Chapter 4 Spinal cord injury
Incidence and aetiology
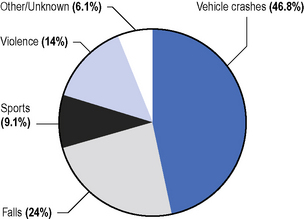
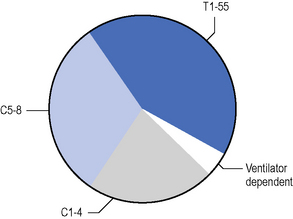
Data detailed are taken from the summary in De Vivo (2007). The ratio of male to female cases is approximately 3:1, with greater male preponderance in young age groups. Spinal cord damage can be traumatic or non-traumatic. The main causes of traumatic injury are shown in Figure 4.1. Gunshots and stabbings also make small but increasing contributions (Harrison, 2000; Whalley Hammell, 1995). A significant number of patients with mental health problems will sustain injury from jumping from a height. The level of injury at the time of discharge from hospital is illustrated in Figure 4.2.
The American Spinal Injury Association (ASIA) has produced a classification system for SCI, which is explained below and the classification of injury at discharge is also detailed below (see ‘Diagnosis’).
Non-traumatic aetiology is more common than traumatic. Reported incidence of nontraumatic SCI varies depending on which conditions are included in the study population. Non-traumatic most commonly result from degenerative disc disease and spinal canal stenosis, developmental anomalies (e.g. spina bifida) and congenital anomalies (e.g. angiomatous malformations); inflammation (e.g. multiple sclerosis); ischaemia (e.g. cord stroke); pressure on the cord due to expanding lesions (e.g. abcess or tumour extrinsic or intrinsic to the spinal cord) (New & Sundrajan, 2008). Each condition has distinct management needs and features. Their management will benefit from the knowledge and skills derived from an understanding of traumatic SCI, which is the focus of this chapter.
Terminology
Terms used to describe these patients indicate the general level of the spinal injury listing body functions and structure and a list of domains of activity and participation (International Classification of Functioning, Disability and Health; World Health Organization, 2001).
Types of spinal cord injury
SCI damages a complex neural network involved in transmitting, modifying and coordinating motor, sensory and autonomic control of organ systems. This dysfunction of the spinal cord causes variable loss of homeostatic and adaptive mechanisms which keep people naturally healthy. Any damage to the spinal cord results in deficits that can only be partly predicted, as described below. The pathology of the cord will influence the presenting impairment and the resulting prognosis. It can be precisely correlated with the neurological picture because of the segmental nature of the spinal cord (Kakulas, 2004). Complete transection of the cord is uncommon. It is useful to note that whilst the incidence of SCI in the population as a whole has largely remained the same, the overall prevelence is increasing. There are an increasing number of older people with SCI, reflecting the increasing ageing of the general population, in addition there are more people surviving with SCI into old age (Box 4.1 & Box 4.2).
Over the years there has been a significant reduction in mortality and preservation of neurology in new lesions (Grundy & Swain, 2002; Whalley Hammell, 1995). There are many reasons accounting for this, including:
A previous trend towards increasing incomplete lesions has now lessened. Recent incidence at time of injury has moved closer to equal numbers of incomplete and complete lesions. It is suggested that people with the more serious injuries are surviving, including a higher number of long-term ventilator-dependant patients. It is thought that the accumulative benefits of improved advanced life support and early interventions have now become fully established. It appears that there are no further influences currently increasing the trend towards an incomplete presentation, although the outcomes of new early restorative interventions will be eagerly awaited. There is a significant trend in the reduction in length of stay in the American Model Managed Care Systems, which also reflects a lower Functional Independence Measure (FIM) score at time of discharge and an increase in complications during the first year post discharge (De Vivo, 2007).
Pathogenesis
A brief outline of the pathological changes that occur with SCI is now given; further details can be found in other texts such as Tator (1998).
Later problems
After some weeks, there is evidence of astroglial scarring with cyst formation producing distorted neural architecture. In some cases, months or years later, a syrinx, an expanding cavity within the spinal cord probably associated with disordered cerebrospinal fluid (CSF) flow, may extend rostrally to produce further spinal cord damage. This posttraumatic syringomyelia may require drainage by shunt to prevent further extension. In view of this possibility, the neurological status should be reassessed periodically (Illis, 1988) and appropriate magnetic resonance imaging (MRI) scanning performed at intervals to minimize further neurological loss.
Spinal cord plasticity
When peripheral nerve is damaged, repair can lead to significant return of function (Battiston et al., 2009; Dahlin, 2008). It has been demonstrated that the central nervous system (CNS) has the capacity to regenerate and recover. It has similarly been hypothesized that there is capacity within the spinal cord to regenerate through a number of mechanisms. Research is ongoing to identify axonal budding, unmasking and interspinal spinal circuits (central pattern generators). For further reading on neuroplasticity see Chapter 11, Adkins et al. (2006), Kleim (2009), and Schwartz and Begley (2003). A summary of research aiming to establish new treatments in the management of spinal cord damage is discussed briefly below.
Treatment approaches in immediate post-injury management
There are four main approaches currently being considered to develop treatments for SCI (Ronsyn et al., 2008):
Understanding axonal guidance systems that will be required for directed outgrowth and functional reconnection will be essential if useful functional activity is to be regained. Although much progress has been made in the laboratory setting, no techniques applied to humans, despite having been through well researched trials (Fawcett et al., 2007; Lammertse et al., 2007; Steeves et al., 2007; Tuszynski et al., 2007) have yet to emerge showing any consistent results (Johnston, 2001; Ramer et al., 2000; Ronsyn et al., 2008; Tator, 1998).Claims for successful regeneration of the chronically injured spinal cord through late repair, remain contentious. Most studies lack robust pre- and post-intervention measures with many appearing to rely on associated intensive rehabilitation to demonstrate small functional improvements.
Diagnosis
Incomplete versus complete injury classification
ASIA Impairment Scale
In terms of diagnosis and prognosis, the classification of SCI has important ramifications. The ASIA Impairment Scale (ASIA, 2008) is the latest updated criteria for assessing and classifying functional levels of SCI, including the definitions of complete and incomplete lesions. The assessment is completed with the patient in supine, to enable testing in the acutely unstable injured person. The assessment comprises of 10 key myotomes and 28 dermatomes (Figure 4.3). Each dermatome is tested for light touch and pin prick sensation. The full description for these classifications will not be detailed here and it is available at www.asia-spinalinjury.org, with the impairment scale and classification outlined in Figure 4.4.
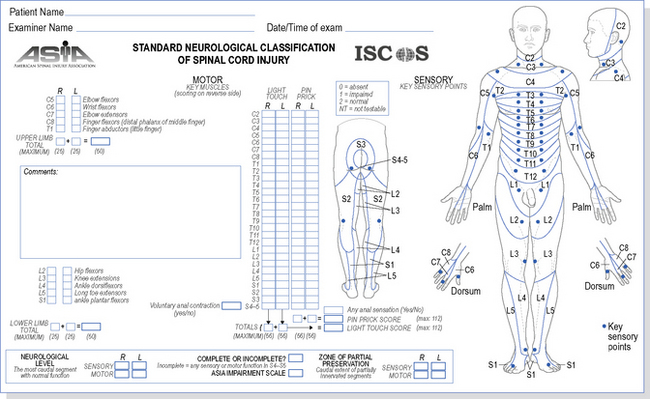
Figure 4.3 The American Spinal Injury Association (ASIA) Dermatome Chart and Impairment Scale.
American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, reprint 2008; Chicage, Il. Reprinted with permission.
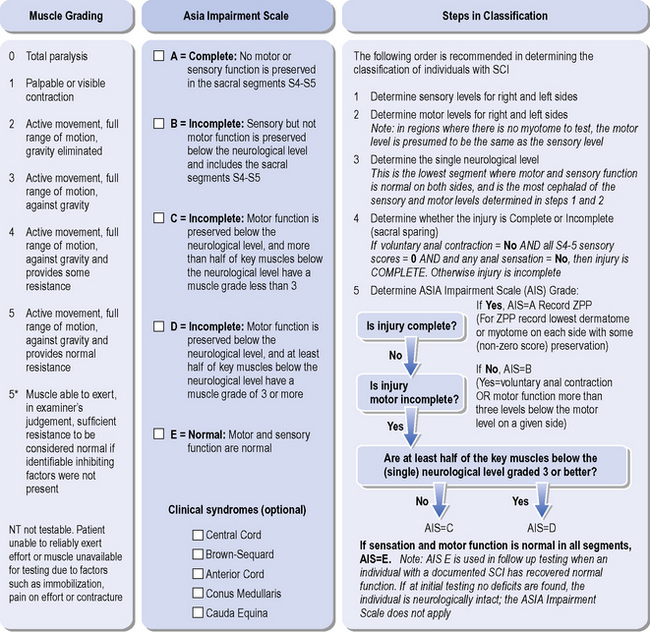
Figure 4.4 ASIA classification guide.
