 55-2). The spinal epidural space is located between the dura mater and the periosteum of the vertebral bodies, and contains an internal vertebral venous plexus, fat, and loose areolar tissue.
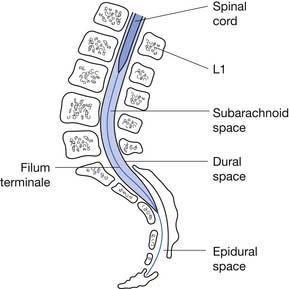
FIGURE 55-2 A sagittal schematic showing the relationship between the dura, subarachnoid space, and the epidural space.
(Redrawn from Pansky B: Review of gross anatomy, ed 5, New York, 1984, Macmillan, with permission of Macmillan.)
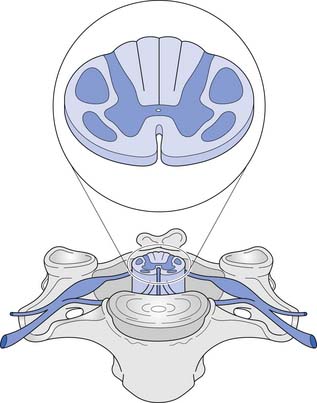
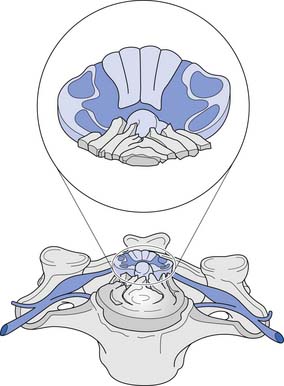
A cross-sectional view of the spinal cord (Figure 55-3) reveals a central butterfly-shaped region of gray matter consisting of neuronal cell bodies, their processes, supporting glial cells, and small blood vessels surrounded by white matter consisting of neuronal fiber tracts and supporting glial cells. The gray matter is subdivided into two horns on each side called the anterior (ventral) and posterior (dorsal) horns. The posterior horn contains cell bodies of sensory neurons, whereas the anterior horn contains cell bodies of interneurons and motor neurons.
The white matter is subdivided into three columns on each side called the anterior, lateral, and posterior columns. The columns are further subdivided into tracts. The gracilis tract, located in the medial posterior column, contains fibers from the T7–S5 dermatomes that relay touch, vibration, and position sense. The cuneatus tract, located in the lateral posterior column rostral to T6, contains fibers from dermatomes above T7 that relay touch, vibration, and position sense. These tracts, comprising the posterior columns, ascend ipsilaterally to the medulla. The lateral spinothalamic tract, located peripherally in the lateral column, contains fibers that relay pain and temperature sensations; this tract ascends contralaterally to the thalamus. The lateral corticospinal tract is located centrally and posteriorly in the lateral column. This tract contains fibers, most of which emanate from the motor cortex, that are responsible for voluntary and reflex movement. Approximately 90% of the corticospinal fibers cross midline in the caudal medulla, forming the pyramidal decussations, and descend contralaterally in the lateral corticospinal tract to terminate on interneurons and α- and γ-motor neurons in the spinal cord. The remaining corticospinal fibers, located in the medial anterior column, do not cross midline in the medulla but descend ipsilaterally in the anterior corticospinal tract. These fibers ultimately cross midline segmentally near their terminations on interneurons and alpha and gamma motor neurons in the spinal cord. About 55% of the corticospinal fibers terminate in the cervical cord, 20% in the thoracic cord, and 25% in the lumbosacral cord.461 A Brown-Séquard syndrome refers to an injury of the spinal cord in which one side is damaged more than the other (Figure 55-4), resulting in relatively greater ipsilateral weakness and position sense loss, but with contralateral pain and temperature sensation loss.
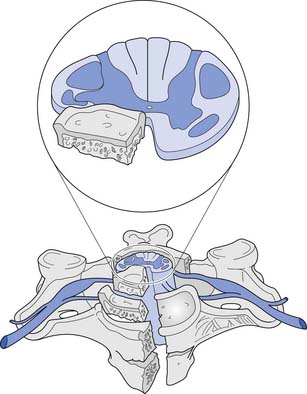
A corticospinal neuron is known as an upper motor neuron (UMN). The motor neuron to which it synapses in the spinal cord, which exits the spinal cord to innervate muscle, is known as a lower motor neuron (LMN). If damage to the UMNs and LMNs within the spinal cord is localized to a few segmental levels anywhere rostral to the conus medullaris (see below), a constellation of signs and symptoms develops, often called the UMN syndrome. This includes loss of voluntary movement, spasticity, hyperreflexia, clonus, and development of Babinski’s sign.265 If, in addition, there is damage to a significant number of LMNs below the level of injury, loss of voluntary movement occurs without the subsequent development of the other components of the UMN syndrome. Examples of this, defined as LMN injuries, include an SCI caused by an extensive vascular insult to the spinal cord, an injury occurring at the conus medullaris, or an injury occurring at the cauda equina. The conus medullaris syndrome refers to an injury of the sacral spinal cord and the lumbar nerve roots within the spinal canal (Figure 55-5), resulting in an areflexic bladder, bowel, and lower limbs. Conus medullaris lesions localized to the proximal sacral cord can occasionally show a preserved sacral reflex, such as the bulbocavernosus reflex. The cauda equina syndrome refers to an injury to the lumbosacral roots within the spinal canal (Figure 55-6), resulting in an areflexic bladder, bowel, and lower limbs.
After an acute UMN-predominant SCI, initial development of the UMN syndrome is delayed by a process called spinal shock, whereby there is a transient suppression and gradual return of reflex activity below the level of injury. Ditunno et al.150 have proposed a four-phase model of spinal shock. During phase 1, occurring 0 to 24 hours postinjury, there is motor neuron hyperpolarization, manifesting clinically as hyporeflexia. During phase 2, occurring on days 1 to 3 postinjury, there is denervation supersensitivity and receptor upregulation, manifesting clinically with reflex return. During phase 3, occurring 1 to 4 weeks postinjury, there is interneuron synapse growth, manifesting clinically as early hyperreflexia. And finally, during phase 4, occurring 1 to 12 months postinjury, there is long axon synapse growth, manifesting clinically as late hyperreflexia.
Blood is supplied to the spinal cord through two posterior spinal arteries, a single anterior spinal artery, and several segmental radicular arteries (Figure 55-7). The posterior spinal arteries branch from the vertebral arteries and travel along the posterior surface of the spinal cord to supply the posterior one third of the spinal cord. Two anterior spinal arteries also branch from the vertebral arteries, but these quickly unite to form a single artery that travels along the anterior surface of the spinal cord to supply the anterior two thirds of the spinal cord. The anterior spinal artery and the posterior spinal arteries are dependent on contributions from the segmental radicular arteries along the spinal cord to maintain an adequate blood supply to the spinal cord. The segmental radicular arteries travel through the intervertebral foramina from the aorta and divide into anterior and posterior branches that eventually anastomose with their respective spinal arteries. These radicular arteries are not all identical in size or distribution. In the upper thoracic region between T1 and T4, there is little overlap between radicular arterial supplies. Between T12 and L2, there is an anterior radicular artery that is more dominant than its neighbors, called the artery of Adamkiewicz. This artery, usually found on the left side of the body, is an important blood supply to the caudal two thirds of the spinal cord. On reaching the anterior surface of the spinal cord, the artery of Adamkiewicz divides into a small ascending and larger descending branch. The latter travels down to the level of the conus medullaris, where it forms an anastomotic circle with the terminal branches of the posterior spinal arteries. The regions between T1 and T4, and T12 and L2 are areas particularly prone to ischemic damage, because of the importance of individual radicular arteries. The ischemic damage often affects the anterior portion of the spinal cord more than the posterior portion because of the nature of the single anterior and dual posterior blood supplies. In this situation the corticospinal and spinothalamic tracts are affected, while the gracilis tract is often spared. This leads to a syndrome of paraplegia, with loss of pain and temperature sensation, and relative sparing of touch and position sensation, called the anterior cord syndrome (Figure 55-8).
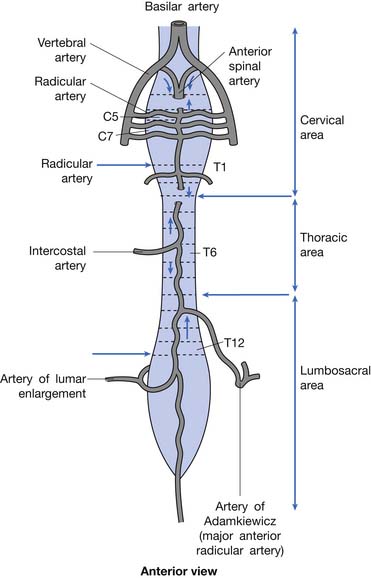
FIGURE 55-7 The spinal cord blood supply.
(Redrawn from Pansky B, Allen D, Budd G: Review of neuroscience, ed 2, New York, 1998, McGraw-Hill, with permission of McGraw-Hill.)
