CHAPTER 43 Spinal cord and spinal nerves: gross anatomy
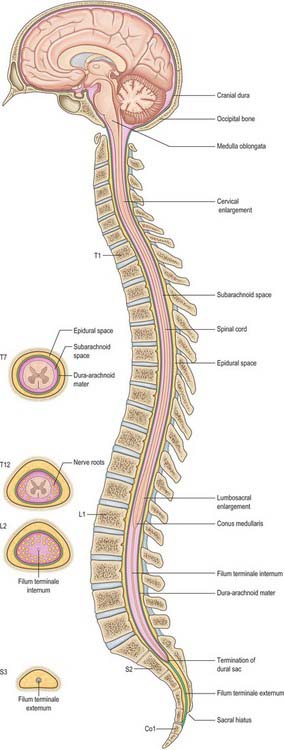
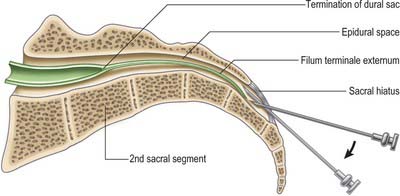
This chapter deals with the gross anatomy of the structures which lie within the vertebral canal and its extensions through the intervertebral foramina, the spinal nerve or radicular (‘root’) canals. The spinal cord, its blood vessels and nerve roots lie within a meningeal sheath, the theca, which occupies the central zone of the vertebral canal and extends from the foramen magnum, where it is in continuity with the meningeal coverings of the brain, to the level of the second sacral vertebra in the adult. Distal to this level the dura extends as a fine cord, the filum terminale externum, which fuses with the posterior periosteum of the first coccygeal segment. Tubular prolongations of the dural sheath extend around the spinal roots and nerves into the lateral zones of the vertebral canal and out into the root canals, eventually fusing with the epineurium of the spinal nerves. Between the theca and the walls of the vertebral canal is the epidural (spinal extradural) space, which is loosely filled with fat, connective tissue containing small arteries and lymphatics, and an important venous plexus. Three-dimensional appreciation of the anatomy of the spinal theca and its surroundings is essential for the efficient management of spinal pain and of spinal injuries, tumours and infections. Equally significant clinically is the anatomy of the often precarious blood supply of the spinal cord and its associated structures. The increasing application and refinement of diagnostic imaging and endoscopic procedures lend a new importance to topographical detail here.
SPINAL CORD (MEDULLA)
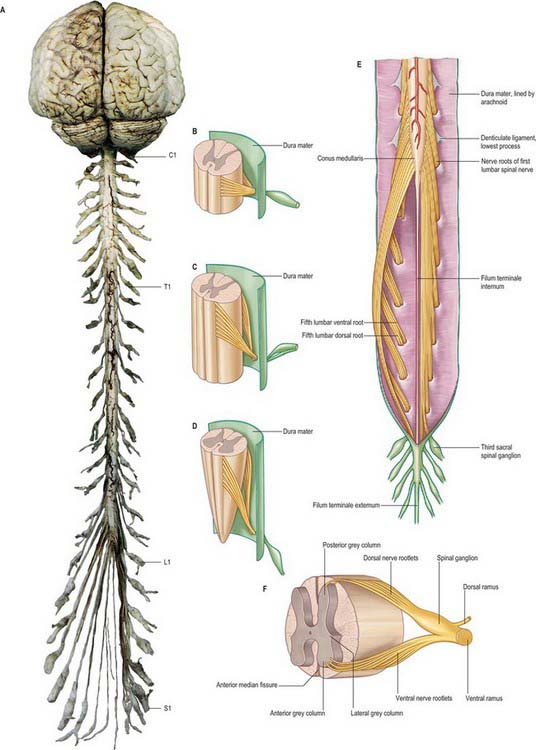
The spinal cord occupies the superior two-thirds of the vertebral canal (Fig. 18.1, Fig. 43.1). It is continuous cranially with the medulla oblongata, and narrows caudally to the conus medullaris, from whose apex a connective tissue filament, the filum terminale, descends to the dorsum of the first coccygeal vertebral segment. The cord extends from the upper border of the atlas to the junction between the first and second lumbar vertebrae: its average length in European males is 45 cm, its weight approximately 30 g. (For dimensional data consult Barson & Sands 1977.)
During development, the vertebral column elongates more rapidly than the spinal cord, so that there is an increasing discrepancy between the anatomical level of spinal cord segments and their corresponding vertebrae. At stage 23, the vertebral column and spinal cord are the same length, and the cord ends at the last coccygeal vertebra: this arrangement continues until the third fetal month. At birth, the spinal cord terminates at the lower border of the second lumbar vertebra, and may sometimes reach the third lumbar vertebra. In the adult, the spinal cord is said to terminate at the level of the disc between the first and second lumbar vertebral bodies, which lies a little above the level of the elbow joint when the arm is by the side, and also lies approximately in the transpyloric plane (p. 1054). However, there is considerable variation in the level at which the spinal cord ends. It may end below this level in as many as 40% of subjects, or opposite the body of either the first or second lumbar vertebra: very occasionally it ends as high as the caudal third of twelfth thoracic or as low as the disc between the second and third lumbar vertebrae. Its position rises slightly in vertebral flexion, and there is some correlation with the length of the trunk, especially in females. The spinal cord varies in transverse width, gradually tapering craniocaudally, except at the levels of the enlargements. It is not cylindrical, being wider transversely at all levels, especially in the cervical segments.
