39 Spinal Cord Injury
 Epidemiology
Epidemiology
Spinal cord injury typically occurs in males at the peak of their productive lives. The incidence of traumatic SCI is approximately 11,000 new cases each year in the United States,1 with a prevalence of 191,000. The prevalence of SCI patients is increasing steadily owing to improved survival in both the acute and chronic stages of the disease. The amount spent on the treatment of spinal cord injuries in the United States is approximately 5.6 billion dollars each year and rising annually.2 The cost of caring for the individual spinal cord–injured patient is directly related to the injury level of the spinal cord and to the patient’s age, with the highest costs associated with older quadriplegic patients who are dependent on a ventilator.2
 Etiology
Etiology
Most spinal injuries result from high-speed motor vehicle accidents (Figure 39-1). Falls and work-related injuries are other important contributors. Spinal cord injury that is due to violence is on a dramatic rise secondary to increased incidence of assaults. These injuries include both blunt and penetrating injuries, such as gun and knife wounds. Sports-related injuries, which include football, horseback riding, and hockey injuries, are relatively rare but have received recent media attention.3–4 Finally, recreational injuries from jet skis, snowmobiles, snow skiing, snowboarding, and parachuting, to name but a few, appear to be on the rise as “extreme sports” become more prevalent.
 Immobilization and Diagnostic Evaluation
Immobilization and Diagnostic Evaluation
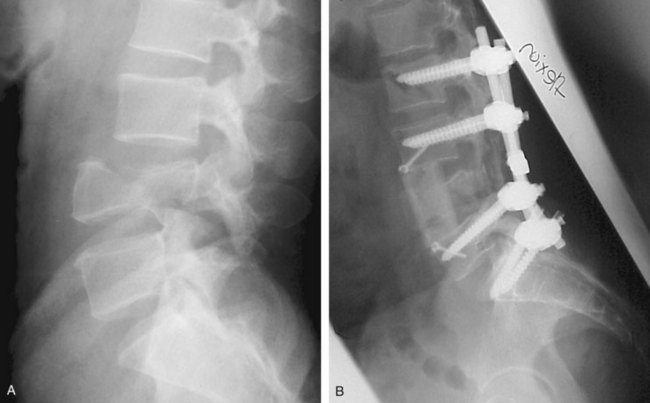
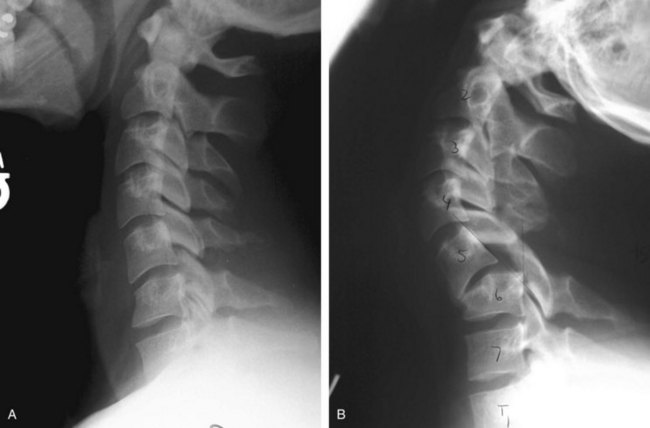
Further diagnostic studies will be dictated by the findings of the initial and secondary surveys as well as findings of initial diagnostic studies. Several points are important to keep in mind. First, important information can be obtained from studies performed for other reasons. For example, routine chest and abdominal radiographs may provide important information regarding the presence of significant thoracic/lumbar spine injury. Although these do not replace subsequent “formal” spine studies, they are often obtained as part of the routine trauma workup and provide early clues to the presence of spine trauma and may help prioritize subsequent imaging studies (Figure 39-2). Radiographs and particularly the CT are the most sensitive tools in detecting a fracture of the spine, but occasionally it is difficult to clear the spine—even in the absence of a fracture—because an unstable ligamentous injury without fracture may exist.