Pathophysiology of Acute Spinal Cord Injury
The secondary injury cascade is a term that refers to a series of biochemical processes that occur after an SCI, and that tend to cause further neuronal damage beyond the mechanical damage caused at the moment of impact. Ischemia of the gray matter at the site of injury occurs almost immediately after SCI. This ischemia appears to result from vasoconstriction of blood vessels supplying the cord, and is mediated by the rapid release of various vasoactive substances such as serotonin, thromboxanes, platelet-activating factor, peptidoleukotrienes, and opioid peptides after SCI.341,393 Ischemia is followed by the development of edema at the site of injury.334 At a cellular level, there is a marked rise in intraneuronal calcium concentrations. This begins within minutes after injury, reaching a peak at about 8 hours postinjury and remaining elevated for at least 1 week.319 Elevated levels of excitatory amino acids, such as glutamate and aspartate, acting at their receptors have been noted to play a role in increasing the intracellular calcium concentrations.100,275 Intracellular calcium facilitates the activation of phospholipases A2 and C, which leads ultimately to the production of free radicals and free fatty acid metabolites, which cause damage to local cell membranes.78,167,370 There is also a rapid rise in potassium in the extracellular space, directly related to cell membrane damage, which causes depolarization of other neuronal cells and conduction block.479Microhemorrhages appear in the central gray matter at the site of impact. Iron in this hemorrhaged blood catalyzes the peroxidation of lipids, leading to further tissue damage as well as catalyzing the further production of oxygen free radicals.393
Initially, neutrophils migrate to the site of injury, where they can contribute to cellular injury by producing lysosomal enzymes and oxygen radicals. These are followed by macrophages that phagocytose cell debris.351 Schwann cells appear that modify myelin sheaths and produce neurotrophic factors, while fibroblasts produce basic fibroblast growth factor and promote angiogenesis and neovascularization at the site of injury.61,271 Small CSF-filled cysts eventually form that are partially surrounded by demyelinated nerve fibers. These coalesce over months and can give rise to myelomalacia and syringomyelia.281 Demyelination of white matter tracts begins within 24 hours of injury and increases thereafter, with Wallerian degeneration occurring by 3 weeks.460 Remyelination seems to occur after SCI, although in an inadequate fashion, with abnormally short internodal distances and notably thin myelin.62,172
Spinal Mechanics and Stability
There is no universally accepted definition of spinal stability. White and Panjabi467 defined clinical instability as “the loss of the ability of the spine under physiologic loads to maintain relationships between vertebrae in such a way that there is not initial damage or subsequent irritation to the spinal cord or nerve roots and, in addition, there is no development of incapacitating deformity or pain due to structural changes.” A commonly accepted model for thoracolumbar stability, which is often used in the middle and lower cervical spine as well, was developed by Denis129,130 and modified by Ferguson and Allen.173 The model divides the spine into three columns: anterior, middle, and posterior (Figure 55-9). The anterior column is composed of the anterior longitudinal ligament, the anterior two thirds of the vertebral body, and the anterior two thirds of the annulus fibrosis or disk. The middle column is composed of the posterior one third of the vertebral body, the posterior one third of the annulus fibrosis, and the posterior longitudinal ligament. The posterior column is composed of the pedicles, facet joints, laminae, supraspinous ligament, interspinous ligament, facet joint capsule, and ligamentum flavum. When the integrity of the middle and either the anterior or the posterior column is affected, the spine is likely to be unstable.129,130 The columns can be compromised by either fracture or ligamentous disruption. Gunshot wounds, because of the nature of the injury, can affect more than one column and the spine can still remain stable.262 It should also be noted that SCI can occur without obvious radiographic findings.
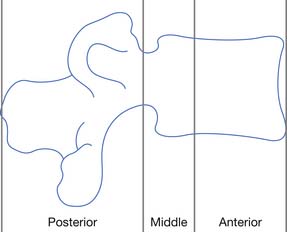
FIGURE 55-9 The three-column concept of spinal anatomy.
(Redrawn from Ferguson RL, Allen BL Jr: A mechanistic classification of thoracolumbar spine fractures, Clin Orthop 189:77-88, 1984, with permission.)
Fractures or dislocations in the thoracic and lumbar spine most commonly involve the T12 and the L1 vertebrae, respectively. Common mechanisms include compression-flexion, distraction-flexion, translation, and torsion-flexion.386 Axial loading of a flexed spine can cause several different patterns of injury depending on the vector of force. There might be only compression of the anterior column leading to a compression fracture or, with a greater compressive force, compression of the anterior column with distraction of the posterior elements. If the vector of force causes the axis of rotation to be anterior to the vertebral body, a Chance-type distraction can occur with distraction of all three columns, through the bony vertebra alone (Figure 55-10), through the ligamentous structures alone, or through a combination of bony and ligamentous structures. In addition, there can be compression of all three columns with retropulsion of the middle column into the spinal canal. The latter often causes SCI. Translation of adjacent vertebrae, as occurs for example when a person falls from a height and strikes part of the torso on an immovable object, is the injury pattern most likely to cause SCI. If there is translation more than 25% of the width of a vertebra, ligamentous structures in all three columns are probably disrupted.195,228 Compression and rotation of the anterior column, and distraction and rotation of the posterior column, cause a torsion-flexion injury where the facets and the anterior longitudinal ligament are usually disrupted, and SCI is likely.
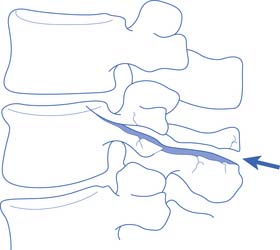
FIGURE 55-10 A Chance fracture.
(Redrawn from Schultz RJ: The language of fractures, Baltimore, 1990, Williams & Wilkins, with permission of Williams & Wilkins.)
A Jefferson fracture, originally described by Sir Geoffrey Jefferson,236 is a burst fracture of the atlas (C1 vertebra). This is caused by axial compression, which can occur, for example, when a football player spears another player with his helmet (Figure 55-11). A hangman’s fracture is a traumatic spondylolisthesis of the axis (C2 vertebra). It is caused by bilateral fractures through the pars interarticularis of the axis that result from hyperextension and axial compression, as can occur in an abrupt deceleration when a person’s forehead strikes the windshield. A fracture of the odontoid process of the axis can be caused by hyperflexion, hyperextension, or excessive lateral bending. The traditional classification of odontoid fractures includes three types.16 Type 1 is a fracture through the tip of the odontoid, type 2 is a fracture through the base of the odontoid, and type 3 is a fracture that extends from the base of the odontoid into the axis proper.
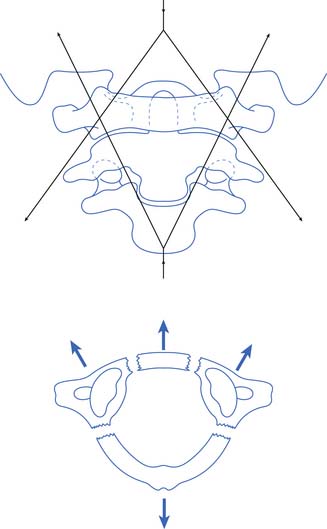
FIGURE 55-11 A Jefferson fracture. A comminuted fracture of the ring of C1.
(Redrawn from Schultz RJ: The language of fractures, Baltimore, 1990, Williams & Wilkins, with permission of Williams & Wilkins.)
Hyperflexion of the subaxial cervical spine (C3–C7) can cause an anterior subluxation, a simple compression fracture, bilateral facet dislocations, a flexion teardrop fracture, or a clay shoveler’s fracture. A flexion teardrop fracture is characterized by retropulsion of the larger portion of a vertebral body into the spinal canal, detached from an anterior fragment (teardrop); it is associated with posterior facet and ligamentous disruption (Figure 55-12). Flexion teardrop fractures are often associated with an anterior cord syndrome, if not a complete SCI. A clay shoveler’s fracture is an avulsion fracture of the spinous process of C6, C7, or T1. It is not typically associated with neurologic injury. Hyperflexion with rotation often causes a unilateral facet dislocation.
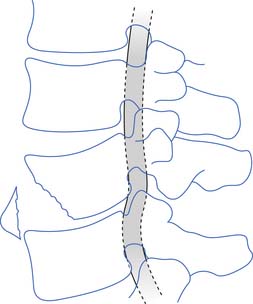
FIGURE 55-12 A flexion teardrop fracture. The spinal cord is compressed by the posteroinferior aspect of the vertebrae.
(Redrawn from Schultz RJ: The language of fractures, Baltimore, 1990, Williams & Wilkins, with permission of Williams & Wilkins.)
Finally, and not uncommonly, significant axial loading of the subaxial cervical spine causes a burst fracture, whereby an intervertebral disk implodes through the superior end plate of the vertebral body below, causing this vertebral body to burst into multiple fragments.391 These fractures usually include at least two columns, are generally unstable, and often are associated with SCI.
Classification of Spinal Cord Injury
The diagnosis of SCI can be made promptly by performing a neurologic examination. The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) provides a procedure for classifying an SCI.286 Online e-learning modules are available through the American Spinal Injury Association (ASIA) website. The examination, which is safe to perform soon after SCI, even in persons with an unstable spine, is performed with the injured individual in the supine position. Subsequent examinations are always performed in the same position. The procedure includes a systematic evaluation of all the dermatomes and extremity myotomes. Because SCI usually affects the spinal cord at a discrete site, determining the last intact sensory and motor level can reliably and accurately determine an NLI. A complete injury is defined within the ISNCSCI as an injury in which there is the lack of any sensory or motor function in the lowest sacral segment; this includes sensation deep within the anus, sensation at the anal mucocutaneous junction, or a voluntary contraction of the external anal sphincter. An incomplete injury is defined as an injury in which there is at least partial sensory or motor function in the lowest sacral segment.