American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, reprint 2008; Chicage, Il. Reprinted with permission.
The ASIA system defines that a patient can have neurological sparing below the injury level, but in the absence of the sacral sparing, this is classified as a complete lesion, ASIA A, with zones of partial preservation. Where there is sensory preservation of S4–S5, the patient is classified sensory incomplete, ASIA B. This implies the preservation of the long tracts through the lesion. The classification of incomplete versus complete lesions indicates the presence of sensation in the lower sacral segments S4-S5, which implies significant prognostic indication of potential for neurological improvement. To be classified ASIA C, the patient must be assessed to have preserved S4-S5 sensation and voluntary anal sphincter motor activity. If the voluntary anal activity is absent then there must be preservation of motor function in some muscles innervated more than three levels below the motor classification level. In addition, more than half the key muscles below the neurological level must be grade 1-2/5. Similarly if half the key muscles are grade 3-5/5 then the classification will be ASIA D. The classification of SCI on discharge from hospital is shown in Figure 4.5.
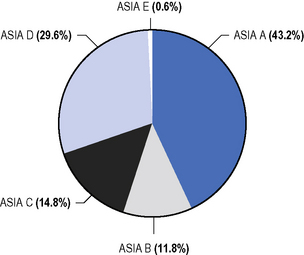
Figure 4.5 American Spinal Injury Association (ASIA) classification at discharge from hospital.
American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, reprint 2008; Chicage, Il. Reprinted with permission.
From a prognostic point of view, research suggests that 72 hours post injury (Brown, 1994; Maynard et al., 1979), and 1 month post injury are good time points for this classification (Waters et al., 1994a, 1994b). Further assessment is advised at 3 months post injury. All patients are assessed immediately on hospital admission, to gain baseline data. The wealth of prognostic statistics is based on data obtained using these time periods.
Incomplete lesions and prognostic indicators
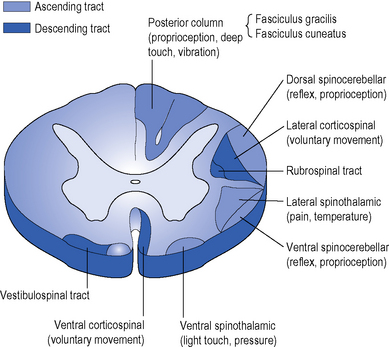
There are recognized patterns of incomplete cord injury which tend to present clinically as combinations of syndromes rather than in isolation. The signs and symptoms are related to the anatomical areas of the cord affected (Figures 4.6 & 4.7). Clinically, patterns of incomplete lesions are referred to as a syndrome.
Recent statistics show the common patterns of incomplete lesions to be the following:
Anterior cord syndrome
Anterior cord syndrome describes the effects of ventral cord damage affecting spinothalamic and corticospinal tracts; there is complete motor loss caudal to the lesion, and loss of pain and temperature sensation as these sensory tracts are located anterolaterally in the spinal cord. Preservation of the posterior columns means that perception of vibration and proprioception on the ipsilateral side are intact. This syndrome can arise from anterior spinal artery embolization. Motor recovery is thought to be less in these patients in comparison with other incomplete lesions (Crozier et al, 1991; Foo, 1986).
Brown–Séquard syndrome
Originally described by Galen, this syndrome describes sagittal hemicord damage with ipsilateral (same side) paralysis and dorsal column interruption, leading to loss of proprioception, in addition to contralateral (opposite side) loss of temperature and pain sensation. The relatively normal pain and temperature sensation on the ipsilateral side is due to the spinothalamic tract crossing over to the opposite side of the cord, at the level they enter the cord. This hemisection injury of the cord is classically caused by stabbing. This syndrome has a favourable prognosis, with almost all patients ambulating successfully (Johnston, 2001). The theory for this is that, despite the loss of pinprick on the one side of the cord, axons in the contralateral cord may facilitate recovery (Little & Habur, 1985).
Central cord lesion
The upper-limbs are more profoundly affected than the lower limbs and the condition is typically seen in older patients with cervical spondylosis. Due to degenerative changes in the spinal column, there are osteophytes and possible disc bulges, combined with spondylitic joint changes in the anterior part of the vertebral column. Posteriorly, the ligamentum flavum is thickened. A hyperextension injury compresses the cord in the narrowed canal and leads to interference of the blood supply. This may be already compromised in an older person and so has less potential for recovery. The central cervical tracts are predominantly affected. There is often flaccid weakness of the arms, due to lower motor neurone (LMN) lesions, and spastic patterning in the arms and legs due to upper motor neurone (UMN) injury. Bowel and bladder dysfunctions are common, but only partial. Research findings vary, showing that 57–86% patients will ambulate, although 97% of younger patients less than 50 years ambulate compared with 41% over 50 years (Foo, 1986).
Prognosis
Recovery of the incomplete spinal cord injury
Ninety per cent of incomplete SCI patients have some recovery of a motor level in their upper-limbs, compared to 70–85% of the complete injuries (Ditunno et al., 2000). Pinprick sparing in a dermatome is an excellent indicator of increased recovery of motor strength (Poynton et al., 1997) and it has been found that pinprick preservation below the level of the injury to the sacral dermatomes is the best indicator of useful recovery, with 75% of patients regaining the ability to walk. Fifty per cent of patients who had no sacral sparing regained some motor recovery but not of functional use (Katoh & El Masry, 1995).
Studies have found that incomplete SCI patients showed ongoing improvement in their motor activity, although this tended to slow during the second year post injury, with the exception of incomplete tetraplegics who lacked sharp/blunt discrimination and failed to demonstrate any lower-limb motor recovery. In incomplete paraplegics, there was evidence of 85% of the muscles recovering from a flicker to an antigravity grade within the first year, but if there was no activity initially, only 26% gained an antigravity grade (Waters et al., 1994a, 1994b).
Ambulation recovery
Between 44 and 76% of people with incomplete SCI, with preserved sensation but no motor function, have been reported to achieve ambulation (Maynard et al., 1979; Waters et al., 1994a, 1994b).
Crozier et al. (1991) reported, using ASIA assessment at 72 hours, that 89% of ASIA B–E patients with pinprick preservation went on to ambulate, compared with 11% having preserved light touch but not pinprick.
Summary of changes in ASIA impairment scale from admission to discharge
Acute general management
Trauma management
Immediate management
Acute hospital management
Acute trauma management guidelines are well established (Moore et al., 1991). Management of the patient with an SCI has special features resulting from spinal cord shock. Full details are described by Grundy and Swain (2002).
Breathing
Paralysis of respiratory muscles may be a feature. Patients with acute cervical cord injuries can fatigue in their breathing. Pulse oximetry is a crude indicator of respiratory distress because it measures only haemoglobin saturation and not partial pressure of oxygen (Po2) (Hough, 2001). Any evidence of desaturation or of falling saturation should be proactively addressed by the critical care team to maintain oxygenation and prevent further cord damage. Monitoring patients’ breathing rate, pattern and colour, and noting agitation, drowsiness or distressed behaviour, is vital. Arterial blood-gas analysis may be the critical factor in deciding whether to provide ventilatory support. Respiratory failure remains one of the main causes of death in acute tetraplegia, whilst pneumonia is the leading cause of death in all persons with SCI (Jackson et al., 1994). The principles of respiratory management are covered in Chapter 15.
Spinal cord shock
This is the phenomenon of cessation of nervous system function below the level of damage to the cord and may be due to the loss of descending neural influences. It is usually expected that after several seconds to months, the flaccid paralysis and areflexia of spinal shock are replaced by hyperexcitability, seen clinically as hyperreflexia, spasticity and spasms. More recently this has been identified as a critical period when the timing of potential interventions can influence recovering neurological systems. Studies have shown that there is competetive synaptic growth into the synaptic spaces that have been vacated. These transient vacant sites become open to repopulation by spared axons. It is at this moment that interventions should ideally target synapse growth mediating voluntary movement rather than local segmental neurons mediating spasticity and hyperreflexia (Ditunno et al., 2004; McDonald et al., 2002).