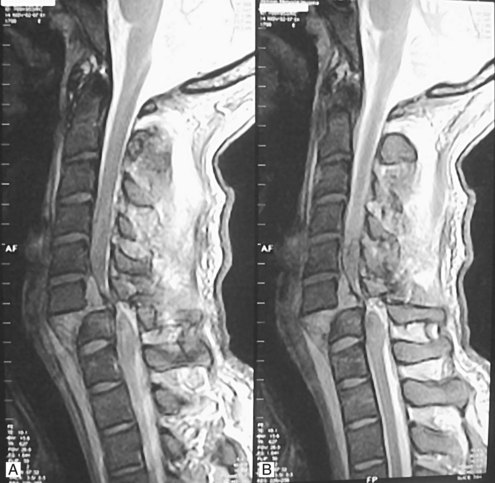
Patients with a suspected spinal column injury who are unconscious, uncooperative, or intoxicated or who have associated traumatic injuries that distract from their assessment will often require further radiographic study of the cervical spine before the discontinuation of cervical spine immobilization. Several options exist and include (1) maintenance of the collar and/or spine precautions until the patient becomes coherent and responsive, (2) dynamic imaging of the spine with physician monitoring, and (3) magnetic resonance imaging (MRI) of the spine to rule out a purely ligamentous injury. Of the three options, we frequently use MRI to clear the spine because a completely negative MR image in the setting of trauma indicates that there is no instability of the cervical spine (Figure 39-3). Malalignment and evidence of spine trauma on these imaging studies frequently determines subsequent management and diagnostic decision making. Cervical subluxations often require the use of traction and/or manual reduction of the fracture-dislocation. Diazepam (Valium) or lorazepam (Ativan), along with careful neurologic monitoring, often in the intensive care unit (ICU) setting, is required because application of traction can realign the spine but can also result in neurologic deterioration.
 Pediatric Spinal Cord Injury
Pediatric Spinal Cord Injury
Pediatric spine trauma is relatively uncommon, representing approximately 5% of all spinal cord injuries.5 For a specific discussion of pediatric SCI, please see Chapter 210. In addition, guidelines have been published on this topic.6
 Pharmacotherapy
Pharmacotherapy
The concepts of primary and secondary SCI are important principles in understanding the pathophysiology and the role of pharmacotherapeutic agents in emergent treatment. The primary injury mechanism results from a mechanical insult that occurs at the time of impact and includes acute compression, impaction, distraction, laceration, and shear.7 Secondary injuries occur after the initial injury and account for some of the progressive pathologic changes associated with SCI.7 A number of drugs have been tested in the laboratory, but only a few of these agents have progressed to clinical trials to evaluate their efficacy. Five randomized controlled trials of pharmacotherapy for acute SCI have been conducted, focusing on the therapeutic effect of either corticosteroids or gangliosides.