The sensory portion of the neurologic examination includes the testing of a key point (Table 55-3) for absent, impaired, or normal sensation in each of the 28 dermatomes on each side of the body for both light touch and pinprick. Pinprick sensation is elicited with a disposable safety pin, whereas touch sensation is elicited by a wisp of cotton or a fingertip. For an inability to distinguish between pinprick and touch, sensation should be graded as absent for pinprick sensation. The motor portion of the neurologic examination includes the testing of a key muscle function for strength on a 6-point scale (Table 55-4 for each of 10 myotomes on each side of the body), as well as testing for contraction of the external anal sphincter.
Table 55-3 International Standards for Neurological Classification of Spinal Cord Injury Neurologic Examination Sensory Testing Points
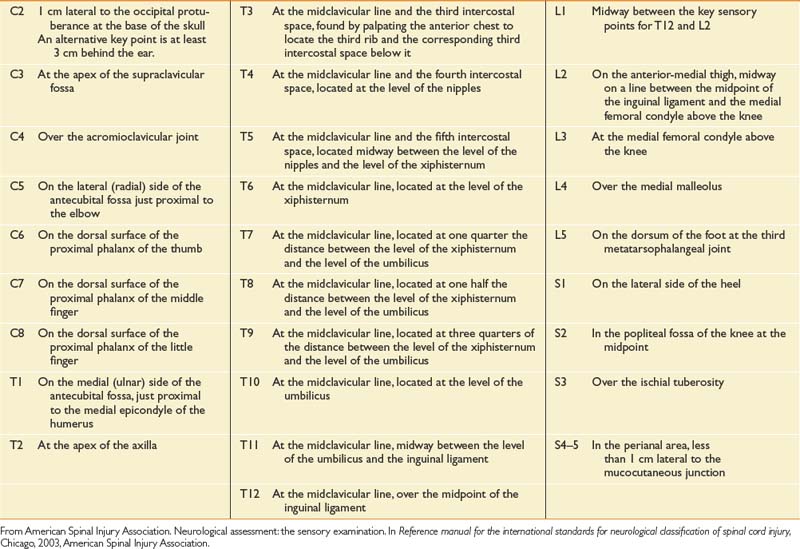
Table 55-4 International Standards for Neurological Classification of Spinal Cord Injury Neurologic Examination Motor Testing Points



The ISNCSCI also includes a scale of impairment called the ASIA Impairment Scale (AIS), which classifies an SCI into five categories of severity, labeled A through E, based on the degree of motor and sensory loss. An SCI that results in the absence of any sensory or motor function in the sacral segments S4–S5 would have an AIS category of A and be designated as complete. For an SCI where sensation is preserved in the sacral segments S4–S5, but there is no motor function caudal to three segments below the NLI, the AIS is B. For an SCI where sensation is preserved in the sacral segments S4–S5, but more than half the key muscles below the NLI have a muscle grade less than 3/5, the AIS is C. For an SCI where sensation is preserved in the sacral segments S4–S5, but at least half the key muscles below the NLI have a muscle grade greater than or equal to 3/5, the AIS is D. When sensory and motor function is normal, the AIS is E. AIS categories B through E designate incomplete injuries.
Nontraumatic Spinal Cord Injury
Unlike the National SCI Model System Database for traumatic SCI, there is no comprehensive database of information about persons with nontraumatic SCI. Nontraumatic SCI can be caused by a variety of diseases, including neoplastic, infectious, inflammatory, vascular, degenerative (spondylotic), congenital, and toxic-metabolic disorders. Persons affected by nontraumatic SCI are clinically quite different from those with traumatic injuries. Those with nontraumatic SCI generally have less severe injuries. Persons with nontraumatic SCI almost always have incomplete injuries, whereas those with traumatic injuries are only slightly more likely to have incomplete injuries. Incomplete injuries are associated with a far better prognosis for neurologic improvement than are complete injuries. Unlike persons with traumatic SCI, persons with nontraumatic SCI are significantly more likely to have paraplegia than tetraplegia.301
Neoplastic Causes of Spinal Cord Injury
Tumors can arise from either the neural elements in the spinal canal, such as the spinal cord or spinal nerves, or the structures comprising the spinal column, most commonly the vertebral bodies. Tumors arising from the spine (extradural tumors) are much more common than those arising from neural elements (intradural tumors). Extradural tumors are most commonly metastatic lesions, being 25 times more common than primary tumors involving the spine.109 After brain metastasis, spinal cord compression is the second most common type of neurologic involvement of cancer.1
Classification of Spinal Tumors
The most common method of classifying tumors relates to the anatomic location of tumor involvement. Spinal tumors are extradural when they arise from structures outside the dura, most commonly the vertebral body. A less likely origin is from the structures of the posterior bony arch or the soft tissues outside the dura. Most tumors metastatic to the spine are extradural, making up 55% of all spinal tumors.270 The most common primary sites of metastatic tumors to the spine are lung, breast, prostate, and kidney.220 The mechanisms of metastasis include direct extension of tumor from adjacent tissues, and hematogenous spread through Batson’s vertebral venous plexus, a valveless venous system draining the thoracic, abdominal, and pelvic viscera.27 Primary spine tumors that are present in the extradural region make up less than 1% of all spinal tumors. These include multiple myeloma, osteogenic sarcoma, vertebral hemangioma, chondrosarcoma, and chordoma.220
Tumors arising within the intradural space include those that are intramedullary (i.e., tumors arising from the parenchyma of the spinal cord) and those that are intradural but extramedullary. Intramedullary tumors are usually primary tumors, most commonly ependymomas and astrocytomas, which together make up 75% of all intramedullary tumors.220 Ependymomas tend to be well encapsulated and regularly shaped in contrast to astrocytomas, which tend to be irregularly shaped with multiple extensions into the cord parenchyma. Most intradural extramedullary tumors are both benign and primary, and include meningiomas and nerve sheath tumors such as schwannomas and neurofibromas.220 Those metastatic tumors that are seen can arise either by hematogenous spread or as “drop metastases,” lesions that directly extend from the CSF in association with malignant brain tumors such as medulloblastomas.270
Clinical Presentation of Spinal Tumors
Acute spinal cord compression is associated with rapid neurologic decline, and constitutes a medical emergency because it can rapidly progress to paraplegia or tetraplegia. When signs and symptoms of acute spinal cord compression related to neoplastic involvement of the spine are present, most patients will have substantial radiographic abnormalities. The syndrome of acute spinal cord compression, when related to spinal tumors, most often results from the invasion of spinal structures by extradural metastases.109
Management of Spinal Cord Compression by Tumor
Acute spinal cord compression is managed with corticosteroids, radiation, and surgical intervention. corticosteroids, typically dexamethasone, are administered to reduce tumor-related inflammatory changes and prostaglandin production.220 Radiation therapy is often used in cases of spinal cord compression resulting from soft tissue encroachment. It can be used alone in the setting of spinal stability, or in combination with surgery. Radiation therapy is less often used for the treatment of intradural or intramedullary tumors, unless such tumors are deemed unresectable or when surgical resection is incomplete.220 Radiosensitive tumors involving the spine include lymphomas, small cell lung cancer, and multiple myeloma, whereas less radiosensitive tumors include breast, prostate, non–small cell lung, and renal cell cancers.220 Complications of radiation directed to the spine include radiation myelopathy and radiation plexopathy. In the setting of acute spinal cord compression, the immediate goal of surgical treatment is decompression of the cord to preserve or improve neurologic function.