Stauffer (1983) noted that it is rare to see patients in total spinal shock and totally areflexic. Strong spasticity almost immediately post injury is indicative of an incomplete SCI. In these patients assessment of voluntary movement requires careful differentiation. In the authors’ experience, development of increased muscle tone and involuntary movements may mislead patients to believe they have functional return of activity. It is important for the therapist to anticipate such reactions and to assess carefully in order to avoid confusion and disappointment.
Spinal stabilization versus conservative management
Spinal fractures may be classified as stable, unstable or quasistable (i.e. currently stable but likely to become unstable in the course of everyday activity). Disagreement continues between protagonists and antagonists of surgical stabilization of the spine, but surgery is increasingly used (Collins, 1995).
Definition of instability or stability of a spinal lesion has now achieved substantial agreement based on the three column principles (Dennis, 1983). There is general agreement that restoration of the anatomy of the canal is sensible in terms of giving the cord the best opportunity for recovery.
It is debated whether neurological recovery or degree of spinal stability in the long term differs with surgical or conservative management. Surgery aims to minimize neurological deterioration, restore alignment and stabilization, facilitate early mobilization, reduce pain, minimize hospital stay and prevent secondary complications (Johnston, 2001). Review of evidence over the last decade does not identify any specific timing or role for early surgical decompression. Surgical intervention within the first 72 hours after injury has been shown to be safe and a role for urgent decompression has been identified in certain circumstances and may improve neurological outcomes (Fehlings & Perrin, 2006).
Management of acute lesions at T4 and above
Surgical stabilization may be achieved by anterior or posterior fixation, or a combination of the two (e.g. Collins, 1995). Patients managed conservatively are immobilized with bed rest; depending on the degree of instability, they may have to be maintained in spinal alignment by skull traction. Traction is applied usually by halo traction, Gardner–Wells or cone calipers (Grundy & Swain, 2002).
Early mobilization may be indicated and can be achieved using a halo brace (Hossain et al., 2004). Care in handling and positioning during physiotherapy is discussed below (see ‘Acute physical management’).
Special problems in spinal cord injury
Osteoporosis
Osteoporosis is a loss in bone mass without any alteration of the ratio between mineral and the organic matrix. A text by (Jiang et al., 2006) provides a comprehensive overview of osteoporosis. It is thought that immobilization for long periods and a sedentary life lead to an increase in bone reabsorption, thus causing osteoporosis.
Rapid loss of bone minerals occurs during the first 4 months following SCI. A range of data available from studies include: at 1 year bone mass density reduces in the femoral neck by 27%, mid femoral shaft by 25% and distal femur by 43%. The reduction continues in the pelvis and lower limbs over the 10 years after injury and can reduce by 50%. Tetraplegics can lose up to 16% on their bone mass density in their upper limbs. The osteoporosis may cause fractures of long bones during relatively simple manoeuvres, such as transfer or passive movements (Belanger et al., 2000). The rate of incidence of fractures has been documented to be 1% in the first year post injury, 1.3% per year at 1–9 years, 3.4% per year at 10–19 years, 4.6% per year at 20–29 years (Jiang et al., 2006).
It has been shown that early mobilization with weight bearing might prevent or slow bone-mineral loss (De Bruin et al., 1999). Bone mineral density can be preserved in bones below the level of the lesion (Frey-Rindova et al., 2000). The question is frequently asked whether a patient who has not stood for several years should recommence standing. There is a variety of opinion on how to proceed, either returning straight to standing or commencing a weight-bearing programme using a tilt table, usually combined with bone-enhancing agents, and monitoring using bone densitometry, before returning to standing in a frame. Such advice is empirical and, as yet, despite many studies, no clear guidance has been produced.
Heterotopic ossification
Calcification in denervated or UMN-disordered muscle remains an ill-understood process and commonly occurs in patients with SCI (David et al., 1993). It may be confused in the early stages with deep venous thrombosis, when it presents as swelling, alteration in skin colour and increased heat, usually in relation to a joint. During the active process, analysis of plasma biochemistry shows a raised alkaline phosphatase. It can result in loss of range of movement (ROM) and difficulty in sitting. If ossification occurs around the hips it may lead to further skin pressure problems. Treatment of this condition is discussed by David et al. (1993). It must be emphasized that stretching should be gentle, as overstretching may be a predisposing factor for this condition.
The bladder
Urological complications of SCI are major mortal and morbid risks and the reader is referred to Fowler and Fowler (1993) for a review. Spinal cord damage disrupts the neural controls of bladder function. The objectives of bladder management are to provide a system ensuring safety, continence and least social disruption.
Fertility
Fertility is usually maintained in women, with the ovulatory cycle being normal within 9 months after injury. Fertility in men is, however, a problem (Brindley, 1984). Improvements in fertility rates for men after SCI have been made due to several important technical advances. These include improved methods in the retrieval and enhancement of sperm, such as electroejaculation, and improved means of achieving fertilization with limited sperm quality and numbers through in vitro techniques.
Acute physical management
Principles of impairment assessment
Assessment must be carried out as soon after admission as possible to obtain an objective baseline measurement of function, to identify where specific problems are likely to develop and to instigate prophylactic treatment. History of the injury is taken, including the results of relevant tests, e.g. lung function. Medical history is also noted. It is important to be aware of associated injuries, as these may influence management. The principles of assessment are discussed in Chapter 1 and a physiotheray assessment database is shown in Table 4.1.
| Database |
FVC, forced vital capacity.
Treatment objectives in the acute phase
Spasticity: a reminder!
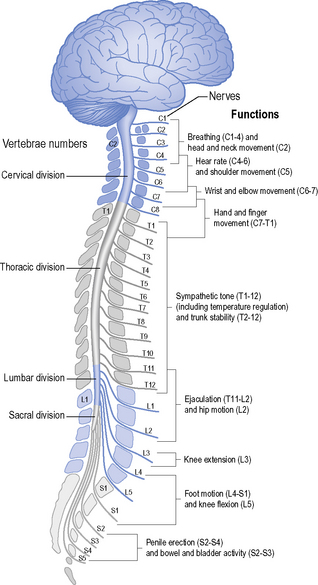
Since the spinal cord is shorter than the vertebral column, lower-vertebral injuries will not involve damage to the cord, but there will be nerve root damage which will determine the presence of spasticity. The spinal cord usually extends to the first or second lumbar vertebra (Williams, 1995). Below this level, the nerve roots descend as the cauda equina and emerge from their respective vertebral levels. Injuries at these lower levels, therefore, are peripheral nerve (LMN) injuries.
Cervical injuries and potential for brachial plexus lesions
In the immediate period of patient assessment, after an admission following a SCI, it is very difficult to determine the pathology causing the resulting loss of movement. This is especially difficult in cervical lesions where there is a reasonable possibility of brachial plexus involvement. On assessment, muscles of the upper limb could be weak or flaccid and may be presumed to result from spinal shock. If the mechanism of injury or MRI scans suggest more extensive tissue damage, it would be highly advisable to assess for a LMN injury. This can be done extensively using nerve conduction studies but may be difficult to obtain in the acute/intensive therapy unit (ITU) setting. A more rapid and broad test is to apply neurotrophic electrical stimulation to the muscles innervated by the injured nerve roots. Only an intact peripheral nerve will conduct and produce a muscle contraction. Hence this is a useful diagnostic tool in determining a brachial plexus lesion. It is most important to identify such an injury at the earliest opportunity in order to facilitate a primary repair of the brachial plexus (Birch, 1993).
Respiratory management
Effect of cord injury on the respiratory system
Respiration is a complex motor activity using muscles at various levels (see below). Patients with lesions of T1 and above will lose some 40–50% of their respiratory function, but most patients with cervical injuries have an initial vital lung capacity of only 1.5 litres or less. Thus, all patients with cervical injuries should be fully evaluated for respiratory efficiency by monitoring spirometry and Po2 in the initial weeks after injury. For an overview of respiratory physiology with an explanation of the tests mentioned here and normal values, the reader is referred to relevant textbooks (e.g. Hough, 2001; Smith & Ball, 1998).
Atelectasis is common in patients with SCI. Subsequent infection and pneumonia still account for considerable morbidity and some mortality in tetraplegics. Prophylactic tracheostomy is often advised to assist in effective clearance of secretions. High cervical injuries are prone to bronchospasm and bronchial hypersecretion due to disrupted sympathetic response. Appropriate treatment with bronchodilators in conjunction with manual techniques will be required to maintain adequate ventilation (see Ch. 15). Chest and head injuries are commonly associated with spinal injury and provide their own respiratory problems, which must also be assessed and treated appropriately (see Ch. 3).