Corticosteroids
A number of studies have shown improved neurologic recovery in animals with spinal cord injuries that have received either dexamethasone or methylprednisolone.8–12 Corticosteroid treatment initially held promise as a potential therapeutic agent for its putative role in reducing white matter edema and inflammation. Current evidence, however, suggests that the major mechanism of action is reduction of the effects of secondary injury—in particular, the destructive effects of lipid peroxidation on cell membranes.2 Other actions include improving spinal cord blood flow, enhancing the postinjury activity of Na+/K+-ATPase, and facilitating the recovery of extracellular calcium ion.8,13
The first NASCIS trial (NASCIS I) examined low- (100 mg) and high- (1000 mg) dose methylprednisolone given for 10 days. Unfortunately, this trial had no control group, and no significant difference in outcome was found except for an increased number of wound infections among patients in the high-dose group.14 The second NASCIS trial (NASCIS II) was a prospective, randomized, double-blind, multicenter trial that demonstrated improved neurologic outcomes after 6 weeks, 6 months, and 1 year in patients with nonpenetrating SCI who had received a regimen of methylprednisolone, which included a bolus dose of 30 mg/kg.15 Improvement in motor and sensory scores associated with administration of methylprednisolone was only observed if the drug was given within 8 hours of injury when compared with naloxone or a placebo. Results of this study have been criticized.16,17 Some of the criticisms relate to difficulties in randomization, reporting methods, analysis of benefit limited to small subgroups within the larger study, and lack of replication of results by a completely independent group of investigators, among others. However, the administration of methylprednisolone is believed to reduce the amount of secondary injury that occurs after SCI and has become an important tool in the treatment of SCI in most North American centers. The results of NASCIS III have been published and compared the dosage of methylprednisolone used in the NASCIS II protocol with a longer dosing regimen (48 hours) as well as with a 21-aminosteroid. The 21-aminosteroids (lazaroids), a new class of steroids that are potent inhibitors of lipid peroxidation, lack much of the glucocorticoid activity of many of the traditional steroid compounds. Results of the study suggested that when patients are seen within 3 hours of their injury, they should receive a bolus dose of methylprednisolone (30 mg/kg intravenously [IV]) followed by 23 hours of treatment (5.4 mg/kg/h IV). Patients seen between 3 and 8 hours should receive the same bolus followed by a longer dosing regimen (48 hours). Complications from 48 hours of treatment included a significant increase in severe sepsis and pneumonia.18 Neurosurgical guidelines for management of spine trauma recommend methylprednisolone as a treatment option, recognizing that the risks of use have been more clearly demonstrated than benefit.19,20
Gangliosides
Gangliosides are complex sialic acid–containing glycosphingolipids which are present in high concentrations in neural membranes. These compounds are involved in a variety of cell-surface phenomena such as cell-substrate binding and receptor functions.21 Basic research in the past 15 years has demonstrated that these compounds can (1) promote the survival of neurons in cell culture; (2) increase the number, length, and branching of neuronal processes in cell culture; and (3) improve functional recovery after a variety of traumatic and ischemic insults to the peripheral and central nervous system. A limited number of animal studies have examined the role of gangliosides after SCI and have shown only a modest effect on the regeneration of serotonergic neurons.22 A recent prospective, randomized, double-blind, single-center study found a beneficial effect in functional neurologic outcomes when the ganglioside GM1 was administered within 72 hours of human SCI.23 However, a multicenter trial demonstrated no statistically significant benefit with administration of this agent at 26 and 52 weeks after injury.24
 Hypothermia
Hypothermia
Two recent studies were published on the safety and feasibility of mild to moderate intravascular cooling for SCI. Levi et al. reported on a series of 14 patients with American Spinal Injury Association (ASIA) classification A complete cervical cord injuries who underwent a protocol to achieve temperatures of 33.5°C via a closed-loop delivery system. Researchers found good correlation between intravascular and intrathecal cerebrospinal fluid temperature.25 Average time between inductions of hypothermia was 9.17 ± 2.24 hours (mean ± SEM); time to target temperature was 2.72 ± 0.42 hours; duration of cooling at target was 47.6 ± 3.1 hours; and average total length of time of cooling was 93.6 ± 4 hours. A subsequent paper summarized the complications and neurologic outcomes seen in the SCI patients treated with hypothermia and compared them to age- and injury-matched controls. The hypothermia group ASIA conversion rate to a higher grade was approximately 42%, with a similar frequency of complications to institutional controls.26 This pilot study suggested that systemic intravascular cooling can be accomplished with minimal variations in temperature and few adverse events, and may pave the way for larger multicenter SCI trials to test the efficacy of mild to moderate hypothermia in SCI.27
 Intensive Care Unit Management
Intensive Care Unit Management
Spinal cord injury is associated with profound effects on all vital systemic functions. Through primarily class III medical evidence, numerous reports indicate lower morbidity and mortality rates in patients with SCI managed with ICU monitoring and aggressive medical management of these changes.28–36 At the least, these studies taken together indicate that a systematic approach must be taken to evaluate and treat each of the potential complications. Early and late complications will be seen, and the degree of involvement of each system is usually correlated with the level and severity of injury.