For most intramedullary and intradural extramedullary tumors, surgical treatment is the most effective. Ependymomas, because of their encapsulated nature, often can be completely resected with good preservation of neurologic function.162,295 In contrast, because astrocytomas are irregular and invasive without a clear plane for resection, the goal of surgery is a subtotal resection of clearly abnormal tissue.110,295 Intradural extramedullary meningiomas arise from the dura and are resected along with the involved dura after being accessed through a laminectomy.294 Nerve sheath tumors can be entirely intradural or, in the case of the neurofibroma, can have extradural extension through an enlarged neural foramen. In neurofibromatosis type 2, extensive intradural involvement throughout much of the spinal cord can be present. In such cases, tumor debulking rather than complete resection is usually the surgical goal.301
Infectious and Inflammatory Causes of Spinal Cord Injury
Bacterial Infection
Bacteria can invade a vertebral body either hematogenously or by direct extension from a contiguous focus of infection, causing vertebral osteomyelitis. Persons at increased risk for bacterial vertebral osteomyelitis include persons who use intravenous drugs; immunosuppressed individuals; persons with diabetes; or renal persons with disease who are receiving dialysis.31,371 Children are relatively susceptible to bacterial diskitis alone, probably because of a relatively robust blood supply to the intervertebral disk198 The bacteria most commonly implicated in vertebral osteomyelitis is Staphylococcus aureus, which accounts for more than half of all infections.382 Although infection can be seen in any portion of the spine, the lumbar spine is the most common area.246 Spine pain is by far the most common symptom of vertebral osteomyelitis, seen in greater than 90% of persons affected.371 Other symptoms can include fever or a neurologic deficit related to spinal cord compression from vertebral body collapse, or the presence of an epidural abscess. Laboratory markers of inflammation, such as the erythrocyte sedimentation rate and C-reactive protein, are very frequently found to be elevated with active infection.371,383 Isolation of the etiologic pathogen is vital to treatment success. This can be accomplished by recovery of the organism in blood cultures or through cultures of tissue obtained from the spine, either by needle or open biopsy. Treatment of vertebral osteomyelitis involves intravenous antibiotic administration for at least 4 weeks. Surgical treatment is indicated when appropriate antibiotics have been ineffective, when there is spinal cord or nerve root compression causing a neurologic deficit, or when there is spinal instability or spinal deformity.31,246
Tuberculosis of the spine, also known as Pott’s disease, results from hematogenous spread of the bacterium Mycobacterium tuberculosis to the spine, typically from a pulmonary focus.352 Spinal tuberculosis is treated with at least two and as many as four antituberculous agents for a 6- to 12-month duration.
Human Immunodeficiency Virus and Human T-Lymphotropic Virus Infection
Persons with human immunodeficiency virus (HIV) infection are susceptible to spinal cord disease, which can occur as vacuolar myelopathy, as primary HIV myelitis, or as a result of opportunistic infections of the spinal cord. A clinically evident myelopathy is found in 7% to 20% of persons with HIV infection.141,260 Vacuolar myelopathy presents with an incomplete spastic paraplegia with loss of proprioception and vibration sense.141 Vacuoles are found within the dorsolateral white matter tracts of the spinal cord in 40% to 55% of persons with HIV infection at autopsy, most commonly in the mid to low thoracic cord.202,363
Human T-lymphotropic virus type 1 (HTLV-1) is another retrovirus that causes a progressive chronic myelopathy. The clinical condition is a slowly progressive spastic paraplegia, which is referred to as both tropical spastic paraparesis and HTLV-1–associated myelopathy. The virus is transmitted through blood, sexual contacts, and from mother to child in breast milk. It occurs in the Caribbean, southern Japan, central and south Africa, and regions of South America.192
Transverse Myelitis
When a person has a rapidly evolving myelopathy with no history of trauma or physical or radiographic evidence of a structural lesion, the differential diagnosis should include an autoimmune disease such as systemic lupus erythematosus, multiple sclerosis, neuromyelitis optica, a paraneoplastic syndrome, nutritional deficiency, vascular insufficiency, and infection. If evidence for any of these conditions cannot be identified, transverse myelitis could be the etiology. Transverse myelitis is a myelopathic process of unknown cause, resulting in inflammation of the spinal cord. It can progress over the course of several hours or up to 2 to 3 weeks. The etiology can be infectious, but usually no organism is isolated. Although transverse myelitis can occur in any region of the spinal cord, the thoracic region is most common.120 In persons with transverse myelitis, magnetic resonance imaging (MRI) scanning often shows spinal cord swelling, with a region of increased signal on T2-weighted images that correlates with the patient’s clinical level.35 Treatment of transverse myelitis usually involves administration of corticosteroids, which have been shown to be associated with a greater and more rapid recovery than that in patients not treated with corticosteroids.263,397 Plasmapheresis can also be helpful.462
Vascular Causes of Spinal Cord Injury
Ischemia of the spinal cord, although less common than ischemia of the brain, is a well-known cause of SCI. It is most commonly associated with the anterior cord syndrome, and can occur as the result of systemic or local spinal cord hypoperfusion, embolization, or rarely thrombosis. Ischemia can also result from the presence of a type I spinal arteriovenous malformation (AVM). This is a dural arteriovenous fistula that arises when a single dural arterial feeder, either spontaneously or after trauma, develops a fistula to the spinal venous circulation. This fistula causes venous congestion and hypertension, which results in hypoperfusion of the spinal cord. Symptoms caused by type I AVMs are usually of gradual onset, with a progressive course, although there can be stepwise episodes of deterioration interspersed with periods of clinical stability. Sensory symptoms are the most common initial presenting symptoms, but weakness and sphincter disturbances are often present by the time the diagnosis has been made.242
Outcomes of Traumatic Spinal Cord Injury
Neurologic Recovery in Complete Tetraplegia
Persons with motor complete tetraplegia have a poor prognosis for recovering the ability to walk. Only 2% to 3% of persons initially classified as having an AIS of A convert to AIS D by 1 year.287 The prognosis for recovery of motor strength and sensation in myotomes and dermatomes close to the NLI is not nearly so dire. Overall, between 30% and 80% of persons with motor complete tetraplegia recover a single motor level, meaning gaining functional motor strength at that level, within 1 year of injury. Within this group, the most important prognostic factor for single motor level recovery is the initial presence of nonfunctional muscle strength (grade 1 or 2) at the level. A muscle with grade 1 or 2 strength at 1 week has a 70% to 80% chance of reaching grade 3 by 1 year. In contrast, for a muscle with grade 0 strength, there is only a 30% to 40% chance that such a muscle will reach grade 3 or better by 1 year.151 When examined at 1 month postinjury, a muscle with grade 1 or 2 strength just distal to the level of injury has a greater than 95% chance of reaching a grade of 3 or better, whereas only 25% of muscles with grade 0 strength reach grade 3.456 The chance of functional recovery of a muscle two levels below the motor level of injury, when the first muscle below the motor level is grade 0, is exceedingly rare. The stronger a muscle is within the zone of injury, the stronger it will probably become within 1 year. The speed of recovery is also correlated with strength within the zone of injury. A muscle with grade 2 strength just distal to the motor level will probably gain more strength and do so sooner than a muscle graded 0 in the same location.149 Several studies have also shown a strong correlation between motor function and self-care skills.72,148
Neurologic Recovery and Ambulation in Incomplete Tetraplegia
In the early 1970s, Bosch et al.66 reported that 90% of persons with central cord syndrome, but only a few of those with anterior cord lesions, were able to ambulate after SCI. Maynard et al.293 subsequently reported that 87% of persons with motor incomplete tetraplegia initially were walking by 1 year, whereas 47% of persons with sensory incomplete, but motor complete, tetraplegia were walking by 1 year. Among sensory incomplete patients, the type of sensation preserved below the level of injury is prognostically important. Persons with preservation of pinprick sensation near the anus have a greater than 70% chance of regaining ambulatory ability, while persons who have spared light touch sensation only in the same region are unlikely to regain ambulatory ability.113 Among persons with motor incomplete SCI, age and initial motor strength seem to be major determinants of ambulation. In one study of 105 persons with incomplete motor tetraplegia, in which the age of 50 years was arbitrarily chosen as a cutoff, 91% of all persons younger than 50 years, either AIS C or D, ambulated at 1 year. All persons older than 50 years and AIS D ambulated, whereas only 40% of persons older than 50 years and AIS C ambulated.87
Neurologic Recovery and Ambulation in Paraplegia
Recovery of lower limb function in persons with paraplegia is dependent on the completeness of injury and the level of injury. Among persons with complete paraplegia, about 75% retain the same NLI at 1 year that they had at 1 month postinjury, 20% gain a single level, and 7% gain two neurologic levels.459 When the gained levels are truncal in distribution, there is usually no functional significance or change. Persons with T1–T8 complete paraplegia do not recover lower limb voluntary movement. However, 15% of persons with complete paraplegia between T9 and T11, and 55% of persons with paraplegia at T12 and below, recover some lower limb function.459
Persons with incomplete paraplegia have the best prognosis for ambulation among all the groups of persons with traumatic SCI. Eighty percent of individuals with incomplete paraplegia regain functional hip flexion and knee extension within 1 year of injury, making both indoor and community-based ambulation possible.456
Functional Recovery
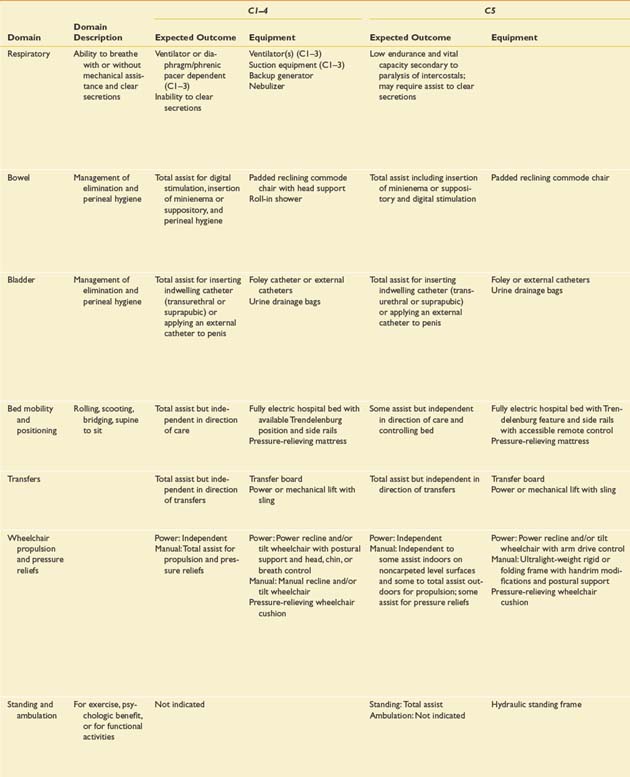
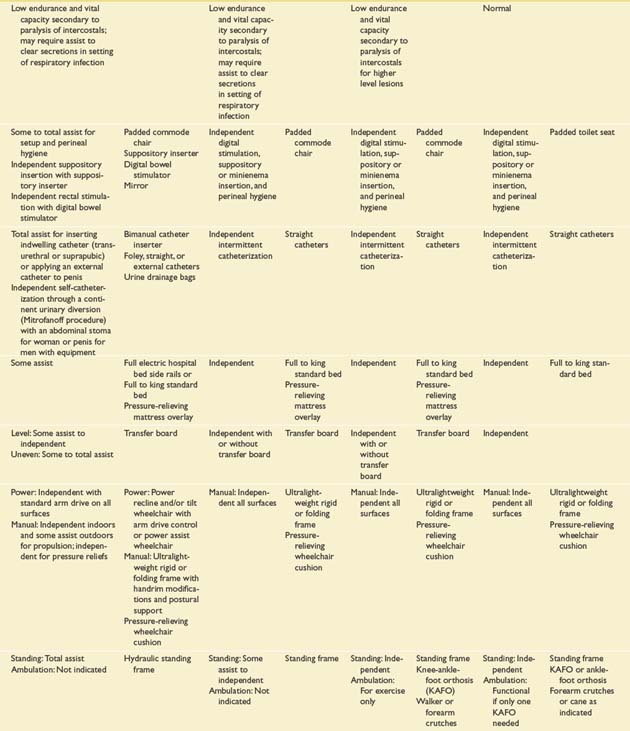
Prognostication of functional outcome depends on the physical examination findings, familiarity with the published functional outcomes of persons with SCI of different NLI, and an ability to integrate into a prognosis a host of other factors. These factors include, but are not limited to, preexisting medical conditions, concomitant injuries, secondary complications, cognitive impairments, age, body habitus, availability of financial resources and insurance coverage, psychologic factors, social factors, and cultural factors.468 The information in Table 55-5 is modified from the Outcomes Following Traumatic Spinal Cord Injury clinical practice guideline, which was first published by the Paralyzed Veterans of America in 1999.468 The expected outcomes are stratified by NLI and described for several different domains. They reflect the level of independence that can be expected of an average individual with a motor complete SCI under optimal circumstances 1 year after injury.