A posterolateral sulcus exists from 1.5 to 2.5 mm lateral to each side of the posterior median sulcus. Dorsal roots (strictly rootlets) of spinal nerves enter the cord along the sulcus. The white substance between the posterior median and posterolateral sulcus on each side is the posterior funiculus. In cervical and upper thoracic segments a longitudinal posterointermediate sulcus marks a septum dividing each posterior funiculus into two large tracts: the fasciculus gracilis (medial) and fasciculus cuneatus (lateral). Between the posterolateral sulcus and anterior median fissure is the anterolateral funiculus. This is subdivided into anterior and lateral funiculi by ventral spinal rootlets which pass through its substance to issue from the surface of the cord. The anterior funiculus is medial to, and includes, the emerging ventral rootlets, whilst the lateral funiculus lies between the roots and the posterolateral sulcus. In upper cervical segments, nerve rootlets emerge through each lateral funiculus to form the spinal accessory nerve which ascends in the vertebral canal lateral to the spinal cord and enters the posterior cranial fossa via the foramen magnum (Fig. 28.11).
DORSAL AND VENTRAL ROOTS
The paired dorsal and ventral roots of the spinal nerves are continuous with the spinal cord (Fig. 43.1F; see also p. 754). They cross the subarachnoid space and traverse the dura mater separately, uniting in or close to their intervertebral foramina to form the (mixed) spinal nerves. Since the spinal cord is shorter than the vertebral column, the more caudal spinal roots descend for varying distances around and beyond the cord to reach their corresponding foramina. In so doing they form a divergent sheaf of spinal nerve roots, the cauda equina, which is gathered round the filum terminale in the spinal theca, mostly distal to the apex of the cord.
Ventral spinal roots contain efferent somatic and, at some levels, preganglionic sympathetic, axons which extend from neuronal cell bodies in the ventral horns and intermediolateral columns respectively. There are also afferent nerve fibres in these roots. The rootlets comprising each ventral root emerge from the anterolateral sulcus in groups over an elongated vertical elliptical area (Fig. 43.1F). Dorsal spinal roots bear ovoid swellings, the spinal ganglia, one on each root proximal to its junction with a corresponding ventral root in an intervertebral foramen. Each root fans out into six to eight rootlets before entering the cord in a vertical row in the posterolateral sulcus. Dorsal roots are usually said to contain only afferent axons (both somatic and visceral) which are the central processes of unipolar neurones in the spinal root ganglia, but they may also contain a small number (3%) of efferent fibres and autonomic vasodilator fibres.
MENINGES
DURA MATER
Epidural space
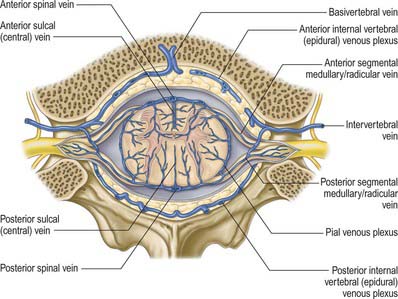
The epidural space lies between the spinal dura mater and the tissues which line the vertebral canal (Fig. 43.2). It is closed above by fusion of the spinal dura with the edge of the foramen magnum, and below by the posterior sacrococcygeal ligament which closes the sacral hiatus. It contains loosely packed connective tissue, fat, a venous plexus, small arterial branches, lymphatics and fine fibrous bands which connect the theca with the lining tissue of the vertebral canal. These bands, the meningovertebral ligaments, are best developed anteriorly and laterally. Similar bands tether the nerve root sheaths or ‘sleeves’ within their canals. There is also a midline attachment from the posterior spinal dura to the ligamentum nuchae at atlanto-occipital and atlanto-axial levels (Dean & Mitchell 2002). The venous plexus consists of longitudinally arranged chains of vessels, connected by circumdural venous ‘rings’. The anteriorly placed vessels receive the basivertebral veins.
Epidural injections
Contrast media and other fluids injected into the epidural space at the sacral level can spread up to the cranial base (p. 760). Local anaesthetics injected near the spinal nerves, just outside the intervertebral foramina, may spread up or down the epidural space to affect the adjacent spinal nerves or may pass to the opposite side. The paravertebral spaces of each side communicate via the epidural space, particularly at lumbar levels.
For a review of the morphology of the epidural space and a discussion of the nature of the lining layer of the vertebral canal, see Newell (1999).
Subdural space
The subdural space is a potential space in the normal spine because the arachnoid and dura are closely apposed (Haines et al 1993). It does not connect with the subarachnoid space, but continues for a short distance along the cranial and spinal nerves. Accidental subdural catheterization may occur during epidural injections. Injection of fluid into the subdural space may either damage the cord by direct toxic effects or by compression of the vasculature.
ARACHNOID MATER
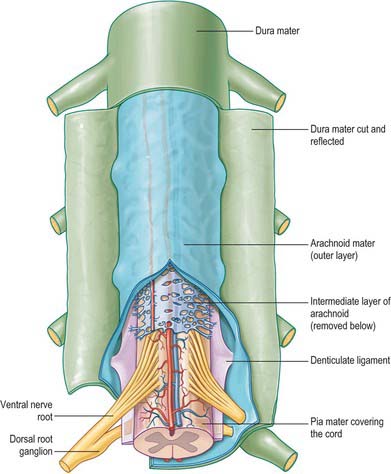
The spinal arachnoid mater, which surrounds the spinal cord, is continuous with the cranial arachnoid mater (Fig. 43.3). It is closely applied to the deep aspect of the dura mater. At sites where vessels and nerves enter or leave the subarachnoid space, the arachnoid mater is reflected on to the surface of these structures and forms a thin coating of leptomeningeal cells over the surface of both vessels and nerves. Thus a subarachnoid angle is formed as nerves pass through the dura into the intervertebral foramina. At this point, the layers of leptomeninges (arachnoid and pia) fuse and become continuous with the perineurium. The epineurium is in continuity with the dura. Such an arrangement seals the subarachnoid space so that particulate matter does not pass directly from the subarachnoid space into nerves. The existence of a pathway of lymphatic drainage from the CSF is controversial.