Respiratory System
Respiratory complications are a major source of morbidity and mortality after SCI, with an 18% to 30% mortality rate reported in patients with tetraplegia.32,37 In a study by Hachen and associates,28,30 most early deaths were related to pulmonary complications, with the likelihood of severe insufficiency related to SCI severity. Whereas most cervical spinal cord injuries occur below C4, with the phrenic nerves continuing to innervate the diaphragm, the respiratory system is frequently severely affected, particularly after cervical spinal cord injuries. Specifically, marked reductions in (forced) vital capacity, inspiratory capacity, and expiratory flow rates frequently result in hypoxemia.28,32,38–41 These changes may be attributed to variable paralysis of the intercostal muscles and accessory muscles of respiration. Loss of abdominal muscle tone and ileus also reduce the mechanical efficiency of breathing.
The most common respiratory complications include atelectasis, pneumonia, pulmonary embolus, pulmonary edema, and acute respiratory distress syndrome. In addition to difficulty with taking deep breaths and coughing, patients are often unable to clear airway secretions. Accumulation of secretions and/or mucus plugs can result in respiratory failure. Prevention includes respiratory treatment with bronchodilators, frequent pulmonary toilet, chest physiotherapy, increasing airway humidity, intubation, and mechanical ventilation including the use of continuous positive airway pressure. The use of the RotoRest bed significantly decreases pulmonary complications associated with SCI32,42 because it improves pulmonary blood flow and reduces the incidence of pulmonary emboli.
Most patients can be discontinued or weaned from the ventilator after they have been medically stabilized, which usually means treatment of pulmonary infections, reestablishment of euvolemia, enhancement of respiratory muscle function, and nutritional supplementation to offset the high caloric requirements of the trauma. Initially, weaning the intermittent mandatory ventilation rate is followed by weaning of the positive airway pressure (either continuous or end-expiratory). With prolonged periods of ventilation (>2 weeks), and/or multiple failed extubations, one should consider a tracheostomy. The likelihood of requiring a tracheostomy increases after a high SCI, preexisting pulmonary disease, and the age of the patient. Tracheostomy effectively reduces the physiologic dead space. Northrup and colleagues43 have demonstrated that a tracheostomy can be performed before anterior cervical instrumentation of the spine, with a low risk of infection; but in our patient population, early surgery for stabilization is advocated, and consequently, few patients undergo tracheostomy before anterior cervical stabilization surgery.
Cardiovascular System
Animal studies indicate that ischemia underlies many of the secondary mechanisms of post SCI, often dictating the resultant deficits.28,44–46 Human studies suggest a direct correlation between the severity of SCI and the incidence and severity of cardiovascular problems.28,47 Together, this suggests that reducing the magnitude of secondary injury should be at the forefront of medical management of SCI.
The typical patient with SCI without associated vascular or visceral injury presents to the emergency department with a mean arterial blood pressure of 80 mm Hg and a heart rate of 65 beats/min.35 Persistent bradycardia is a frequent finding and is often profound enough to produce hemodynamic compromise.28,48 The patient’s blood pressure may respond to volume resuscitation, but often these patients require low-dose pressors. Aggressive medical management, including volume expansion and maintenance of mean arterial blood pressure greater than 85 mm Hg, is believed to potentially enhance neurologic outcome by maximizing spinal cord perfusion at the injury site and thus reducing the likelihood of secondary injury.7 Invasive hemodynamic monitoring will demonstrate a normal cardiac index with a low systemic vascular resistance. In the elderly patient with SCI, careful attention to volume replacement is required so as not to precipitate heart failure.