Acute Phase of Injury
Prehospital Care
The first 24 hours after trauma are the deadliest. In this period, primary and secondary injuries to the central nervous system are the leading cause of death.2 The first step in the treatment of a person with a suspected spinal injury is ensuring an adequate airway, breathing, and circulation. Patients with cervical cord injuries are at high risk for respiratory failure and must be monitored closely for the need for ventilatory support. Even if intubation is not needed urgently, arterial blood gas and vital capacity measurements are useful tools in identifying delayed respiratory muscle fatigue. When intubation is necessary, it must be done carefully in the setting of suspected or confirmed cervical spine trauma to avoid secondary cord injury. The standard technique for urgent intubation in this setting is rapid sequence induction with cricoid pressure and manual inline stabilization.108 Alternatively, awake fiberoptic intubation is an appropriate alternative, and may be the preferred technique, in a cooperative patient.
All persons with suspected acute SCI should have their spines immobilized. Persons with altered mental status, evidence of intoxication, suspected limb fracture or distracting injury, focal neurologic deficit and spine pain or tenderness should also be suspected of having a traumatic SCI.152 The entire spine should be immobilized in a neutral supine position regardless of the position the individual was found in after the accident. This is best accomplished with the use of a combination of a rigid cervical collar with supportive blocks on a backboard that has straps to secure the body.21,123 On the spine board, an occipital pad for an adult or an occipital recess for a child younger than 6 years can be used to compensate for the different sizes of the head relative to the body in persons of different age-groups.176
Once on the spine board, the individual is transported to a trauma center where the initial goals are to establish hemodynamic stability, to prevent hypoxemia and aspiration of stomach contents, and to maintain spinal immobilization until definitive management is accomplished. Maintenance of adequate blood pressure is critical because hypotension and shock are extremely deleterious to the injured spinal cord.21 A target mean arterial blood pressure of 85 mm Hg for a minimum of 7 days has been associated with favorable outcomes.269,450 Although neurogenic shock is associated with cervical and high thoracic injuries, it is important to evaluate the patient fully for other causes of shock including hemorrhage, pneumothorax, myocardial infarction, cardiac tamponade, sepsis from intraabdominal injury, or even acute adrenal insufficiency in patients with concomitant brain injury. Neurogenic shock is the result of sympathetic denervation and is characterized by hypotension and bradycardia in the setting of flaccid paralysis. Bradycardia results from unopposed parasympathetic input to the heart, but can also be stimulated by endotracheal suctioning. Neurogenic shock is treated with restoration of intravascular volume and vasopressor agents. The ideal pressor agents have both α- and β-adrenergic actions, to counter the loss of sympathetic tone and provide chronotropic support to the heart.108 Atropine is helpful to rapidly reverse the bradycardia. A temporary or permanent cardiac pacemaker insertion is rarely necessary.105 Hemorrhagic shock is treated by controlling bleeding and vigorous fluid resuscitation. Core temperature should be monitored because persons with cervical or high thoracic injuries can become relatively poikilothermic because of autonomic nervous system disruption. Placement of a nasogastric tube in the acute period is important to prevent emesis and aspiration of gastric contents, while an indwelling bladder catheter ensures adequate bladder drainage in a situation where urinary retention is the rule. Early contact between the receiving trauma center and a specialized SCI center should be established, with arrangements made for transfer to the spinal injury center once medical stability has been secured.
Once stabilized medically, a thorough evaluation of neurologic status and spinal stability is performed. The neurologic status is determined using the ISNCSCI. Serial examinations should be performed to detect neurologic deterioration or improvement, particularly in the first 3 days after injury and after manipulation such as transport, closed reduction or surgical treatment. Spinal stability is assessed for the entire spine, not just the clinically likely area of injury, because there is an approximately 20% chance of finding noncontiguous spine fractures.448 Computed tomography (CT) imaging of the entire spine is recommended because of the lack of sensitivity of plain film protocols, particularly in the craniocervical region and at the cervicothoracic junction.296,448 MRI evaluation is essential for evaluating nonbony tissues including the spinal cord, nerve roots, ligaments, and intervertebral disks, and should be performed to evaluate the area of a known or suspected SCI. MRI evaluation is particularly important for identification of spinal pathology in persons with a neurologic deficit not identified by CT and for those persons who are unconscious or obtunded.
Surgical Management
Closed reduction of a cervical dislocation is performed by applying a series of increasing distracting forces through the long axis of the body by means of a two-point attachment to the skull with a tong device. The tong device is attached to a rope that passes through a pulley and is attached to a weight. Up to 140 lb of weight has been applied to achieve cervical spine realignment in an awake and cooperative patient.111,447 When the obstruction to normal alignment is overcome with the applied distraction force, and the spine is realigned (e.g., a jumped facet), the distracting forces are reduced again. Typically 10 to 15 lb of traction is kept in place to maintain alignment.
Operative treatment of acute spinal injury is generally performed either to stabilize an unstable spine or to decompress compressed neural elements—for example, spinal cord or nerve roots. The timing of surgical treatment has been controversial, except in the setting of a progressively worsening neurologic deficit when immediate surgery is usually indicated. Studies have supported both immediate and delayed decompression.288,449 One large multicenter study of approximately 800 persons compared outcomes for persons in three groups: those with early surgery (defined as either within 24 hours of injury or between 24 and 72 hours from injury), late surgery (>72 hours after injury), or no surgery.300 In comparing the early and delayed surgery groups, those undergoing later surgery had significantly increased acute care and total hospital lengths of stay, as well as a higher incidence of pneumonia and atelectasis. There were no differences in neurologic or functional outcomes between the early and delayed surgery groups.
Most practitioners of SCI medicine agree that persons with incomplete injuries and spinal cord compression are best served by performing a decompressive procedure to maximize the potential for neurologic recovery. Decompression of the spinal cord in the setting of a neurologically complete injury has been more controversial. It is well established that decompression of the cord in a complete injury is not usually associated with a change from complete to incomplete status. Therefore the role of surgery in neurologically complete lesions is to provide early stability and rapid involvement of the injured person in rehabilitation, potentially minimizing the occurrence of medical complications in the early phase of the postinjury period. Decompression of the cord in complete injuries might also reduce the incidence of posttraumatic cystic myelopathy.
Penetrating injuries to the spinal cord are overwhelmingly caused by gunshot wounds. Stab wounds as a cause of SCI are relatively rare.233 Surgical management for such injuries is only rarely indicated. As a rule, neither of these mechanisms of injury cause spinal instability. Removal of bullets or bullet fragments is typically performed only if their presence in the spinal canal is associated with progressive neurologic deterioration.