Muscles affecting respiratory function
The diaphragm is the main inspiratory muscle but relies on other muscles to maximize efficiency. The accessory muscles (innervated by C1–C8 nerves and cranial nerve XI) include the trapezius, sternomastoid, levator scapulae and scalenii muscles; they can act as sole muscles of inspiration for short periods, but if the diaphragm is paralysed they cannot maintain prolonged adequate ventilation unassisted (see inspiratory muscle training in sections ‘Acute respiratory care and management of complications’ and ‘Rehabilitation: ongoing respiratory management’, below) (see Box 4.3).
| Accessory muscles | C1 – C8 |
| Diaphragm | C3 – C5 |
| Intercostal muscles | T1 – T11 |
| Abdominal muscles | T6 – L1 |
Chest movement
The use of accessory muscles and diaphragm function can be assessed by palpation at the lower costal border. Muscle paralysis results in altered mechanics of respiration. Studies have shown chest wall compliance in tetraplegic patients can be reduced by more than 50% of the normal value (De Troyer & Estenne, 1995). In lesions involving paralysis of the abdominal and intercostal muscles, the lower ribs will be drawn in on inspiration in a paradoxical movement (Pryor & Webber, 1998). These abnormalities reduce the efficiency of the diaphragm in producing negative intrathoracic pressure, hence causing reductions in lung volume and efficiency of ventilation. On assessment any asymmetry of chest wall movement and respiratory rate is noted.
Extreme ventilatory compromise in spinal injury is caused by one or more of the complications listed in Table 4.2.
Table 4.2 Spinal cord lesions and the effects on forced vital capacity
| Spinal cord lesion | Complication | FVC % of normal |
|---|---|---|
| Lumbar and low thoracic |
FVC, forced vital capacity.
Forced vital capacity
The forced vital capacity (FVC) is a readily available objective measurement of respiratory muscle function, as is peak expiratory flow rate. As mentioned earlier, it is used acutely to monitor respiratory status. If the FVC is less than 1 L, the therapist may choose to instigate either intermittent positive-pressure breathing (IPPB), e.g. the Bird respirator, or bilevel intermittent positive airway pressure (BIPAP), as discussed by Hough (2001). This assisted ventilation can be used prophylactically to maintain and increase inspiratory volume and aid clearance of secretions. It is a useful adjunct to active manual techniques for patients with sputum retention and lung collapse, and can be used to administer bronchodilators (Pryor & Webber, 1998). Elective ventilation is normally undertaken if the FVC falls below 500 ml but may be considered in some patients if FVC is around 1 L, depending on other complications that can impair the active cycle of breathing.
In cases of severe pain from rib fractures and associated soft-tissue injuries, a mixture of nitrous oxide and oxygen (Entonox) may be used and, if applicable, entrained into the IPPB circuit. Trancutaneous electrical nerve stimulation (TENS) has also been found to be effective in assisting pain management (see Ch. 12).
Cough
A patient with a lesion above T6 will not have an effective cough as he or she will have lost the action of the abdominal muscles. The physiotherapist can compensate for this loss by the use of assisted coughing, in order that the patient can clear secretions (Bromley, 2006). The Emerson Cough Assist Insufflator-Exsufflator is used to produce a cough by introduction of positive pressure then withdrawing negative pressure via a facemask, in order to assist secretion clearance.
Precautions in treating unstable spinal cord injury
These precautions in treating unstable SCI are outlined in Box 4.4 (CSP, 1997) and are for guidance only. They are widely accepted in many centres, but the point 2 lacks clear evidence to support this guidance. In a recent informal survey there was no agreement nationally or internationally, amongst the spinal surgeons questioned, to determine whether it is necessary to hold down the patient’s shoulder (termed a shoulder hold) during some treatment techniques. There are some centres that advocate that the patient’s head is stabilized and their shoulders held down onto the bed, whilst leg movements are performed and during chest physiotherapy. This stabilization is also recommended when moving the shoulders above 90°, particularly if the patient has high levels of spasticity.
Acute respiratory care and management of complications
Ventilation
Assisted ventilation may be necessary. Proactive intervention before the patient becomes exhausted will make subsequent management easier. Elective intubation is potentially less damaging to the spinal cord than intubation following cardiac arrest. Respiratory therapy for the spinally injured ventilated patient is similar to that for other ventilated patients (Hough, 2001; Smith & Ball, 1998), apart from added vigilance to protect the fracture site.
Weaning from the ventilator should start as soon as the patient’s condition stabilizes. It is important to avoid fatigue whilst weaning, so careful monitoring ensures that FVC does not fall by more than 20%, or respiratory rate rise above 25–30 breaths/minute. Patients who fail to wean – usually those with a greater degree of diaphragm paralysis – require long-term ventilation (see ‘The long-term ventilated patient’, below).
Suctioning
In patients with lesions above T6, the thoracolumbar sympathetic outflow is interrupted. During suction the vagus nerve is unopposed and the patient may become hypotensive and bradycardiac, possibly resulting in cardiac arrest. Endotracheal intubation may produce a similar response. Suction causes vagal stimulation via the carotid bodies, which pass impulses to the brain via the glossopharyngeal nerves and are sensitive to lack of oxygen (Williams, 1995). It is, therefore, wise to preoxygenate the patient, monitor heart rate and have atropine on standby.
Active assisted facilitation of movement and passive movements
In order to produce a remembered coordinated movement pattern during assisted movements, attempts should be made to position joint girdles in functional alignment, prior to moving the limbs. During a period of sustained bed rest, an SCI individual’s body schema, the internal three-dimensional, dynamic representation of the spatial and biomechanical properties of one’s body, is lost from the parietal area of the brain. This means that the different joints of the body are not clearly identified during movement. On attempting a functional activity, the individual cannot differentiate the different parts of a movement resulting in an abnormal mass pattern. Care should be taken to activate each joint movement to achieve the maximum outcome possible and feed into the preparation of the body for the changes in postural adjustments resulting from voluntary movement (Mouchnino et al., 1992; Schepens et al., 2008).
Cortical mapping has demonstrated change, by the performance of passive movements with the patient visualizing the movement and in the presence of some sensory feedback (Reddy et al., 2001).
Functional electrical stimulation (FES) is a useful adjunct to improve a movement where only a flicker is first available (see Ch. 12). Similarly, electromyographic (EMG) biofeedback can assist the patient to move in the absence of full sensation (see Ch. 12). The aims of all such movements are to:
Movements are commenced immediately after injury and features specifically important for SCI patients are now discussed. Shoulder movements are usually performed at least twice a day and leg movements once a day, in order to monitor any return of movement. For lumbar and low thoracic fractures, hip flexion should be kept to below 30°, to avoid lumbar flexion, until stability is established. Knee flexion must, therefore, be performed in Tailor’s position, i.e. ‘frogging’ (Figure 4.8).
Special emphasis should be put on the following:
During recovery, the handling principles apply to facilitate normal movement and not to elicit spasm and reinforce the spastic pattern. Extreme ROM must be avoided, especially at the hip and knee, as microtrauma may be a predisposing factor in the formation of periarticular ossification (see above). Passive movements of paralysed limbs are continued until the patient is mobile and thus capable of ensuring full mobility through his or her own activities, unless there are complications, such as excessive spasm or stiffness. See Box 4.5 for key points in acute management.
Turning and positioning/tone management
Waring and Maynard (1991) reported that 75% of tetraplegics had shoulder pain, 60% lasting 2 weeks or more. Of the patients with pain, 39% had unilateral and 61% bilateral symptoms. In over one-third, onset was within the first 3 days postinjury and 52% within the first 2 weeks postinjury. Pain may result from muscle imbalance, spasticity, and direct trauma to the shoulder girdle, combined with the joint immobilization, central and peripheral sources of nerve pain.
Scott and Donovan (1981) described special positioning to prevent loss of range: 90° abduction, combined with other positioning techniques, leads to decreased frequency and severity of shoulder pain.
Positioning is used to minimize spasticity similarly to patients with other conditions. When the patient is exhibiting mass muscle tone in flexion or extension, the limbs and trunk may be placed into reflex-inhibiting positions. Some examples of positioning are shown in Figures 4.8 and 4.9. Positioning is also discussed in Chapter 14 and by Pope (2002).
Rehabilitation
The following section outlines management from the start of the mobilization phase through to discharge. Much of this information has been gained from the authors’ experience; procedures may vary between centres but the principles are similar (Bromley, 2006; Whalley Hammell, 1995).