Gastrointestinal System
Patients with spinal cord injuries are at high risk of developing gastric and duodenal stress ulcers. Use of steroids compounds the risk of developing significant gastrointestinal hemorrhage. All patients with spinal cord injuries should receive at minimum an H2 blocker to prevent this dreaded complication. The reported risk of gastrointestinal hemorrhage in NASCIS II for the control group was 3% and for the methylprednisolone group, 4.5%.15
Integument
The SCI patient is extremely susceptible to developing decubiti. Frequent log rolling is invaluable in preventing skin breakdown. Specialized beds to turn SCI patients (e.g., RotoRest42 [KCI,] can reduce the incidence of skin breakdown by preventing pressure on a single area. Early intervention for skin breakdown frequently involves application of the DuoDERM patch (ConvaTec, Princeton, New Jersey) to prevent progression.
Thromboembolic Complications
Patients with SCI are at high risk of lower-extremity venous thromboembolism, which may manifest as deep vein thrombosis (DVT) in the lower or upper extremities and lead to leg swelling and/or pulmonary embolism. Depending on injury severity, age, and diagnostic methods, incidence of thromboembolic events ranges from 7% to 100%.49 The majority of these events occur within the first 3 months after injury, except in patients who are elderly, obese, or who have had prior thromboembolic events.49
Numerous studies have addressed the issue of preventive measures for DVT. Prevention has traditionally included the administration of low doses of heparin (5000 units subcutaneously) twice daily or more. However, meta-analysis of available literature suggests that better alternatives include the combination of pneumatic compression stockings with low-molecular-weight heparin (Lovenox) or adjusted-dose heparin.49
Current recommendations for evaluation of suspected thromboemboli include use of Doppler ultrasound for suspected DVT and venography if a strong clinical suspicion exists for DVT despite a negative ultrasound or if pulmonary embolism is suspected.49,50 Treatment of pulmonary emboli or above-knee DVT requires heparinization. Should there be a contraindication to heparinization, an inferior vena cava filter should be placed. Prophylactic placement of inferior vena cava filters has been advocated,27,49,51,52 but these procedures are not without risk, and no study thus far compares success rates to the aforementioned conservative prevention modalities.49,53
 Prognostic Factors for Recovery
Prognostic Factors for Recovery
The clinician uses the neurologic examination, patient age, and appearance of the spinal cord on MRI as well as other clinical data to guide the patient and his or her family on the expected outcome for a specific injury. In any traumatic SCI, it is important to ascertain whether the patient has a functionally complete or incomplete neurologic deficit. The distinction is important because the prognosis for neurologic recovery differs for these two conditions. Patients with no evidence of motor or sensory function below their spinal column injury are considered to have functionally complete injuries. Patients with no voluntary motor control and only slight sensory preservation in their lowest sacral dermatomes or some anal tone are still considered to have incomplete injuries. Functionally, patients with complete cervical spinal cord injuries who remain complete within the first 24 hours of admission are unlikely to regain significant ambulatory function (1% to 3%).54,55 However, most patients who enter the hospital with an incomplete neurologic injury obtain some degree of recovery. The level and degree of an incomplete injury also provides important prognostic information. Cervical injuries have a higher potential for recovery when compared with thoracic and/or thoracolumbar injuries. The less severe the SCI, the more likely the patient will recover.56
The majority of injuries occur in males, with well over half the injuries occurring in the 16- to 30-year-old age group. Prognosis for recovery is inextricably linked to age, with younger patients fairing much better than their older counterparts for regaining neurologic function after SCI.57,58 The two most important potential neurologic explanations are the capacity of the “young” spinal cord to function with major deficiencies in the neural circuitry and the possibility of some spontaneous regeneration of the CNS after injury.59 The reverse also appears to be true. It is well recognized that patients with stable incomplete injuries who age may lose function, and this may simply be the result of the loss of the last few functioning neurons or axons within the damaged region of spinal cord.60 Neuronal loss is a normal part of the aging process for both the brain and spinal cord, and the clinical deterioration observed after SCI may be likened to the postpolio syndrome.
MRI after SCI allows visualization of the spinal cord in a noninvasive manner. The images provide immediate feedback to the surgeon about the degree of spinal cord compression, as well as information regarding the stability of the spinal column through an assessment of the integrity of the ligaments, disks, and surrounding soft tissues. In addition, intramedullary hemorrhage may be easily discerned, providing important prognostic information. Intramedullary hemorrhage is more commonly observed after neurologically complete injuries, and hemorrhage signifies a worse neurologic and functional outcome.61,62 MRI of SCI is discussed in greater detail in Chapter 40.