PIA MATER
The spinal pia mater (Fig. 43.3) closely invests the surface of the spinal cord and passes into the anterior median fissure. As in the cranial region, there is a subpial ‘space’, however over the surface of the spinal cord the subpial collagenous layer is thicker than in the cerebral region, and it is continuous with the collagenous core of the ligamentum denticulatum.
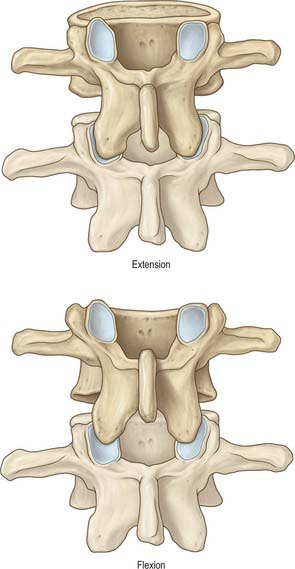
The ligamentum denticulatum is a flat, fibrous sheet which lies on each side of the spinal cord between the ventral and dorsal spinal roots. Its medial border is continuous with the subpial connective tissue of the cord and its lateral border forms a series of triangular processes, the apices of which are fixed at intervals to the dura mater. There are usually 21 processes on each side. The first crosses behind the vertebral artery where it is attached to the dura mater, and is separated by the artery from the first cervical ventral root. Its site of attachment to the dura mater is above the rim of the foramen magnum, just behind the hypoglossal nerve: the spinal accessory nerve ascends on its posterior aspect (Fig. 28.11). The last of the dentate ligaments lies between the exiting twelfth thoracic and first lumbar spinal nerves and is a narrow, oblique band which descends laterally from the conus medullaris. Changes in the form and position of the dentate ligaments during spinal movements have been demonstrated by cine-radiography.
Beyond the conus medullaris, the pia mater continues as a coating of the filum terminale.
COVERINGS AND RELATIONS OF THE SPINAL ROOTS AND NERVES IN THE RADICULAR CANAL
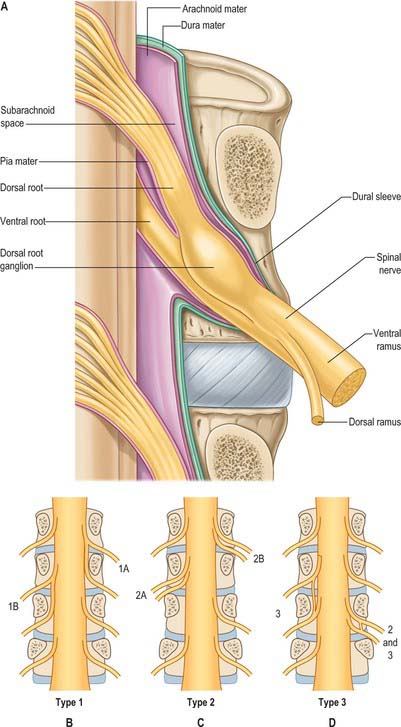
Tubular prolongations of the spinal dura mater, closely lined by the arachnoid, extend around the spinal roots and nerves as they pass through the lateral zone of the vertebral canal and through the intervertebral foramina (Fig. 43.3, Fig. 43.4A). These prolongations, the spinal nerve sheaths or root sheaths, gradually lengthen as the spinal roots become increasingly oblique. Each individual dorsal and ventral root runs in the subarachnoid space with its own covering of pia mater. Each root pierces the dura separately, taking a sleeve of arachnoid with it, before joining within the dural prolongation just distal to the spinal ganglion. The dural sheaths of the spinal nerves fuse with the epineurium, within or slightly beyond the intervertebral foramina. The arachnoid prolongations within the sheaths do not extend as far distally as their dural coverings, but the subarachnoid space and its contained CSF extend sufficiently distally to form a radiologically demonstrable root sleeve for each nerve. Shortening or obstruction of this sleeve seen on MRI indicates compression of the spinal nerve. At the cervical level, where the nerves are short and the vertebral movement is greatest, the dural sheaths are tethered to the periosteum of the adjacent transverse processes. In the lumbosacral region there is less tethering of the dura to the periosteum, though there may be an attachment posteriorly to the facet joint capsule.
CEREBROSPINAL FLUID (CSF)
The cerebrospinal fluid is described in detail on page 242. Although there is free communication between the spinal and cerebral subarachnoid spaces, the mode of circulation of the spinal CSF and the contribution that it makes to the overall circulation of CSF remains uncertain in man: CSF may be absorbed from the spinal subarachnoid space, and spinal arachnoid granulations and villi have been described (Kiddo et al 1976).