Pharmacologic Treatment of Acute Spinal Cord Injury
Because motor and sensory recovery after traumatic SCI often is poor, there has been an intense interest in finding an effective treatment for SCI. A number of large multicenter clinical trials have investigated potential chemical treatments for acute traumatic SCI, including methylprednisolone, GM-1 ganglioside, gacyclidine, tirilazad, and naloxone.69,71,72 Implantation of autologous activated macrophages to the inferior border of an acute cord lesion has also been investigated. None of these treatments have been definitively demonstrated to improve neurologic outcomes. Therapeutic hypothermia, which gained national attention in 2007 when associated with a substantial recovery in a professional football player, is presently a clinically unproven treatment.
Of the treatments investigated, only high-dose methylprednisolone sodium succinate (MPSS) has been regularly administered, and for a time was considered the standard of care. MPSS was reported in the National Acute Spinal Cord Injury Studies (NASCIS II and III) to improve motor and sensory scores when administered in extremely high doses within 8 hours of injury according to a protocol in which patients received a loading dose of 30 mg/kg over the first hour of administration, followed by a maintenance dose of 5.4 mg/kg for the next 23 hours.71 If MPSS administration is begun more than 2 hours after injury, it was recommended to continue the maintenance dose for an additional 24 hours.72 The positive outcomes noted in these trials were subsequently criticized because of the failure to identify and control for potential confounding factors, and because post hoc analysis failed to demonstrate improvement in the primary outcome measures of motor and sensory scores. At this time, therefore, the neuroprotective effects of high-dose MPSS are uncertain. Certainly, MPSS should never be administered for neuroprotection in neurologically intact patients or in any patient beyond 8 hours from the time of SCI.
Rehabilitation Phase of Injury
The Interdisciplinary Team
In the acute hospital setting, staff members who are not fully familiar with treating persons with SCI need to be educated about the potential secondary complications of SCI and how to prevent them. The patient and family members need to be educated about the nature of an SCI and the patient’s prognosis and the uncertainty of such. Transfer to a specialized SCI rehabilitation unit should also be facilitated, because patients treated in a specialized SCI center have increased overall survival rates, decreased complication rates for pressure ulcers and other problems, a decreased length of hospital stay, greater functional gains during rehabilitation, a greater likelihood of home discharge, and lower rehospitalization rates.53,137 Physical and occupational therapists in the acute hospital should facilitate prevention of secondary complications such as contractures, pressure ulcers, and disuse atrophy. This is done through maintenance of joint ROM, splinting, positioning, and selective muscle strengthening. ROM of all joints is performed and taught by the therapists to persons with SCI and their caregivers as soon as it is medically safe to do so. Performance of an adequate daily stretching program can prevent joint contractures. Splinting of joints, with either an off-the-shelf or a custom splint fabricated by an occupational therapist, is also often used to provide a prolonged stretch, to facilitate a functional joint position, and to prevent skin breakdown.
The person with SCI and the family members are essential members of the team. If the person with SCI does not participate in the SCI rehabilitation program, it is not likely to be of much benefit. Rehabilitation nurses, in addition to performing their standard nursing duties, provide education on prevention and treatment of secondary complications, as well as training in bowel and bladder management. Psychologists help to reduce depression and anxiety, as well as facilitate adjustment to a catastrophic and life-altering injury, by supporting persons with SCI (and their families) through the grieving process. This is achieved by providing individual psychotherapy, cognitive-behavioral techniques to enhance adaptive coping, and group psychotherapy to provide additional support and information sharing. Social workers or case managers help individuals with SCI, their families, or their caregivers to obtain needed available resources, benefits, and services. They facilitate the transition from an inpatient rehabilitation unit to the home or another facility, and provide family support. Other physician consultants are typically involved at various points in the rehabilitation process, especially if secondary complications develop. Speech therapists evaluate and treat the swallowing and communicating problems that are common in individuals with tracheotomies, high cervical neurologic levels of injury, and anterior approach cervical spinal surgeries. They commonly perform bedside swallowing evaluations as well as participate in modified barium swallow tests.
Physical Skill Training
Training in activities that are performed on a therapy mat are commonly begun as soon as a patient is able to tolerate being out of bed. These activities, which are often composed of separately performed parts of a more complex functional skill, are typically sequenced from the easiest to the most difficult. In progressing through these graduated skills, persons with SCI, who are often able to do little for themselves initially, move to a level of stability within a specific training posture. Finally, they are able to move in a safe and effective fashion to complete functional tasks.390 When the tasks are mastered on the mat, they are performed in other more real-life environments, such as in bed.
Transfer training for a person with a complete paraplegia or lower tetraplegia is usually first taught with a sliding board. For a transfer into or out of a manual wheelchair, the wheelchair is positioned at an angle of 30 to 45 degrees from a parallel position to the mat, with the front of the seat nearest the mat. This allows clearance of the rear wheels by the buttocks during the transfer. For a transfer into or out of a power wheelchair, the wheelchair is positioned parallel to the mat, because the high wheels that are present on a manual wheelchair are not in the way on a power chair. Next, the individual scoots forward in the chair, removes the armrest, inserts the sliding board deep under the leg closest to the mat, and then rocks the head and shoulders away from the mat while simultaneously pushing up and toward the mat with the arm furthest from the mat. This causes the buttocks to move onto the sliding board. The rocking and pushing is repeated until the individual is safely on the mat, at which time the sliding board is removed. Leg rests might need to be removed for the transfer. A popover transfer is similar, except that a sliding board is not used. In addition to these techniques, several other different types of transfer techniques are used by persons with varying levels of neurologic function. These include the dependent lift transfer, mechanical lift transfer, stand-pivot transfer, sit-pivot transfer, and floor-to-chair transfer. The mechanical lift transfer uses a mechanical device attached to a sling. The dependent lift transfer and the mechanical lift transfer are used mainly for individuals who are unable to physically assist in the transfers. The stand-pivot and sit-pivot transfers require weight-bearing on the lower limbs, and are useful only if a person has significant lower extremity extensor tone or adequate lower extremity strength to briefly squat or stand. The floor-to-chair transfer is important for anyone who falls out of the wheelchair or otherwise ends up on the floor, and needs to get back into the chair or another higher surface. Standing can be initiated on a standing frame or a tilt table. Standing seems to help lessen bone loss after an acute injury, improves physical self-concept, and improves self-reported health.121,214,239,261
Standing should be implemented only with caution in individuals with chronic SCI, because of osteoporosis. Individuals with osteoporosis have a risk for fracture even without weight-bearing. Although ambulation is an expressed goal of most people who have experienced an SCI, recovery of ambulation is variable (see discussion above). For persons with incomplete motor SCI, gait training can be facilitated by body weight support (BWS). For persons with complete thoracic level injuries who wish to undergo ambulation training, orthoses that stabilize the knees and ankles are required. A swing-through gait pattern (Figure 55-13) is taught in several steps similar to the mat activities described above. This begins in the parallel bars and includes going from sit to stand, balancing with extended hips, push-ups in the standing position, turning while standing, recovery from a flexed hip position, advancement of the lower extremities with hip hiking, performance of a step-to gait, and finally a swing-through gait pattern. After the swing-to or swing-through gait pattern is mastered in the parallel bars, it is performed with a walker or crutches.
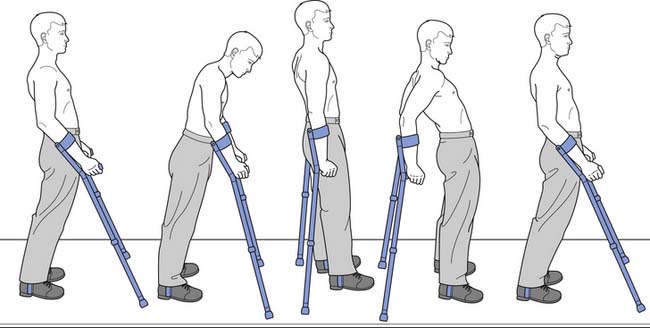
FIGURE 55-13 The swing-through gait pattern used by a person with complete paraplegia with long leg braces.
(Redrawn from Schmitz TJ: Traumatic spinal cord injury. In O’Sullivan SB, Schmitz TJ, editors: Physical rehabilitation: assessment and treatment, Philadelphia, 2001, FA Davis, with permission of FA Davis.)
Wheelchair Skills
Physical and occupational therapists not only train persons with SCI in wheelchair mobility, but also help select the proper seating systems to ensure proper sitting position. Wheelchair users are taught to manage or to direct the management of all wheelchair components, including the brakes, armrests, footrests, wheels, and seat cushion. They are taught how to fold or break down the chair so it can be placed properly in a vehicle. They are taught wheelchair propulsion, first indoors over level surfaces, then outdoors over uneven terrain. Proper body mechanics are taught to achieve efficient wheelchair propulsion patterns, including an ideal propulsive stroke and an ideal recovery stroke. An ideal propulsive stroke is one that occurs at a steady speed that maximizes the handrim “contact” or “push” angle (angle along the arc of the pushrim) while keeping stroke frequency and forces to a minimum.122,377 Although self-selected wheelchair propulsion patterns are often not the most efficient ones, experienced wheelchair users often significantly improve propulsion biomechanics from early to late during extended propulsion. Individually selected wheelchair propulsion patterns are often influenced by poor wheelchair sitting positions.377 Of several propulsion patterns that have been described, differing primarily in their recovery phase, a semicircular wheelchair propulsion pattern has been shown to be the most efficient and least stressful on the shoulders and nerves crossing the wrist.64,65 Another basic wheelchair mobility skill is performance of a wheelie, in which the individual in the wheelchair balances on the rear two wheels. This is an important skill that needs to be mastered, to become independent in curb and single-step climbing in a wheelchair.