Aims of rehabilitation
Goal-planning and outcome measures
Evaluation of progress by review of goal achievement is advised. The goals can be divided into achievable targets and should be patient-focused, appropriate and objective. It is recommended that they are created in a team environment, led by the patient, with interdisciplinary cooperation. It is at this point that patients are fully encouraged to take the locus of control for their rehabilitation. This theme is extended into the philosophy of their future reintegration. Chapter 11 discusses the issues of patient-centred practice in goal-setting and treatment, using a problem-solving approach.
There are several measures used to evaluate patient progress, applicable to an intervention or management technique. In general the World Health Organization recognizes Functional Independence Measures (FIM; Hamilton & Granger, 1991), Spinal Cord Independance Measure (SCIM: Catz A et al., 2007) and Craig Handicap Assessment and Reporting Technique (CHART; Whiteneck et al., 1992) as appropriate validated measures. Other outcome measures are discussed by Stokes (2009).
Objectives of rehabilitation
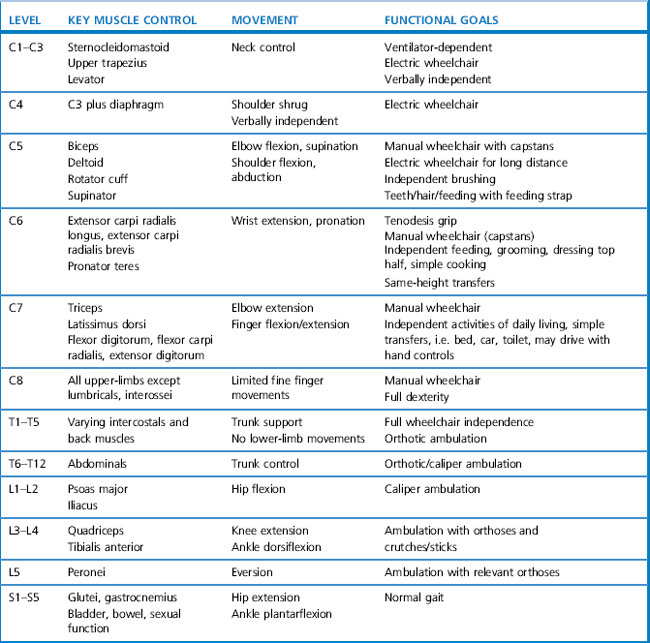
The progression of objectives as the patient gains more ability is outlined below. These objectives need to be set in relation to the level of spinal injury and the appropriate functional goals (Table 4.3). These expectations for function, depending on level of SCI, can only be a guide, especially in the light of the prevalence of incomplete lesions.
Key elements of the rehabilitation process are considered below.
Psychological aspects
Although all the members of the MDT will play a role in supporting a patient, advice and guidance from a qualified psychologist are essential and, at times, one-to-one direct patient therapy by the psychologist is necessary. The process of adaptation to spinal paralysis, reflected as integration back into the community, is a gradual one. Maintaining a positive approach with realistic expectations is essential for the patient’s wellbeing (see Ch. 17).
Pain management
Pain can be a problem initially during movements, notably due to neurodynamics, which is impairment of movement and/or elasticity of the nervous system (Shacklock, 2005). Chronic pain is a common problem in SCI. Between 65 and 85% of patients will experience significant pain and one-third of these will be classified as severe. A modern classification of pain following spinal cord injury has been proposed by Siddall (2009). This divides pain into nociceptive and neuropathic. Nociceptive is subdivided into musculoskeletal and visceral, whilst neuropathic is divided into above, at level and below level. The comprehensive review paper by Siddall (2009) goes on to describe the mechanisms of neuropathic pain and its management with sections on general principles, surgical approaches, pharmacological options, neurostimulation, and psychological and environmental management. With such a wide range of approaches it is clear that none are uniformly successful in pain control. Further research into management systems, with particular emphasis on cognitive systems, is proceeding.
Spasticity
SCI patients presenting with an upper motor neurone syndrome will exhibit both negative and positive symptoms. Spasticity will be a feature in this patient group, presenting a challenge in all aspects of patient management (Satkunam, 2003). It is a very large subject and requires more consideration than this chapter allows (see Ch. 14). Spasticity is difficult to define and more recently is described as a sensori-motor phenomenon related to the integration of the nervous system motor responses to sensory input. It is related to the hypersensitivity of the reflex arc resulting from the loss of descending inhibition (Ivanhoe & Reistetter, 2004). A further definition states that the disordered sensory-motor control presents as intermittent or sustained involuntary activation of muscles (Pandyan et al., 2005).
In incomplete cord lesions, depending on the pattern, spasticity tends to occur earlier and may present immediately. When severe, it will inhibit any underlying voluntary movement and create ‘wrong’ synaptic connections in the recovering nervous system. In complete spinal cord lesions, it most commonly becomes apparent about 3 months after the injury. It tends to reach a maximum between 6 and 12 months after injury and then diminishes, becoming more manageable. However, in a minority it remains at a high level and presents a major problem affecting function, posture and joint movement (Sheean, 1998).
Spasticity management
The management of spasticity should be undertaken by a coordinated multidisciplinary team rather than by clinicians in isolation (Barnes et al., 2001). Spinal cord spasticity presents in different patterns to that usually seen in stroke patients (Ch. 2).
The best management is prevention in the first few months after injury (Ditunno et al., 2004). Essentially in the first few days the opportunities for functional connections should be maximized.
It is also important to avoid triggering factors, such as urinary tract infection, constipation or skin breakdown. A collaborative assessment and evaluation tool to determine the best management of spasticity in SCI patients is currently being developed to accompany the document by Barnes et al. (2001).
The main medical approach is through pharmacology, although none of the drugs commonly used (baclofen, dantrolene and tizanidine) is universally effective or indeed predictable in its effect. Nerve blocks have been used for many years, usually using either phenol or alcohol. All of the above drugs have significant and numerous side-effects (see Martindale, 2007). An Intra thecal baclofen pump system can be used to deliver the baclofen directly into the spinal fluid. A small refillable pump is inserted under the skin delivering a regular treatment dose to maintain reduced levels of spasticity. As the use of intramuscular botulinum toxin increases, the use of other nerve blocks will diminish.
Functional mobility
Standing programme
Care must be taken when initiating standing. The autonomic disturbance present in patients with cervical and thoracic injuries can result in significant problems with hypotension which in the early stages may affect cord perfusion. Blood pressure studies and monitoring of pressures in sitting and gradual tilt table standing have been suggested (Kassioukov et al., 2009). These problems will usually resolve as the venous return improves.
Bone loss after SCI is greatest in the lower limbs. There is some evidence, although limited, to support standing by any means can improve preservation of bone mass density in the femoral shaft and proximal femur of complete SCI individuals (Goemaere et al., 1994). However the early weight-bearing mobilization of patients might be important in preventing or slowing the bone mineral loss after injury (de Bruin et al., 1999). In practice tilt table standing is commenced as soon as possible. This has many other benefits: respiratory psychological and improved systemic body functions. Standing is of great value in retaining neuromuscular flexibility and in reduction of spasticity (Bohannon & Larkin, 1985; Eng et al., 2001; Goemaere et al., 1994; Golding, 1994).
There has been a variety of studies to identify the time and regularity necessary for a patient to stand. It is suggested that the amount of time will influence the reduction in spasticity and at least half an hour every day is recommended (Walter et al., 1999).
Upper-limb management
This activity is continued from the acute phase using a variety of techniques (see Chs 12 & 18). Once the patient has begun to mobilize in the wheelchair there are many options for strengthening during functional activities.
The evidence of shoulder pain in wheelchair users is undeniable – 78% of tetraplegics and 59% of paraplegics, although studies vary in numbers. Specific exercises to address muscle imbalance and maintenance of full range have been shown to reduce this incidence (Curtis et al., 1999).
Strengthening and cardiovascular fitness
The rehabilitation process incorporates components of strengthening and fitness, as part of restoration of function. An average SCI patient is eight times less active than a middle-aged sedentry man. Wheelchair users have an increased risk of secondary disabilities such as coronary heart disease, obesity, hypertension and diabetes (Finley et al., 2002). The changes in muscle function resulting from SCI lead to reduced energy expenditure and decreased strength.
There are many charities and organizations available to promote these opportunities and to support equipment funding for SCI individuals. Activities can eventually progress to more competitive sports and clubs, as well as encouraging attendance at the many fully integrated fitness centres available. Cardiovascular fitness after SCI has been reviewed (Finley et al., 2002; Jacobs & Nash, 2001) and in general for neurological conditions in Chapter 18.