SPINAL NERVES
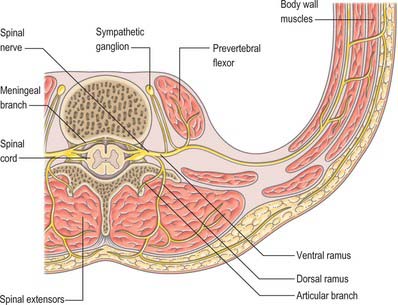
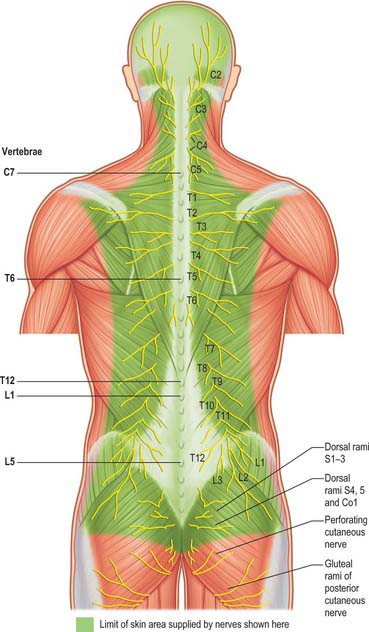
In those body segments which largely retain a metameric (segmental) structure, e.g. the thoracic region, spinal nerves show a common plan (Fig. 43.5). The dorsal, epaxial, ramus passes back lateral to the articular processes of the vertebrae and divides into medial and lateral branches which penetrate the deeper muscles of the back: both branches innervate the adjacent muscles and supply a band of skin from the posterior median line to the lateral border of the scapula (Fig. 43.6). The ventral, hypaxial, ramus is connected to a corresponding sympathetic ganglion by white and grey rami communicantes. It innervates the prevertebral muscles and curves round in the body wall to supply the lateral muscles of the trunk. Near the midaxillary line it gives off a lateral branch which pierces the muscles and divides into anterior and posterior cutaneous branches. The main nerve advances in the body wall, where it supplies the ventral muscles and terminates in branches to the skin.
Spinal nerves are united ventral and dorsal spinal roots, attached in series to the sides of the spinal cord. The term spinal nerve strictly applies only to the short segment after union of the roots and before branching occurs. This segment, the spinal nerve proper, lies in the intervertebral foramen: it is sometimes mistakenly called the ‘nerve root’. There are 31 pairs of spinal nerves: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, 1 coccygeal. The abbreviations C, T, L, S and Co, with appropriate numerals, are commonly applied to individual nerves. The peripheral nerves emerge through the intervertebral foramina. At thoracic, lumbar, sacral and coccygeal levels the numbered nerve exits the vertebral canal by passing below the pedicle of the corresponding vertebra, e.g. L4 nerve exits the intervertebral foramen between L4 and L5. However, in the cervical region, nerves C1–7 pass above their corresponding vertebrae. C1 leaves the vertebral canal between the occipital bone and atlas and hence is often termed the suboccipital nerve. The last pair of cervical nerves does not have a correspondingly numbered vertebra and C8 passes between the seventh cervical and first thoracic vertebrae. Each nerve is continuous with the spinal cord by ventral and dorsal roots; the latter each bears a spinal ganglion (‘dorsal root ganglion’).
SPINAL ROOTS AND GANGLIA
Dorsal (posterior) roots
Little is known of the detail of the regions of entry and emergence of afferent and efferent rootlets in humans, but these zones of transition between the central and peripheral nervous systems have been extensively described in rodents (Fraher 2000) (see also p. 63).
Appearance and orientation of roots at each spinal level
The size and direction of spinal nerve roots vary. The upper four cervical roots are small, the lower four are large. Cervical dorsal roots have a thickness ratio to the ventral roots of 3 : 1, which is greater than in other regions. The first dorsal root is an exception, being smaller than the ventral and it is occasionally absent. The conventional view is that the first and second cervical spinal roots are short, running almost horizontally to their exits from the vertebral canal, and that from the third to the eighth cervical levels the roots slope obliquely down. Obliquity and length increase successively, although the distance between spinal attachment and vertebral exit never exceeds the height of one vertebra. An alternative view (Kubik & Müntener 1969) states that upper cervical roots descend, the fifth is horizontal, the sixth to eighth ascend, the first two thoracic roots are horizontal, the next three ascend, the sixth is horizontal and the rest descend. This view is based on the observation that the cervicothoracic part of the spinal cord grows more in length than other parts.
Lower lumbar and upper sacral roots are the largest, and their rootlets are the most numerous. Coccygeal roots are the smallest. Kubik & Müntener (1969) confirm that lumbar, sacral and coccygeal roots descend with increasing obliquity to their exits. The spinal cord ends near the lower border of the first lumbar vertebra, and so the lengths of successive roots rapidly increase: the consequent collection of roots is the cauda equina (Fig. 43.1A). The largest roots, and hence the largest spinal nerves, are continuous with the spinal cervical and lumbar enlargements and innervate the upper and lower limbs.
Spinal ganglia (dorsal root ganglia)
Spinal ganglia are large groups of neurones on the dorsal spinal roots. Each is oval and reddish; its size is related to that of its root. A ganglion is bifid medially where the two fascicles of the dorsal root emerge to enter the cord. Ganglia are usually sited in the intervertebral foramina, immediately lateral to the perforation of the dura mater by the roots (Fig. 43.1B). However, the first cervical ganglion lies on the vertebral arch of the atlas, the second lies behind the lateral atlantoaxial joint, the sacral lie inside the vertebral canal, and the coccygeal ganglion usually lies within the dura mater. The first cervical ganglia may be absent. Small aberrant ganglia sometimes occur on the upper cervical dorsal roots between the spinal ganglia and the cord.