Spinal Cord Injury Education
During the early phases of SCI, most patients and their families have little knowledge or understanding of the injury; its multiple consequences; the myriad of interventional and management options, community resources, and equipment needs; and the prognosis for life, health, and function. They are often overwhelmed by the gravity of the situation and unable to adjust to a changed lifestyle and self-image. A comprehensive education program is an essential part of any SCI rehabilitation program and, if properly designed, helps the person with SCI and the family members not only to gain knowledge, but also to emotionally adjust and prepare for a successful community reintegration. Although some of the learning occurs in formal education classes, group discussions, reading specific educational materials published by various SCI organizations, or extracting information on the Internet, one-on-one instruction by health care professionals is the most helpful in addressing individual needs and concerns.216 With proper education and the ability to access appropriate information readily, the person with SCI becomes best able to manage successfully the various impairments and ensure the highest possible function and quality of life.
The curriculum of a structured SCI education program should be as broad as possible and include the anatomy, physiology, and classification of SCI, as well as the various medical consequences of SCI, psychosocial adjustments that need to be made, the effect of SCI on sexual health and fertility, assistive technology available for persons with SCI, nutritional needs with SCI, available community resources, and ongoing research to improve neurologic function.
Home and Environmental Modifications
The ADA does not demand removal of architectural barriers in private homes, most of which remain inaccessible for wheelchair users. A home evaluation is best performed before a new wheelchair user returns home. This begins with a review of the floor plan, followed by a home visit, recommendations for architectural changes, and contracting with architects and builders. The main home areas of concern include the main entrance, bathroom, bedroom, and kitchen. The home must also have an exit that the person with SCI can use in an emergency.154
Driver Training
Some people with C5 tetraplegia are able to drive, but usually not within 1 year of injury. Most such individuals use a power wheelchair for mobility and are not able to transfer to and from the wheelchair and the car. They require a van with power door openers, automatic lift or ramp, and extensive modifications of the control mechanisms. Occasionally, they require a multiaccess driving system in which the steering, accelerator, and brake are operated by a single control lever.13
Over the years, major advances in assistive driving technology have made it possible for more people with disability to safely operate a vehicle. The primary controllers of a vehicle, such as steering and braking, can even be concentrated in a complete system that can be operated with only one hand, through a tripin or joystick terminal device. This can incorporate the secondary controllers—for example, the gear shift, turn signals, hazard warning lights, horn, dimmers, cruise control, washer, wipers, radio, air conditioner, heater, defroster, doors, lift, and steering tilt.82,404 All drivers must use seat belts, but those with reduced trunk control must also use safety belts to secure trunk stability, such as shoulder, chest, or lap belts.
All persons with disability wanting to drive should undergo a driving evaluation by a specialist, usually an occupational therapist certified by the Association of Driver Educators for the Disabled (ADED). The ADED website can help to locate certified driving evaluators in the United States and Canada. A predriving evaluation includes an assessment of the person’s medical and driving history, as well as functional capabilities. Interactive driving simulators can present the users with diverse challenges in a safe environment, and can provide objective measurement of driving behaviors. Ultimately, however, an actual on-the-road driving evaluation is essential. Selection of the proper vehicle and appropriate modifications to fit the user’s ability can involve input from various members of the rehabilitation team. After the vehicle has been modified, the driving educator ensures that all the equipment is appropriate and that the driver is able to use the controls. Behind-the-wheel training by the driving educator can be a lengthy process for persons with high-level tetraplegia, because of the complexity of the equipment and the impairment of the learner. The high cost of vehicles and modifications, and the complexity of training are the most common reasons some people with high-level tetraplegia (C5–C6) choose not to drive. Finally, it should be noted that persons with physical disability do not have worse safety records than other drivers.316
Vocational Training
Persons who have SCI and are employed report higher levels of psychologic adjustment, satisfaction with life, independence, and general health than those who are unemployed.241,252,253 Nevertheless, only about one quarter of all individuals with SCI are employed. Among persons listed in the National SCI Database, approximately 63% were employed at the time of their SCI, 19% were students, and 17% were unemployed. The relatively high number of unemployed persons at the time of injury is recognized as a factor negatively affecting postinjury employment.255
Predictors of employment after SCI include being employed before SCI, having a less physically demanding occupation before SCI, being younger at the time of SCI, having a less severe SCI, having lived more years with SCI, having more education before SCI, being motivated to work, and being white.139,201,252,441 Education has been found to be the factor most strongly associated with postinjury employment. Only 5% of persons in the National SCI Database with less than 12 years of education were found to be employed, but with each successive educational milestone completed, employment numbers improved, reaching a high of 68.8% employment of persons with doctoral degrees.253 In general, by postinjury year 10, 31.8% of persons with paraplegia and 26.4% of those with tetraplegia are employed.23
Vocational rehabilitation is concerned with supporting efforts by a person with a disability to return to and maintain employment. Rehabilitation counselors, who facilitate this process, usually have master’s degrees in rehabilitation counseling and optimally are accredited by the Council on Rehabilitation Education. Vocational rehabilitation typically begins with an evaluation of the person’s functional limitations, barriers to employment, transferable skills, career interests, and prior achievement. The assessment might also include an assessment of performance during simulated or actual work. Assessment might be followed by counseling and support with regard to educational or vocational reentry, job accommodation, and supported employment. Educational or vocational reentry is often facilitated by a rehabilitation counselor who can liaise with employers, because most employers have little experience interacting with persons with disability and can have difficulty imagining how a person with an SCI could perform a specific job.241 Job accommodation, or the modification of a job to make it accessible to a person with SCI, might involve modification of the job site, use of adaptive equipment, or job policy changes.437 Modifications of the job site can range from the simple, such as adjustment of desk height to accommodate a wheelchair, to the complex, such as redesign of an assembly line.437 Adaptive equipment can include tools with special handles or sit-stand workstations. Job policy changes can include reassignment of physical tasks or changes in the number and length of workday breaks. Supported employment refers to the need by the individual with SCI for additional assistance in the workplace, ranging from a need for a full-time personal care assistant to the occasional need for manipulation of work materials.
Several legislative initiatives have been implemented with a goal of promoting employment of persons with disabilities. The Rehabilitation Act of 1973 provides federal funding for vocational rehabilitation programs in each state. ADA attempts to prevent discrimination in employment against qualified individuals who are able to perform essential functions of a job with or without accommodation.12 Workers’ compensation programs in most states provide a vocational rehabilitation program for workers who are injured at work, including retraining of those who cannot return to a previous or similar job.