Functional electrical stimulation effects on muscle and fitness
Many studies have investigated the effects of using FES to support exercise in SCI individuals. The positive effects of FES exercise are well established, although intensity, duration and frequency of these interventions all vary (Giangregorio & McCartney, 2006). FES exercise can produce isometric contractions resulting in an increase in muscle cross-sectional area. FES cycle ergonometry (Figure 4.10) has demonstrated increases in muscle fibre area and capillary bed (Belanger et al., 2000) in addition to increasing whole body metabolism and cardiovascular fitness (Davis et al., 2008).
Functional electrical stimulation in restoration of function and gait
Research and technologies have influenced our clinical practice, e.g. body weight support treadmill gait training (Figure 4.11) is based around the central pattern generator (CPG) theory (see below). Research to incorporate FES with this training is underway. In the future methods that use the concept of activity-dependent neuroplasticity will most likely play an increasing role in the rehabilitation of SCI.
FES and its role in functional activities and gait requires further evaluation. A comprehensive review by Ragnarsson (2008) identifies the current state-of-the-art and future therapeutic potential. It has been used as a neuroprosthesis in activities of daily living (ADL) to restore upper-limb function. Surface FES systems that aim to assist paraplegic patients to walk are already approved in the USA – Parastep System (Sigmedics, Inc., IL, USA) and for incomplete injuries the Odstock Drop Foot Stimulator is widely used. The implanted devices for foot drop have been developed and are curently being used in clinical practice (Chae et al., 2000).
Despite promising advances in technology, the physiological limitations of the neuromuscular system prohibit the clinical use of FES alone to achieve a realistic and successful functional outcome. Gait remains inefficient, needing high energy levels. It has been demonstrated that function achieved through an external locus of control will always be of limited value to patients (Bradley, 1994). The recent research is seeking to provide cortical patterns of activity to trigger the stimulation for movement (Grill et al., 2001).
Ongoing respiratory management
Tetraplegic and some high thoracic paraplegic patients will benefit from ongoing respiratory monitoring and from respiratory training. Respiratory capacity is impaired by motor weakness, spasticity and pain. Inspiratory muscle-training devices provide resistance through a variety of devices or valves. Some studies have demonstrated some limited benefit for improving FVC (Hough, 2001; Van Houtte et al., 2006). More recently devices have been developed to produce training through the ‘Test of Incremental Respiratory Endurance (TIRE) protocol. This system produces inspiratory muscle training working at 80% of the maximum performance (Figure 4.12). The visual display provides a goal-orientated programme for the individual to try to beat during each exercise session (www.trainair.co.uk).
Wheelchairs
The variety of wheelchairs available increases each year, including some that enable the patient to rise into standing. Initially, patients are mobilized in a standard wheelchair offering greatest support and stability, following a comprehensive assessment to ensure it is correctly fitted and adjusted. Adaptations are made to provide a well-supported, evenly balanced seating position (Harvey, 2008; Pope, 2002). Tension adjustable canvas systems and modular backrests are all valuable in producing postural control.
The key to good stability is achieved by support and alignment at the pelvis. The cushion is equally important and should be assessed in a similar way, also taking into account the need for protection of pressure areas. Various pressure assessment tools are used to evaluate skin viability when sitting and these aid cushion prescription (Barbenel et al., 1983). Later in rehabilitation, it will be appropriate to try a variety of wheelchairs to offer greater mobility and independence according to an individual’s needs. In view of the incidence of shoulder pain in wheelchair users (Ballinger et al., 2000), it is appropriate to consider adaptations and weight of the wheelchairs. There are many light-weight wheelchairs available and the use of assisted wheeling systems can ease the effort of wheeling. A battery-powered third wheel or trike adaptation can be fitted onto the front of a light weight manual wheelchair to provide assisted mobility outdoors.
Rehabilitation of incomplete lesions
Treatment may include: facilitation of normal movement patterns; muscle strengthening (see Ch. 18); addressing muscle imbalance and compensations; and inhibition of spasticity (see Ch. 14). Balance re-education, gait re-education, wheelchair skills and functional activities are performed as appropriate to the patient’s level of ability.
Research suggests that patterned neural activity may be an important mechanism for developing and maintaining inhibitory circuitry (McDonald et al., 2002). There have been many studies exploring gait facilitation using a partial weight-bearing system on the treadmill. This approach is based on the principles of CPGs and repeated exercise of gait motion to increase strength, coordination and endurance (Ladouceur et al., 1997). There is evidence from animal studies that neural networks in the isolated spinal cord are capable of generating rhythmic output (reciprocally organized between agonists and antagonists) in the absence of efferent descending and movement-related afferent sources (Duysens & Van de Crommert, 1998). This is postulated to be similar in humans. Spinal systems contribute to the control of locomotion by local segmental and intersegmental spinal circuits (Grillner & Wallen, 1985).
In normal walking, it has been shown that muscle activity patterns are not centrally generated by reflex-induced activity, e.g. through stretch reflexes (Prochazka et al., 1979). The gait facilitation on the treadmill system is thought to be influenced by three main sensory sources acting on the CPGs:
Some outcome measures being assessed as evaluation tools for this work in the national spinal injuries centres are: Walking Index for Spinal Cord Injury (WISCI; Ditunno et al., 2000) and WISCI II (Ditunno et al., 2001).
There have been concerns from physiotherapists against gait exercise too early, causing the development of ‘wrong patterns’. The motion of the hip is helped by the harness so as to move almost entirely within normal range limits. Therapists helping to guide foot placement manually found this an effective way of controlling motion and blocking abnormal harmful patterns. FES has been introduced as an adjunct to assist with the gait pattern (see Ch. 12). The advantages of this gait training would be to improve systemic functions and stimulate the vegetative nervous sequelae. Early training with some weight-bearing may help reduce osteoporosis and patients have been shown to need less support after training (Abel et al., 2002).
Assisted gait, calipers and orthoses
It should be recognized that, even if a patient has the ability to walk with calipers, he or she may not choose to or may be advised not to try. Techniques used for gait training have been discussed in detail by Bromley (2006) and an outline of the progression of training is given below.
The long-term ventilated patient
Once past the acute phase, their individual needs are ascertained. Specialized wheelchairs are available which can be mouth- or head-controlled. Prophylactic chest management is vital, with tracheostomy, regular bagging and suction to reduce atelectasis and prevent infection (see Ch. 15). FES for the abdominal muscles has been evaluated as a method to assist coughing (Linder, 1993).
Assessment involves nerve conduction studies and may include fluoroscopic examination to visualize diaphragmatic excursion (Zejdlik, 1992). Electrodes are placed around the phrenic nerve either in the thorax or neck. An external pacing box is attached to external transmitter antennae; these are placed over the implanted receivers and when stimulated provide diaphragmatic contraction (Buchanan & Nawoezenski, 1987). There is no set regime for training but some patients have managed to achieve 24-hour pacing. Others use the ventilator part-time.
Initially, physiotherapy will aim to maximize strength in all innervated muscles to assist in head control and strengthen accessory respiratory muscles. Glossopharyngeal breathing techniques and use of a biofeedback training system can allow the patient to manage for short periods off the ventilator. Regular tilt table standing is recommended in addition to passive movements. Management of spasticity is also an important consideration (see Ch. 14) and may involve medication.
Children with spinal cord injury
The number of children with spinal cord damage which is non-congenital is thankfully quite low. Numbers vary between studies but a study gives an example that less than 2% of children admitted with all forms of traumatic injury have an SCI (Brown et al., 2001). Traumatic causes are mostly traffic accidents and falls.
In very young children, the head is proportionally larger and heavier and, therefore, injuries are commonly cervical. The range of maximum flexion of the cervical spine tends to move lower as the child gets older (Grundy & Swain, 2002). The review provided by Vogel and Anderson (2003) offers a comprehensive overview of the management of children with SCI. Other texts include Osenback and Menezes (1992) and Short et al. (1992).
Special considerations for children with SCI include the following:
Case Study
Clinical reasoning in a case for later intervention more than 2 years post spinal cord lesion.
Patient: JG (date of birth 1975)
Pathology
Social history
Body structure and function (impairments)
Participation restrictions
Main problem
Compensations
Hypothesis
Treatment progression summary
Abel R., Schablowski M., Rupp R., Gemer H.J. Gait analysis on the treadmill – monitoring exercise in the treatment of paraplegia. Spinal Cord. 2002;40:17-22.
Adkins D.L., Boychuk J., Remple M.S., et al. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J. Appl. Physiol.. 2006;101(6):1776-1782.
American Spinal Injuries Association (ASIA). International Standards for Neurological and Functional Classification of Spinal Cord Injury, reprint 2008. Chicago, IL: American Spinal Injuries Association, 2008.