 Research
Research
Spinal cord injury research is an absolute priority of the National Institutes of Health. Models of SCI, mechanisms of secondary injury, treatment of the acute phase of SCI, and development of transplantation strategies to repair a damaged spinal cord are ongoing across North America and around the world. The treatment arms of research can be divided into two categories: (1) agents that can be given during the acute phase of injury to limit secondary injury mechanisms or (2) strategies to promote regeneration. Two of the most promising drugs, methylprednisolone and ganglioside GM,1 have only yielded modest results. Methylprednisolone, which is used in almost all major SCI centers, is coming under closer scrutiny as to its effectiveness.17 Drugs of the future include neurotrophins, which can promote survival and regeneration of injured nerve cells, drugs that prevent the inflammatory response to SCI,63 and drugs that prevent apoptotic cell death.64 In the transplantation arena, cellular therapies to treat chronic injury are important. Cells of interest include Schwann cells, olfactory ensheathing glia, embryonic spinal cord, and neural progenitor cells. Antibodies that neutralize inhibitory proteins within myelin have also demonstrated promise. Strategies that combine a number of the aforementioned treatments are most likely to have a beneficial effect in the future.
Key Points
2002 Deep venous thrombosis and thromboembolism in patients with cervical spinal cord injuries. Neurosurgery. 2002;50(3 Suppl):S73-S80.
Northrup BE, Vaccaro AR, Rosen JE, et al. Occurrence of infection in anterior cervical fusion for spinal cord injury after tracheostomy. Spine (Phila Pa 1976). 1995;20(22):2449-2453.
Schaefer DM, Flanders AE, Osterholm JL, et al. Prognostic significance of magnetic resonance imaging in the acute phase of cervical spine injury. J Neurosurg. 1992;76(2):218-223.
Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15-26.
Vale FL, Burns J, Jackson AB, et al. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87(2):239-246.
1 Harvey C, Rothschild BB, Asmann AJ, et al. New estimates of traumatic SCI prevalence: a survey-based approach. Paraplegia. 1990;28(9):537-544.
2 Berkowitz M. Assessing the socioeconomic impact of improved treatment of head and spinal cord injuries. J Emerg Med. 1993;11(Suppl 1):63-67.
3 Torg JS, Naranja RJJr, Palov H, et al. The relationship of developmental narrowing of the cervical spinal canal to reversible and irreversible injury of the cervical spinal cord in football players. J Bone Joint Surg Am. 1996;78(9):1308-1314.
4 Tator CH, Carson JD, Edmonds VE. Spinal injuries in ice hockey. Clin Sports Med. 1998;17(1):183-194.
5 Proctor MR. Spinal cord injury. Crit Care Med. 2002;30(11 Suppl):S489-S499.
6 Management of pediatric cervical spine and spinal cord injuries. [No Authors Listed]. Neurosurgery. 2002;50(3 Suppl):S85-S99.
7 Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15-26.
8 Young W. Secondary CNS injury. J Neurotrauma. 1988;5(3):219-221.
9 Braughler JM, Hall ED. Correlation of methylprednisolone levels in cat spinal cord with its effects on (Na+ + K+)-ATPase, lipid peroxidation, and alpha motor neuron function. J Neurosurg. 1982;56(6):838-844.
10 Ducker TB, Hamit HF. Experimental treatments of acute spinal cord injury. J Neurosurg. 1969;30(6):693-697.
11 Green BA, Kahn T, Klose KJ. A comparative study of steroid therapy in acute experimental spinal cord injury. Surg Neurol. 1980;13(2):91-97.
12 Hansebout RR, Kuchner EF, Romero-Sierra C. Effects of local hypothermia and of steroids upon recovery from experimental spinal cord compression injury. Surg Neurol. 1975;4(6):531-536.