Reconstructive Surgery of the Upper Limbs
Functional upper limb surgical reconstruction has historically been delayed for at least 1 year postinjury to allow for neurologic recovery in targeted muscles.456,457 Muscles are chosen for transfer that have a strength grade of 4 or 5, because one grade of muscle strength is usually lost with the transfer. Transferred muscles with a strength grade of less than 3 generally do not improve function. Muscles should not be chosen for transfer if their loss would result in a functional decline. After a tendon transfer procedure, the tendon constructs are typically immobilized for several weeks in a nonstretched position, followed by gradual mobilization, strengthening, and reeducation. Functional electrical stimulation (FES) is often used to facilitate neuromuscular reeducation. Common tendon transfers and tenodesis procedures are shown in Table 55-6 stratified to ISNCSCI motor levels and the more specific International Classification for Surgery of the Hand in Tetraplegia (ICSHT) motor groups.298,299 The ICSHT has a sensory component as well, for which the presence or absence of two-point discrimination of 10 mm or less on the pulp of the thumb is determined. A two-point discrimination threshold of 10 mm or less has been demonstrated to be predictive of sufficient proprioception in the hand to allow for hand function without visual cues.315
Table 55-6 International Classification for Surgery of the Hand in Tetraplegia, International Standards for Neurologic Classification of Spinal Cord Injury, and Possible Tendon Transfers
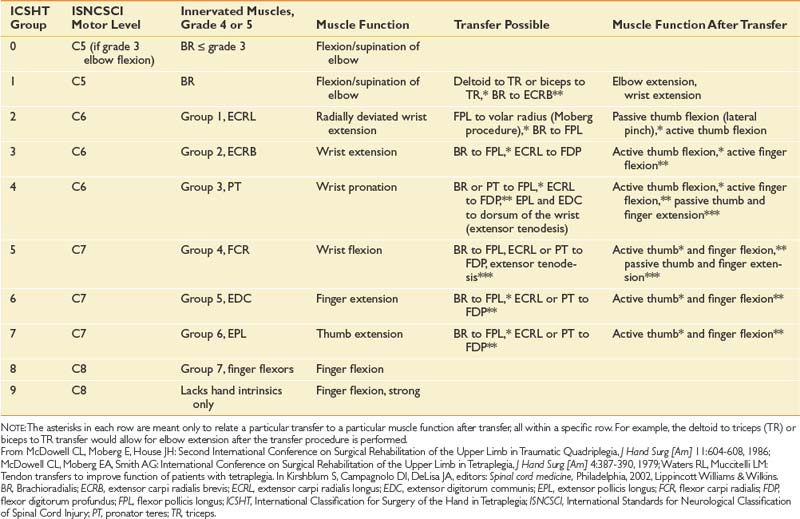
Functional improvements achieved after a deltoid or biceps to triceps transfer primarily occur through an increased ability to stabilize the arm and reach overhead. Improvements can be seen in feeding, grooming, pressure relief performance, and writing.322,364 Functional improvements after a brachioradialis (BR) to extensor carpi radialis brevis transfer can include an increased ability to pick up objects, feed, groom, write, and type.240 Functional improvements after a BR to flexor pollicis longus (FPL) transfer can include an increased ability to pick up a pen and write, more efficient grooming, and less dependence on a wrist-hand orthosis for grasp.455 The combination of BR to FPL and extensor carpi radialis longus to flexor digitorum profundus (FDP) tendon transfers has been shown to lead to improved key pinch, grasp strength, and subjective ADL performance.277 Functional improvements after the combination of BR to FPL and pronator teres to FDP tendon transfers have been noted in manual wheelchair propulsion, lower limb dressing, opening of jars, and the transferring of objects with the reconstructed hands.4,188
Functional Electrical Stimulation for Therapeutic Exercise
Sedentary lifestyle and impaired autonomic function in persons with SCI lead to many degenerative physiologic changes that can affect their health and wellness.366 Muscle bulk, strength, endurance, and bone density are lost in the paralyzed limbs; cardiovascular fitness, vital capacity (VC), and lean body mass are reduced; and certain endocrine functions are altered.47 First introduced more than 20 years ago, FES-assisted cycle ergometers have been clinically used by persons with SCI, both those with paraplegia and with tetraplegia, to effectively reverse many of these secondary effects, leading to improved muscular endurance, exercise tolerance, oxidative metabolism, cardiovascular fitness, glucose homeostasis, and lipid lipoprotein profiles, as well as decreased muscle atrophy.238,317,356,367,423,424
FES is not effective in stimulating muscles paralyzed by LMN damage (i.e., damage affecting the anterior horn cells, motor nerve roots, or both), but such damage usually occurs at the level of the SCI lesion. FES is usually a relatively safe intervention, but its application in the presence of cardiac pacemakers or implanted defibrillators is best avoided. Caution is also warranted in the presence of various heart conditions (especially dysrhythmia and congestive heart failure) and during pregnancy.204
Body Weight Support Ambulation Training on a Treadmill
The spinal cord was viewed in the past as being a large nerve solely for conducting information between the brain and the periphery. It was thought to be capable of processing only simple spinal reflexes. Through the pioneering work of Barbeau and Rossignol36 and Edgerton et al.156 during the 1980s, it has been shown that after SCI, the spinal cord can generate motor control to allow full weight-bearing locomotion without any supraspinal influence. The human spinal cord has also been demonstrated to be capable of processing complex proprioceptive information, including information generated during standing and stepping. The level of motor function achieved after SCI is in large part user-dependent, and it improves and is maintained by training.155 Clinical studies have shown that specific intensive walking training of persons with incomplete SCI significantly improves walking capabilities.465,466 Such training consists of upright walking on a treadmill, with partial BWS provided by a suspending harness, with a therapist guiding and setting the limbs. BWS training has been shown to be effective in improving ambulatory function after SCI, although not more effective than equivalent overground mobility training.422
Chronic Phase of Injury
Adjustment to Disability
SCI is a catastrophic event with a profound effect on the injured person as well as on family members. Different personal, interpersonal, and cultural factors ultimately determine how successfully an individual with SCI will adjust or adapt to the disability.321 Successful adjustment is thought to have occurred when the disability is no longer the dominant concern in the person’s life.223 Such adjustment is highly individually variable and has been estimated by some to occur over 2 to 5 years, whereas others propose that adjustment is a lifelong process.144,146,443 Adjustment has been considered by many to consist of sequential psychologic reactions according to stage theories, beginning with denial and followed by anger, bargaining, depression, and finally acceptance or adjustment.174,259,464 Although such sequential reactions are often seen in persons with SCI, they tend to be highly individualized.418
Depression and anxiety are usually present early after SCI, and have been reported to occur in more than 30% of persons for 2 or more years postinjury.112,182 Risk factors for depression are similar to those in able-bodied individuals, including a family or personal history of depression. Factors specific to the SCI, such as the presence of complete neurologic injury, or medical comorbidities can increase the likelihood of depression. Depression and anxiety can play a role in the relatively high incidence of substance abuse, divorce, and suicide after SCI.146,254,389 Depression after SCI needs to be managed by the entire rehabilitation team using cognitive-behavioral treatments, and even psychopharmacology if needed. Members of the rehabilitation team need to communicate appropriately with a person with SCI by clearly answering all questions, addressing effectively the medical concerns, working with the person with SCI on realistic goals, and nurturing hopeful thinking.174 Pharmacologic treatment of depression should be considered for those whose symptoms interfere with performance in social, self-care, recreational, and vocational activities.174 A variety of antidepressant drugs can be used. These are best prescribed by a psychiatrist for those most severely affected—for example, those with suicidal thoughts or psychotic symptoms.
Quality of Life
Quality of life is a concept that is difficult to define but that can be described as a determination of the individual’s satisfaction with life—for example, whether the individual is able to do the things he or she personally wants to do. Compared with nondisabled people, persons with SCI tend to report decreased well-being and a poorer state of health, and score lower on physical, emotional, and social health domains of life.482 A metaanalysis has shown no relationship between the neurologic level, the completeness of SCI, and the subjective quality of life. Some factors are thought to affect quality of life positively, such as mobility and ADL independence, emotional support, good overall health, self-esteem, absence of depression, physical and social activities and integration, being married and employed, having completed more years of education, and living at home.144,145,223,257 In general, dissatisfaction with life after SCI seems more related to social disadvantages than to physical limitations.
Recovery-Enhancing Therapies
Most patients with SCI, even those with neurologically complete lesions, experience some degree of spontaneous neurologic recovery. This occurs as a result of resolution of the acute pathology, with recovery of nerve roots and spinal cord at the level of the lesion.149
Despite major efforts to find effective treatments to enhance neurologic recovery during chronic SCI, no reports of scientifically conducted clinical trials exist that show effectiveness of any specific pharmacologic agents.297 At best, active exercise, such as BWS ambulation, might promote recovery of motor function in persons with incomplete SCI (and even in one person with C2 AIS A injury).39,206 The drug 4-aminopyridine, which blocks potassium channels in nerve membranes and enhances conduction in demyelinated axons, has been extensively tested in clinical trials. Although it might improve some symptoms associated with SCI, especially spasticity, it has not been reported to improve motor or sensory function.435
Late Neurologic Decline
New motor or sensory deficits are reported to develop in approximately 20% to 30% of persons with chronic SCI.88,274 Most common is entrapment of a peripheral nerve (10%), especially of the median nerve at the carpal tunnel, or of the ulnar nerve at the elbow. Other entrapments can occur as well.88 Further damage of the spinal cord can be caused by posttraumatic syringomyelia (PTS), also called posttraumatic cystic myelopathy, by tethering or by compression of the cord.
Although it is common to find MRI evidence of a cyst within the spinal cord at the level of the injury, only 5% of all people with SCI develop PTS. It becomes a problem when the cyst expands longitudinally and damages the cord, causing clinical symptoms such as pain, sensory loss, weakness, altered muscle tone, and a variety of autonomic symptoms.274 The exact cause of PTS is unknown, but it might be related to obstruction of the normal flow of CSF because of scarring or canal narrowing. This leads to abnormal increases in CSF pressure during coughing and straining, ultimately causing a longitudinal dissection of the cord.274
The diagnosis of PTS is confirmed by MRI or CT myelogram with delayed images. The clinical symptoms of PTS and their progression vary greatly. If no neurologic decline is noted on regular follow-up examinations, the treatment can be symptomatic. Activity restrictions are usually advisable, especially avoiding strenuous exercise that could raise venous and CSF pressure. When continuous neurologic decline or intractable pain is associated with PTS, surgical treatment is usually indicated to reduce the size of the syrinx or to prevent its expansion. Surgical approaches include placement of a shunt in the syrinx to drain the fluid into the peritoneal, pleural, or subarachnoid spaces; marsupialization of the syrinx; and duraplasty.274 When treatment is successful, strength might increase, and pain and spasticity might diminish.
Tethering of the spinal cord as a result of meningeal or arachnoid scar formations can occur after SCI and prevent normal rostrocaudal sliding of the cord within the spinal canal on motion. Tethering of the cord in the cervical spine can generate enough cord traction with flexion of the neck to cause cord or brainstem displacement and neurologic symptoms, such as weakness, sensory deficits, and pain. If this occurs, surgical untethering can be considered.410