Ballinger D.A., Rintala D.H., Hart K.A. The relation of shoulder pain and range of motion problems to functional limitations, disability and perceived health of men with spinal cord injury: a multifaceted longitudinal study. Arch. Phys. Med. Rehabil.. 2000;81:1575-1581.
Barbenel J.C., Forbes C.D., Lowe G.D.O. Pressure Sores. London: Macmillan Press, 1983.
Barnes M., Bhakta B., Moore P., et al. The Management of Adults with Spasticity using Botulinum Toxin. A Guide to Clinical Practice. Byfleet: Harvard Health, 2001.
Battiston B., Papalia I., Tos P., Geuna S. Peripheral nerve repair and regeneration research. Int. Rev. Neurobiol.. 2009;87:1-7.
Belanger M., Stein R.B., Wheeler G.D., et al. Electrical stimulation:can it increase muscle strength and reverse osteopenia in spinal cord individuals? Arch. Phys. Med. Rehabil.. 2000;81(8):1090-1098.
Birch R. Management of brachial plexus injuries. In: Greenwood R., Barnes M.P., McMillan T.M., et al, editors. Neurological Rehabilitation. London: Churchill Livingstone; 1993:587-606.
Bohannon R.W., Larkin P.A. Passive ankle dorsiflexion increases in patients after a regime of tilt table-wedge board standing. A clinical report. Phys. Ther.. 1985;65:1676-1678.
Bohannon R.W., Smith M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther.. 1987;67:206-207.
Bradley M. The effects of participating in a functional electrical stimulation exercise programme on affect in people with FES. Arch. Phys. Med. Rehabil.. 1994;75:676-679.
Brindley G.S. The fertility of men with spinal injury. Paraplegia. 1984;22:337-348.
Bromley I. Tetraplegia and Paraplegia, sixth ed. London: Churchill Livingstone, 2006.
Brown P. Pathophysiology of spasticity. J. Neurol. Neurosurg. Psychiatry. 1994;57:773-777.
Brown R.L., Brunn M.A., Garcia V.F. Cervical spine injuries in children: a review of 103 patients treated consecutively at a level 1 pediatric trauma center. J. Pediatr. Surg.. 2001;36:1107-1114.
Buchanan L.E., Nawoezenski D.A. Spinal Cord Injury Concepts and Management Approaches. London: Williams & Wilkins, 1987.
Burns A.S., Ditunno J.F. Establishing prognosis and maximizing functional outcomes after spinal cord injury. Spine. 2001;26:S137-S145.
Catz A., Itzkovich M., Tesio L., et al. A Multicentre international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:275-291.
Chae J., Kilgore K., Triolo R., et al. Functional neuromuscular stimulation in spinal cord injury. Phys. Med. Rehabil. Clin. N. Am.. 2000;11:209-226.
Chartered Society of Physiotherapy (CSP). Standards of Physiotherapy Practice for People with Spinal Cord Lesions. London: CSP, 1997.
Crozier K.S., Groziani V., Ditunno J.F., et al. Spinal cord injury, prognosis for ambulation based on sensory examination in patients who are initially motor complete. Arch. Phys. Med. Rehabil.. 1991;72:119-121.
Collins W. Surgery in the acute treatment of spinal cord injury: a review of the past forty years. J. Spinal Cord Med.. 1995;18:3-8.
Curtis K.A., Tyner T.M., Zachary L., et al. Effect of a standard exercise protocol on shoulder pain in long term wheelchair users. Spinal Cord. 1999;37:421-429.
Dahlin L.B. Techniques of peripheral nerve repair. Scand. J. Surg.. 2008;97(4):310-316.
David O., Sett P., Burr R.G., et al. The relationship of heterotopic ossification to passive movements in paraplegic patients. Disabil. Rehabil.. 1993;15:114-118.
Davis G.M., Hamzaid N.A., Fornusek C. Cardiorespiratory, metabolic and biomechanical responses during functional electrical stimulation leg exercise: health and fitness benefits. Artif. Organs.. 2008;32(8):625-629.
Dennis F. Three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
De Bruin E.D., Frey-Rindova P., Herzog R.E., Dietz V., Dambacher M.A., Stussi E. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch. Phys. Med. Rehabil.. 1999;80(Suppl. 2):214-220.
De Troyer A., Estenne M. The Respiratory System in Neuromuscular Disorders. In: Roussos C., editor. The Thorax: Disease Edition: Part C. Marcel Dekker; 1995:2177-2317.
De Vivo M.J. Trends in spinal cord injury rehabilitation outcomes from Model systems in the United States; 1973-2006. Spinal Cord. 2007;45:713-721.
Ditunno J.F., Young W., Donovan W.H. The International Standards booklet for neurological and functional classification of spinal cord injury. Paraplegia. 1994;32:70-80.
Ditunno J.F., Ditunno P.L., Graziani V., et al. Walking index for spinal cord injury (WISCI). An international multi centre validity and reliability study. Spinal Cord, 38. 2000: 234-243. Spinal Cord. 2001;39:654-656. Revision: (WISCI II)
Ditunno J.F., Little J.W., Tessler A., et al. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42:383-395.
Duysens J., Van de Crommert H.W.A.A. Neural control of locomotion: Part 1: The central pattern generator from cats to humans. Gait Posture. 1998:131-141.
Eng J.J., Levins S.M., Townson A.F., et al. Use of prolonged standing for individuals with spinal cord injuries. Phys. Ther.. 2001;81(8):1392-1399.
Fawcett J.W., Curt A., Steeves J.D., et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45(3):190-205. Epub 2006 Dec 19 (Review)
Fehlings M.G., Perrin R.G. The timing of sugical intervention the treatment of acute spinal cord injury: a systematic review of clinical evidence. Spine. 2006;31(Suppl. 11):S28-S35.
Finley M.A., Rodgers M.M., Keyser R. Impact of physical exercise on controlling secondary conditions associated with spinal cord injury. Neurology Report. 2002.
Foo D. Spinal cord injury in forty four patients with cervical spondylosis. Paraplegia. 1986;24:301-306.
Fowler C.J., Fowler C.G. Neurogenic bladder dysfunction and its management. In: Greenwood R., Barnes M.P., McMillian T.M., et al, editors. Neurological Rehabilitation. London: Churchill Livingstone; 1993:269-277.
Frey-Rindova P., de Bruin E.D., Stussi E., et al. Bone Moneral density in Upper and Lower Extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38(Suppl. 1):26-32.
Giangregorio L., McCartney N. Bone Loss and Muscle Atrophy in Spinal Cord Injury: Epidemiology, Fracture Prediction and Rehabilitation Strategies. J. Spinal Cord Med.. 2006;29(5):489-500.
Goemaere S., Van Laere M., De Nerve P., et al. Bone mineral status in paralegics patients who do not perform standing. Osteoporos. Int.. 1994;4:138-143.
Golding J.S. The mechanical factors which influence bone growth. Eur. J. Clin. Nutr.. 1994;48(Suppl. 1):S178-S185.
Grill W.M., McDonald J.W., Peckham P.H., et al. At the interface: convergence of neural regeneration and neuroprostheses for restoration of function. J. Rehabil. Res. Dev.. 2001;38:633-639.
Grillner S., Wallen P. Central pattern generators for locomotion, with special reference to vertebrates. Annu. Rev. Neurosci.. 1985;8:233-261.
Grundy D., Swain A. ABC of Spinal Cord Injury, fourth ed. London: British Medical Journal Publications, 2002.
Hamilton B.B., Granger C.V. A Rehabilitation Uniform Data System. New York: Buffalo Publications, 1991.
Harrison P. The First 48 Hours. London: Spinal Injuries Association, 2000.
Harvey L. Management of Spinal Cord Injuries. Edinburgh: Churchill Livingstone, 2008.
Hough A. Physiotherapy in Respiratory Care: An Evidence Based Approach to Respiratory Management and Cardiac Conditions, third ed. Cheltenham: Nelson Thornes, 2001.
Hossain M., McLean A., Fraser M.H. Outcome of halo immobilisation of 104 cases of cervical spine injury. Scott. Med. J.. 2004;49(3):90-92.
Illis L.S. Spinal Cord Dysfunction Assessment. Oxford: Oxford University Press, 1988.
Ivanhoe C.B., Reistetter T.A. Spasticity: The misunderstood part of the upper motor neuron syndrome. Am. J. Phys. Med. Rehabil.. 2004;83(Suppl.):S3-S9.
Jackson A.B., Groomes T.E. Incidence of respiratory complications following spinal cord injury. Arch. Phys. Med. Rehabil.. 1994;75(3):270-275.