13 Lewin MG, Hansebout RR, Pappius HM. Chemical characteristics of traumatic spinal cord edema in cats. Effects of steroids on potassium depletion. J Neurosurg. 1974;40(1):65-75.
14 Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251(1):45-52.
15 Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
16 Shapiro SA. Methylprednisolone for spinal cord injury. J Neurosurg. 1992;77(2):325-327.
17 Nesathurai S. Steroids and spinal cord injury: Revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45(6):1088-1093.
18 Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597-1604.
19 Hurlbert RJ. Strategies of medical intervention in the management of acute spinal cord injury. Spine (Phila Pa 1976). 2006;31(11 Suppl):S16-S21. discussion S36 15
20 Hadley MN, Walters BC, Grabb PA, et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg. 2002;49:407-498.
21 Rodden FA, Wiegandt H, Bauer BL. Gangliosides: The relevance of current research to neurosurgery. J Neurosurg. 1991;74(4):606-619.
22 Commissiong JW, Toffano G. The effect of GM1 ganglioside on coerulospinal, noradrenergic, adult neurons and on fetal monoaminergic neurons transplanted into the transected spinal cord of the adult rat. Brain Res. 1986;380(2):205-215.
23 Geisler FH, Dorsey FC, Coleman WP. Correction: recovery of motor function after spinal-cord injury–a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med. 1991 Dec 5;325(23):1659-1660.
24 Geisler FH, Coleman WP, Grieco G, et al. The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976). 2001;26(24 Suppl):S87-S98.
25 Management of acute spinal cord injuries in an intensive care unit or other monitored setting. [No Authors Listed]. Neurosurgery. 2002;50(3 Suppl):S51-S57.
26 Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
27 Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
28 Wilson JT, Rogers FB, Wald SL, et al. Prophylactic vena cava filter insertion in patients with traumatic spinal cord injury: preliminary results. Neurosurgery. 1994;35(2):234-239. discussion 1994;35(2):239
29 Gschaedler R, Dollfus P, Molé JP, et al. Reflections on the intensive care of acute cervical spinal cord injuries in a general traumatology centre. Paraplegia. 1979;17(1):58-61.
30 Hachen HJ. Idealized care of the acutely injured spinal cord in Switzerland. J Trauma. 1977;17(12):931-936.
31 Levi L, Wolf A, Belzberg H. Hemodynamic parameters in patients with acute cervical cord trauma: description, intervention, and prediction of outcome. Neurosurgery. 1993;33(6):1007-1016. discussion 1993;33(6):1016-17
32 Reines HD, Harris RC. Pulmonary complications of acute spinal cord injuries. Neurosurgery. 1987;21(2):193-196.
33 Tator CH. Vascular effects and blood flow in acute spinal cord injuries. J Neurosurg Sci. 1984;28(3-4):115-119.
34 Tator CH, Rowed DW, Schwartz ML, et al. Management of acute spinal cord injuries. Can J Surg. 1984;27(3):289-293. 296
35 Vale FL, Burns J, Jackson AB, et al. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87(2):239-246.
36 Zach GA, Seiler W, Dollfus P. Treatment results of spinal cord injuries in the Swiss Paraplegic Centre of Basle. Paraplegia. 1976;14(1):58-65.
37 Silver NG, Gibbon NO. Prognosis in tetraplegia. Br Med J. 1968;4(5623):79-83.
38 Ledsome JR, Sharp JM. Pulmonary function in acute cervical cord injury. Am Rev Respir Dis. 1981;124(1):41-44.
39 Lu K, Lee TC, Liang CL, et al. Delayed apnea in patients with mid- to lower cervical spinal cord injury. Spine (Phila Pa 1976). 2000;25(11):1332-1338.
40 Mansel JK, Norman JR. Respiratory complications and management of spinal cord injuries. Chest. 1990;97(6):1446-1452.
41 McMichan JC, Michel L, Westbrook PR. Pulmonary dysfunction following traumatic quadriplegia: Recognition, prevention, and treatment. JAMA. 1980;243(6):528-531.