SPINAL NERVES PROPER
Immediately distal to the spinal ganglia, ventral and dorsal roots unite to form spinal nerves (see Fig. 43.5, Fig. 15.15). These very soon divide into dorsal and ventral rami, both of which receive fibres from both roots. At all levels above the sacral, this division occurs within the intervertebral foramen. Division of the sacral spinal nerves occurs within the sacral vertebral canal, and the dorsal and ventral rami exit separately through posterior and anterior sacral foramina at each level. Spinal nerves trifurcate at some cervical and thoracic levels, in which case the third branch is called a ramus intermedius. At or distal to its origin each ventral ramus gives off recurrent meningeal (sinuvertebral) branches and receives a grey ramus communicans from the corresponding sympathetic ganglion. The thoracic and first and second lumbar ventral rami each contributes a white ramus communicans to the corresponding sympathetic ganglia. The second, third and fourth sacral nerves also supply visceral branches, unconnected with sympathetic ganglia, which carry a parasympathetic outflow direct to the pelvic plexuses.
Meningeal nerves
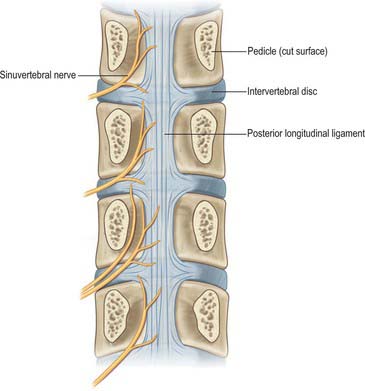
Recurrent meningeal (or sinuvertebral) nerves (Fig. 43.7) occur at all vertebral levels. They are mixed sensory and sympathetic nerves, represented by numerous fine filaments amongst which one, or two to four, larger trunks may be evident. At cervical levels the autonomic roots arise from the grey rami that form the vertebral nerve (p. 461). At thoracic and lumbar levels, each nerve is formed by a somatic root from the ventral ramus and by an autonomic root from the grey ramus communicans of that segment. Each nerve pursues a recurrent course through the intervertebral foramen, passing ventral to the spinal nerve, to enter the vertebral canal, where it divides into ascending, descending, and transverse branches. These branches communicate with corresponding branches from the segments above and below, and from the opposite side, forming arcades along the floor of the vertebral canal. Meningeal branches of the arcades form a plexus on the ventral surface of the dural sac and nerve root sleeves which attenuates laterally; the posterior paramedian dura is devoid of nerve endings. Skeletal branches are distributed to the posterior longitudinal ligament, the periosteum of the vertebral bodies, and to the posterior and posterolateral aspects of the intervertebral discs. Vascular branches accompany the veins and arteries of the vertebral canal and those of the vertebral bodies. The upper three cervical meningeal nerves ascend through the foramen magnum into the posterior cranial fossa, where they innervate the dura mater that covers the clivus. En route, they innervate the median atlanto-axial joint and its ligaments.
VARIATIONS OF SPINAL ROOTS AND NERVES
The courses of spinal roots and nerves in relation to the thecal sac and vertebral and radicular canals may be aberrant. An individual intervertebral foramen may contain a duplicated sheath, nerve and roots, which will then be absent at an adjacent level. Abnormal communications between roots may occur within the vertebral canal. These anomalies have been described and classified for the lumbosacral spine by Neidre & Macnab (1983) (Fig. 43.4).
RAMI OF THE SPINAL NERVES
Dorsal (posterior primary) rami of spinal nerves are usually smaller than the ventral rami and are directed posteriorly. Retaining a segmental distribution, all, except for the first cervical, fourth and fifth sacral and the coccygeal, divide into medial and lateral branches which supply the muscles and skin of the posterior regions of the neck and trunk (Fig. 43.6).
Cervical dorsal spinal rami
First cervical dorsal ramus (suboccipital nerve)
The first cervical dorsal ramus, the suboccipital nerve, is larger than the ventral (Fig. 42.59). It emerges superior to the posterior arch of the atlas and inferior to the vertebral artery and enters the suboccipital triangle to supply rectus capitis posterior major and minor, obliquus capitis superior and inferior, and semispinalis capitis. A filament from the branch to the inferior oblique joins the second dorsal ramus. The suboccipital nerve occasionally has a cutaneous branch which accompanies the occipital artery to the scalp, and connects with the greater and lesser occipital nerves. It may also communicate with the accessory nerve.
Second cervical dorsal ramus
The second cervical dorsal ramus is slightly larger than the ventral and all the other cervical dorsal rami (Fig. 42.59, Fig. 29.15). It runs between the lamina of the axis and the inferior oblique, below which it divides into a large medial and smaller lateral branch. The dorsal ramus or its medial branch receives communicating branches from the first cervical dorsal ramus which pass both through and around inferior oblique. A descending communicating branch crosses the C2–3 facet joint to reach the third cervical dorsal ramus.
Third cervical dorsal ramus
The third cervical dorsal ramus is intermediate in size between the second and fourth. It courses back round the articular pillar of the third cervical vertebra, medial to the posterior intertransverse muscle, and divides into medial and lateral branches. The lateral branch passes dorsally across the surface of semispinalis capitis to supply it and the overlying longissimus capitis and splenius capitis. It receives a communicating branch from the lateral branch of the second cervical dorsal ramus, and sends a similar branch to the lateral branch of the fourth cervical dorsal ramus. The deep medial branch curves dorsally and medially around the waist of the articular pillar before ending in multifidus. It sends an articular branch to the C3–4 facet joint, and may also send a branch into semispinalis capitis. The superficial medial branch, the third occipital nerve, curves around the lateral and dorsal surfaces of the C2–3 facet joint, which it supplies. It continues transversely, deep to semispinalis, which it supplies, and sends a communicating branch to the greater occipital nerve. Just above the second cervical spinal process, the third occipital nerve turns dorsally to pierce semispinalis capitis, splenius capitis and trapezius, and becomes cutaneous over a small area immediately below the superior nuchal line. Communicating branches join the cutaneous branches of the greater and lesser occipital nerves.