Jacobs P.L., Nash M.S. Modes, benefits and risks of voluntary and electrically induced exercise in persons with spinal cord injury. J. Spinal Cord Med.. 2001;24:10-18.
Jiang S.-D., Dai L.-Y., Jiang L.-S. Osteoporosis after a spinal cord injury. Osteoporos. Int.. 2006;17:180-190.
Johnston L. Human spinal cord injury: new and emerging approaches to treatment. Spinal Cord. 2001;39:609-613.
Kakulas B.A. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42(10):549-563.
Kassioukov A., Eng J.J., Warburton D., Teasell R. A Systematic Review of the Management of Orthostatic Hypotension after Spinal Cord Injury. Arch. Phys. Med. Rehabil.. 2009;90(5):876-885.
Katoh S., El Masry W.S. Motor recovery of patients presenting with motor paralysis and sensory sparing following cervical spinal cord injury. Paraplegia. 1995;33:506-509.
Kleim J.A. Lennon S., Stokes M., editors. Pocketbook of Neurological Physiotherapy. Edinburgh: Churchill Livingstone. 2009:41-50.
Ladouceur M., Pepin A., Norman K.E., et al. Recovery of walking after spinal cord injury. Adv. Neurol.. 1997;72:249-255.
Lammertse D., Tuszynski M.H., Steeves J.D., et al. International Campaign for Cures of Spinal Cord Injury Paralysis. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord. 2007;45(3):232-242. Epub 2006 Dec 19 (Review)
Linder S.H. Functional electrical stimulation to enhance cough in spinal cord injury. Chest. 1993;103:166-169.
Little J.W., Habur E. Temporal course of motor recovery after Brown Séquard spinal cord injury. Paraplegia. 1985;23:39-46.
Martindale: the complete Martindale: the complete drug reference, thirtyfifth ed. London: Pharmaceutical Press. 2007.
Mc Donald J.W., Becker D., Sadowsky M.D., et al. Late recovery following spinal cord injury. J. Neurosurg. (Spine2). 2002;97:252-265.
McKinley W., Santos K., Meade M., et al. Incidence and outcomes of spinal cord injury clinical syndromes. J. Spinal Cord Med.. 2007;30(3):215-224.
Maynard F.M., Reynolds G.G., Fountain S., et al. Neurological prognosis after traumatic quadraplegia, three years experience of California Regional Spinal Cord Injury Care System. J. Neurosurg.. 1979;50:611-616.
Medical Research Council (MRC) of the United Kingdom. Aids to Examination of the Peripheral Nervous System. 1978. Memorandum No 45. Palo Alto, Calif: Pedragon House
Moore E.E., Mattox K.L., Feliciano D.V. Trauma, second ed. Connecticut: Appleton & Lange, 1991.
Mouchnino L., Aurenty R., Massion J., et al. Coordination between equilibrium and head-trunk orientation during leg movement: a new strategy build up by training. J. Neurophysiol.. 1992;67(6):1587-1598.
New P.W., Sundrajan V. Spinal Cord. 2008;46:406-411.
Osenback R.K., Menezes A.M. Paediatric spinal cord and vertebral column injury. Neurosurgery. 1992;30:385-390.
Pandyan A.D., Gregoric M., Barnes M.P., Wood D., Van Wijck F., Burridge J., Hermens H., Johnson G.R. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil. Rehabil.. 2005;27:2-6.
Pope P.M. Postural management and special seating. In: Edwards S., editor. Neurological Physiotherapy: A Problem Solving Approach. second ed. London: Churchill Livingstone; 2002:189-217.
Poynton A.R., O’Farrel D.A., Shannon F., et al. Sparing of sensation to pinprick predicts motor recovery of a motor segment after injury to the spinal cord. J. Bone Joint Surg. Br.. 1997;79:952-954.
Prochazka A., Stephens J.A., Wand P. Muscle spindle discharge in normal and obstructed movements. J. Physiol.. 1979;287:57-66.
Pryor J.A., Webber B.A. Physiotherapy for Respiratory and Cardiac Problems, second ed. Edinburgh: Churchill Livingstone, 1998.
Ragnarsson K.T. Functional Electrical stimulation after spinal cord injury; current use, therapeutic effects and future direction. Spinal Cord. 2008;46:255-274.
Ramer M.S., Harper G.P., Bradbury E.J. Progress in spinal cord research. A refined strategy for the International Spinal Research Trust. Spinal Cord. 2000;38:449-472.
Reddy H., Floyer A., Donaghy M., et al. Altered cortical activation with finger movements after peripheral denervation: comparison of active and passive tasks. Exp. Brain Res.. 2001;138(4):484-491.
Ronsyn M.W., Berneman Z.N., Van Tendeloo V.F.I., et al. Can cell therapy heal a spinal cord injury. Spinal Cord. 2008;46:532-539.
Satkunam L.E. Rehabilitation medicine: 3. Management of adult spasticity. Can. Med. Assoc. J.. 2003;169(11):1173-1179. Review
Scott J.A., Donovan W.H. The prevention of shoulder pain and contracture in the acute tetraplegia patient. Paraplegia. 1981;19:313-319.
Schepens B., Stapley P., Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J. Neurophysiol.. 2008;100(4):2235-2253.
Schwartz J.M., Begley S. The Mind and the Brain: Neuroplasticity and the Power of Mental Force. New York: Harper Collins, 2003.
Shacklock M. Clinical Neurodynamics: a new system of musculoskeletal treatment. London: Elsevier Health, 2005.
Sheean G., editor. Sasticity Rehabilitation. London: Churchill Communications Europe, 1998.
Short D.J., Frankel H.L., Bergström E.M.K. Injuries of the Spinal Cord in Children. London: Elsevier Science, 1992.
Siddall P.J. Management of neuropathic pain following spinal cord injury; now and in the future. Spinal Cord. 2009;47:352-359.
Spinal Injuries Association (SIA). Preserving and developing the national spinal cord injury service. London: Spinal Injuries Association, 2009. Research report May 2009
Smith M., Ball V. Cardiovascular/Respiratory Physiotherapy. London: Mosby, 1998.
Stauffer E.S. Rehabilitation of posttraumatic cervical spinal cord quadraplegia and pentaplegia. In: The Cervical Spine. Philadelphia: Lippincott; 1983.
Steeves J.D., Lammertse D., Curt A., et al. International Campaign for Cures of Spinal Cord Injury Paralysis. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45(3):206-221. Epub 2006 Dec 19 (Review)
Stokes E.K. Outcome measurement. In: Lennon S., Stokes M., editors. Pocketbook of Neurological Physiotherapy. Edinburgh: Churchill Livingstone; 2009:192-201.
Tator C.H. Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery. 1998;42:696-707.
Tuszynski M.H., Steeves J.D., Fawcett J.W., et al. International Campaign for Cures of Spinal Cord Injury Paralysis. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord. 2007;45(3):222-231. Epub 2006 Dec 19 (Review)
Van Houtte S., Vanlandewijke Y., Gosselink R. Respiratory muscle training in persons with spinal cord injury: a systematic review. Respir. Med. 2006.
Vogel L.C., Anderson C.J. Spinal Cord Injury in children and adolescents: a review. Spinal Cord Med.. 2003;26(3):193-203.
Waring W.P., Maynard F.M. Shoulder pain in acute traumatic quadraplegia. Paraplegia. 1991;29:37-42.
Waters R.L., Adkins R.H., Yakura J.S., et al. Motor and sensory recovery following incomplete paraplegia. Arch. Phys. Med. Rehabil.. 1994;75:67-72.
Waters R.L., Adkins R.H., Yakura J.S., et al. Motor and sensory recovery following incomplete tetraplegia. Arch. Phys. Med. Rehabil.. 1994;75:306-311.
Walter J.S., Sola P.G., Sacks J., et al. Indications for a home standing program for individuals with spinal cord injury. J. Spinal Cord Med.. 1999;22(3):152-158.
Whalley Hammell K. Spinal Cord Injury Rehabilitation. Canada: Chapman & Hall, 1995.
Whiteneck G.G., Charlifue S.W., Gerhart K.A., et al. Quantifying handicap: a new measure of long-term rehabilitation outcomes. Arch. Phys. Med. Rehabil.. 1992;73:519-526.
Williams P. Gray’s anatomy, thirtyeighth ed. Edinburgh: Churchill Livingstone, 1995.
World Health Organization. International Classification of Functioning, Disability and Health. Geneva: World Health Organization, 2001. Available online at: http://www.who.int/classification/icf
Zejdlik C.P. Management of Spinal Cord Injury, second ed. Boston: Jones & Bartlett, 1992.