42 Green BA, Green KL, Klose KJ. Kinetic therapy for spinal cord injury. Spine (Phila Pa 1976). 1983;8(7):722-728.
43 Northrup BE, Vaccaro AR, Rosen JE, et al. Occurrence of infection in anterior cervical fusion for spinal cord injury after tracheostomy. Spine (Phila Pa 1976). 1995;20(22):2449-2453.
44 Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44(5):1027-1039. discussion 1999;44(5):1039-40
45 Tator CH. Ischemia as a secondary neural injury. In: Salzman SK, editor. Neurobiology of Central Nervous System Trauma. New York: Oxford University Press; 1994:209-215.
46 Tator CH. Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med. 1996;19(4):206-214.
47 Piepmeier JM, Lehmann KB, Lane JG. Cardiovascular instability following acute cervical spinal cord trauma. Cent Nerv Syst Trauma. 1985;2(3):153-160.
48 Lehmann KG, Lane JG, Piepmeier JM, et al. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J Am Coll Cardiol. 1987;10(1):46-52.
49 Deep venous thrombosis and thromboembolism in patients with cervical spinal cord injuries. Neurosurgery. 2002;50(3 Suppl):S73-S80.
50 Prevention of thromboembolism in spinal cord injury. Consortium for Spinal Cord Medicine. [No Authors Listed]. J Spinal Cord Med. 1997;20(3):259-283.
51 Jarrell BE, Posuniak E, Roberts J, et al. A new method of management using the Kim-Ray Greenfield filter for deep venous thrombosis and pulmonary embolism in spinal cord injury. Surg Gynecol Obstet. 1983;157(4):316-320.
52 Khansarinia S, Dennis JW, Veldenz HC, et al. Prophylactic Greenfield filter placement in selected high-risk trauma patients. J Vasc Surg. 1995;22(3):231-235. discussion 1995;22(3):235-36
53 Quirke TE, Ritota PC, Swan KG. Inferior vena caval filter use in U.S. trauma centers: a practitioner survey. J Trauma. 1997;43(2):333-337.
54 Levi L, Wolf A, Rigamonti D, et al. Anterior decompression in cervical spine trauma: does the timing of surgery affect the outcome? Neurosurgery. 1991;29(2):216-222.
55 Heiden JS, Weiss MH, Rosenberg AW, et al. Management of cervical spinal cord trauma in Southern California. J Neurosurg. 1975;43(6):732-736.
56 Tator CH. Spine-spinal cord relationships in spinal cord trauma. Clin Neurosurg. 1983;30:479-494.
57 Cifu DX, Seel RT, Kreutzer JS, et al. A multicenter investigation of age-related differences in lengths of stay, hospitalization charges, and outcomes for a matched tetraplegia sample. Arch Phys Med Rehabil. 1999;80(7):733-740.
58 Prusmack C, Rochman AS, Levi AD. The effect of age on survival following traumatic spinal cord injury. Top Spinal Cord Inj Rehabil. 2006;12(1):49-57.
59 Inoue T, Kawaguchi S, Kurisu K. Spontaneous regeneration of the pyramidal tract after transection in young rats. Neurosci Lett. 1998;247(2-3):151-154.
60 Wang D, Bodley R, Sett P, et al. A clinical magnetic resonance imaging study of the traumatised spinal cord more than 20 years following injury. Paraplegia. 1996;34(2):65-81.
61 Schaefer DM, Flanders AE, Osterholm JL, et al. Prognostic significance of magnetic resonance imaging in the acute phase of cervical spine injury. J Neurosurg. 1992;76(2):218-223.
62 Kulkarni MV, McArdle CB, Kopanicky D, et al. Acute spinal cord injury: MR imaging at 1.5 T. Radiology. 1987;164(3):837-843.
63 Bethea JR, Castro M, Keane RW, et al. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998;18(9):3251-3260.
64 Emery E, Aldana P, Bunge MB, et al. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89(6):911-920.