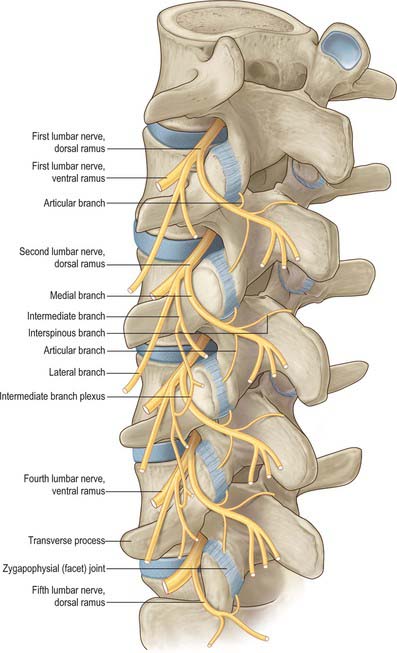
Lumbar dorsal spinal rami
After leaving its spinal nerve, each of the first to fourth lumbar dorsal rami passes through an aperture in the dorsal leaf of the intertransverse ligament just above a transverse process (Fig. 43.8). Each dorsal ramus supplies the overlying medial intertransverse muscle before dividing into lateral and medial branches. The lateral branches supply the longissimus and iliocostalis components of the lumbar erector spine. The upper three lumbar lateral branches emerge from the lateral border of iliocostalis lumborum and pierce the aponeurosis of latissimus dorsi to become cutaneous, They cross the iliac crest to reach the gluteal skin, some reach as far as the level of the greater trochanter. Medial branches cross the junction of the superior articular process and transverse process and hook medially between the mammillary and accessory processes, deep to the mammillo-accessory ligament. Medial to the ligament, each medial branch sends articular branches to the facet joints above and below its course, before entering multifidus, which it supplies. Terminal branches reach interspinalis at each segment.
VASCULAR SUPPLY OF SPINAL CORD, ROOTS AND NERVES
ARTERIES
The spinal cord, its roots and nerves are supplied with blood by both longitudinal and segmental vessels (Fig. 43.9). Three major longitudinal vessels, a single anterior and two posterior spinal arteries (each of which is sometimes doubled to pass on either side of the dorsal rootlets), originate intracranially from the vertebral artery and terminate in a plexus around the conus medullaris. The anterior spinal artery forms from the fused anterior spinal branches of the vertebral artery, and descends in the anterior median fissure of the cord. Each posterior spinal artery originates either directly from the ipsilateral vertebral artery or from its posterior inferior cerebellar branch, and descends in a posterolateral sulcus of the cord. The segmental arteries are derived in craniocaudal sequence from spinal branches of the vertebral, deep cervical, intercostal and lumbar arteries. These vessels enter the vertebral canal through the intervertebral foramina and anastomose with branches of the longitudinal vessels to form a pial plexus on the surface of the cord. The segmental spinal arteries send anterior and posterior radicular branches to the spinal cord along the ventral and dorsal roots. Most anterior radicular arteries are small, and end in the ventral nerve roots or in the pial plexus of the cord. The small posterior radicular arteries also supply the dorsal root ganglia: branches enter at both ganglionic poles to be distributed around ganglion cells and nerve fibres. (See also Crock 1996.)
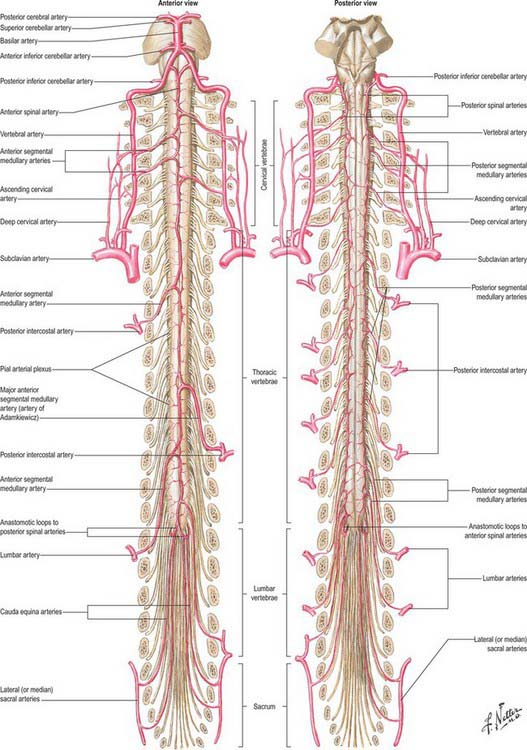
Fig. 43.9 Arteries of the spinal cord.
(Reprinted from Netter Anatomy Illustration Collection, © Elsevier Inc. All Rights Reserved.)
Segmental medullary feeder arteries
Some radicular arteries, mainly situated in the lower cervical, lower thoracic and upper lumbar regions, are large enough to reach the anterior median sulcus where they divide into slender ascending and large descending branches. These are the anterior medullary feeder arteries (Dommisse 1975). They anastomose with the anterior spinal arteries to form a single or partly double longitudinal vessel of uneven calibre along the anterior median sulcus. The largest anterior medullary feeder, the great anterior segmental medullary artery of Adamkiewicz, varies in level, arising from a spinal branch of either one of the lower posterior intercostal arteries (T9–11), or of the subcostal artery (T12), or less frequently of the upper lumbar arteries (L1 and L2). It most often arises on the left side (Carmichael & Gloviczki 1999). Reaching the spinal cord, it sends a branch to the anterior spinal artery below and another to anastomose with the ramus of the posterior spinal artery which lies anterior to the dorsal roots. It may be the main supply to the lower two-thirds of the cord. Central branches of the anterior spinal artery enter the anterior median fissure, and then turn right or left to supply the ventral grey column, the base of the dorsal grey column, including the dorsal nucleus, and the adjacent white matter (Fig. 43.9).
Intramedullary arteries
The central branches of the anterior spinal artery supply about two-thirds of the cross-sectional area of the cord. The rest of the dorsal grey and white columns and peripheral parts of the lateral and ventral white columns are supplied by numerous small radial vessels which branch from posterior spinal arteries and the pial plexus. In a microangiographic study of the human cervical spinal cord, up to six anterior, and eight posterior, radicular spinal arteries were described, and up to eight central branches arose from each centimetre of the anterior spinal artery (Turnbull et al 1966).
VEINS
The venous drainage of the spinal cord (Fig. 43.11) follows a similar pattern to that of its arterial supply (Gillilan 1970). Intramedullary veins within the substance of the cord drain into a plexus of surface veins, the coronal plexus. There are six tortuous longitudinal channels within this plexus, one in each of the anterior and posterior median fissures, and four others which run on either side of the ventral and dorsal nerve roots. Only the anterior median vein, which drains the central grey matter, is consistently complete. These vessels connect freely and drain superiorly into the cerebellar veins and cranial sinuses, and segmentally mainly into medullary veins. The segmental veins drain into the intervertebral veins and thence into the external vertebral venous plexuses, the caval and azygos systems.
THE EFFECTS OF INJURY
SPINAL CORD INJURY AND VERTEBRAL COLUMN INJURY
In estimating the vertebral levels of cord segments in the adult, a useful approximation is that in the cervical region the tip of a vertebral spinous process corresponds to the succeeding cord segment (i.e. the sixth cervical spine is opposite the seventh spinal segment); at upper thoracic levels the tip of a vertebral spine corresponds to the cord two segments lower (i.e. the fourth spine is level with the sixth segment), and in the lower thoracic region there is a difference of three segments (i.e. the tenth thoracic spine is level with the first lumbar segment). The eleventh thoracic spine overlies the third lumbar segment and the twelfth is opposite the first sacral segment. In making this estimate by palpation of the vertebral spines, the relationship of the individual spines to their vertebral bodies should be remembered (p. 745).
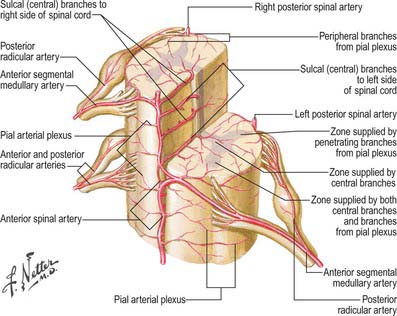
Fig. 43.10 Arterial disposition within the spinal cord.
(Reprinted from Netter Anatomy Illustration Collection, © Elsevier Inc. All Rights Reserved.)
Complete division above the fourth cervical segment causes respiratory failure because of the loss of activity in the phrenic and intercostal nerves. Lesions between C5 and T1 paralyse all four limbs (quadriplegia), the effects in the upper limbs varying with the site of injury: at the fifth cervical segment paralysis is complete; at the sixth, each arm is positioned in abduction and lateral rotation, with the elbow flexed and the forearm supinated, due to unopposed activity in the deltoid, supraspinatus, rhomboid and brachial flexors (all supplied by the fifth cervical spinal nerves). In lower cervical lesions upper limb paralysis is less marked. Lesions of the first thoracic segment paralyse small muscles in the hand and damage the sympathetic outflow to the head and neck, resulting in contraction of the pupil, recession of the eyeball, narrowing of the palpebral fissure and loss of sweating in the face and neck (Horner’s syndrome). However, sensation is retained in areas innervated by segments above the lesion, thus cutaneous sensation is retained in the neck and chest down to the second intercostal space, because this area is innervated by the supraclavicular nerves (C3 and C4). At thoracic levels, division of the cord paralyses the trunk below the segmental level of the lesion, and both lower limbs (paraplegia). The first sacral neural segment is approximately level with the thoracolumbar vertebral junction: injury, which commonly occurs here, paralyses the urinary bladder, the rectum and muscles supplied by the sacral segments, and cutaneous sensibility is lost in the perineum, buttocks, the back of the thighs and the legs and soles of the feet. The roots of lumbar nerves descending to join the cauda equina may be damaged at this level, causing complete paralysis of both lower limbs. Lesions below the first lumbar vertebra may divide or damage the cauda equina, but severe nerve damage is uncommon and is usually confined to the spinal roots at the level of the trauma. Neurological symptoms may also occur as a result of interference with the spinal blood supply, particularly in the lower thoracic and upper lumbar segments.
LESIONS OF THE CONUS AND CAUDA EQUINA
Lesions of the conus and cauda equina, e.g. tumours, cause bilateral deficit, often with pain in the back extending into the sacral segments and to the legs. Loss of bladder and erectile function can be early features. There are lower motor neurone signs in the legs with fasciculation and muscle atrophy. Sensory loss usually involves the perineal or ‘saddle area’ as well as involving other lumbar and sacral dermatomes. There may be congenital abnormalities, e.g. spina bifida, lipomata or dystematomyelia, and the conus may extend below the lower border of L1, often with a tethered filum terminale. Extramedullary lesions include prolapsed intervertebral discs. A midline (central) disc protrusion in the lumbar region may present with involvement of only the sacral segments.
CLINICAL PROCEDURES
ACCESS TO CEREBROSPINAL FLUID
The safest approach to the cerebrospinal fluid (CSF) is to enter the lumbar cistern of the subarachnoid space in the midline, well below the level at which the spinal cord normally terminates (see above). The fine needle employed is unlikely to damage the mobile nerve roots of the cauda equina. This procedure is called lumbar puncture. It is also possible to access the CSF by midline puncture of the cerebello-medullary cistern (cisterna magna) (Ch. 16): this is cisternal puncture.
Lumbar puncture: adult
Lumbar puncture in the adult may be performed with the patient either sitting or lying on the side on a firm flat surface. In each position, the lumbar spine must be flexed as far as possible, in order to separate the vertebral spines maximally and expose the ligamentum flavum in the interlaminar window (Fig. 43.12). A line is then taken between the highest points of the iliac crests: this line intersects the vertebral column just above the palpable spine of L4. With the spines now identified, the skin is anaesthetized and a needle is inserted between the spines of L3 and L4 (or L4 and L5). Exact identification of the level by palpation is difficult (Broadbent et al 2000). The soft tissues which the needle will ultimately traverse should also be anaesthetized, though care should be taken lest the injection of an excessive amount of local anaesthetic compromises appreciation of the structures being traversed. These include the subcutaneous fat, and supraspinous and interspinous ligaments down to the ligamentum flavum itself. The lumbar puncture needle may then be inserted in the midline or just to one side, and angled in the horizontal and sagittal planes sufficiently to pierce the ligamentum flavum in or very near the midline. There is then a slight loss of resistance as the needle enters the epidural space, and careful advancement will next pierce the dura and arachnoid to release CSF.
ACCESS TO THE EPIDURAL SPACE
Caudal epidural
The route of access to the caudal epidural space is via the sacral hiatus. The space is therefore entered below the level of termination of the dural sac, i.e. the subarachnoid space and its contained CSF, which usually extends to the level of the second sacral segment. Occasionally the dural sac ends as high as the fifth lumbar vertebra, and very rarely it may extend to the third part of the sacrum, in which case it is occasionally possible to enter the subarachnoid space inadvertently during the course of a sacral nerve block.
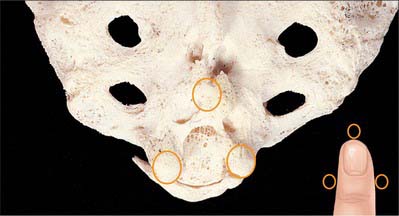
With the patient in the lateral position or lying prone over a pelvic pillow, the sacral hiatus is identified by palpation of the sacral cornua (Fig. 43.13). These are felt at the upper end of the natal cleft approximately 5 cm above the tip of the coccyx. Alternatively, the sacral hiatus may be identified by constructing an equilateral triangle based on a line joining the posterior superior iliac spines: the inferior apex of this triangle overlies the hiatus. After local anaesthetic infiltration, a needle is introduced at 45° to the skin, to penetrate the posterior sacrococcygeal ligament and enter the sacral canal. Once the canal is entered, the hub of the needle is lowered so that the needle may pass along the canal (Fig. 43.2, Fig. 43.14). If the needle is angled too obliquely it will strike bone; if it is placed too superficially it will lie outside the canal. The latter malposition can be confirmed by careful injection of air while palpating the skin over the lower sacrum.
Barson AJ, Sands J. Regional and segmental characteristics of the human adult spinal cord. J Anat. 1977;123:797-803.
Bogduk N. Clinical Anatomy of the L Spine and Sacrum, 4th edn. Edinburgh: Elsevier Churchill Livingstone, 2005.
Broadbent CR, Maxwell WE, Ferrie R, Wilson DJ, Gawne-Cain M, Russell R. Ability of anaesthetists to identify a marked lumbar interspace. Anaesthesia. 2000;55:1122-1126.
Carmichael SW, Gloviczki P. Anatomy of the blood supply to the spinal cord: the artery of Adamkiewicz revisited. Perspect Vasc Surg. 1999;12:113-122.
Crock HV. An Atlas of Vascular Anatomy of the Skeleton and Spinal Cord. London: Martin Dunitz, 1996.
Dean NA, Mitchell BS. Anatomic relation between the nuchal ligament (ligamentum nuchae) and the spinal dura mater in the craniocervical region. Clin Anat. 2002;15:182-185.
Dommisse GF. The Arteries and Veins of the Human Spinal Cord From Birth. Edinburgh: Churchill Livingstone, 1975.
Fraher JP. The transitional zone and CNS regeneration. J Anat. 2000;196:137-158.
Gillilan LA. Veins of the spinal cord. Anatomic details; suggested clinical applications. Neurology. 1970;20:860-868.
Haines DE, Harkey HL, Al-Mefty O. The ‘subdural’ space: a new look at an outdated concept. Neurosurgery. 1993;32:111-120.
Kiddo DK, Gomez DG, Pavese AM, Potts DG. Human spinal arachnoid granulations. Neuroradiol. 1976;11:221-228.
Kubik S, Müntener M. Zur Topographie der spinalen Nervenwurzeln. II Der Einfuss des Wachstums des Duralsackes, sowie der Krümmagen und der Bewegungen der spinalen Nervenwurzeln. Acta Anat. 1969;74:149-168.
Neidre A, Macnab I. Anomalies of the lumbosacral nerve roots. Spine. 1983;8:294-299.
Newell RLM. The spinal epidural space. Clin Anat. 1999;12:375-379.
Turnbull IM, Brieg A, Hassler O. Blood supply of cervical spinal cord in man. A microangiographic cadaver study. J Neurosurg. 1966;24:951-965.